Abstract
The zebrafish is a useful model organism for developmental and genetic studies. The morphology and function of zebrafish myeloid cells were characterized. Adult zebrafish contain 2 distinct granulocytes, a heterophil and a rarer eosinophil, both of which circulate and are generated in the kidney, the adult hematopoietic organ. Heterophils show strong histochemical myeloperoxidasic activity, although weaker peroxidase activity was observed under some conditions in eosinophils and erythrocytes. Embryonic zebrafish have circulating immature heterophils by 48 hours after fertilization (hpf). A zebrafish myeloperoxidase homologue (myeloid-specificperoxidase; mpx) was isolated. Phylogenetic analysis suggested it represented a gene ancestral to the mammalian myeloperoxidase gene family. It was expressed in adult granulocytes and in embryos from 18 hpf, first diffusely in the axial intermediate cell mass and then discretely in a dispersed cell population. Comparison of hemoglobinized cell distribution,mpx gene expression, and myeloperoxidase histochemistry in wild-type and mutant embryos confirmed that the latter reliably identified a population of myeloid cells. Studies in embryos after tail transection demonstrated that mpx- and peroxidase-expressing cells were mobile and localized to a site of inflammation, indicating functional capability of these embryonic granulocytes. Embryonic macrophages removed carbon particles from the circulation by phagocytosis. Collectively, these observations have demonstrated the early onset of zebrafish granulopoiesis, have proved that granulocytes circulate by 48 hpf, and have demonstrated the functional activity of embryonic granulocytes and macrophages. These observations will facilitate the application of this genetically tractable organism to the study of myelopoiesis.
Introduction
Zebrafish (Danio rerio) have emerged as a useful model organism for studying a wide variety of physiological systems. Approximately 26 zebrafish mutants have genetic lesions primarily affecting hematopoiesis. Most of these were recognized on the basis of anemia1,2; hence, it is not surprising that as the mutated genes underpinning these mutants were cloned, it was noted that they are genes primarily involved with erythropoiesis. Zebrafish mutants exist with lesions in genes encoding heme biosynthetic enzymes,3-5 a structural protein,6 and a novel iron transporter.7Another mutant has defective vasculogenetic and hematopoietic function,8 suggesting a genetic lesion at the level of the embryonic hemangioblast. The study of early hematopoietic commitment and erythropoiesis in these mutants has generated a useful range of reagents.9 10
Unlike erythropoiesis, which generates one mature cell type, myelopoiesis is a complex process that generates several cell types: monocytes–macrophages and several types of granulocytes. Teleosts, including cyprinids such as Danio, also have a process of multilineage myelopoiesis for host defense.11 However, less is known about zebrafish myelopoiesis than about erythropoiesis.
Macrophages have been recognized in zebrafish as early as the 13-somite stage (15 hours after fertilization [hpf]). They emerge from the anterior lateral plate mesoderm, migrate over the yolk sac, phagocytose cell corpses, and clear bacteria from the circulation.12Two markers of early macrophage commitment were characterized:draculin, which had an expression pattern overlapping that of early markers of erythroid commitment and also marked the rostral population of mobile macrophages, and L-plastin, which marked an early macrophage population as it spread over the yolk sac and a dispersed axial population of cells presumed to be tissue macrophages.12 A zebrafish homologue of c-fms, the receptor for colony-stimulating factor-1, has been isolated. Zebrafish csf1r shows several differences from its murine counterpart: in zebrafish, csf1r is expressed in neural crest cells and in macrophages; unlike the murineosteopetrosis mutant lacking colony-stimulating factor-1, the zebrafish csf1r mutant panther does not have adult macrophage deficiency.13
The kidney is the primary adult hematopoietic organ for zebrafish granulopoiesis14 and a site of granulopoiesis from as early as 96 hpf.15 Granulocytes circulate in adult and embryonic zebrafish.15,16 Like other teleosts,11 adult zebrafish have at least 2 granulocyte lineages.16 In the zoological literature, these are usually called heterophil or neutrophil granulocytes (presumed to be functionally orthologous with the mammalian neutrophil) and eosinophil granulocytes. Teleost basophil granulocytes are also described in some species.11,17 Tissue myeloblasts have been identified in zebrafish embryos on the second day of life in axial tissues in the vicinity of the yolk sac, apparently entering the circulation by 34 hpf.15 No molecular marker of zebrafish granulocytes has yet been described, though the recently cloned zebrafish CCAAT–enhancer-binding protein homologue c-ebp1 is a candidate,18 because mammalian C-EPBε is seen primarily in myeloid and lymphoid cells and plays an important role in mammalian granulocyte development. However, c-ebp1expression largely overlaps that ofL-plastin18; hence, it is unlikely to be granulocyte specific.
Our ultimate goal was to exploit the strengths of zebrafish genetics to study developmental myelopoiesis, particularly granulopoiesis. Therefore, it was necessary to comprehensively characterize embryonic and adult zebrafish myelopoiesis. In this report, we describe 2 types of adult zebrafish granulocytes and embryonic zebrafish granulocytes and macrophages. Histochemical detection of granulocytes by myeloperoxidase histochemistry was evaluated. For more specific identification, we cloned and characterized a myeloid-specific zebrafish peroxidase gene. Rather than presume that morphologic and enzymatic parallels indicate a parallel physiological function, as they do in other vertebrates, we devised simple functional tests of granulocytes and macrophages in zebrafish embryos. Our studies provide a basis for further exploiting the strengths of this model organism in the study of myelopoiesis.
Materials and methods
Zebrafish
Wild-type zebrafish stocks obtained from a local pet shop were held in the Ludwig Institute Aquarium Facility, and standard husbandry practices were used. Embryos were grown in 0.003% 1-phenyl-2-thiourea (P-7629; Sigma, Castle Hill, New South Wales, Australia) to suppress melanization. The m39 cloche deletion allele19 and the b104 spadetail null allele20 were used.
Collection of zebrafish tissues
Adult zebrafish were anesthetized in water containing 25 to 100 mg/L benzocaine (E-1505; Sigma), then were dried and killed by cervical transection. Smears of approximately 1 μL blood (collected as it pooled near the still beating heart) were stained with May-Grünwald-Giemsa (BDH, Kilsyth, Victoria, Australia). Organs were dissected under a Leitz dissecting microscope. The posterior kidney was displayed by opening the peritoneal cavity and removing the abdominal organs, and then it was removed by scraping it from the ventral surface of the upper abdominal cavity with fine forceps. To prepare single-cell suspensions, kidneys or spleens collected from 4 to 8 animals were pooled in 500 μL 0.9× phosphate-buffered saline (PBS) and were passed through a 70-μm sieve (Falcon 2350; Becton Dickinson, Franklin Lakes, NJ) using a 1-mL syringe plunger handle. Cell counts in trypan blue confirmed cell viability. Cytospin preparations were of 104 to 105 cells resuspended in 200 μL 50% fetal calf serum in 0.9× PBS. Tissues were fixed in 10% formaldehyde for histology, 4% paraformaldehyde for in situ hybridization, and 2.5% glutaraldehyde for electron microscopy.
Histochemical staining
Myeloperoxidase staining of whole zebrafish embryos was based on the method of Kaplow.21 Briefly, embryos were fixed for 60 seconds (1 in 10 dilution of 37% formaldehyde stock in 100% ethanol) and washed for 15 to 30 seconds in water; excess water was carefully removed. Fixed embryos were placed in freshly prepared incubation mixture, made by mixing a pre-prepared stock solution (30% ethanol with 3 mg/mL benzidine dihydrochloride [B-3383; Sigma], 1.32 mM ZnSO4, 0.123 M sodium acetate, and 0.0146 M sodium hydroxide) with hydrogen peroxide (20 vol [6%]) in a ratio of 25 mL stock solution to 87.5 μL H2O2. The stain reaction was allowed to proceed under observation for 1 to 10 minutes and, when focal staining of cells was evident, was stopped by removing embryos and washing them repeatedly in tap water. Myeloperoxidase-positive cells were characterized by a blue–black precipitate immediately after staining, but within the first 24 hours of storage the color changed to brown, with some leaching of the precipitate during further storage in 4% paraformaldehyde in PBS. To stain cells on glass slides, the same reagents were used with a staining period of 30 seconds; slides were counterstained with Giemsa stain and were examined under oil without coverslips. For histochemical staining of hemoglobin, embryos were placed in freshly prepared o-dianisidine stain solution (40% ethanol with 0.01 M sodium acetate, 0.65% H2O2, and 0.6 mg/mL o-dianisidine [D-9143; Sigma]) for 15 minutes and then were washed in water.
Electron microscopy
Embryos and tissues fixed in freshly prepared 2.5% glutaraldehyde were processed as described.22 For peroxidase electron microscopy, the conditions selected for studies of carp leukocytes17 were used, except that fixation was in 2.5% glutaraldehyde with a reaction mixture containing 0.05 M Tris and 0.01% H2O2 saturated with 3,3′-diaminobenzidine hydrochloride, pH 7.6. Postfixation was with 1% OsO4 in 0.1 M phosphate buffer (1 hour, 4°C), and then samples were processed as previously described.22Measurements of cytoplasmic granule dimensions were made on photographic prints at 17.5 × 103 and 157.5 × 103 magnification. Data presented are mean ± SD (range) for the number of measurements indicated.
Isolation of a zebrafish peroxidase gene
Database searching identified 2 EST clones with homology to mammalian leukocyte peroxidases (fj81h09 and fj80f04), which, though similar in their partially overlapping 5′ sequences (GenBank accession numbers AW419670 and AW34911, respectively), had unrelated 3′ sequences (AW420468 and AW420365). Assuming that the 5′ sequences of fj81h09 and fj80f04 represented transcripts of the same gene, the 2 primer pairs (5′-CGGTTCTGTGGATTGTCT-3′ with 5′-CACGACCACCAGGAGCAA-3′; and 5′-GGATTGTCTGCTCCTCAGA-3′ with 5′-GCCACCGTCACCAGTCTC-3′) were used to amplify 125 and 385 nt fragments, respectively, from an adult zebrafish kidney cDNA library (gift of L. Zon, Boston, MA). Sequencing confirmed them to be fragments of a cDNA with homology to mammalian leukocyte peroxidases. The 385 nt fragment was used for generating riboprobes and for serial screening the adult kidney cDNA library under high-stringency conditions (0.1 × SSC and 0.1% sodium dodecyl sulfate at 62°C), resulting in 16 positive clones. These clones were internally sequenced to confirm their identities—clone 16 was sequenced bidirectionally in full, and selected clones were sequenced regionally to define several transcript variations. Intron–exon boundaries were compared between zebrafish and murine genes (GenBank accession number X15378).23 Potential single nucleotide polymorphisms were distinguished from sequencing errors either by their representation in several clones or by bidirectional sequencing, but they were not confirmed on genomic DNA. GenBank accession numbers for the zebrafish nucleotide sequences areAF326958 and AF378824-6. Linkage group assignment of fj80f04 was based on GenBank sequence AW420365 using the primers 5′-TGGTTAGAGAATGCCTTATGT-3′ and 5′-CACAGCATGGACTACCGA-3′ and polymerase chain reaction conditions of 94°C (30 seconds), 55°C (30 seconds), and 72°C (60 seconds) for 40 cycles. The map location was calculated with SAMapper1.0.
Phylogenetic analysis
Sequence identity values were determined using the CLUSTAL algorithm in MegAlign application of the DNASTAR suite of programs (Madison, WI; http://www.dnastar.com) with PAM250 residue weight tables and no manual adjustments. Dendrograms of peroxidase protein domains were based on a previous analysis24 using catalytic and region 1 domains as demarcated therein. For the zebrafish peroxidase, the sequences of the conceptual translation used were residues 110 to 622 (catalytic domain) and 58 to 109 (region 1). The dendrogram was constructed based on an alignment generated from Clustal X 1.8125 using default settings and viewed with Treeview, using linoleate diol synthase from Gaeumannomyces graminisas an outgroup. Bootstrap values derive from 1000 bootstrap trials. GenBank accession numbers of proteins included in the analysis are as listed previously.24
In situ hybridization analyses
Whole-mount in situ hybridization analyses were performed as previously described using a hybridization temperature of 70°C.10 26 The 385 nucleotide fragment of the zebrafishmpx catalytic domain was subcloned into pBluescript (Stratagene, La Jolla, CA), and riboprobes corresponding to it were transcribed using T7 polymerase with EcoRV-linearized template (antisense) and T3 polymerase with BamHI-linearized template (sense). Controls with sense riboprobes prepared in parallel with initial mpx antisense analyses showed no staining and, hence, subsequently were not repeated.
Leukocyte function assays
For the minor trauma assay, zebrafish embryos (2-7 days after fertilization [dpf]) were anesthetized in egg water with 2.5 to 10 mg/L benzocaine, and the tail was transected near its tip. Embryos were then placed in egg water without anesthetic until analysis at different time points up to 2 days after trauma.
To demonstrate phagocytic function, embryos were microinjected with india ink (Hunt Manufacturing, Statesville, NC) diluted approximately 1:10 in PBS so as to flow freely through the micro-injection needle, using a finely drawn glass capillary tube and Narishige micromanipulators (Tokyo, Japan). The most successful outcomes resulted from injections directly into the chambers of the heart or the veins as they converged toward the heart. Immediately after a successful injection, the circulation was outlined by black ink; these embryos were selected for analysis.
Results
Morphology of adult zebrafish leukocytes
Adult zebrafish have 2 types of circulating granulocytes (Figure1A). The more common had a pale cytoplasm and multilobulated segmented nucleus, typical of the heterophil granulocyte of other cyprinid teleosts (Figure 1A-B).11,17The less common had an eosinophilic cytoplasm with a peripheral nonsegmented nucleus (Figure 1A, C), typical of the cyprinid eosinophil granulocyte.11 17 Heterophil granulocytes comprised more than 95% of circulating granulocytes in most animals (n > 12), though in 2 animals with no apparent disease eosinophils comprised 24% and 49% of blood leukocytes.
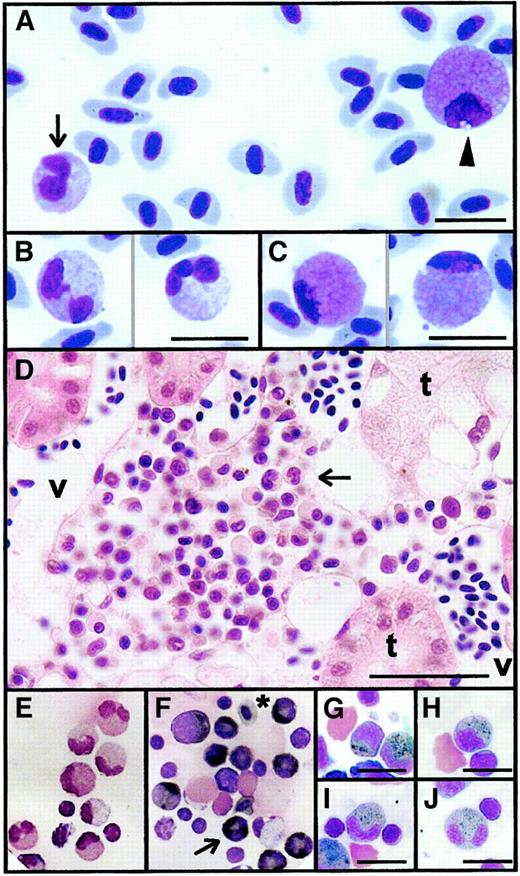
Light microscope appearance of adult zebrafish granulocytes.
(A) A heterophil (black arrow) and an eosinophil (arrowhead) in a single field of a May-Grünwald-Giemsa–stained zebrafish blood smear. (B) Detail of mature heterophils with double- and triple-lobed nuclei (May-Grünwald-Giemsa–stained blood smear). (C) Detail of mature eosinophils, illustrating characteristic pink cytoplasm and peripheral nucleus (May-Grünwald-Giemsa–stained blood smear). (D) Hematoxylin and eosin section of posterior kidney showing an interstitial niche of myelopoiesis lying between renal tubules (t) and blood vessels (v), including several heterophil granulocytes recognizable by their indented or segmented nuclei (arrow). (E-J) Myeloperoxidase histochemical staining of cytospin preparations of single-cell suspensions prepared from adult zebrafish kidneys. Giemsa-stained preparation (E) showing mature heterophil granulocytes serving as negative control for (F), which was stained histochemically for myeloperoxidase and counterstained with Giemsa (to display nuclear morphology), showing myeloperoxidase-positive cells with black cytoplasmic staining at various stages of heterophil development (eg, arrow). *Erythrocyte with weakly peroxidase-staining gray cytoplasm. Myeloperoxidase-positive heterophil granulocytes of various stages of development: promyelocyte (G), myelocyte (H), metamyelocyte (I), and segmented mature form (J). Myeloperoxidase positivity is strongest at the myelocyte and metamyelocyte stages of development. Scale bar = 10 μm in all panels except D, where it equals 24 μm.
Light microscope appearance of adult zebrafish granulocytes.
(A) A heterophil (black arrow) and an eosinophil (arrowhead) in a single field of a May-Grünwald-Giemsa–stained zebrafish blood smear. (B) Detail of mature heterophils with double- and triple-lobed nuclei (May-Grünwald-Giemsa–stained blood smear). (C) Detail of mature eosinophils, illustrating characteristic pink cytoplasm and peripheral nucleus (May-Grünwald-Giemsa–stained blood smear). (D) Hematoxylin and eosin section of posterior kidney showing an interstitial niche of myelopoiesis lying between renal tubules (t) and blood vessels (v), including several heterophil granulocytes recognizable by their indented or segmented nuclei (arrow). (E-J) Myeloperoxidase histochemical staining of cytospin preparations of single-cell suspensions prepared from adult zebrafish kidneys. Giemsa-stained preparation (E) showing mature heterophil granulocytes serving as negative control for (F), which was stained histochemically for myeloperoxidase and counterstained with Giemsa (to display nuclear morphology), showing myeloperoxidase-positive cells with black cytoplasmic staining at various stages of heterophil development (eg, arrow). *Erythrocyte with weakly peroxidase-staining gray cytoplasm. Myeloperoxidase-positive heterophil granulocytes of various stages of development: promyelocyte (G), myelocyte (H), metamyelocyte (I), and segmented mature form (J). Myeloperoxidase positivity is strongest at the myelocyte and metamyelocyte stages of development. Scale bar = 10 μm in all panels except D, where it equals 24 μm.
The kidney is the primary hematopoietic organ of adult zebrafish.14 Sections of adult kidney showed nests of hematopoietic tissue between renal tubules and blood vessels (Figure1D). The adult posterior kidney contained myeloid cells at all stages of development, with the heterophil granulocyte lineage the more abundant (Figure 1E).
To determine whether zebrafish heterophils contained granules analogous to those of mammalian neutrophils, histochemical staining for myeloperoxidase (an enzyme characteristic of neutrophil primary granules) was performed. Heterophil series cells showed strong myeloperoxidase activity (Figure 1E-F). Histochemical demonstration of myeloperoxidase-containing granules facilitated recognition of immature heterophil granulocyte cells of promyelocyte, myelocyte, and metamyelocyte and segmented stages of development (Figure 1G-J, respectively). However, histochemical staining for myeloperoxidase activity was not totally specific to heterophils. Weak peroxidase activity was evident in erythrocytes (Figure 1F). Eosinophil granulocytes, recognizable by their characteristic nuclear morphology and location, were negative for histochemical myeloperoxidase activity under cytospin staining conditions. Myeloperoxidase-stained cytospin preparations showed a 5:1 heterophil–eosinophil ratio (n = 3), confirming that heterophils were the more numerous zebrafish granulocyte. Large vacuolated macrophages and immature monocytoid cells were also negative for histochemical myeloperoxidase activity.
Electron microscopy confirmed the presence of 2 distinct types of zebrafish granulocytes. Heterophils were characterized by electron-dense, elongated, cigar-shaped, cytoplasmic granules (Figure2A-D). Immature heterophils contained numerous mitochondria and a prominent endoplasmic reticulum (Figure2B). In mature heterophils, the elongated and sometimes segmented nucleus was typically peripheral. Heterophils contained up to 110 granules in their cytoplasmic cross-section. These highly distinctive elongated granules were 0.42 ± 0.13 μm in length (range, 0.23-0.8; n = 139) and contained 1 or 2 axes of regularly arrayed electron-dense lamellations with a periodicity of 3.9 ± 0.4 nm (n = 20) in groups of 6.0 ± 1.8 (range, 2-10; n = 20) lamellae (Figure 2D). Eosinophils were characterized by a cytoplasm packed with larger round or elliptical granules of longest diameter 0.66 ± 0.26 μm (range, 0.17-1.42, n = 99) with a broad, marbled variation in electron density (Figure 2A, E-G). Immature eosinophils were densely packed with rough endoplasmic reticulum (Figure 2E). The correlation between electron microscope granule appearance and light microscope cell type was secure by virtue of the relative abundance of the 2 cell types (Figure 2A) and by their characteristic nuclear morphology and location (Figure 2C, F). Both granulocyte types were also identified in electron micrographs of adult zebrafish spleen (data not shown).
Electron microscope appearance of adult zebrafish granulocytes and macrophages.
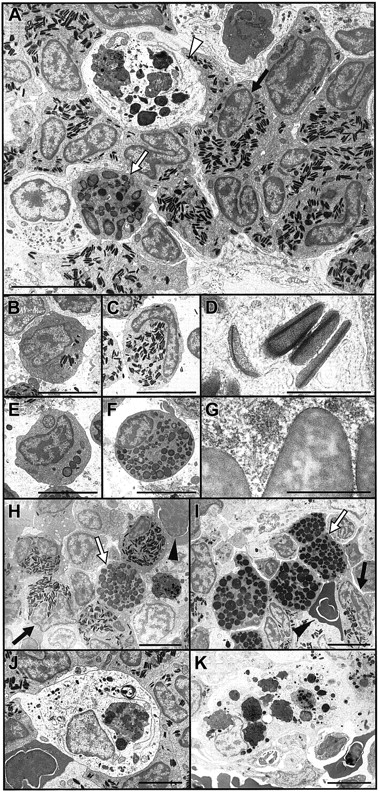
(A) Electron micrograph overview of hematopoietic area of adult posterior zebrafish kidney, illustrating heterophil granulocytes (eg, black arrow) as the most prevalent cell, a rarer eosinophil granulocyte (white arrow), and a macrophage (white arrowhead) containing numerous cytoplasmic phagosomes including electron-dense material of similar appearance to erythrocyte cytoplasm. (B-D) Electron microscope appearance of zebrafish heterophil granulocytes. (B) Immature heterophil promyelocyte with large nucleus and few electron-dense, cigar-shaped cytoplasmic granules. (C) Heterophil metamyelocyte with cytoplasm densely packed with electron-dense granules and peripheral nonsegmented nucleus. (D) Higher-power view of the cigar-shaped, electron-dense heterophil granulocyte cytoplasmic granules, showing their axial electron-denser lamellations. (E-G) Electron microscope appearance of zebrafish eosinophil granulocytes. (E) Immature eosinophil promyelocyte with large nucleus and few round cytoplasmic granules of variable electron density. (F) Eosinophil metamyelocyte with cytoplasm densely packed with round and oval-shaped granules and peripheral nonsegmented nucleus. (G) Higher-power view of the characteristic granules of eosinophils, larger than heterophil granules (D) and with marbled variable electron density. (H, I) Electron micrograph incubated for peroxidase (I) and negative control (H) showing the peroxidase reactivity of eosinophil granules (white arrow), evidenced by their darker color in panel I than in panel H, and erythrocyte cytoplasm (black triangle) under conditions of this stain. The already electron-dense heterophil granules (black arrow) are not discernibly darker under the peroxidase reaction conditions. (J, K) Macrophage in kidney (J) and spleen (K) of adult zebrafish, with phagosomes suggestive of erythrophagocytosis. Scale bar = 5 μm in all panels except D and G, where it equals 0.5 μm.
Electron microscope appearance of adult zebrafish granulocytes and macrophages.
(A) Electron micrograph overview of hematopoietic area of adult posterior zebrafish kidney, illustrating heterophil granulocytes (eg, black arrow) as the most prevalent cell, a rarer eosinophil granulocyte (white arrow), and a macrophage (white arrowhead) containing numerous cytoplasmic phagosomes including electron-dense material of similar appearance to erythrocyte cytoplasm. (B-D) Electron microscope appearance of zebrafish heterophil granulocytes. (B) Immature heterophil promyelocyte with large nucleus and few electron-dense, cigar-shaped cytoplasmic granules. (C) Heterophil metamyelocyte with cytoplasm densely packed with electron-dense granules and peripheral nonsegmented nucleus. (D) Higher-power view of the cigar-shaped, electron-dense heterophil granulocyte cytoplasmic granules, showing their axial electron-denser lamellations. (E-G) Electron microscope appearance of zebrafish eosinophil granulocytes. (E) Immature eosinophil promyelocyte with large nucleus and few round cytoplasmic granules of variable electron density. (F) Eosinophil metamyelocyte with cytoplasm densely packed with round and oval-shaped granules and peripheral nonsegmented nucleus. (G) Higher-power view of the characteristic granules of eosinophils, larger than heterophil granules (D) and with marbled variable electron density. (H, I) Electron micrograph incubated for peroxidase (I) and negative control (H) showing the peroxidase reactivity of eosinophil granules (white arrow), evidenced by their darker color in panel I than in panel H, and erythrocyte cytoplasm (black triangle) under conditions of this stain. The already electron-dense heterophil granules (black arrow) are not discernibly darker under the peroxidase reaction conditions. (J, K) Macrophage in kidney (J) and spleen (K) of adult zebrafish, with phagosomes suggestive of erythrophagocytosis. Scale bar = 5 μm in all panels except D and G, where it equals 0.5 μm.
We also performed peroxidase electron microscopy with diaminobenzidine (DAB) substrate to localize peroxidase activity within leukocytes.17 It was difficult to discern whether the density of the already electron-dense granules of heterophil granulocytes increased under the peroxidase–DAB staining conditions used. However, there was marked increase in the relative electron density of the granules of eosinophil granulocytes, indicating that they contained a peroxidasic activity (Figure 2H-I). In addition, under these staining conditions, an increase occurred in the relative electron density of the cytoplasm of erythrocytes, confirming the presence of a weak peroxidasic activity in their cytoplasm.
To search for zebrafish basophil granulocytes and tissue mast cells, we surveyed toluidine blue-stained tissue sections of whole adult zebrafish. No cells with positive cytoplasmic granules were observed in any organ.
Macrophages were also evident in sections of adult kidney and spleen (Figure 2A, J-K). They were large cells with numerous cytoplasmic phagosomes. Large phagosomes containing material of a density and appearance similar to those of erythrocyte cytoplasm were commonly observed in splenic and kidney macrophages, suggesting that hemophagocytosis is not unusual in normal adult zebrafish.
Initiation of granulopoiesis in zebrafish embryos
Zebrafish embryos of 24 hpf and older were surveyed by electron microscopy to determine when granulocytes first appeared during zebrafish development. Cells containing characteristic heterophil granules were reliably found in tissues of 48-hpf zebrafish embryos (Figure 3A-B) and within axial vessels (Figure 3C-D). This indicates that primitive granulocytes circulate in zebrafish embryos at 48 hpf and that cells within embryos containing these granules are indeed granulocytic leukocytes. No cells containing the granules of an eosinophil granulocyte were detected in embryos of up to 5 dpf.
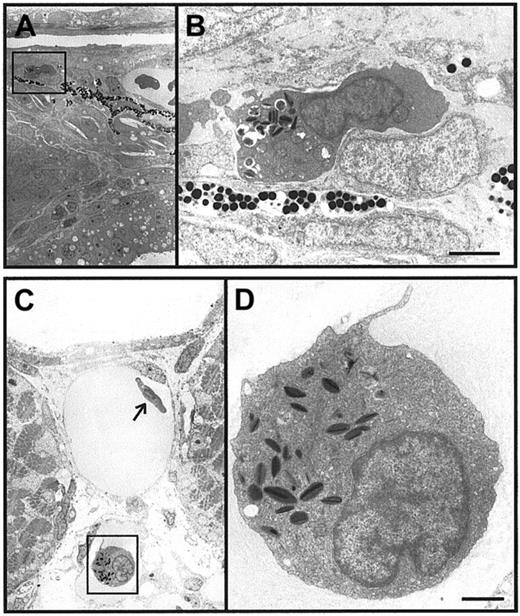
Initiation of granulopoiesis in zebrafish embryos.
(A-D) Immature heterophil granulocytes in a 48-hpf zebrafish embryo. Panels A and C are low-power electron microscope views of immature heterophil granulocytes in axial tissue (A) and in an axial vessel (C). The heterophils boxed in panels A and C are recognizable by the characteristic cigar-shaped, electron-dense granules in cytoplasm and are shown at higher power in panels B and D. Scale bars = 2 μm (A), 1 μm (B).
Initiation of granulopoiesis in zebrafish embryos.
(A-D) Immature heterophil granulocytes in a 48-hpf zebrafish embryo. Panels A and C are low-power electron microscope views of immature heterophil granulocytes in axial tissue (A) and in an axial vessel (C). The heterophils boxed in panels A and C are recognizable by the characteristic cigar-shaped, electron-dense granules in cytoplasm and are shown at higher power in panels B and D. Scale bars = 2 μm (A), 1 μm (B).
These observations in fixed embryos correlated with observations made in vivo in 1-phenyl-2-thiourea–treated living embryos of 2 dpf and older and in adults under Nomarski illumination. Particularly when the heart rate was slowed by anesthesia, large round cells that rolled slowly along the vessel walls were observed within the ventral venous plexus, occasionally lodging momentarily, while erythrocytes streamed past in the center of the vessel.
Embryos were surveyed for peroxidase-positive cells by myeloperoxidase histochemical staining of whole zebrafish embryos (Figure4A-E). Peroxidase enzymatic activity was not detected in 24-hpf embryos (Figure 4A), but peroxidase-positive cells were scattered throughout 33-hpf embryos (Figure 4B), particularly over the surface of the yolk and in the ventral vein region. By 48 hpf and beyond, peroxidase-positive cells were most evident in the ventral venous plexus (Figure 4C-D) but were scattered throughout the entire embryo. Peroxidase positivity was cellular (Figure 4E). Consistent with the weak peroxidasic activity observed in adult erythrocytes, in embryos of 33 hpf and older, weaker peroxidase activity was evident in the pooled red blood cells in the region of the heart (Figure 4B-C). Parallel staining with myeloperoxidase ando-dianisidine for hemoglobin revealed that the strongly peroxidase-positive cells never pooled within vessels as did erythrocytes and were significantly larger (Figure 4F-G) than erythrocytes, indicating that the dispersed population of strongly peroxidase-positive cells was different from that of hemoglobinized erythrocytes.
Initiation of granulopoiesis in zebrafish embryos.
(A-E) Whole-mount myeloperoxidase histochemistry of zebrafish embryos at 26 (A), 31 (B), and 48 (C) hpf, showing discrete histochemically positive myeloperoxidase reactive cells over the yolk sac and in the ventral venous plexus (B-E, black arrows). Discrete cellular staining is shown at higher power under Nomarski illumination in the ventral venous plexus of 48-hpf embryos (D, E). Diffuse, less intense staining over the surface of the yolk (arrowhead B, C) is believed to be nonspecific staining in pooled erythrocytes. (F, G) Comparison of histochemical staining for myeloperoxidase (F) ando-dianisidine staining (G) for hemoglobin in the head and gill region of 6-dpf embryos. Myeloperoxidase-positive cells are scattered and discrete, whereas hemoglobin-positive cells are pooled in large blood vessels. (H-K) Comparison of histochemical staining for myeloperoxidase (J, K) and o-dianisidine staining (H, I) for hemoglobin in wild-type (H, J) and cloche (I, K) 3-dpf (H, I) and 2-dpf (J, K) embryos. Cloche (clo) embryos retain some globin expression in the ventral vein region (H, I, arrowhead). Myeloperoxidase-positive cells were fewer and more sparsely scattered and were generally located in the posterior ICM immediately caudal of the tip of the yolk sac extension (K, arrow). (L, M) Myeloperoxidase histochemical staining of a 48-hpf spadetail(spt) embryo (M) and age-matched sibling wild-type embryo (L), showing myeloperoxidase-positive cells (arrows) scattered throughout the spt embryo.
Initiation of granulopoiesis in zebrafish embryos.
(A-E) Whole-mount myeloperoxidase histochemistry of zebrafish embryos at 26 (A), 31 (B), and 48 (C) hpf, showing discrete histochemically positive myeloperoxidase reactive cells over the yolk sac and in the ventral venous plexus (B-E, black arrows). Discrete cellular staining is shown at higher power under Nomarski illumination in the ventral venous plexus of 48-hpf embryos (D, E). Diffuse, less intense staining over the surface of the yolk (arrowhead B, C) is believed to be nonspecific staining in pooled erythrocytes. (F, G) Comparison of histochemical staining for myeloperoxidase (F) ando-dianisidine staining (G) for hemoglobin in the head and gill region of 6-dpf embryos. Myeloperoxidase-positive cells are scattered and discrete, whereas hemoglobin-positive cells are pooled in large blood vessels. (H-K) Comparison of histochemical staining for myeloperoxidase (J, K) and o-dianisidine staining (H, I) for hemoglobin in wild-type (H, J) and cloche (I, K) 3-dpf (H, I) and 2-dpf (J, K) embryos. Cloche (clo) embryos retain some globin expression in the ventral vein region (H, I, arrowhead). Myeloperoxidase-positive cells were fewer and more sparsely scattered and were generally located in the posterior ICM immediately caudal of the tip of the yolk sac extension (K, arrow). (L, M) Myeloperoxidase histochemical staining of a 48-hpf spadetail(spt) embryo (M) and age-matched sibling wild-type embryo (L), showing myeloperoxidase-positive cells (arrows) scattered throughout the spt embryo.
We evaluated this histochemical assay for granulocyte identification in 2 zebrafish mutants with perturbed hematopoiesis. The mutantcloche (clo) fails to initiate hematopoiesis in the lateral plate mesoderm and intermediate cell mass.8,10,26 However, because peroxidase activity was not detectable histochemically before 33 hpf in wild-type embryos, embryos were studied at 2 and 3 dpf, when clo homozygous mutant embryos were unequivocally identifiable by their pericardial edema. Although clo embryos showed a marked reduction in the number of peroxidase-positive cells, a few positive cells were detected in some clo embryos, particularly in the regions of the posterior intermediate cell mass and the ventral venous plexus (Figure 4J-K). Because there is residual expression of some markers of erythropoiesis in clo at this stage,10,26embryos were stained in parallel for hemoglobin usingo-dianisidine. Although numbers of hemoglobinized cells in the clo embryos were reduced (Figure 4H-I), the reduction in number of peroxidase positive-cells was greater (Figure 4I, K). The mutant spadetail (spt) fails to initiate erythropoiesis,10 but our studies confirmed that it initiates myelopoiesis (G.J. Lieschke et al, manuscript submitted). Consistent with this, histochemically myeloperoxidase-positive cells were demonstrated scattered throughout 2-dpf spt embryos (Figure 4L-M).
Isolation and characterization of a zebrafish peroxidase gene
To address the nonspecificity of the histochemical myeloperoxidase stain and to develop an independent way of identifying zebrafish granulocytes, we isolated a zebrafish peroxidase gene fragment. Three overlapping clones (clones 9, 15, 16) combined to describe a 2814-nucleotide cDNA fragment encoding the carboxyl terminal 678 amino acids of a peroxidase protein, embracing the region 1 and catalytic domains. Sequence comparison over the catalytic domain showed 51% and 52% amino acid identity with that of human and murine myeloperoxidases (Figure 5A) and 53% identity with human and murine eosinophil peroxidases. There was also a higher degree of identity in the region 1 domain (58%-64%).
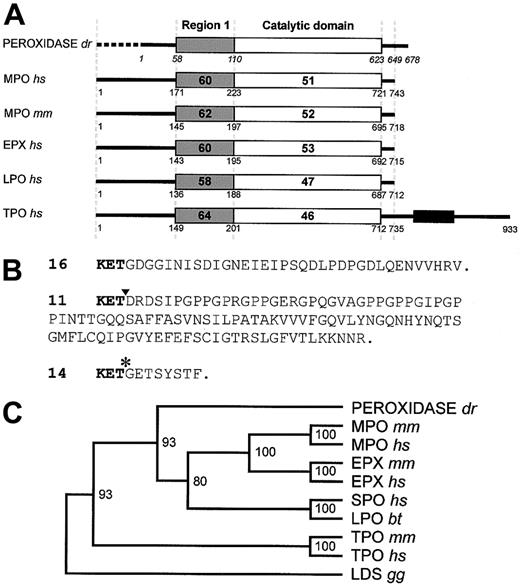
A zebrafish myeloid-specific peroxidase(mpx) gene.
(A) Domain homology alignment of Danio rerio (dr) peroxidase, compared with human (hs) and murine (mm) myeloperoxidase (MPO), eosinophil peroxidase (EPX), lactoperoxidase (LPO), and thyroid peroxidase (TPO). Bold numbers within domains indicate percentage amino acid identity to the zebrafish peroxidase in region 1 (shaded) and the catalytic domain (open). Numbers under the box diagrams representing each protein indicate the amino acid number of the junction shown, counted from the initiation methionine, except for zebrafish peroxidase, where the italicized numbering starts from the first amino acid of the conceptual translation of the incomplete cDNA clone 16. (B) Three variant carboxyl termini of the Danio rerioperoxidase. The upper sequence (exemplified by clone 16) represents the most prevalent cDNA form recovered from library screening (GenBankAF378824). Clone 11 contained a 38-nucleotide deletion of the 38 nucleotides 1930-1967 (position marked by ▾), resulting in a change in reading frame and the variant conceptual translation shown (GenBankAF378825). Clone 14 differed from clones 16 and 11, again diverging after nucleotide 1929 (marked by *), resulting in the variant conceptual translation shown (GenBank AF378826). Six clones appeared to have a retained intron of 587-91 nucleotides after nucleotide 1735, and other clones identified 3 more apparently retained introns (length)—after nucleotide 1065 (91 nt), after nucleotide 1325 (90 nt), and after nucleotide 1496 (92 nt). (C) Phylogenetic analysis of theDanio rerio peroxidase with its closest mammalian homologues. Analysis was confined to the catalytic domain of each protein. The dendrogram was constructed using Clustal X and Treeview, building on the analysis of the entire peroxidase family as given in Daiyasu and Toh,24 using linoleate diol synthase (LDS) from Gaeumannomyces graminis (gg) as an outgroup. Bootstrap values (n = 1000) are indicated at nodes as percentages. SPO, salivary peroxidase; bt, bovine.
A zebrafish myeloid-specific peroxidase(mpx) gene.
(A) Domain homology alignment of Danio rerio (dr) peroxidase, compared with human (hs) and murine (mm) myeloperoxidase (MPO), eosinophil peroxidase (EPX), lactoperoxidase (LPO), and thyroid peroxidase (TPO). Bold numbers within domains indicate percentage amino acid identity to the zebrafish peroxidase in region 1 (shaded) and the catalytic domain (open). Numbers under the box diagrams representing each protein indicate the amino acid number of the junction shown, counted from the initiation methionine, except for zebrafish peroxidase, where the italicized numbering starts from the first amino acid of the conceptual translation of the incomplete cDNA clone 16. (B) Three variant carboxyl termini of the Danio rerioperoxidase. The upper sequence (exemplified by clone 16) represents the most prevalent cDNA form recovered from library screening (GenBankAF378824). Clone 11 contained a 38-nucleotide deletion of the 38 nucleotides 1930-1967 (position marked by ▾), resulting in a change in reading frame and the variant conceptual translation shown (GenBankAF378825). Clone 14 differed from clones 16 and 11, again diverging after nucleotide 1929 (marked by *), resulting in the variant conceptual translation shown (GenBank AF378826). Six clones appeared to have a retained intron of 587-91 nucleotides after nucleotide 1735, and other clones identified 3 more apparently retained introns (length)—after nucleotide 1065 (91 nt), after nucleotide 1325 (90 nt), and after nucleotide 1496 (92 nt). (C) Phylogenetic analysis of theDanio rerio peroxidase with its closest mammalian homologues. Analysis was confined to the catalytic domain of each protein. The dendrogram was constructed using Clustal X and Treeview, building on the analysis of the entire peroxidase family as given in Daiyasu and Toh,24 using linoleate diol synthase (LDS) from Gaeumannomyces graminis (gg) as an outgroup. Bootstrap values (n = 1000) are indicated at nodes as percentages. SPO, salivary peroxidase; bt, bovine.
Several other clones appeared likely to represent splice variants (Figure 5B). Two clones (11, 13) contained a 38-nucleotide deletion after nucleotide 1929 that resulted in a frame shift and a 121-amino acid variant carboxyl tail (Figure 5B). Clone 14 also diverged after nucleotide 1929 for its entire remaining length of 743 nucleotides, resulting in a short, 8-amino acid carboxyl terminus (Figure 5B). These variations occurred near the point at which mammalian myelosinophil and eosinophil peroxidases end when the sequences are aligned.
The remaining 10 partially sequenced clones contained multiple instances of 4 apparently retained introns that aligned exactly with the boundaries between exons 9 and 10, 10 and 11, 11 and 12, and 12 and 13 of murine myeloperoxidase,23 though the introns were of different sizes in the 2 species. This indicates a high degree of conservation in the genomic structure of this region of these peroxidase genes.
To determine whether zebrafish peroxidase was orthologous to a particular mammalian peroxidase gene, we undertook phylogenetic analysis on the basis of their catalytic domains (Figure 5C). The zebrafish peroxidase lay basal to the 3 closely related mammalian peroxidases (myeloperoxidase, eosinophil peroxidase, and lactoperoxidase), thereby complicating the naming of the zebrafish peroxidase we isolated. This phylogeny suggests that the gene duplication and diversification that occurred in mammals to create these various peroxidases occurred after the evolutionary divergence of fish and tetrapods. We also built a phylogenetic tree for the region 1 domains—this, too, placed zebrafish peroxidase outside the group of closely related mammalian peroxidases, including outside the thyroid peroxidases, on a node with a bootstrap value of 69.4%, supporting this evolutionary hypothesis. We have, therefore, called the zebrafish peroxidase gene we isolated myeloid-specificperoxidase (mpx), taking into account its expression pattern described below and avoiding suggestion of the simple orthologous relation that might be inferred from the name myeloperoxidase.
The EST clone fj80f04, which corresponded to our mpx clone 16 at the 5′ and 3′ ends, was mapped on the T51 radiation hybrid map to zebrafish linkage group 10 between the SSLP markers z8146 and z9473, flanked by the mapped ESTs fj59e03 and fa97h07. There are no closely mapped genes or annotated ESTs to suggest a syntenic relation between this part of the zebrafish linkage group 10 and the human genome in the vicinity of myeloperoxidase on human chromosome 17q23.1.27 28
We also identified 11 potential single-nucleotide polymorphisms; all lay within the protein-coding sequence, though this is not a complete analysis because not all clones were sequenced in full. This variation probably reflects the fact that the library was prepared from RNA from multiple animals of a noninbred strain. Nine of 11 were conservative polymorphisms [nt# (nt/nt)]: 765(C/T), 777(T/C), 879(C/G), 897 (C/T), 903(A/G), 984(C/T), 1008(C/A), 1050(C/T), and 1890(A/G). Two nonconservative variations were 16(G/C), changing Arg54 to Thr, and 1576(G/A), changing Gln526 to Lys.
Expression of zebrafish mpx
The expression pattern of mpx was evaluated by whole-mount in situ hybridization. Cells scattered throughout the adult kidney and spleen showed strong cytoplasmic expression (Figure6A-B); no mpx-expressing cells were seen in other tissues such as the gut (not shown) or gill arches (Figure 6C). Erythrocytes within vessels were negative formpx expression (Figure 6A, C), indicating greater specificity of this method of detection of leukocyte peroxidase gene expression.
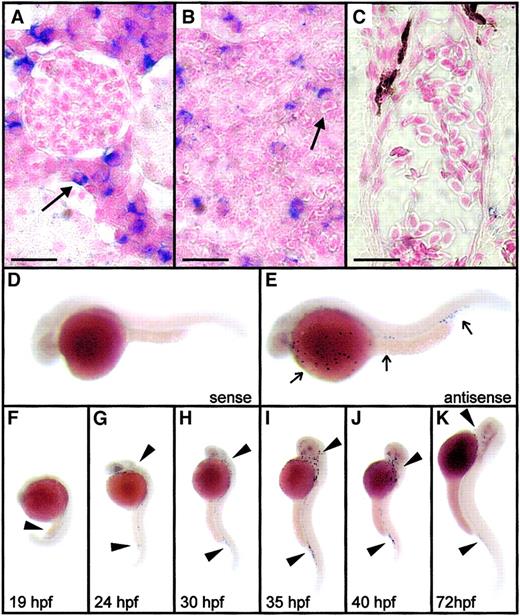
Expression of zebrafish mpx in adult and embryonic zebrafish by in situ hybridization.
(A-C) Whole-mount in situ hybridization analysis of mpxexpression in adult kidney (A), spleen (B), and gill vasculature (C). Black arrows indicate blue cytoplasmic staining indicating gene expression in heterophils of the adult kidney and spleen but not in erythrocytes in the gill vessels. Control sections with sense riboprobes showed no staining under identical hybridization and development conditions. Scale bar (A-C) = 14 μm. (D-E) Expression of mpx in a 30-hpf embryo (E) as detected by an antisense riboprobe (E, arrow), along with a control embryo hybridized to a sense riboprobe (D). Panels D and E are composite images bringing several focal planes together to demonstrate at higher magnification the typical appearance of the scattered mpx-positive cells indicated by arrows in panels G to K. (F-K) Expression ofmpx in zebrafish embryos from 19 to 72 hpf. Note that expression occurs first diffusely throughout the intermediate cell mass at 19 hpf (F, black arrowhead), but from 24 hpf expression is discrete in cells scattered throughout the embryo, particularly over the surface of the yolk and in relation to the axial vasculature (G-K, black arrowheads).
Expression of zebrafish mpx in adult and embryonic zebrafish by in situ hybridization.
(A-C) Whole-mount in situ hybridization analysis of mpxexpression in adult kidney (A), spleen (B), and gill vasculature (C). Black arrows indicate blue cytoplasmic staining indicating gene expression in heterophils of the adult kidney and spleen but not in erythrocytes in the gill vessels. Control sections with sense riboprobes showed no staining under identical hybridization and development conditions. Scale bar (A-C) = 14 μm. (D-E) Expression of mpx in a 30-hpf embryo (E) as detected by an antisense riboprobe (E, arrow), along with a control embryo hybridized to a sense riboprobe (D). Panels D and E are composite images bringing several focal planes together to demonstrate at higher magnification the typical appearance of the scattered mpx-positive cells indicated by arrows in panels G to K. (F-K) Expression ofmpx in zebrafish embryos from 19 to 72 hpf. Note that expression occurs first diffusely throughout the intermediate cell mass at 19 hpf (F, black arrowhead), but from 24 hpf expression is discrete in cells scattered throughout the embryo, particularly over the surface of the yolk and in relation to the axial vasculature (G-K, black arrowheads).
Expression patterns of mpx in zebrafish embryos are shown in Figure 6D-K. Expression was first seen at 18 to 19 hpf, diffusely through the axial intermediate cell mass (Figure 6F). Prolonged staining incubations of younger embryos did not detect earliermpx expression. In older embryos, mpx-expressing cells were scattered throughout the embryo, particularly over the yolk sac, related to the axial blood vessels above the posterior yolk sac extension and the posterior ventral vein plexus, and in the head and pharyngeal regions of the embryo (Figure 6D-E, G-K). Althoughmpx mRNA expression was demonstrated at 19 hpf (12-14 hours before the first detection of enzymatic activity by histochemical staining), after 30 hpf the distribution of mpx-expressing cells essentially recapitulated the pattern demonstrated by myeloperoxidase histochemistry.
Functional studies of phagocytes in zebrafish embryos
To determine whether the peroxidase-positive granulocytes of zebrafish embryos were functional, we devised an acute inflammation assay in which the tip of the embryo's tail was sectioned and the resultant process of inflammation followed. We initially evaluated the behavior of histochemically detected peroxidase-positive cells in this assay using embryos at 6 dpf. Before and immediately after the trauma, there was no aggregation of peroxidase activity at the trauma site (Figure 7A-B), but after 8 hours, peroxidase activity accumulated at the trauma site (Figure 7C). The assay was highly reproducible (Figure 7D). Accumulated peroxidase activity was punctate and related to cells (Figure 7E), though there appeared to be some spread of enzymatic activity beyond the margins of cells. Patterns of o-dianisidine and peroxidase histochemical staining were significantly different at 6 hours and 2 days after trauma (Figure 7F-G). Hemoglobinized cells were smaller than peroxidase-positive cells, and, after 2 days, peroxidase-positive cells remained, despite less hemoglobinized cell accumulation. The specificity of this effect for the peroxidase-positive cell population was further confirmed when embryos were evaluated for mpxmRNA expression after trauma. In 2-dpf embryos, before and immediately after trauma, there were no mpx-expressing cells at the trauma site, but by 8 hours after trauma, mpx-expressing cells had accumulated (Figure 7H-J). Heterophil granulocytes were readily located on electron microscope examination of transverse sections immediately proximal to the trauma site. Immature heterophils were found within vessels (Figure 7K) and at sites otherwise unusual for such cells, such as between muscle fibers (eg, Figure 7L), suggesting migration of these immature heterophil granulocytes through tissues and toward the inflammatory site.
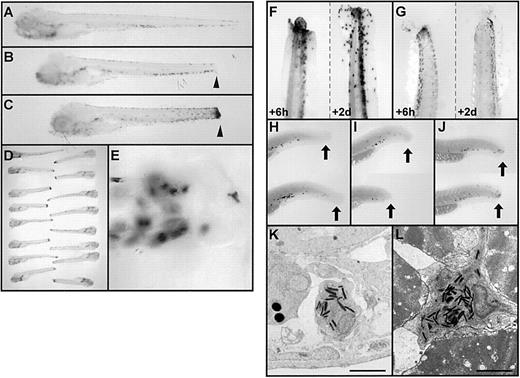
Functional studies of granulocytes in zebrafish embryos.
(A-E) Acute inflammation assay for granulocyte function in embryonic zebrafish at 6 dpf, based on tail transection and whole-mount histochemical staining for myeloperoxidase. Whole-mount zebrafish embryos were subjected to myeloperoxidase histochemical staining before (A), immediately after (B), and 8 hours after (C) transection near the tail tip. Note the scattered population of darkly staining peroxidase-positive cells in the ventral vein region in nontraumatized embryos and the accumulation of peroxidase activity at the site of acute inflammation (B and C arrowheads). Panel D displays an array of 16 embryos, indicating the assay is highly reproducible. At higher power, under Nomarksi illumination, the aggregated myeloperoxidase activity is seen to be distinctly cellular (E). (F, G) Comparison of the pattern of myeloperoxidase histochemical staining (F) ando-dianisidine staining (G) for hemoglobin in embryos 6 hours (left tail in panels) and 2 days (right tail in panels) after tail transection, showing the longer persistence of peroxidase-positive cells compared with hemoglobin-containing cells. (H-J) Identical assay to those in panels A to C, performed in 48-hpf embryos but using whole-mount in situ hybridization for mpx-expressing cells rather than histochemistry. The pattern of mpx-expressing cells (black dots) is shown before (H), immediately after (I), and 8 hours after (J) tail transection. Note the accumulation ofmpx-expressing cells at the site of transection (shown by the black arrow) after (J), but not before (H, I), 8 hours. (K, L) Electron micrographs showing granulocytes, recognizable by their characteristic cytoplasmic cigar-shaped, electron-dense granules, in the vicinity of the trauma site in embryos subjected to tail transection 8 hours earlier. Granulocytes are seen within a vessel (K) and at an unusual site between skeletal muscle fibers (L). Scale bar = 2 μm (K, L).
Functional studies of granulocytes in zebrafish embryos.
(A-E) Acute inflammation assay for granulocyte function in embryonic zebrafish at 6 dpf, based on tail transection and whole-mount histochemical staining for myeloperoxidase. Whole-mount zebrafish embryos were subjected to myeloperoxidase histochemical staining before (A), immediately after (B), and 8 hours after (C) transection near the tail tip. Note the scattered population of darkly staining peroxidase-positive cells in the ventral vein region in nontraumatized embryos and the accumulation of peroxidase activity at the site of acute inflammation (B and C arrowheads). Panel D displays an array of 16 embryos, indicating the assay is highly reproducible. At higher power, under Nomarksi illumination, the aggregated myeloperoxidase activity is seen to be distinctly cellular (E). (F, G) Comparison of the pattern of myeloperoxidase histochemical staining (F) ando-dianisidine staining (G) for hemoglobin in embryos 6 hours (left tail in panels) and 2 days (right tail in panels) after tail transection, showing the longer persistence of peroxidase-positive cells compared with hemoglobin-containing cells. (H-J) Identical assay to those in panels A to C, performed in 48-hpf embryos but using whole-mount in situ hybridization for mpx-expressing cells rather than histochemistry. The pattern of mpx-expressing cells (black dots) is shown before (H), immediately after (I), and 8 hours after (J) tail transection. Note the accumulation ofmpx-expressing cells at the site of transection (shown by the black arrow) after (J), but not before (H, I), 8 hours. (K, L) Electron micrographs showing granulocytes, recognizable by their characteristic cytoplasmic cigar-shaped, electron-dense granules, in the vicinity of the trauma site in embryos subjected to tail transection 8 hours earlier. Granulocytes are seen within a vessel (K) and at an unusual site between skeletal muscle fibers (L). Scale bar = 2 μm (K, L).
To evaluate whether macrophages were functional at an early age of development (2 dpf) and to develop an assay for displaying functional macrophages in vivo, we micro-injected embryos with a suspension of carbon particles. Circulating carbon was cleared and within 1 hour had accumulated in axial cells of the ventral venous plexus (Figure8A-B). Histologic examination of embryos confirmed that the carbon had been taken up into the cytoplasm of phagocytic cells (Figure 8C-D) and not merely localized in intravascular embolic aggregates.
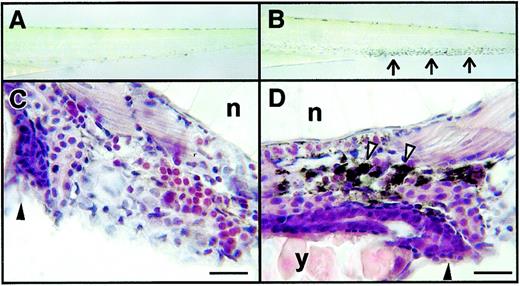
Functional studies of macrophages in zebrafish embryos.
(A, B) Dissecting microscope appearance of embryos before (A) and 2 hours after (B) intravascular micro-injection with a carbon particle suspension. Arrows in panel B indicate the accumulation of black carbon within cells in the ventral venous plexus. (C, D) Light microscope appearance of hematoxylin and eosin-stained sections of uninjected embryos (C) and embryos injected previously with carbon suspension (D). Cell with carbon in the cytoplasm, indented by the nuclei, indicated by white arrowheads. Other structures are gut and anal canal (black arrowhead), notochord (n), and yolk (y). Embryos were aged 2 dpf. Scale bars = 10 μm (C, D).
Functional studies of macrophages in zebrafish embryos.
(A, B) Dissecting microscope appearance of embryos before (A) and 2 hours after (B) intravascular micro-injection with a carbon particle suspension. Arrows in panel B indicate the accumulation of black carbon within cells in the ventral venous plexus. (C, D) Light microscope appearance of hematoxylin and eosin-stained sections of uninjected embryos (C) and embryos injected previously with carbon suspension (D). Cell with carbon in the cytoplasm, indented by the nuclei, indicated by white arrowheads. Other structures are gut and anal canal (black arrowhead), notochord (n), and yolk (y). Embryos were aged 2 dpf. Scale bars = 10 μm (C, D).
Discussion
The nomenclature of piscine granulocytes has long been a source of confusion.11 Our studies indicate that like other cyprinid teleosts,17,29 adult zebrafish have at least 2 types of granulocytes, a neutrophilic–heterophilic granulocyte and an eosinophilic granulocyte. The term “acidophilic granulocyte” has also been used,30 but we have avoided it because it has been confused with eosinophilic granulocytes and the more abundant heterophilic–neutrophilic granulocytes. Even within the recent literature, these terms for granulocytes have been applied inconsistently or even incorrectly.16 It is very important that these names, which refer to the staining reaction of the cells, not result in mistaken inferences about the function of these cells in host defense.
The electron-dense, lamellated, cigar-shaped granules of the more prevalent zebrafish heterophil closely resemble those of the carp heterophil.11,17,29 Because zebrafish heterophils display myeloperoxidase activity, it is probable that this enzyme is located in these granules, though we have not proven this. Our observation that mature heterophils from adults show weaker histochemical peroxidase activity than immature heterophils (Figure 1G-J) is also consistent with that of Bielek,17 who found reduced peroxidase activity in mature carp heterophils compared with immature carp heterophils. Cyprinid heterophils are implicated in the processes of acute inflammation and antibacterial defense. Adult carp heterophils showed respiratory burst and bactericidal activity to Aeromonas salmonicida.31 Peripheral blood granulocytosis occurs in zebrafish experimentally infected with Listeria spp, though the type of granulocyte is not described.32 Our studies in zebrafish embryos demonstrate mobilization of peroxidase-expressing cells to a site of acute inflammation within several hours of traumatization, and they confirm the presence of heterophil granulocytes in tissues and circulatory areas proximal to the site of trauma. Hence, we propose that the zebrafish heterophil indeed plays a role analogous to that of the mammalian neutrophil in these respects.
The functional role of the zebrafish eosinophil is less certain. Even observations made in other teleosts must be extrapolated with caution in light of the considerable variation between species in cell and granule morphology of granulocytes with eosinophilic cytoplasm. We have not yet identified a molecular marker for zebrafish eosinophils, nor have we determined the point in development at which production of eosinophil granulocytes is initiated. In due course, it will be interesting to isolate zebrafish orthologues of genes important for eosinophil functions in mammals,33 such as major basic protein, eosinophil-derived neurotoxin, and eosinophil cationic proteins, and to determine with which zebrafish granulocyte they are associated. Although mammalian eosinophils have their own unique peroxidase, we have not identified a second leukocyte peroxidase in zebrafish. Despite annotations in GenBank suggesting that EST clones exist that may represent a zebrafish myeloperoxidase and an eosinophil peroxidase, we have shown this to be false. One clone (fj80f04) is the same as the cDNA we have called mpx. The other (fj81h09) does not contain the peroxide sequence AW419670, though it contains AW420468, leading us to think that AW419670 has been mistakenly linked to fj81h09 in GenBank.
We isolated a zebrafish peroxidase gene expressed in myeloid cells that we called myeloid-specific peroxidase, or mpx. Phylogenetic analyses of mpx gene on the basis of the catalytic and the region 1 domains placed it at the base of Daiyasu and Toh24 subfamily 12. This led us to hypothesize that the gene duplication and diversification in tetrapods that created myeloperoxidase, eosinophil peroxidase, lactoperoxidase, and salivary peroxidase postdate the last common ancestor of tetrapods and zebrafish. Therefore, we suggest that mpx represents an ancestral subfamily 12 gene. Consistent with our hypothesis of an evolutionarily recent duplication of this family in tetrapods, mammalian eosinophil peroxidase, lactoperoxidase, and myeloperoxidase lie within 100 kb on human chromosome 17q23.1. In addition, within this region, lactoperoxidase and myeloperoxidase lie head to head with minimal intervening sequence,34 an arrangement consistent with 2 recent intrachromosomal gene duplications. Similarly, in mice, myeloperoxidase and eosinophil peroxidase both map to chromosome 11,35 36 (lactoperoxidase is not mapped in mice). Because we have identified only one zebrafish peroxidase thus far, it is not possible to say whether fish have undergone their own independent process of duplication and diversification of this gene family, though we hypothesize this to be the case. If this has occurred, there is no reason to presume that the pattern of peroxidase gene duplication in fish will resemble that in mammals. Indeed, it is possible that the great diversity of morphology of granulocytes and their granules within fish reflects this opportunity for divergent evolution. Given the close location of these 3 peroxidases in the mammalian genome, it will be interesting to search the genomic sequence of linkage group 10 in the region of mpx for other peroxidase genes as it becomes available from the zebrafish genome project. It is also possible that, in the absence of extensive gene duplication, the alternate splice forms we have described may be deployed for different functions in zebrafish granulocytes.
Expression of mpx was first observed diffusely in the intermediate cell mass (ICM) at 18 to 19 hpf. This is the region undergoing active erythroid commitment at this time, as indicated by the expression of molecular markers of erythroid commitment. A few hours later, discrete mpx-expressing cells are observed over the yolk sac and in the axis of the embryo. The significance of the early diffuse mpx expression in the ICM is unclear, nor is the mechanism by which dispersed cells arise over the yolk sac with strong expression of mpx, a marker of terminal myeloid–granulocytic differentiation. We (G.J. Lieschke et al, manuscript submitted) have characterized the expression pattern of zebrafish spi1, an orthologue of mammalian PU.1 that has been shown to function as a molecular antagonist of Gata-1 and can direct cells toward a myeloid fate. Expressed in the caudal lateral plate mesoderm before its convergence to form the axial ICM,spi1 was never expressed in the axial structure itself. Interestingly, the earliest site of spi1 expression is in the rostral lateral plate mesoderm anterior to the heart field, a site from which the early macrophage population, described by Herbomel et al,12 arises but not from which granulocytes might be thought to arise, from the later expression patterns of eitherc-ebp118 or mpx. However, our fate-mapping studies confirmed that cells from this early anterior location end up in the nascent circulation early on the second day of life and look like the circulating granulocytes we visualized by electron microscopy (Figure 3D). Whether a subset of these earliest rostrally arising myeloid phagocytes are those that later expressmpx or whether these earliest mpx-expressing cells arise from a separate site is uncertain. The expression ofspi1 from 14 to 20 hpf in the caudal lateral plate mesoderm and its later expression from 26 to 30 hpf in the posterior ICM, immediately caudal to the posterior yolk extension, leave open the possibility that myeloid commitment is directed at these sites from the moment these posterior regions of spi1 expression are first observed.
In mammals, monocytes also contain myeloperoxidase granules,37 but this is not typical of quiescent tissue macrophages.38 Although mature macrophages and their immediate precursors are myeloperoxidase-positive in the cyprinidCarassius auratus L.,39 we did not observe histochemical myeloperoxidase activity in morphologically identified macrophages and their precursors in cytospin preparations of adult zebrafish kidney leukocytes, nor did we detect mpxexpression in a pattern corresponding to the first wave of zebrafish macrophage development.12 Hence, our observations collectively indicate that most cells with myeloperoxidase activity in adult zebrafish are granulocytes and that there are defined populations of adult and embryonic zebrafish macrophages that do not express this enzyme. It remains possible, however, that in zebrafish a minor population of the myeloperoxidase- or mpx-expressing cells is monocytic rather than granulocytic, as in goldfish and mammals. Clarification of the precise lineage specificities of myeloperoxidase activity and mpx expression awaits the generation of additional independent markers of the several zebrafish myeloid lineages.
The capacity to generate mutant zebrafish is large, and the tools to translate mutants into identified genes, including the complete sequence of the zebrafish genome, are now rapidly being collected. Existing zebrafish mutants demonstrate the genetic dissociability of myeloid and erythroid development in the early zebrafish embryo. In mutants exemplified by spadetail, erythropoiesis fails (evidenced by a lack of gata1expression), but myelopoiesis initiates, because myeloid cells have been demonstrated morphologically (G.J. Lieschke et al, manuscript submitted) and histochemically (Figure 3M). A recent report alluded to the generation of a mutant that initiated erythropoiesis but failed to initiate myelopoiesis on the basis of a loss of L-plastinand c-ebp1 expression.18 In other mutants typified by the mutant cloche, the first wave of erythropoiesis and myelopoiesis is absent, though even in them39 deletion mutant, erythroid cells and myeloid cells have now been identified in the ventral vein region after the first day of life (our studies, Rowley et al,10 and Liao et al26). With a basic understanding of zebrafish myelopoiesis and an increasing repertoire of tools to study zebrafish myelopoiesis specifically, it may be hoped that the progressive generation and study of mutants with selective lineage-specific defects in hematopoiesis and myelopoiesis will contribute significantly to a comprehensive genome-based understanding of the genetic regulation of these developmental processes.
We thank Sony Varma for excellent technical assistance, Nadine Watson for electron micrographs, Yi Zhou for radiation hybrid mapping, Nathan Hall for bioinformatics advice, Ashley Dunn and the Ludwig Institute Molecular Biology Laboratory for helpful comments early in this project, Janna Stickland and Pierre Smith for help with photography and figures, and Cuong Do and Bill Robinson for comment and discussion. G.J.L. thanks Tony Burgess for encouragement to apply zebrafish methodologies to the study of myelopoiesis.
Supported by National Health and Medical Research Council of Australia project grant 134510 (A.C.W.). G.J.L. is a Wellcome Senior Research Fellow in Medical Sciences in Australia. A.C.O. was the recipient of a Ludwig Institute Postdoctoral Fellowship. M.O.C. is the recipient of an Australian Postgraduate Research Award. A.C.W. is the recipient of a Viertel Senior Research Fellowship.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Graham J. Lieschke, Ludwig Institute for Cancer Research, PO Box 2008, The Royal Melbourne Hospital, Parkville, Victoria, 3050, Australia; e-mail: graham.lieschke@ludwig.edu.au.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal