Abstract
After non-T-cell–depleted allogeneic hematopoietic stem cell transplantation (HSCT), both alloreactive and homeostatic signals drive proliferation of donor T cells. Host-reactive donor T cells, which proliferate on alloantigen stimulation, are responsible for the life-threatening graft-versus-host disease. Non–host-reactive donor T cells, which proliferate in response to homeostatic signals, contribute to the beneficial peripheral T-cell reconstitution. The elimination of alloreactive T cells is a major therapeutic challenge for HSCT and would greatly benefit from their specific identification. After T-cell transfer in lymphopenic recipients, the present results show that alloreactive T cells rapidly divided; up-regulated CD69, CD25, and CD4 molecules; and down-regulated CD62L. In contrast, nonalloreactive T cells started to divide later and did not up-regulate CD69, CD25, and CD4. Thus, these 2 cell populations can be effectively discriminated. This should facilitate the specific depletion of alloreactive T cells in allogeneic HSCT.
Introduction
After non-T-cell–depleted allogeneic hematopoietic stem cell transplantation (HSCT), T cells transferred together with hematopoietic stem cells in lymphopenic recipients can proliferate on different types of stimulation. Host-reactive donor T cells proliferate on alloantigen stimulation and are responsible for the major complication encountered after allogeneic HSCT, that is, graft-versus-host disease.1 In lymphopenic recipients, donor T cells also undergo homeostasis-driven proliferation,2-6 and likewise contribute to the immune reconstitution that protects against opportunistic infectious agents. Thus, within the transferred donor T cells, deleterious alloreactive T cells should be eliminated, whereas the beneficial nonalloreactive T cells should be spared. The discrimination between these different cells should allow therapeutic intervention and remains a major challenge. To address this question, we compared the characteristics of allogeneic versus homeostasis-driven T-cell proliferation by analyzing kinetics of cell division and phenotypic changes that occur in donor CD8+ and CD4+ T cells, after transfer in semiallogeneic or syngeneic lymphopenic mice.
Study design
Mice
The C57BL/6 (B6, H-2b) and (B6xDBA2)F1 (B6/D2, H-2bxd) mice were obtained from Iffa Credo (L'Arbresle, France). Human (h) CD4 transgenic mice express the hCD4 protein under the control of regulatory sequences of the hCD4 gene. Transgene expression is selectively observed at the cell surface of mouse (m) CD4 and mCD8 cells.7 Mice were manipulated according to European Economic Community guidelines.
Experimental model
The T cells (107) collected from spleen and lymph nodes of B6 hCD4 transgenic mice were labeled with carboxyfluorescein diacetate succinimidyl ester (CFSE) and injected into syngeneic (B6) or semiallogeneic (B6/D2), lethally irradiated mice (11 Gy). At different time points after T-cell transfer, splenocytes from grafted animals were collected and incubated with 2.4 G2 anti-Fc receptor monoclonal antibody (mAb). Cells were stained with combinations of the following mAbs: phycoerythrin-labeled anti-CD4 and anti-CD8 (BD Pharmingen, San Diego, CA), allophycocyanin-labeled anti-hCD4 (Caltag Laboratories, San Francisco, CA), biotinylated anti-CD44 and anti-CD25 (Caltag), and anti-CD62L and anti-CD69 (Pharmingen). Biotinylated mAbs were revealed with tricolor-labeled streptavidin (Caltag). To detect intracellular interferon-γ (IFN-γ), collected cells were incubated in the presence of anti-CD3 mAb and Golgi plug (BD Pharmingen) for 4 hours. The cells were then surface stained with antibodies to hCD4 and mCD4 or mCD8, then resuspended in Cytofix/Cytoperm (BD Pharmingen), and stained for intracellular IFN-γ (BD Pharmingen). Appropriate isotypic controls were used. Events were acquired on a FACSCalibur (Becton Dickinson, San Jose, CA) and analyzed using CellQuest software (Becton Dickinson). Cell proliferation was studied as the sequential loss of CFSE fluorescence on cell division after FACS analysis of the hCD4 population.
Results and discussion
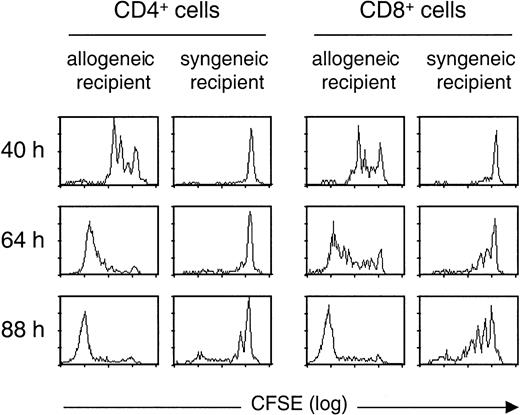
To identify donor T cells after their infusion in lethally irradiated recipients, we used T cells obtained from transgenic mice expressing a hCD4 marker molecule on both CD4+ and CD8+ T cells.7 Using CFSE staining of donor T cells, we analyzed T-cell division at 3 different time points following semiallogeneic or syngeneic transplantation (Figure1). When T cells were transferred in allogeneic recipients, both CD4+ and CD8+ T cells rapidly proliferated, with 3 rounds of division already visualized at 40 hours after infusion. At 88 hours, most donor T cells present in the spleen had divided more than 8 times. In contrast, when infused in irradiated syngeneic recipients, T cells divided more slowly. For CD4+ T cells, no division could be observed at 64 hours. At 88 hours, most of the CD4+ cells had made no or only one round of division, whereas a minor fraction had divided several times. Similarly, the kinetics of CD8+ T-cell division was delayed in syngeneic as compared to allogeneic recipients. These differences in cell division kinetics provide a first means to discriminate alloreactive T cells from other T cells proliferating on homeostatic signals in lymphopenic recipients.
CD4+ and CD8+ cells proliferate more rapidly in semiallogeneic than in syngeneic irradiated hosts.
CFSE-labeled T cells from hCD4 transgenic B6 mice were transferred into semiallogeneic (B6/D2) or syngeneic (B6) lethally irradiated mice. Data show proliferation (as examined by CFSE dilution pattern) of donor (hCD4+ gated) CD4+ and CD8+ T cells recovered from spleen of recipient mice at 3 different time points (40, 64, and 88 hours) following transfer. Similar results were obtained for 3 allogeneic and 3 syngeneic recipient mice at each time point.
CD4+ and CD8+ cells proliferate more rapidly in semiallogeneic than in syngeneic irradiated hosts.
CFSE-labeled T cells from hCD4 transgenic B6 mice were transferred into semiallogeneic (B6/D2) or syngeneic (B6) lethally irradiated mice. Data show proliferation (as examined by CFSE dilution pattern) of donor (hCD4+ gated) CD4+ and CD8+ T cells recovered from spleen of recipient mice at 3 different time points (40, 64, and 88 hours) following transfer. Similar results were obtained for 3 allogeneic and 3 syngeneic recipient mice at each time point.
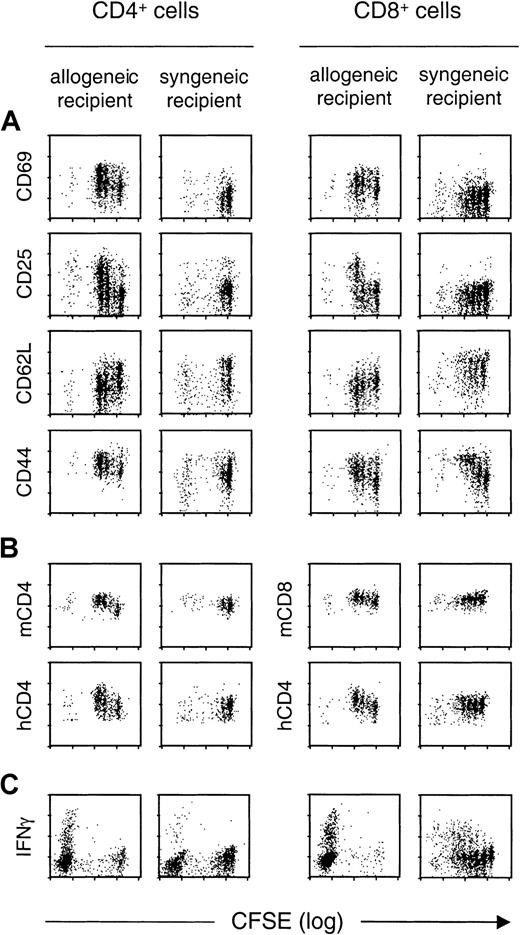
We next examined changes in the expression of various activation markers during allogeneic versus homeostatic T-cell proliferation, in correlation with cell division (Figure2A). Analyses were performed by flow cytometry from 40 hours to day 7 after infusion. To assess phenotypic changes for a similar number of divisions, we compared the results obtained at 40 hours and 88 hours after transfer in allogeneic or syngeneic hosts, respectively. In allogeneic hosts, cell divisions were associated with increased expression of activation markers for both CD4+ and CD8+ T cells: CD69 became overexpressed even before the first cell division occurred, whereas CD25 (the interleukin-2 receptor α chain) was up-regulated after the second cell division. In marked contrast, in syngeneic hosts, division of CD4+ and CD8+ T cells was not associated with increased expression of CD69 or CD25, even at day 7 at which 8 divisions could be observed (not shown).
Expression of T-cell phenotypic markers as a function of cell division during allogeneic versus homeostatic proliferation.
CFSE-labeled T cells from hCD4 transgenic B6 mice were transferred into semiallogeneic (B6/D2) or syngeneic (B6) lethally irradiated mice. CD4+ and CD8+ donor (hCD4+ gated) T cells recovered from spleens of recipient mice were analyzed for expression levels of (A) indicated T-cell activation markers, (B) mCD4 and mCD8 molecule, as well as the hCD4 marker for both cell types, and (C) intracellular IFN-γ production. The figure compares results obtained 40 hours after allogeneic transfer versus 88 hours after syngeneic transfer. For IFN-γ staining experiments (C), cells were collected from allogeneic or syngeneic recipients 8 days after transplantation. The control group was constituted by nonirradiated mice receiving syngeneic T cells in which no T-cell divisions were observed. In these mice, the level of expression for the different markers examined was comparable to the one observed on T cells freshly isolated from donor mice (not shown). Similar results were obtained in 3 allogeneic and 3 syngeneic recipient mice, at each time point.
Expression of T-cell phenotypic markers as a function of cell division during allogeneic versus homeostatic proliferation.
CFSE-labeled T cells from hCD4 transgenic B6 mice were transferred into semiallogeneic (B6/D2) or syngeneic (B6) lethally irradiated mice. CD4+ and CD8+ donor (hCD4+ gated) T cells recovered from spleens of recipient mice were analyzed for expression levels of (A) indicated T-cell activation markers, (B) mCD4 and mCD8 molecule, as well as the hCD4 marker for both cell types, and (C) intracellular IFN-γ production. The figure compares results obtained 40 hours after allogeneic transfer versus 88 hours after syngeneic transfer. For IFN-γ staining experiments (C), cells were collected from allogeneic or syngeneic recipients 8 days after transplantation. The control group was constituted by nonirradiated mice receiving syngeneic T cells in which no T-cell divisions were observed. In these mice, the level of expression for the different markers examined was comparable to the one observed on T cells freshly isolated from donor mice (not shown). Similar results were obtained in 3 allogeneic and 3 syngeneic recipient mice, at each time point.
We also analyzed markers commonly used to identify naive (CD62L) and memory (CD44) T cells (Figure 2A). CD62L was rapidly down-regulated on cell division on CD4+ and CD8+ T cells transferred into allogeneic hosts, whereas it started to be down-regulated only after 8 divisions in syngeneic hosts (not shown). CD44 was up-regulated on CD8+ T cells in both allogeneic and syngeneic recipients, whereas CD44 up-regulation was preferentially observed in the allogeneic setting for CD4+ T cells.
Recent reports have raised the possibility of identifying activated CD4+ T cells, on the basis of their increased expression of the CD4 molecule following antigen,8 or alloantigen9 stimulation. We indeed observed an up-regulation of mCD4 on cell division in the allogeneic but not the syngeneic setting (Figure 2B), whereas mCD8 expression was not increased on cell division. The hCD4 molecule, whose expression is under the control of CD4 regulatory sequences lacking the CD4 silencer, is expressed on both CD4+ and CD8+ T cells in the donor transgenic mice.7 Interestingly, after allogeneic stimulation, hCD4 was up-regulated on both T-cell types. Thus, CD4, which is specifically up-regulated in alloreactive CD4+ T cells, is another marker that could be used to track alloreactive T cells.
We next examined the production of IFN-γ by CD4+ and CD8+ donor T cells. Figure 2C shows that a large proportion of CD4+ and CD8+ T cells that had proliferated for 8 days in allogeneic recipients produced IFN-γ after in vitro stimulation for 4 hours with an anti-CD3 mAb. CD4+ and CD8+ T cells that had undergone homeostatic proliferation during 8 days also produced IFN-γ on restimulation in vitro. These results suggest that alloreactive T cells cannot be specifically identified by their expression of IFN-γ.
Several independent studies have analyzed T-cell proliferation after transfer to syngeneic or allogeneic hosts, in normal or lymphopenic mice.10-15 Our study compares for the first time the division kinetics and activation markers of a polyclonal population of T cells transferred in irradiated syngeneic or allogeneic hosts, an experimental model of T-cell behavior during HSCT. By analyzing kinetics of cell division, we identified a time frame—from transfer to 88 hours—during which alloreactive T cells have all divided several times while the majority of the nonalloreative T cells have not started to divide. This finding supports strategies of selective elimination of alloreactive T cells based on their rapid proliferation within the first 3 to 4 days after HSCT.16 17
Recent studies using clonal populations of CD8+ T cells from T-cell receptor transgenic mice have found that antigen, but not homeostasis-driven T-cell proliferation, is associated with up-regulation of activation markers such as CD25 and CD69.10,12 We confirmed and extended these results using the transfer of polyclonal T cells in lethally irradiated recipients, a setting closer to HSCT. Indeed, CD69 and CD25, but also CD62L and CD4, are clearly differentially expressed on allogeneic and homeostatic T-cell proliferations. Such differences suggest that strategies of ex vivo alloreactive depletion based on the expression of activation markers such as CD25,18-20 CD69,21 or both antigens22 on alloantigen-stimulated T cells could also be considered in vivo after T-cell transfer.
We acknowledge B. Bellier for assistance with the intracellular IFN-γ assay, S. Bruel and G. Gavory for their technical assistance, G. Boisserie and F. Baillet for the irradiation of mice, and O. Boyer and C. Frisen for careful reading of the manuscript.
Supported by Université Pierre et Marie Curie, Centre National de la Recherche Scientifique, Association Française contre la Myopathie, Association pour la Recherche contre les Déficits Immunitaires Viro-Induits, Génopoı̈étic S.A. J.L.C. is supported by La Fondation pour la Recherche Médicale.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
José L. Cohen, CNRS/UPMC ESA 7087, Hôpital Pitié-Salpêtrière, 83 bd de l'Hôpital, F-75651 Paris Cedex 13, France; e-mail:jose.cohen@chups.jussieu.fr.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal