Abstract
CD146 is a cell-surface molecule belonging to the immunoglobulin superfamily and expressed in all types of human endothelial cells. Confocal and electron microscopic analysis of confluent human umbilical vein endothelial cells (HUVECs) were used to demonstrate that CD146 is a component of the endothelial junction. Double immunolabeling with vascular endothelial cadherin showed that CD146 is localized outside the adherens junction. Moreover, CD146 expression is not restricted to the junction, since part of the labeling was detectable at the apical side of the HUVECs. Interestingly, cell-surface expression of CD146 increased when HUVECs reached confluence. In addition, the paracellular permeability of CD146-transfected fibroblast cells was decreased compared with that of control cells. Finally, CD146 colocalized with actin, was partly resistant to Triton X-100 extraction, and had its expression altered by actin-disrupting agents, indicating that CD146 is associated with the actin cytoskeleton. These results show the regulated expression of CD146 at areas of cell-cell junction and strongly suggest involvement of CD146 as a mediator of cell-cell interaction.
Introduction
The vascular endothelium forms a continuous monolayer on the inner surface of the vessel wall and plays a pivotal role in regulating blood flow, vascular permeability, thrombogenesis, and hematogenous metastasis.1 Positioned at the interface between blood and tissues, quiescent endothelial cells (ECs) generate an antithrombotic surface equipped to respond quickly to biologic needs.2 The endothelial monolayer requires highly effective intercellular junctions that control the contact between adjacent cells and the trafficking of circulating blood cells.3,4 At least 2 types of cell-cell junctional structures have been identified in the endothelium: adherens junctions (AJs) and tight junctions (TJs). These play a central part in the control of paracellular permeability and maintenance of cell polarity.5-7 The junctions are tightly regulated structures composed of several adhesion molecules interacting with cytoskeletal proteins. Among the adhesive molecules, the endothelium-specific cadherin 5 or vascular endothelial cadherin (VE-cadherin)8,9 is localized in AJs, whereas the junctional adhesion molecule (JAM)10 was reported to be present in TJs. Other molecules, such as platelet endothelial cell adhesion molecule 1 (PECAM-1)/CD31, are not restricted to one type of junctional structure, and their specific localization appears to be important to their vascular functions.11 12
The S-Endo 1–associated antigen (CD146), also referred to as MelCAM or MUC18,13 is a transmembrane glycoprotein that is constitutively expressed in the whole human endothelium, irrespective of its anatomical site or vessel caliber.14,15 CD146 expression is not restricted to ECs; it has also been observed on several other cell types, including melanoma cells,13smooth muscle cells, and follicular dendritic cells.14Using optical microscopy, we previously showed that, in ECs, CD146 is localized at the intercellular boundaries.16 Although the exact role of CD146 in endothelium is unknown, several findings support the possibility that CD146 acts as an adhesion molecule.17CD146 belongs to the immunoglobulin superfamily18 and shares structural and sequence similarities with a subgroup of adhesion molecules including gicerin,19 HEMCAM,20 and BEN/SC1/DM-GRASP/ALCAM.21,22 In melanoma cells, CD146 was found to be a cell-cell adhesion molecule23 with a possible role in melanoma invasion and metastasis.24 CD146 mediates a cation-independent adhesion between melanoma cells through interaction with an unknown heterophilic ligand.23 25
The specific localization of CD146 and its role in the intercellular junction have not been investigated in ECs. In this study, we found CD146 localized at the cell-cell junction. Because of the regulated expression of CD146 during monolayer formation and its involvement in the control of paracellular permeability, we propose a role for CD146 in the control of the cell-cell contacts.
Materials and methods
Materials
Anti-CD146 murine S-Endo 1 monoclonal antibody (mAb; IgG1, Biocytex, Marseille, France) was described previously.26The other mAbs used were anti–PECAM-1 mAb (mouse IgG1; Novocastra Laboratories, Newcastle, United Kingdom), anti–cadherin-5 mAb (mouse IgG1; Immunotech, Marseille, France), anti–major histocompatibility complex (MHC) class I (mouse IgG1; Immunotech), anti–VE-cadherin polyclonal antibody (D. Gulino, Grenoble, France), antitransferrin receptor (A. Le Bivic, Marseille, France), antiplacental alkaline phosphatase (Dako, Trappes, France), ST4 used as a negative control (mouse IgG1, Biocytex), and IgG1 irrelevant mAb (E. Vivier, Marseille, France). Fluorescein isothiocyanate–conjugated (FITC) F(ab′)2 sheep anti–mouse IgG was from Silenus (Hawthorn, Australia) and horseradish peroxidase anti–mouse IgG was from Jackson Laboratories (Palo Alto, CA). Tetrarhodamine isothiocyanate (TRITC)–coupled goat anti–mouse and FITC-coupled goat anti–rabbit IgG were from Immunotech. Goat anti–mouse IgG fractions coupled with 5 nm colloidal gold (GAM-5) and goat anti–rabbit IgG fractions coupled with 10 nm colloidal gold (GAR-10) were purchased from British Biocell (Cardiff, United Kingdom). Chemicals were from Sigma (St Louis, MO) and cell cultures were from Life Technologies (Paisley, United Kingdom).
Cell culture
Human umbilical vein endothelial cells (HUVECs) were obtained as described previously.27 Briefly, HUVECs were harvested by means of collagenase type I digestion. The cells were maintained and subcultured in RPMI 1640 (Gibco-BRL, Cergy Pontoise, France) containing 20% heat-inactivated fetal-calf serum (FCS), penicillin (0.1 IU/L), streptomycin (100 μg/mL), and Fungizone (Roche Diagnostics, Mannheim, Germany) (2.5 mg/mL). Cells were cultured on glass coverslips precoated with 0.2% gelatin for fluorescence microscopy studies. In some experiments, confluent ECs were incubated with 2 mM cytochalasin B (CCB) for 1 hour or with 3 μM/mL ethyleneglycoltetraacetic acid (EGTA) for 5 minutes in serum-free culture medium. To allow studies at various stages of confluence, ECs were seeded at different initial densities as described previously.28
Confocal microscopy
HUVECs were fixed with 3% formaldehyde in phosphate-buffered saline (PBS) for 30 minutes at room temperature and permeabilized with 0.2% Triton X-100. After being washed with PBS and incubated with blocking reagent (5% nonfat milk in PBS) for 15 minutes, cells were incubated for 30 minutes at room temperature with anti-CD146 or irrelevant IgG1 mAb (20 μg/mL). Secondary labeling was then done for 30 minutes with FITC F(ab′)2 sheep anti–mouse IgG (1:100) alone or with TRITC-labeled phalloidin (10 mM), which stained actin filaments. The samples were mounted in Mowiol and examined with a Leica confocal microscope. Cells were visually sectioned to 0.5 μm by the microscope.
Electron microscopy
HUVECs grown to confluence were fixed in 1% glutaraldehyde (Ladd Research, Burlington, VT) in 0.1 M phosphate buffer (pH 7.4) for 1 hour at 22°C. They were washed 3 times in PBS, scraped from the culture dish with a rubber policeman, embedded in sucrose, and frozen in liquid nitrogen. The immunochemical reactions were then done on thin sections collected on copper grids according to the method described previously.29 30 Briefly, the sections were labeled by a first incubation with a mouse mAb to CD146 or a rabbit polyclonal antibody to VE-cadherin; both antibodies were used at a 1:50 dilution in PBS containing 1% bovine serum albumin (BSA) for 20 minutes at 22°C. Sections were washed and then incubated with GAM-G5 or GAR-G10, with the gold conjugates used at a dilution of 1:30 for 20 minutes at 22°C. Double labeling was done by a first incubation with a mouse mAb to CD146 and a rabbit polyclonal antibody to VE-cadherin. Sections were washed and then incubated with both GAM-G5 and GAR-G10 for 20 minutes at 22°C. They were then counterstained with 2% uranyl acetate (pH 7) and methyl cellulose uranyl and observed under a Phillips CM10 electron microscope.
Quantitative flow cytometry
The level of membrane expression of CD146 in HUVECs was determined. All detached ECs were labeled with anti-CD146 mAb or an isotype-matched control antibody (10 μg/mL) for 1 hour at 4°C. After 2 washes in PBS containing 0.1% BSA and 0.1% sodium azide, cells were incubated with FITC F(ab′)2 sheep anti–mouse IgG (1:100) for 45 minutes at 4°C. After washing, samples were analyzed by flow cytometry (Epics Profile; Coultronics, Margency, France). The number of antigenic sites per cell was counted by using a quantitative indirect immunofluorescence (QIFI) assay31 32based on the linear relation between antigen expression and the mean fluorescence intensity. When mAbs are used in a saturating concentration, they bind to cell-surface antigen monovalently and the antibody-binding capacity is equivalent to the number of antigenic sites per cell. Antigen expression was quantified by using latex beads (QIFI kit, Dako) bearing an increasing number of mAb molecules labeled with the secondary antibody, which were used to construct a standard regression line between mean fluorescence intensity and number of antigenic sites per cell.
Selective extraction with Triton X-100 and immunoblotting
HUVEC monolayers were rinsed twice with PBS and scraped at 4°C with a rubber policeman in 1 mL lysis buffer (20 mM Tris [pH 8], 150 mM sodium chloride, 5mM EDTA, and 200 mM sucrose) supplemented with a mixture of protease inhibitors in the absence of detergent.33 Cells were broken by 20 passages through a G3 needle. After centrifugation in a microcentrifuge at 750 rpm/minute at 4°C for 10 minutes to remove intact cells and nuclei, the supernatant was incubated with Triton X-100 at 1% for 5 minutes at 4°C. Soluble and insoluble materials were separated by ultracentrifugation for 1 hour at 100 000g at 4°C. Supernatant and pellets were collected, 1 vol 2X sample buffer was added to the supernatant, and 2 vol 1X sample buffer was added to the pellet. Lysates were boiled for 10 minutes, analyzed on a 10% sodium dodecyl sulfate–polyacrylamide gel under nonreducing conditions, and then electrophoretically transferred to nitrocellulose membranes (Amersham, Les Ulis, France). Nonspecific reactivity was reduced by incubating the membrane with 5% nonfat milk and 0.5% Tween 20 in PBS for 2 hours at 37°C. The nitrocellulose membrane was incubated for 1 hour with anti-CD146 mAb (1 μg/mL), washed with 0.5% Tween 20 in PBS, and incubated for 1 hour with 1:10 000 horseradish peroxidase anti–mouse IgG. The blots were washed and revealed with chemiluminescent substrate by using a method described by the manufacturer (Valbiotech, Paris, France).
Cell lines transfected with CD146 complementary DNA
The full-length cDNA of CD146 (drop 7.4; J. Johnson, Munich, Germany) was inserted in the EcoRI sites of the mammalian expression vector PCIneo. Sense and antisense orientations of the inserted cDNA were checked by PvuI digestion. The L929 cell line grown in Dulbecco modified Eagle medium–Ham F12 medium supplemented with 10% FCS was transfected with 4 μg of the construct by using the Lipofectamine method (Life Technologies). After transfection, the transfected cells were selected by resistance to Geneticin (800 μg/mL; Life Technologies). Stable clones from independent transfected cells were obtained by limit dilution and subcloned 3 fold. Two independent clones in the sense orientation (B9-5 and L4B11) and one clone in the antisense orientation (D12) were selected on the basis of CD146 expression determined by immunoblotting and fluorescence-activated cell-sorter scanning analysis with anti-CD146 mAb. An isotype-matched IgG1 mAb was used as a negative control.
Determination of monolayer permeability
L929 cells transfected with CD146 cDNA in the sense or antisense orientation were grown to confluence on cell-culture inserts (12 mm with 0.4-μm pore size; Costar, Brumath, France). These cells were used to assay the passage of macromolecules through the monolayer 7 days after seeding. Each assay was done in duplicate. At the beginning of the experiment, the upper compartments of the inserts were filled with 100 μL 0.5% Hanks balanced salt solution (HBSS)–BSA medium containing 50 μM FITC-dextran (71 kd, Sigma) and the lower compartments with 500 μL 0.5% BSA-HBSS medium. Samples (100 μL) were removed from the lower compartments at 15-minute intervals for 45 minutes. This volume was immediately replaced with HBSS-BSA to prevent changes in hydrostatic pressure. The levels of fluorescence in the samples were determined (Cytofluor; Millipore, Bedford, MA) (excitation at 492 nm and emission at 520 nm) and compared with a standard curve of fluorescence created by using various dilutions for the FITC-dextran to determine micromoles of FITC-dextran at all points. Results were expressed as the permeability coefficient (or rate of flux across the monolayer), which was obtained from the slope of the linear curve regression relating time and FITC-dextran content.
Statistical analysis
Results are expressed as mean values ± SEM. Differences between groups were tested for significance by using the Studentt test for paired observations.
Results
CD146 is expressed at the endothelial cell-cell junction
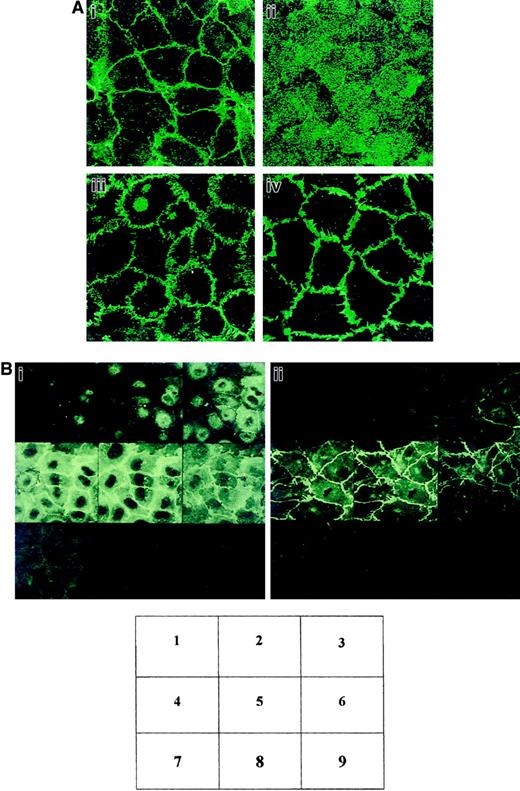
We studied the localization of CD146 by using fluorescence confocal microscopy after labeling of confluent HUVEC monolayers with anti-CD146 mAb. CD146 was detected mainly at the endothelial cell-cell junction (Figure 1Ai), although a faint labeling of CD146 was also detectable in the cytoplasm (Figure 1Ai). The junctional staining patterns of VE-cadherin (Figure 1Aiii) and PECAM-1 (Figure 1Aiv) were used as positive controls for the cell-cell junction. However, the thin continuous staining obtained with anti-CD146 mAb was different from the more segmented aspects of anti–VE-cadherin staining or the broader anti–PECAM-1 staining, suggesting a different localization of these molecules in the area of the cell-cell endothelial junction. In contrast, labeling of the HUVEC monolayer with anti–MHC class I mAb used as a control confirmed a diffuse staining pattern (Figure 1Aii). To better document CD146 localization, serial optical sections (0.5 μm) were obtained from the apical to the basal sides of the endothelial monolayer by using confocal microscopical analysis (Figure 1Bi). Fluorescent labeling for VE-cadherin (Figure 1Bii), known to be restricted to the endothelial junction, was used as a control. The data indicated that the CD146 staining was above the plane of the staining for VE-cadherin (Figure 1Bi, sections 2 and 3, versus Figure 1Bii, sections 2 and 3), showing that CD146 was partly recovered in the apical side of the ECs.
CD146 localization at the cell-cell contacts on cultured ECs examined by confocal microscopy.
(A) HUVECs were grown to confluence on coverslips. The cells were stained with anti-CD146 (i), anti–MHC class I used as a control (ii), anti–VE-cadherin (iii), or anti–PECAM-1 (iv). Magnification × 100. (B) Optical sections of endothelial monolayer were obtained with confocal microscopy from the apical (1-3), intermediate (4-6), and basal (7-9) sides of the cells. (i) Labeling for CD146. (ii) VE-cadherin. Magnification × 100.
CD146 localization at the cell-cell contacts on cultured ECs examined by confocal microscopy.
(A) HUVECs were grown to confluence on coverslips. The cells were stained with anti-CD146 (i), anti–MHC class I used as a control (ii), anti–VE-cadherin (iii), or anti–PECAM-1 (iv). Magnification × 100. (B) Optical sections of endothelial monolayer were obtained with confocal microscopy from the apical (1-3), intermediate (4-6), and basal (7-9) sides of the cells. (i) Labeling for CD146. (ii) VE-cadherin. Magnification × 100.
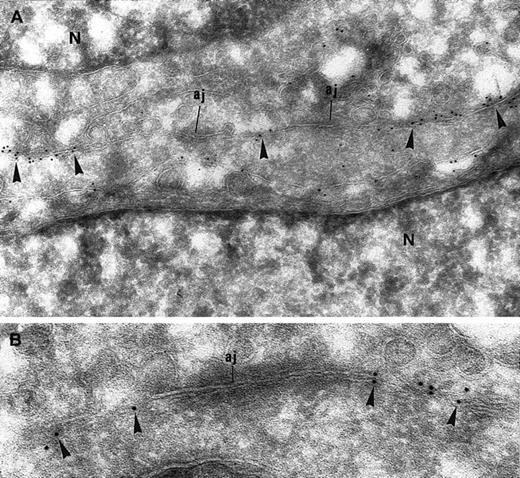
CD146 localization was further investigated by electron microscopy. Consistent with the results of the confocal microscopy analysis, CD146 labeling was present in cell-cell contact areas (Figure2A). Notably, gold particles were occasionally also detectable over the cytoplasm, suggesting an intracytoplasmic pool of CD146 (Figure 2A). CD146 immunolabeling was clearly excluded from the electron-dense zones that showed a typical pattern of cell-cell AJs (Figure 2A-B). Immunolabeling for VE-cadherin, a specific marker of AJs, was observed in these electron-dense areas, which were thus definitively identified as AJs (Figure3A). To confirm that CD146 was absent from the AJs, double staining using immunogold particles of different sizes was done with an anti-CD146 mAb and an anti–VE-cadherin polyclonal antibody. This experiment showed that CD146 and VE-cadherin did not colocalize, demonstrating that CD146, which was present in the endothelial junction, was excluded from the AJ (Figure 3B).
Immunogold labeling for CD146 of ECs grown to confluence.
(A) CD146 immunolabeling appears along the intercellular junctions (arrowheads) but is excluded from the electron-dense area corresponding to AJs. A few gold particles are scattered over the cytoplasm, showing the intracellular pool of CD146 (N indicates nucleus; magnification × 63 000). (B) Higher magnification of an AJ. The immunogold labeling for CD146 is absent from the AJ (magnification × 90 000).
Immunogold labeling for CD146 of ECs grown to confluence.
(A) CD146 immunolabeling appears along the intercellular junctions (arrowheads) but is excluded from the electron-dense area corresponding to AJs. A few gold particles are scattered over the cytoplasm, showing the intracellular pool of CD146 (N indicates nucleus; magnification × 63 000). (B) Higher magnification of an AJ. The immunogold labeling for CD146 is absent from the AJ (magnification × 90 000).
Immunogold labeling of endothelial junction for CD146 and VE-cadherin.
(A) Immunogold labeling of endothelial junction for VE-cadherin, a specific marker of AJs. Gold particles appear at the electron-dense zone of the interendothelial junction, which is thus identified as an AJ (arrowheads; magnification × 71 000). (B) Double immunolabeling for CD146 (5 nm gold) and VE-cadherin (10 nm gold). CD146 is detected apart from the AJ (arrows) and identified as such by the presence of VE-cadherin (magnification × 71 000).
Immunogold labeling of endothelial junction for CD146 and VE-cadherin.
(A) Immunogold labeling of endothelial junction for VE-cadherin, a specific marker of AJs. Gold particles appear at the electron-dense zone of the interendothelial junction, which is thus identified as an AJ (arrowheads; magnification × 71 000). (B) Double immunolabeling for CD146 (5 nm gold) and VE-cadherin (10 nm gold). CD146 is detected apart from the AJ (arrows) and identified as such by the presence of VE-cadherin (magnification × 71 000).
CD146 expression varies with endothelial junction formation
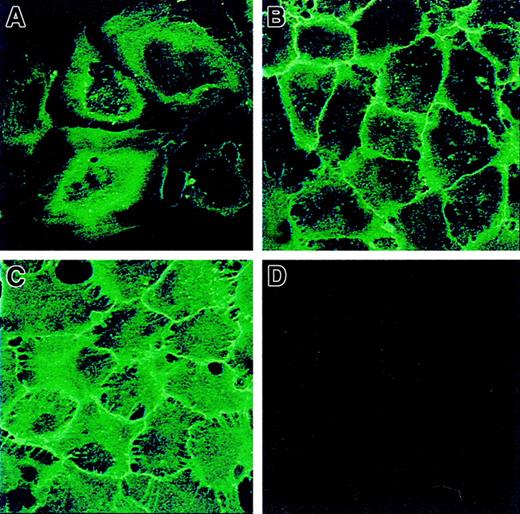
To investigate the dynamics of CD146 cell-surface expression during establishment of cell-cell contacts, CD146 localization was analyzed in HUVECs at different degrees of confluence. Cells were stained with anti-CD146 mAb and examined by confocal microscopy (Figure 4). On sparse HUVECs, a diffuse perinuclear CD146 staining with some concentrated patches at the cell periphery was observed (Figure 4A). When the ECs reached confluence, CD146 labeling appeared as a sharp continuous line along the cell borders (Figure 4B). Once confluence was firmly established, strong CD146 staining at the cell surface was concentrated at the cell-cell junction, with deep intercellular digitations (Figure 4C). To confirm the increase in CD146 membrane expression, CD146 antigenic sites were assessed by quantitative flow cytometry analysis after indirect immunofluorescence labeling of subconfluent or late confluent cells. The number of membrane-associated anti-CD146 antigenic sites per cell was increased in late confluent cells compared with subconfluent cells (975 ± 53 × 103 sites/cell versus 507 ± 24 × 103 sites/cell; n = 5). These data show that CD146 cell-surface expression increases when the cells reach confluence.
CD146 localization on HUVECs at different stages of confluence by confocal microscopy.
In subconfluent HUVECs (A), CD146 was initially perinuclear with some peripheral patches. With the progression of intercellular contacts, an increase in CD146 was observed—as a fine line along the cell periphery at the beginning of confluence (B, recently confluent) and as a thicker line after prolonged confluence, with intercellular digitations (C). At late confluence (D), the cells were also stained with ST4, an irrelevant mAb used as a control. Magnification × 100.
CD146 localization on HUVECs at different stages of confluence by confocal microscopy.
In subconfluent HUVECs (A), CD146 was initially perinuclear with some peripheral patches. With the progression of intercellular contacts, an increase in CD146 was observed—as a fine line along the cell periphery at the beginning of confluence (B, recently confluent) and as a thicker line after prolonged confluence, with intercellular digitations (C). At late confluence (D), the cells were also stained with ST4, an irrelevant mAb used as a control. Magnification × 100.
Role of CD146 in establishment of cell-cell contact
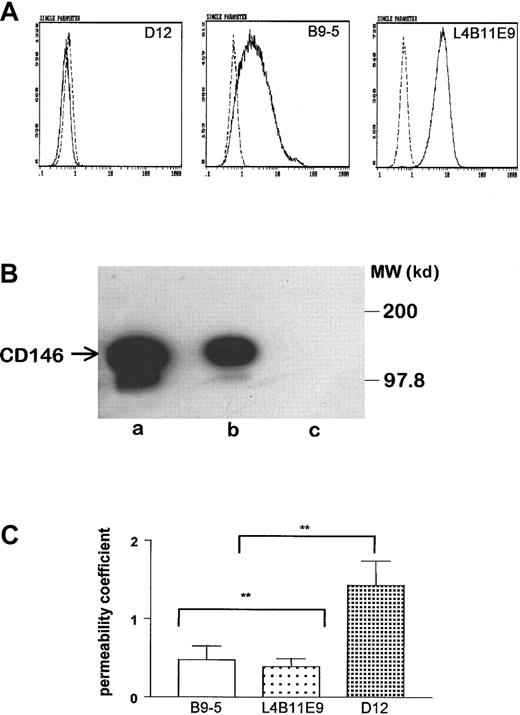
The intercellular localization of CD146 and the dynamics of its cell-surface expression prompted us to investigate whether CD146 plays a role in the control of cell-cell cohesion. Thus, we generated CD146-transfected fibroblast clones (L4B11E9 and B9-5). Control cells consisted of fibroblasts transfected with antisense CD146 (D12). Cell-surface expression of CD146 on transfected cells was confirmed by flow cytometry (Figure 5A). Measurement of antigenic sites of CD146 per cell showed that the 2 independent clones expressed CD146 at different levels: 36 000 sites/cell for B9-5 and 120 000 sites/cell for L4B11E9. Anti-CD146 immunoblotting confirmed the presence in B9-5 cell lysates of a band at a molecular weight (120 kd) identical to that found on HUVECs (Figure 5B, lanes a and b). In contrast, no bands were detected on D12 cell lysates (Figure5B, lane c). The paracellular permeability of CD146-transfected cells was evaluated and compared with that of controls. As shown in Figure5C, the paracellular permeability coefficients of L4B11E9 and B9-5 CD146-transfected cell lines were significantly reduced compared with those of D12 cells. The decrease in permeability in CD146-transfected cell lines indicates that cell-surface expression of CD146 correlated with an increase in cell-cell cohesion.
Characterization of L929 transfected cells.
(A) Expression of CD146 was analyzed by flow cytometry. The 2 independent clones of CD146-transfected cells in sense orientation, B9-5 and L4B11E9, expressed CD146 at different levels, whereas the CD146-transfected clone in antisense orientation, D12, did not express CD146. (B) In B9-5 cell lysate (lane b), Western blotting revealed a band with a molecular weight identical to in HUVEC lysate (lane a) at 120 kd, whereas no band was observed in D12 cell lysate (lane c). (C) Determination of the paracellular permeability coefficient showed a significant decrease for B9-5 and L4B11E9 cells compared with D12 cells (P = .02; n = 6). When EGTA (5 mM for 30 minutes at 37°C) was added, a retraction of transfected cells was observed, thereby excluding the possibility of measuring the permeability coefficient after a reduction in calcium concentration.
Characterization of L929 transfected cells.
(A) Expression of CD146 was analyzed by flow cytometry. The 2 independent clones of CD146-transfected cells in sense orientation, B9-5 and L4B11E9, expressed CD146 at different levels, whereas the CD146-transfected clone in antisense orientation, D12, did not express CD146. (B) In B9-5 cell lysate (lane b), Western blotting revealed a band with a molecular weight identical to in HUVEC lysate (lane a) at 120 kd, whereas no band was observed in D12 cell lysate (lane c). (C) Determination of the paracellular permeability coefficient showed a significant decrease for B9-5 and L4B11E9 cells compared with D12 cells (P = .02; n = 6). When EGTA (5 mM for 30 minutes at 37°C) was added, a retraction of transfected cells was observed, thereby excluding the possibility of measuring the permeability coefficient after a reduction in calcium concentration.
Interaction of CD146 with the actin cytoskeleton
Because the molecular organization of cell-cell contact sites requires interactions between junction proteins and cytoskeletal elements,4 we studied the association between CD146 and the actin-containing cytoskeleton. Confluent HUVECs were stained with both FITC–anti-CD146 mAb and TRITC-phalloidin. Confocal microscopy revealed yellow areas indicating that CD146 partly colocalized with the actin cytoskeleton (Figure 6A). As a control, HUVECs were also stained with both anti–MHC class I mAb and phalloidin (Figure 6B), and as expected, no colocalization with the actin filaments was detected.
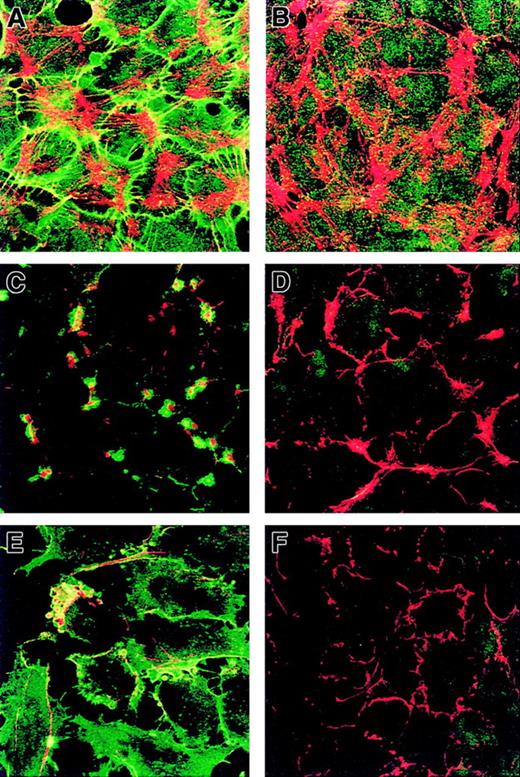
Codistribution of CD146 and endothelial cytoskeleton and effect of cytoskeleton-disrupting agents on CD146 distribution.
HUVECs were double stained with anti-CD146 antibody (green) and phalloidin (red) at confluence. (A) CD146 was associated with the actin cytoskeleton. (B) HUVECs double stained for MHC class I (green) and F-actin (red) used as a control. Confluent monolayers of HUVECs were incubated with CCB for 1 hour (C,D) or with EGTA (3 mM) for 5 minutes (E,F). Cells were then double stained with both anti-CD146 antibody (green) and phalloidin (red) (C,E) or with both anti–MHC class I (green) and phalloidin (red) (D,F). Disruption of microfilaments with CCB or EGTA led to a redistribution of CD146 (C,D) compared with findings in untreated cells (A). Magnification × 100.
Codistribution of CD146 and endothelial cytoskeleton and effect of cytoskeleton-disrupting agents on CD146 distribution.
HUVECs were double stained with anti-CD146 antibody (green) and phalloidin (red) at confluence. (A) CD146 was associated with the actin cytoskeleton. (B) HUVECs double stained for MHC class I (green) and F-actin (red) used as a control. Confluent monolayers of HUVECs were incubated with CCB for 1 hour (C,D) or with EGTA (3 mM) for 5 minutes (E,F). Cells were then double stained with both anti-CD146 antibody (green) and phalloidin (red) (C,E) or with both anti–MHC class I (green) and phalloidin (red) (D,F). Disruption of microfilaments with CCB or EGTA led to a redistribution of CD146 (C,D) compared with findings in untreated cells (A). Magnification × 100.
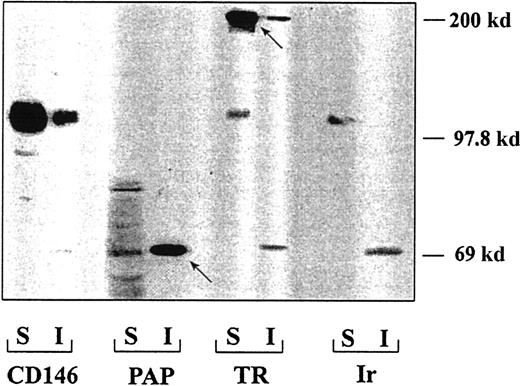
To test the hypothesis that CD146 interacts with the actin cytoskeleton, we investigated CD146 selective extraction with Triton X-100 because Triton insolubility is commonly interpreted as an indicator of cytoskeletal association. Detergent-soluble and insoluble fractions prepared from confluent HUVECs were immunoblotted with anti-CD146 mAb. The percentage of CD146 recovered in the insoluble fraction was 25% ± 2.5% (n = 4; Figure7), indicating that although most of the molecule was detected in the soluble fraction, a fraction of CD146 molecules might be associated with the actin cytoskeleton. As a control, placental alkaline phosphatase, a molecule known to be partly resistant to extraction, was recovered mainly in the insoluble fraction, and transferrin receptor (TR), a molecule known to be almost entirely extractable, was recovered in the soluble fraction (Figure 7). The percentage of TR recovered in the insoluble fraction was 13% ± 1.5%, a value 2-fold lower than the percentage of CD146, thus showing that CD146 was partly resistant to Triton X-100 extraction.
Association of CD146 with the cytoskeleton.
The amount of CD146 recovered in the soluble fraction (S) was 75%; that recovered in the insoluble fraction (I) was 25%. As controls for the specificity of the extractions, placental alkaline phosphatase was recovered mostly in the insoluble fraction and TR in the soluble fraction. The arrows represent the major constituents of each protein taken as a control of the triton X100 extraction.
Association of CD146 with the cytoskeleton.
The amount of CD146 recovered in the soluble fraction (S) was 75%; that recovered in the insoluble fraction (I) was 25%. As controls for the specificity of the extractions, placental alkaline phosphatase was recovered mostly in the insoluble fraction and TR in the soluble fraction. The arrows represent the major constituents of each protein taken as a control of the triton X100 extraction.
The interaction between CD146 and the endothelial cytoskeleton was investigated further by using cytoskeleton-disrupting agents and a double labeling of CD146 and cytoskeletal actin. When HUVECs were treated with a disrupting agent such as CCB and stained with both FITC–anti-CD146 and TRITC-phalloidin, a dramatic redistribution of CD146 into dense foci containing clustered actin was observed (Figure6C versus 6D). The association between CD146 and the cytoskeleton was examined further after cell treatment with a calcium chelator. Within 5 minutes after the addition of EGTA (3 mM), a complete redistribution of CD146 into dense foci was observed, although the molecule remained colocalized with actin, as shown by the presence of yellow patches (Figure 6E compared with 6F). Agents disrupting the actin cytoskeleton modified localization of CD146 at the cell-cell junction, suggesting that this localization is regulated by the organization of the actin cytoskeleton. Together, the results of the Triton X-100 insolubility assessment and the redistribution of the CD146 pattern after CCB or EGTA treatment indicate an association of CD146 with the actin cytoskeleton.
Discussion
We reported previously that S-Endo1 antigen (CD146), a member of the immunoglobulin superfamily, already observed on melanoma cells, is also constitutively expressed in all types of human vascular endothelium.14 16 In the current study, we found that CD146 is localized at the endothelial junction. CD146-regulated expression during monolayer formation, its involvement in the control of paracellular permeability, and its coupling with actin cytoskeleton are all consistent with its role in connecting neighboring cells.
ECs have distinct intercellular contact mechanisms involving multiple intercellular receptors that differ in their spatial and temporal organization as well as their functional properties. In this study, we found that CD146 is a component of the endothelial junction. Electron microscopy revealed several features of its junctional pattern. CD146 was found in intercellular areas of the plasma membrane but was not observed in the electron-dense zones of the junction. These areas, which expressed VE-cadherin, where thus identified as AJs, indicating that CD146 was excluded from AJs. This finding was confirmed by double-labeling experiments indicating that CD146 and VE-cadherin did not colocalize. Moreover, CD146 localization was not restricted to the junction, since analyses of different optical sections of endothelial monolayers indicated that CD146 was also recovered at the apical side of the cells, in contrast to VE-cadherin.
The dynamics of CD146 cell-surface expression during monolayer formation showed that its concentration at the endothelial junction depended on establishment of cell-cell contacts. No cell-surface expression of CD146 was detected on sparse HUVECs, whereas CD146 was concentrated progressively at sites of cell-cell contacts when the monolayer reached confluence. The increase in CD146 expression at the cell junction revealed by confocal analysis was confirmed by an increase in the number of CD146 membrane sites measured by quantitative flow cytometry analysis. Together, these results could indicate the existence of an up-regulation in CD146 synthesis or its redistribution at the cell surface from an intracytoplasmic pool during monolayer formation. Interestingly, a pool of CD146 was detected in the cytoplasm of HUVECs. Localization of CD146 both in the cytoplasm and at the plasma membrane has also been observed in lymphoid and nonlymphoid thymus cells.34 We cannot currently determine whether cell-surface expression of CD146 is a consequence of HUVEC proliferation or of establishment of cell-cell contacts.
Our data suggest involvement of CD146 in the organization of cohesive contacts between adjacent cells. Indeed, 2 independent clones of CD146 transfectants showed reduced paracellular permeability in comparison with antisense CD146-transfected cells. Thus, overexpression of CD146 correlates with a more cohesive monolayer, suggesting that CD146 promotes cell-cell adhesion. In the same experimental system, other molecules known to mediate homophilic adhesion, such as VE-cadherin9 and JAM,10 also caused a reduction in paracellular permeability when transfected in fibroblasts. A role for CD146 in the control of cell-cell cohesion is consistent with (1) CD146 involvement in homotypic aggregation of melanoma cells23,25; (2) enhanced cohesion of breast carcinoma expressing CD146, permitting restraint of tumor cells within the primary lesion35; and binding of CD146 to its putative receptor on trophoblast cells, conferring a stationary phenotype that prevents trophoblastic migration and invasion within the endometrium.36
Formation and maintenance of endothelial cell-cell contacts require a complex interplay among plasma membrane proteins, signaling molecules, and cytoskeleton components.37,38 For VE-cadherin, the effect on cell-cell cohesion requires its anchorage to catenin and the actin cytoskeleton.39 We previously showed that CD146 is also a signaling molecule. CD146 engagement initiates a phosphotyrosine-kinase–dependent signaling pathway that leads to the association of CD146 with the cytoskeleton network through formation of a multiprotein complex including CD146, p59FYN, p125FAK and paxillin.40 Consistent with these data, we found that CD146 colocalized with the actin-containing cytoskeleton, was partly resistant to Triton X-100 extraction, and was redistributed by cytoskeleton-disrupting treatments. Together, these data suggest that CD146 expression and function at the endothelial junction could be controlled by the actin cytoskeleton.
The endothelial junction is composed of several adhesion molecules that could act together in concert by forming transitory or permanent molecular complexes in interaction with proteins that anchor them to the cytoskeleton.4 We attempted to show a molecular association between CD146 and other molecules in the endothelial junction that control monolayer cohesion. Using immunoprecipitation assessments, we found no direct evidence of an association between CD146 and VE-cadherin, PECAM-1, or β1 integrins (data not shown). Nevertheless, a hypothetical cross-talk between CD146 and E-cadherin was suggested by the modulation of CD146 expression on melanoma cells cocultured with keratinocytes.41 In addition, studies have shown that HEMCAM-gicerin, the avian orthologue of CD146, regulates cell adhesion by controlling β1 integrins at the cell surface.42 It is possible that junctional molecules could establish a transitory association or indirect interaction by means of a signaling molecule.
In conclusion, we found that CD146 represents a component of the endothelial junction located outside the AJ and involved in cell-cell cohesion. Although its ligand and mechanism of action are unknown, we propose that CD146 could function as a regulator of endothelial cohesion through the endothelial cytoskeleton network.
We thank the Biocytex company for providing CD146 monoclonal antibodies; Dr E. Vivier for critical reading of the manuscript; Dr D. Gulino for the generous gift of anti–VE-cadherin antibody; Mrs P. Stelmann and M. Nonotte for technical assistance with cell cultures; Mr M. Dehri, R. Pistoresi, and A. Boyer for technical support; and Roche Institute (Faculté de Médecine Nord, Marseille, France) for graphics assistance.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Françoise Dignat-George, Laboratoire d'Hématologie et d'Immunologie, UFR de Pharmacie, 27 Bd Jean Moulin, 13385 Marseille Cedex 5, France; e-mail:hematim@pharmacie.univ-mrs.fr.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal