Abstract
Although erythropoietin (EPO) and its receptor (EPOR) are crucial for the proliferation, survival, and terminal differentiation of erythroid progenitors, it remains to be elucidated whether EPOR-unique signaling is required for erythropoiesis. To address this issue, human granulocyte-macrophage colony-stimulating factor (hGM-CSF) receptor (hGMR)–transgenic mice and heterozygous EPOR mutant mice were crossed by in vitro fertilization. In methylcellulose clonal culture of fetal liver (FL) cells of generated hGMR-expressing EPOR−/− embryos at embryonic day (E) 12.5 of gestation, hGM-CSF stimulated erythroid colony formation under serum-containing and serum-free conditions. Analysis of globin expression in individual erythrocyte-containing colonies formed from E12.5 FL cells showed that hGM-CSF supports primitive and definitive erythropoiesis even in EPOR−/− embryos. In comparison of activities between hGM-CSF and EPO in hGMR-expressing EPOR+/+ embryos, the 2 substances supported the formation of similar numbers of erythroid colonies in clonal culture of E12.5 FL cells; enhanced adult, but not embryonic, globin synthesis; and induced increase of GATA-1 expression and decrease of erythroid Kruppel-like factor and cMyb expression in the FL cells. On the other hand, in E8.0 yolk sac erythropoiesis, both substances had a similar effect on erythroid colony formation, but hGM-CSF induced an increase of β-major globin expression, while EPO did not. All together, the results of the present study demonstrated that hGM-CSF can stimulate the proliferation and differentiation of primitive and definitive erythroid cells independently of EPOR signal if they express hGMR, and the activity is comparable to that of EPO in definitive, but not primitive, erythropoiesis.
Introduction
Erythropoietin (EPO) is a lineage-restricted cytokine and plays a central role in the proliferation,1,2differentiation,3,4 and survival5 of erythroid progenitors. It exerts its function through EPO receptor (EPOR), a member of the class I cytokine receptor family.6Disruption of EPO or the EPOR gene in mice leads to embryonic death at embryonic day (E) 11 to 13.5 of gestation owing to a defect of fetal liver (FL) definitive erythropoiesis.7-9 Even in the absence of EPO or EPOR, FL contains normal numbers of erythroid burst-forming units (BFU-Es) and erythroid colony-forming units (CFU-Es), indicating that EPO and EPOR are not required for the commitment of hematopoietic stem cells to erythroid lineage, but support progenitor cell survival and expansion beyond the CFU-E stage in definitive erythropoiesis.7,9 On the other hand, primitive erythropoiesis, which is the first erythropoiesis to occur in the developing mouse embryo, takes place in blood islands of E7 yolk sac (YS).10 Primitive erythropoiesis yields unique erythrocytes, distinguishable from those in definitive erythropoiesis by their morphology and the hemoglobin types they express. Primitive erythrocytes are nucleated cells containing embryonic as well as adult hemoglobins, whereas definitive erythrocytes are smaller nonnucleated red cells committed only to adult hemoglobin synthesis. In EPO−/− and EPOR−/− mouse embryos, primitive erythropoiesis is partially impaired, but a low level of erythropoiesis in the YS allows the embryos to develop and survive from E7 to E13 of gestation.7-9 Thus, EPOR signaling has different roles in definitive and primitive erythropoiesis.
We previously generated transgenic (Tg) mice expressing human granulocyte-macrophage colony-stimulating factor (hGM-CSF) receptors (hGMRs) consisting of ligand-binding α chain and signal-transducing β chain, so called common β chain, which is also used by interleukin-3 (IL-3) and IL-5 receptors.11 We found that hGM-CSF supports the proliferation and differentiation of erythroid cells in adult bone marrow of the Tg mice, indicating that the development of adult erythroid cells can be stimulated even by hGM-CSF, a myelopoiesis-specific cytokine, as long as hGMR is expressed on the target cells.11 However, it remains to be resolved whether an EPOR-unique signal is essential for erythroid differentiation, or whether the hGM-CSF signal can stimulate embryonic erythropoiesis. To address these issues, we generated hGMR-expressing EPOR−/− embryos, using homozygous hGMR-Tg (hGMR+/+) mice and heterozygous EPOR mutant (EPOR+/−) mice, and examined the effect of hGM-CSF on primitive and definitive erythropoiesis. We also compared hGM-CSF and EPO in terms of their effects on colony formation from E12.5 FL and E8.0 YS cells in methylcellulose clonal culture and the expression of embryonic and adult hemoglobins and transcription factors by reverse transcriptase–polymerase chain reaction (RT-PCR) in hGMR-Tg mouse embryo. The results indicated that hGM-CSF can stimulate colony formation by primitive and definitive erythroid progenitors even if they lack EPOR, as long as they express hGMR, and that hGM-CSF has effects comparable to those of EPO on the differentiation of definitive, but not primitive, erythroid cells.
Materials and methods
Generation of hGMR-expressing EPOR−/− and EPOR+/+ embryos
To generate hGMR-expressing EPOR−/− and EPOR+/+ embryos, hGMR-Tg mice and heterozygous EPOR mutant mice were prepared. The hGMR-Tg mice and heterozygous EPOR mutant mice were generated and maintained as described in the previous reports.7,11 Mice were fertilized in vitro. Then, some of the fertilized eggs were transferred into the oviducts of pseudopregnant ICR females, as described.12 The remaining eggs were stored in liquid nitrogen.13 In some experiments, homozygous hGMR-Tg mice and heterozygous EPOR mutant mice were crossed to efficiently generate hGMR-expressing EPOR+/+ and EPOR−/− embryos.
Cell preparation
Staged embryos were removed on E8.0 for the analysis of YS and on E12.5 for the analysis of FL and were dissected free of maternal tissue without disrupting the placenta. YS was disaggregated individually to single cells by treatment with 3 mg/mL dispase for 30 minutes, and a single-cell suspension of FL was prepared in α–Eagle minimum essential medium (MEM) (Flow Laboratories, Rockville, MD) by mechanical disaggregation. Each suspension was passed through a 38-μm nylon cell strainer (Becton Dickinson Labware, Franklin Lakes, NJ). Cell number and viability were determined by trypan blue exclusion and Turck solution.
Analysis of expression of hGMR and EPOR in embryos
Genomic DNA was prepared from embryos removed from YS or FL by standard methods, to distinguish expression of hGMRα, hGMRβ, and mouse EPOR. The PCR primer sets used are shown in Table1. PCR was performed in PCR buffer, 200 μM each deoxynucleotide, 300 nM each primer and 2 U/mL Taq polymerase in a final volume of 15 μL. The reaction profiles were 28 cycles at 94°C for 30 seconds, 58°C for 40 seconds, and 72°C for 50 seconds for hGMRα and hGMRβ; and 25 cycles at 94°C for 1 minute, 58°C for 1 minute, and 72°C for 2 minutes for EPOR. A Gene Amp PCR System 2400 (Perkin-Elmer, Wellesley, MA) was used. PCR products were run on a 1.2% agarose gel and made visible by ethidium bromide (0.5 μg/mL) staining.
PCR primers of cytokine receptors, globins, and transcription factors
| . | Primers . | References . |
|---|---|---|
| hGMRα | 5′-CGTCTTCCACCTCATGGTTATC-3′ | |
| 5′-CTGCAGACGTCCGCATCTTG-3′ | ||
| hGMRβ | 5′-AGGTCACCAAGGAACAATCCT-3′ | |
| 5′-TTTGAAGAGCTGAATGA-3′ | ||
| EPOR | 5′-GCACTGAGTGTGTTCTG-3′ | |
| 5′-GCCTCACACTCTTCACC-3′ | ||
| 5′-GCTGCTAAAGCGCATGC-3′ | ||
| α-globin | 5′-CTCTCTGGGGAAGACAAAAGCAAC-3′ | 19 |
| 5′-GGTGGCTAGCCAAGGTCACCAGCA-3′ | ||
| β-major globin | 5′-CTGACAGATGCTCTCTTTGGG-3′ | 19 |
| 5′-CACAACCCCAGAAACAGACA-3′ | ||
| βH1 globin | 5′-AGTCCCCATGGAGTCAAAGA-3′ | 19 |
| 5′-CTCAAGGAGACCTTTGCTCA-3′ | ||
| ε-globin | 5′-GGAGAGTCCATTAAGAACCTAGACAA-3′ | 19 |
| 5′-CTGTGAATTCATTGCCGAAGTGAC-3′ | ||
| ζ-globin | 5′-GCTCAGGCCGAGCCCATTGG-3′ | 19 |
| 5′-TAGCGGTACTTCTCAGTCAG-3′ | ||
| GATA-1 | 5′-CATTGGCCCCTTGTGAGGCCAGAGA-3′ | 20 |
| 5′-ACCTGATGGAGCTTGAAATAGAGGC-3′ | ||
| EKLF | 5′-TCGCCGGAGACGCAGGCT-3′ | 19 |
| 5′-CCCAGTCCTTGTGCAGGA-3′ | ||
| c-Myb | 5′-GAGCTTGTCCAGAAAATATGGTCCGAAG-3′ | 19 |
| 5′-GGCTGCCGCAGCCGGCTGAGGGGAC-3′ |
| . | Primers . | References . |
|---|---|---|
| hGMRα | 5′-CGTCTTCCACCTCATGGTTATC-3′ | |
| 5′-CTGCAGACGTCCGCATCTTG-3′ | ||
| hGMRβ | 5′-AGGTCACCAAGGAACAATCCT-3′ | |
| 5′-TTTGAAGAGCTGAATGA-3′ | ||
| EPOR | 5′-GCACTGAGTGTGTTCTG-3′ | |
| 5′-GCCTCACACTCTTCACC-3′ | ||
| 5′-GCTGCTAAAGCGCATGC-3′ | ||
| α-globin | 5′-CTCTCTGGGGAAGACAAAAGCAAC-3′ | 19 |
| 5′-GGTGGCTAGCCAAGGTCACCAGCA-3′ | ||
| β-major globin | 5′-CTGACAGATGCTCTCTTTGGG-3′ | 19 |
| 5′-CACAACCCCAGAAACAGACA-3′ | ||
| βH1 globin | 5′-AGTCCCCATGGAGTCAAAGA-3′ | 19 |
| 5′-CTCAAGGAGACCTTTGCTCA-3′ | ||
| ε-globin | 5′-GGAGAGTCCATTAAGAACCTAGACAA-3′ | 19 |
| 5′-CTGTGAATTCATTGCCGAAGTGAC-3′ | ||
| ζ-globin | 5′-GCTCAGGCCGAGCCCATTGG-3′ | 19 |
| 5′-TAGCGGTACTTCTCAGTCAG-3′ | ||
| GATA-1 | 5′-CATTGGCCCCTTGTGAGGCCAGAGA-3′ | 20 |
| 5′-ACCTGATGGAGCTTGAAATAGAGGC-3′ | ||
| EKLF | 5′-TCGCCGGAGACGCAGGCT-3′ | 19 |
| 5′-CCCAGTCCTTGTGCAGGA-3′ | ||
| c-Myb | 5′-GAGCTTGTCCAGAAAATATGGTCCGAAG-3′ | 19 |
| 5′-GGCTGCCGCAGCCGGCTGAGGGGAC-3′ |
hGMR indicates human granulocyte-macrophage colony-stimulating factor receptor; EPOR, erythropoietin receptor; EKLF, erythroid Kruppel-like factor.
The primer sets are described in the References listed.
In vitro culture
Methylcellulose clonal culture was prepared by means of a modification of a technique reported previously.14Briefly, 1 mL culture medium containing 1 × 104 E12.5 FL cells, αMEM, 1.2% methlycellulose (Shinetsu Chemical, Tokyo, Japan), 30% fetal bovine serum (FBS) (Hyclone Laboratories, Logan, UT), 1% deionized fraction V bovine serum albumin (BSA) (Sigma Chemical, St Louis, MO), 100 μM mercaptoethanol (Eastman Organic Chemicals, Rochester, NY), and cytokines was plated in 35-mm suspension culture dishes (#1008, Becton Dickinson Labware, Franklin Lakes, NJ). In a culture of E8.0 YS cells, 1 mL culture medium contained one half of whole YS cells obtained from one embryo because of a small quantity of available YS cells. Serum-free culture was prepared as described elsewhere.15 The culture medium contained 1% deionized crystallized globulin-free BSA (Calbiochem-Behring, La Jolla, CA), 300 μg/mL fully iron-saturated human transferrin (Sigma Chemical, St Louis, MO), 96 μg/mL cholesterol (Nacalai Tesque, Kyoto, Japan), and 100 nM sodium selenite (Sigma Chemical), instead of FBS and fraction V BSA. The dishes were incubated at 37°C in a humidified atmosphere flushed with 5% O2 and 5% CO2. Colony formation was monitored at appropriate times: day 3 for erythroid colonies and day 7 for erythroid bursts, erythrocyte-containing mixed hematopoietic colonies, and nonerythroid colonies such as granulocyte-macrophage and mast cell colonies. To assess the accuracy of in situ identification of the colonies, individual colonies were lifted by means of an Eppendorf micropipette under direct microscopic visualization, spread on glass slides with the use of a cytocentrifuge (Cytospin 2; Shandon, Pittsburgh, PA), and then stained with May-Grunwald-Giemsa (Muto Chemica, Tokyo, Japan). E8.0 YS cells or E12.5 FL cells were also incubated in suspension culture as reported previously.14 One milliliter of culture mixture containing 1 × 106 cells per milliliter of E12.5 FL cells, one third of whole E8.0 YS cells obtained from one embryo or their progenies in the culture, αMEM, 10% FBS, 1% deionized fraction V BSA, 100 μM mercaptoethanol, and designated cytokines was incubated in 12-well tissue plates (Nunc, Roskilde, Denmark) for 3 days at 37°C in a humidified atmosphere flushed with 5% O2and 5% CO2.
Cytokines used in the present study were human EPO, hGM-CSF, mouse stem cell factor (SCF), and mouse IL-3, which were kindly provided by Kirin Brewery (Tokyo, Japan). All the cytokines were pure recombinant molecules and were used at concentrations that induced an optimal response in methylcellulose clonal culture of murine adult bone marrow cells. These concentrations are 2 U/mL for human EPO, 100 ng/mL for mouse SCF, and 20 ng/mL for mouse IL-3.15-17 To determine the optimal concentration of hGM-CSF, methylcellulose culture was prepared with various concentrations (80, 10, 10/8, 10/82, 10/83, and 0 ng/mL).
Analysis of adult and embryonic globin chains and transcription factors
Poly(adenylic acid) (poly[A]) messenger RNA (mRNA) was prepared from single colonies harvested on day 7 of methylcellulose culture or E8.0 YS and E12.5 FL mouse embryo cells stimulated by hGM-CSF or EPO for 3 days, by means of a commercial kit (QuickPrep Micro mRNA Purification Kit, Amersham Pharmacia Biotech, Buckinghamshire, United Kingdom). For RT-PCR, first-strand complementary DNA (cDNA) synthesis with the use of random hexamer pd(N)6 (Pharmacia Biotech, Cambridge, United Kingdom) and Molony murine leukemia virus reverse transcriptase first-strand cDNA synthesis kit (Toyobo Biochemicals, Tokyo, Japan) was carried out as described elsewhere,18with the use of poly(A) mRNA isolated from single colonies or cultured cells. The PCR reaction mixtures contained quantities of cDNA equivalent to 1 × 10−6, 10−5, 10−4, 10−3, 10−2, and 10−1 of those derived from 1 × 105hGMR-expressing EPOR−/− or EPOR+/+ FL cells, one third of whole YS cells obtained from one hGMR-expressing EPOR+/+ mouse embryos or their progenies in the culture. The PCR reaction was carried out with the use of the primer sets shown in Table 1. Reactions were controlled by including amplification primers for mouse β-actin, and were incubated at 94°C for 30 seconds, 55-65°C for 1 minute, and 72°C for 2 minutes. PCR products were run on a 1.5% agarose gel and made visible by ethidium bromide (0.5 μg/mL) staining.
Results
Colony formation by hGM-CSF and EPO from E12.5 FL cells of hGMR-expressing EPOR−/−mouse embryo
We crossed hGMR-Tg mice with heterozygous EPOR mutant mice and performed genotypic analysis by PCR with the use of genomic DNA of generated embryos. The numbers of E12.5 embryos differentiated into 6 genotypes were as follows: 5 hGMR+/−(hGMRα+/− hGMRβ+/−)EPOR−/−; 12 hGMR+/−EPOR+/−; 6 hGMR+/−EPOR+/+; 4 hGMR−/−(hGMRα−/−hGMRβ−/−)EPOR−/−; 13 hGMR−/−EPOR+/−; and 5 hGMR−/−EPOR+/+ embryos. We then isolated E12.5 FL cells from EPOR+/+hGMR−/−, EPOR+/+hGMR+/−, EPOR−/−hGMR−/−, and EPOR−/−hGMR+/− embryos. FL cells of EPOR+/+ embryos contained both nucleated primitive erythrocytes and nonnucleated definitive erythrocytes, but most of the erythrocytes in FL cells of EPOR−/− embryos were nucleated primitive erythrocytes.
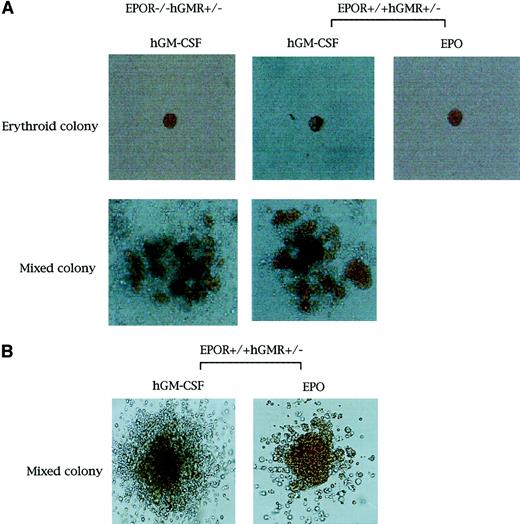
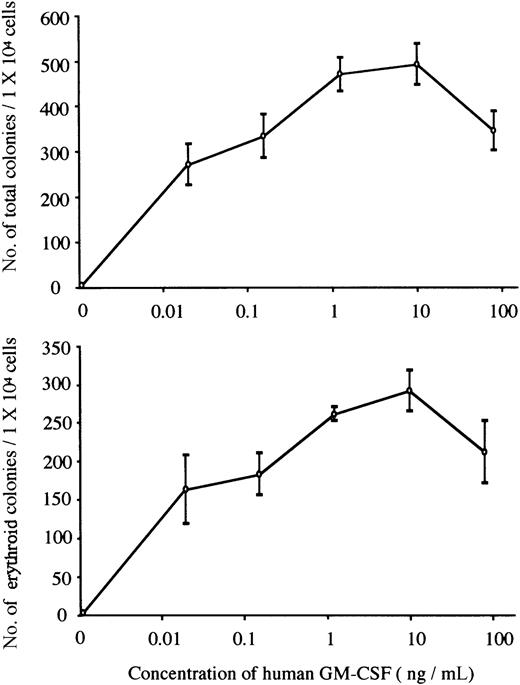
We carried out methylcellulose culture of 1 × 104 E12.5 FL cells with EPO (2 U/mL) or hGM-CSF (10 ng/mL) (Table2; Figure1A). EPO, but not hGM-CSF, stimulated erythroid colony formation in EPOR+/+hGMR−/−embryos. EPO and hGM-CSF induced the formation of similar numbers of erythroid colonies in EPOR+/+ hGMR+/−embryos, although hGM-CSF also induced the formation of erythroid bursts and mixed hematopoietic and nonerythroid colonies, such as granulocyte-macrophage and mast cell colonies. While neither EPO nor hGM-CSF induced erythroid colony formation in EPOR−/−hGMR−/− embryos, hGM-CSF, but not EPO, stimulated the formation of various types of colonies, including erythroid colonies, in EPOR−/−hGMR+/+embryos. This result indicates that hGM-CSF can induce the proliferation and differentiation of FL EPOR−/− erythroid progenitors as long as they express hGMR. To confirm this, FL cells of EPOR−/− hGMR+/− embryos were cultured with various concentrations of hGM-CSF. As shown in Figure2, hGM-CSF stimulated the formation of total and erythroid colonies in a dose-dependent manner. Since the numbers of total and erythroid colonies were maximal at 10 ng/mL hGM-CSF, we used 10 ng/mL hGM-CSF in subsequent experiments.
Colony formation from fetal liver cells by EPO and hGM-CSF
| EPOR . | hGMR . | Cytokine . | No. of colonies/104 fetal liver cells . | ||||
|---|---|---|---|---|---|---|---|
| E . | B . | E-mix . | Non-erythroid . | Total . | |||
| Serum-containing culture | |||||||
| +/+ | −/− | EPO | 488 ± 45 | 0 | 0 | 0 | 488 ± 45 |
| hGM-CSF | 0 | 0 | 0 | 0 | 0 | ||
| +/+ | +/− | EPO | 373 ± 72 | 0 | 0 | 9 ± 6 | 383 ± 78 |
| hGM-CSF | 291 ± 26 | 4 ± 0 | 17 ± 6 | 209 ± 2 | 521 ± 20 | ||
| −/− | −/− | EPO | 0 | 0 | 0 | 0 | 0 |
| hGM-CSF | 0 | 0 | 0 | 4 ± 0 | 4 ± 0 | ||
| −/− | +/− | EPO | 0 | 0 | 0 | 5 ± 2 | 5 ± 2 |
| hGM-CSF | 264 ± 38 | 5 ± 2 | 17 ± 2 | 204 ± 17 | 491 ± 47 | ||
| Serum-free culture | |||||||
| +/+ | +/− | EPO | 189 ± 22 | 0 | 0 | 0 | 189 ± 22 |
| hGM-CSF | 169 ± 19 | 4 ± 0 | 9 ± 6 | 130 ± 23 | 311 ± 27 | ||
| −/− | +/− | EPO | 0 | 0 | 0 | 0 | 0 |
| hGM-CSF | 152 ± 9 | 6 ± 2 | 9 ± 1 | 135 ± 11 | 302 ± 10 | ||
| EPOR . | hGMR . | Cytokine . | No. of colonies/104 fetal liver cells . | ||||
|---|---|---|---|---|---|---|---|
| E . | B . | E-mix . | Non-erythroid . | Total . | |||
| Serum-containing culture | |||||||
| +/+ | −/− | EPO | 488 ± 45 | 0 | 0 | 0 | 488 ± 45 |
| hGM-CSF | 0 | 0 | 0 | 0 | 0 | ||
| +/+ | +/− | EPO | 373 ± 72 | 0 | 0 | 9 ± 6 | 383 ± 78 |
| hGM-CSF | 291 ± 26 | 4 ± 0 | 17 ± 6 | 209 ± 2 | 521 ± 20 | ||
| −/− | −/− | EPO | 0 | 0 | 0 | 0 | 0 |
| hGM-CSF | 0 | 0 | 0 | 4 ± 0 | 4 ± 0 | ||
| −/− | +/− | EPO | 0 | 0 | 0 | 5 ± 2 | 5 ± 2 |
| hGM-CSF | 264 ± 38 | 5 ± 2 | 17 ± 2 | 204 ± 17 | 491 ± 47 | ||
| Serum-free culture | |||||||
| +/+ | +/− | EPO | 189 ± 22 | 0 | 0 | 0 | 189 ± 22 |
| hGM-CSF | 169 ± 19 | 4 ± 0 | 9 ± 6 | 130 ± 23 | 311 ± 27 | ||
| −/− | +/− | EPO | 0 | 0 | 0 | 0 | 0 |
| hGM-CSF | 152 ± 9 | 6 ± 2 | 9 ± 1 | 135 ± 11 | 302 ± 10 | ||
Values are mean ± SD of triplicate plates containing 1 × 104 fetal liver cells of EPO+/+hGMR−/−, EPO+/+hGMR+/−, EPO−/−hGMR−/−, EPO−/−hGMR+/− embryos. The following quantities were used: hGM-CSF, 10 ng/mL; EPO, 2 U/mL.
E indicates erythroid colonies; B, erythroid bursts; E-mix, mixed hematopoietic colonies. See Table 1 for other abbreviations.
Appearance of erythroid and erythrocyte-containing mixed hematopoietic colonies.
Erythroid and erythrocyte-containing mixed hematopoietic colonies (original magnification, × 400 and × 100, respectively. (A) From E12.5 FL cells of EPOR−/−hGMR+/−embryos induced by hGM-CSF, and EPOR+/+hGMR+/−embryos induced by hGM-CSF or EPO. (B) From E8.0 YS cells of EPOR+/+hGMR+/− embryo induced by hGM-CSF or EPO.
Appearance of erythroid and erythrocyte-containing mixed hematopoietic colonies.
Erythroid and erythrocyte-containing mixed hematopoietic colonies (original magnification, × 400 and × 100, respectively. (A) From E12.5 FL cells of EPOR−/−hGMR+/−embryos induced by hGM-CSF, and EPOR+/+hGMR+/−embryos induced by hGM-CSF or EPO. (B) From E8.0 YS cells of EPOR+/+hGMR+/− embryo induced by hGM-CSF or EPO.
The formation of total and erythroid colonies from E12.5 FL cells of hGMR-expressing EPOR−/− embryo at various concentrations of hGM-CSF.
Data represent the means ± SD of triplicate cultures.
The formation of total and erythroid colonies from E12.5 FL cells of hGMR-expressing EPOR−/− embryo at various concentrations of hGM-CSF.
Data represent the means ± SD of triplicate cultures.
We further carried out serum-free methylcellulose clonal culture of hGMR+/−EPOR−/− or hGMR+/−EPOR+/+ FL cells to exclude the possibility that the ability of hGM-CSF to promote colony formation by hGMR-expressing erythroid progenitors is due to the combined actions of hGM-CSF and unknown factors contained in FBS. As shown in Table 2, the result was much the same as with FBS-containing cultures. The number, size, and degree of hemoglobinization of EPOR−/−erythroid colonies supported by hGM-CSF were not different from those by EPO or hGM-CSF in mouse embryos expressing hGM-CSFR and EPOR.
Globin gene expression in erythrocyte-containing colonies formed from hGMR-expressing EPOR+/+or EPOR−/− FL cells by hGM-CSF
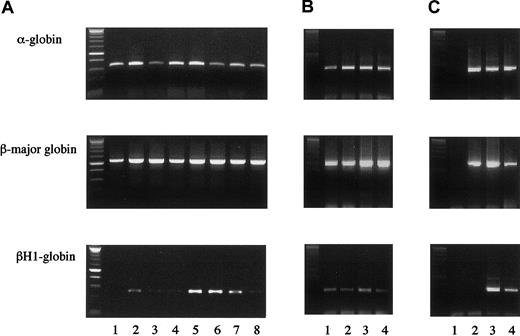
We next examined whether erythrocyte-containing colonies formed from hGMR-expressing EPOR+/+ or EPOR−/− FL cells by hGM-CSF originate from primitive or definitive hematopoiesis. We then randomly chose 4 erythroid bursts/erythrocyte-containing mixed colonies formed from hGMR-expressing EPOR+/+ E12.5 FL cells by hGM-CSF and analyzed the expression of globins in each colony (Figure 3A, lanes 1 to 4). While 1 colony (lane 2) expressed both α- and β-major globins, adult globins, and the embryonic globin βH1, 3 colonies (lanes 1, 3, and 4) expressed α- and β-major globins, but no or little βH1 globin. We also analyzed 4 erythroid bursts/erythrocyte-containing mixed colonies formed from hGMR-expressing EPOR−/− E12.5 FL cells by hGM-CSF (Figure 3A, lanes 5 to 8). Three colonies (lanes 5 to 7) expressed both adult and embryonic globins, and one colony expressed adult globins and a low level of βH1 globin. This result indicates that definitive erythroid progenitors are present even in EPOR−/− embryos, although mature erythrocytes were undetectable, in accordance with previous reports,7-9 and that hGM-CSF stimulates both definitive and primitive erythroid progenitors lacking EPOR but expressing hGMR.
Globin gene expression in single erythrocyte-containing colonies.
(A) Colonies formed by hGM-CSF from E12.5 FL cells of hGMR-expressing EPOR+/+ embryos (lanes 1 to 4) and EPOR−/−embryos (lanes 5 to 8). (B) Colonies formed by hGM-CSF from E8.0 YS cells of hGMR-expressing EPOR+/+ embryos. (C) Globin gene expression of a granulocyte-macrophage colony formed by hGM-CSF from E12.5 FL cells of hGMR-expressing EPOR+/+ embryos as a negative control (lane 1), adult bone marrow cells (lane 2), E12.5 FL cells (lane 3), and E9.5 YS cells of hGMR-expressing mice (lane 4).
Globin gene expression in single erythrocyte-containing colonies.
(A) Colonies formed by hGM-CSF from E12.5 FL cells of hGMR-expressing EPOR+/+ embryos (lanes 1 to 4) and EPOR−/−embryos (lanes 5 to 8). (B) Colonies formed by hGM-CSF from E8.0 YS cells of hGMR-expressing EPOR+/+ embryos. (C) Globin gene expression of a granulocyte-macrophage colony formed by hGM-CSF from E12.5 FL cells of hGMR-expressing EPOR+/+ embryos as a negative control (lane 1), adult bone marrow cells (lane 2), E12.5 FL cells (lane 3), and E9.5 YS cells of hGMR-expressing mice (lane 4).
Colony formation by hGM-CSF and EPO from E8.0 YS cells of mouse embryos expressing hGMR
To compare the effects of hGM-CSF and EPO on primitive erythropoiesis in hGMR-expressing mouse embryos, we carried out the methylcellulose clonal culture of E8.0 YS cells with hGM-CSF or EPO (Table 3). The hGM-CSF and EPO stimulated the formation of similar numbers of erythroid bursts and erythrocyte-containing mixed colonies. RT-PCR showed that all 4 erythroid bursts/erythrocyte-containing mixed colonies formed by hGM-CSF and chosen randomly contained both adult and embryonic globins, confirming that these colonies were derived from primitive erythropoiesis (Figure 3B).
Colony formation from E8.0 yolk sac cells of hGMR-expressing embryo
| Cytokine . | No. of colonies/½ yolk sac cells . | |||
|---|---|---|---|---|
| B . | E-mix . | Non-erythroid . | Total . | |
| EPO | 16 ± 4 | 18 ± 4 | 3 ± 1 | 36 ± 7 |
| GM-CSF | 16 ± 2 | 14 ± 4 | 9 ± 7 | 38 ± 9 |
| Cytokine . | No. of colonies/½ yolk sac cells . | |||
|---|---|---|---|---|
| B . | E-mix . | Non-erythroid . | Total . | |
| EPO | 16 ± 4 | 18 ± 4 | 3 ± 1 | 36 ± 7 |
| GM-CSF | 16 ± 2 | 14 ± 4 | 9 ± 7 | 38 ± 9 |
Values are mean ± SD of 3 samples.
The following quantities were used: hGM-CSF, 10 ng/mL; EPO, 2 U/mL; rSCF, 100 ng/m; mIL-3, 20 ng/mL.
Globin gene expression in E12.5 FL and E8.0 YS cells of hGMR-Tg mice
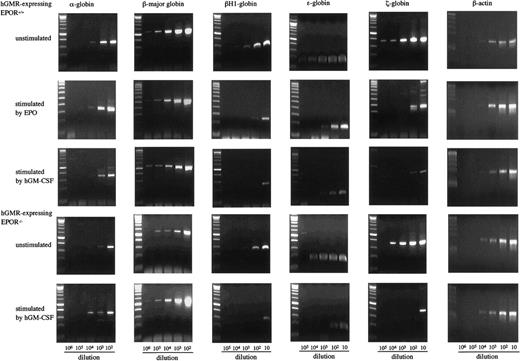
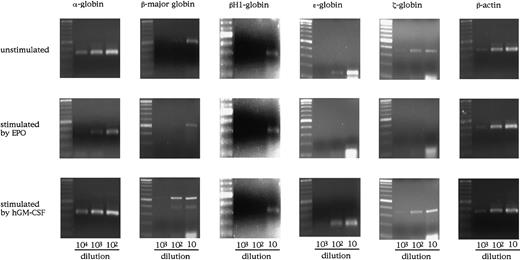
To examine the effects of hGM-CSF and EPO on the differentiation of embryonic erythroid cells, we analyzed the adult globins α- and β-major globins and the embryonic globins, βH1- ε- and ζ-globins by semiquantitative RT-PCR using E12.5 FL cells of hGMR-expressing EPOR+/+ and EPOR−/− embryos. Representative results are presented in Figure4. The α- and β-major globin mRNAs were detected at higher levels in hGMR-expressing EPOR+/+than in EPOR−/− FL cells, and embryonic globin levels were similar or slightly higher in EPOR+/+ FL cells. When hGMR-expressing EPOR+/+ FL cells were stimulated by EPO or hGM-CSF for 3 days in suspension culture, a similar change in the globin expression was observed. The expression of α- and β-major globins showed no remarkable changes or was slightly higher as compared with unstimulated cells, but embryonic globins decreased. When hGMR-expressing EPOR−/− FL cells were stimulated by hGM-CSF for 3 days, the increase of α- and β-major globin RNA levels and the decrease of embryonic globin RNA levels were also observed. This result indicated that hGM-CSF, as well as EPO, induces adult globin expression, but suppresses embryonic globin expression in FL erythropoiesis of hGMR-Tg mouse embryo.
Globin gene expression in E12.5 FL cells of hGMR-expressing EPOR+/+ embryos unstimulated and stimulated by hGM-CSF and EPO, and hGMR-expressing EPOR−/− embryos unstimulated and stimulated by hGM-CSF.
The numbers below each lane indicate the relative dilutions of cDNA prepared from 105 cells. Size marker: 100 base-pair (bp) ladder.
Globin gene expression in E12.5 FL cells of hGMR-expressing EPOR+/+ embryos unstimulated and stimulated by hGM-CSF and EPO, and hGMR-expressing EPOR−/− embryos unstimulated and stimulated by hGM-CSF.
The numbers below each lane indicate the relative dilutions of cDNA prepared from 105 cells. Size marker: 100 base-pair (bp) ladder.
As shown in Figure 3A, however, E12.5 FL cells of hGMR-expressing mouse embryo contained both primitive and definitive erythroid progenitors whether they expressed EPOR or not. Therefore, to examine the effect of hGM-CSF on globin expression by primitive erythroid cells in more detail, we analyzed the expression of globins in E8.0 YS cells of hGMR-expressing EPOR+/+ embryos (Figure5). YS cells expressed all of the globins examined, including adult globin. When YS cells were cultured with hGM-CSF or EPO for 3 days, they induced the expression of globins in a different fashion. The expression of β-major globin was increased by hGM-CSF as well as in the culture of E12.5 FL cells, although EPO showed no remarkable effect on the expression. On the other hand, embryonic globin expression was rather suppressed by EPO.
Globin gene expression in E8.0 YS cells of hGMR-expressing EPOR+/+ embryos unstimulated and stimulated by hGM-CSF and EPO.
The numbers below each lane indicate the relative dilutions of cDNA from one third of whole YS cells obtained from one embryo or their progenies in the culture. Size marker: 100-bp ladder.
Globin gene expression in E8.0 YS cells of hGMR-expressing EPOR+/+ embryos unstimulated and stimulated by hGM-CSF and EPO.
The numbers below each lane indicate the relative dilutions of cDNA from one third of whole YS cells obtained from one embryo or their progenies in the culture. Size marker: 100-bp ladder.
Gene expression of transcription factors in FL cells of hGMR-Tg mice
We also examined the effects of hGM-CSF and EPO on the expression of GATA-1, erythroid Kruppel-like factor (EKLF), and cMyb in E12.5 FL cells of hGMR-expressing EPOR+/+ and EPOR−/−embryos by semiquantitative RT-PCR (Figure6). The expression of GATA-1 in hGMR-expressing EPOR−/− and EPOR+/+ FL cells was similar, but the expressions of EKLF and cMyb were higher in EPOR+/+ embryos. EPO and hGM-CSF showed a similar effect on the expression of transcription factors in hGMR-expressing EPOR+/+ FL cells. Both induced an increase of GATA-1 and a decrease of cMyb. The EKLF expression slightly decreased. When hGMR-expressing EPOR−/− FL cells were stimulated by hGM-CSF for 3 days, the expression of GATA-1 and EKLF increased, but that of cMyb decreased.
Expression of transcription factors GATA-1, EKLF, and cMyb in E12.5 FL cells of hGMR-expressing EPOR+/+ embryos unstimulated and stimulated by hGM-CSF and EPO, and hGMR-expressing EPOR−/− embryos unstimulated and stimulated by hGM-CSF.
The numbers below each lane indicate the relative dilutions of cDNA prepared from 105 cells. Size marker: 100 bp ladder.
Expression of transcription factors GATA-1, EKLF, and cMyb in E12.5 FL cells of hGMR-expressing EPOR+/+ embryos unstimulated and stimulated by hGM-CSF and EPO, and hGMR-expressing EPOR−/− embryos unstimulated and stimulated by hGM-CSF.
The numbers below each lane indicate the relative dilutions of cDNA prepared from 105 cells. Size marker: 100 bp ladder.
Discussion
We previously showed that hGM-CSF can stimulate colony formation from erythroid progenitors in adult bone marrow of Tg mice expressing hGMR.11However, it remains unclear whether the activity of hGM-CSF in this process is mediated by signaling through EPOR. Stopka et al21 demonstrated that erythroid bursts derived from human peripheral blood or bone marrow cells were positive for EPO mRNA, suggesting that erythroid cells proliferate and differentiate in an autocrine manner. It was also reported that β chain associates with EPOR in a murine factor–dependent cell line (Ba/F3) transfected with murine EPOR.22 Furthermore, there is a possibility that β-chain signal interacts with EPOR signal, since kit, a receptor for SCF, was shown to induce phosphorylation of EPOR.23 24 To address this problem, we generated a mouse embryo lacking EPOR, but expressing hGMR.
With regard to this issue, 2 experiments were recently conducted. Socolovsky et al25 have reported that a retrovirally transduced prolactin receptor efficiently supports the differentiation of BFU-E and CFU-E progenitors in FL of EPOR−/− mouse embryos in response to prolactin. Kieran et al8 have shown that a signal transmitted through the thrombopoietin (TPO) receptor MPL, which some FL EPOR−/− erythroid progenitor cells express, stimulates proliferation and terminal differentiation of these progenitors in response to exogenous TPO in combination with either SCF or IL-3 plus IL-11. These reports indicate that the surrogate signal can to some extent rescue erythropoiesis in FL of EPOR−/−mouse embryos, but neither study could clarify the effects of the surrogate signal on primitive and definitive erythropoiesis or the difference in function between the EPO signal and the surrogate signal. In the present study, using generated hGMR-expressing EPOR−/− embryos, we showed that hGM-CSF stimulates the colony growth of primitive and definitive erythroid progenitors. We also showed that hGM-CSF and EPO have a similar effect on the differentiation of definitive, but not primitive, erythroid progenitors in hGMR-expressing embryo.
When E8.0 YS and E12.5 FL cells of hGMR-expressing embryo were cultured with hGM-CSF, various types of colonies were formed in accordance with our previous report that hGM-CSF stimulates various lineages of hematopoietic progenitors in adult bone marrow.11 These results indicate that hGM-CSF functions as a proliferation-promoting factor of hematopoietic progenitors as long as they express hGMR in adult and embryonic hematopoiesis. In particular, analysis of globin types expressed in individual erythrocyte-containing colonies formed from hGMR-expressing EPOR−/− FL cells showed that hGM-CSF stimulates the proliferation of primitive and definitive erythroid progenitors independent of the signal through EPOR. Serum-free culture further confirmed that hGM-CSF signal alone is sufficient for the proliferation of hGMR-expressing embryonic erythroid progenitors. The proliferative activity of hGM-CSF was comparable to that of EPO in hGMR-expressing EPOR+/+ embryos as evaluated from the erythroid colony formation.
We evaluated the differentiation of erythroid cells by examining the expression of globins. Kieran et al8 showed the absence of β-major globin in EPOR−/− FL samples and the requirement of EPOR for β-globin expression. However, using semiquantitative RT-PCR, our system showed that hGMR-expressing EPOR−/− FL cells contained low levels of α- and β-major globin mRNA, as reported previously by Lin et al,9 and that hGM-CSF induced the increase of α- and β-major globin expression and the decrease of embryonic globin expression in the FL cells containing both primitive and definitive erythroid progenitors. Similar changes of globin expression in E12.5 FL cells were induced by hGM-CSF and EPO in hGMR-expressing EPOR+/+ embryos. On the other hand, hGM-CSF induced the increase of adult globin expression in E8.0 YS cells of hGMR-expressing EPO+/+ embryos whereas EPO did not. Since EPO is not essential for primitive erythropoiesis as shown in EPO- or EPOR-deficient mice, hGM-CSF may mimic other cytokines that play a crucial role in primitive erthropoiesis. In any case, hGM-CSF has the effect on the globin synthesis in erythropoiesis of hGMR-expressing mouse embryos.
The differentiation of erythroid cells was also evaluated on the basis of the expression of transcription factors. It was shown that RNA encoding GATA-1 and EKLF was expressed normally in EPOR−/− embryos.8,9 The present study also demonstrated the expression of both in EPOR−/− FL cells although GATA-1 expression was similar to, and EKLF and cMyb expression was lower than, that in EPOR+/+ FL cells. This result is incompatible with previous reports that GATA-1 plays an important role in both primitive and definitive erythropoiesis26-29 and that EKLF and cMyb function predominantly in definitive erythropoiesis.30-32 The hGM-CSF and EPO showed similar effects on the expression of the 3 transcription factors in hGMR-expressing FL cells. Both induced an increase of GATA-1 expression and a decrease of EKLF and cMyb expression, suggesting that the former functions at a later stage and the latter at an earlier stage in the differentiation of erythroid cells. Interestingly, however, hGM-CSF induced an increase of EKLF expression in hGMR-expressing EPOR−/− FL cells, although GATA-1 and cMyb expressions showed changes similar to those in hGMR-expressing EPOR+/+FL cells. Since a number of definitive erythroid cells arrested at the progenitor stage were present in EPOR−/− FL cells, EKLF may function predominantly at this stage.
All together, the results of present study demonstrated that hGM-CSF can stimulate the proliferation and differentiation of primitive and definitive erythroid progenitors independent of EPOR signal if they express hGMR. The prolactin receptor and MPL also have been demonstrated to promote the growth of EPOR−/− erythroid progenitors.8,25 The hGMR and these receptors activate signaling molecules similar to EPOR, such as Janus kinase 2 and signal transducer and activator of transcription 5.33-37Therefore, cytokine receptors, which activate a set of downstream signaling molecules similar to those activated by EPOR, may be able to substitute for EPOR. On the other hand, Wessely et al38analyzed the effect of hGMR signal on erythropoiesis using avian hematopoietic progenitors and found that hGMR only partially substituted for the activities of EPOR. We also observed the differential effects between hGM-CSF and EPO on primitive erythropoiesis of hGMR-expressing EPOR+/+ mice. These observations suggest that there may be a difference in the molecules related to erythropoiesis among species, or between primitive and definitive hematopoiesis. Our mouse model expressing hGMR and/or EPOR should provide a useful tool for the study of the molecular mechanism regulating mammalian embryonic erythropoiesis. We are now examining whether hGM-CSF can rescue the embryonic lethality of hGMR-expressing EPOR−/− mice in vivo.
Supported by the Program for Promotion of Fundamental Studies in Health Sciences of the Organization for Pharmaceutical Safety and Research of Japan.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Kohichiro Tsuji, Department of Clinical Oncology, The Institute of Medical Science, The University of Tokyo, 4-6-1 Shirokanedai, Minato-ku, Tokyo 108-8639, Japan; e-mail:tsujik@ims.u-tokyo.ac.jp.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal