Abstract
Achieving a complete cytogenetic response (CCgR) is a major target in the treatment of chronic myeloid leukemia (CML) with interferon-α (IFN-α), but CCgRs are rare. The mean CCgR rate is 13%, in a range of 5% to 33%. A collaborative study of 9 European Union countries has led to the collection of data on 317 patients who were first seen between 1983 and 1997 and achieved CCgRs with IFN-α alone or in combination with hydroxyurea. The median time to first CCgR was 19 months (95% CI, 17-21; range, 3-84 months). At last contact, 212 patients were still alive and in continuous CCgR; 105 patients had lost CCgR, but 53% of them were still alive and in chronic phase. IFN-α treatment was discontinued permanently in 23 cases for response loss, in 36 cases for chronic toxicity (15 are still in unmaintained continuous CCgR), and in 8 cases because it was believed that treatment was no longer necessary (7 of these 8 patients are still in unmaintained continuous CCgR). The 10-year survival rate from first CCgR is 72% (95% CI, 62%-82%) and is related to the risk profile. High-risk patients lost CCgR more frequently and more rapidly and none survived more than 10 years. Low-risk patients survived much longer (10-year survival probability 89% for Sokal low risk and 81% for Euro low risk). These data point out that a substantial long-term survival in CCgRs is restricted mainly to low-risk and possibly intermediate-risk patients and occurs significantly less often in high-risk patients.
Introduction
Treatment of Philadelphia-positive (Ph+) chronic myeloid leukemia (CML) with interferon-α (IFN-α) was introduced in the early 1980s,1-3 was developed and expanded at the M. D. Anderson Hospital, Houston, TX,4,5 but did not become widely accepted as standard treatment until several randomized studies6-9 showed, with one exception,10 that the survival of the patients who received IFN-α was longer than the survival of the patients who received conventional chemotherapy. Soon after, the role of IFN-α was confirmed by a meta-analysis of the randomized trials.11From the initial studies it was surprising to find that, using IFN-α, it was possible to obtain a cytogenetic response (CgR) without inducing a phase of marrow aplasia, as was necessary with nonconventional intensive chemotherapy (reviewed by Talpaz et al12) and with allogeneic bone marrow transplantation (alloBMT).13-16 That surprise quickly transformed into a strong interest when it was realized that CgRs associated with IFN-α tended to increase and to improve with time and to become stable.5,17-19 Achieving a complete CgR (CCgR) is one of the main targets of treatment and could become a surrogate marker for a clinical end point such as survival duration.20 In CML a clear relationship between CgR and long-term survival has been shown with alloBMT but not with standard chemotherapy. With IFN-α several studies have already focused on the importance of CgR, but, for many reasons, the relationship between survival and the rate and the degree of IFN-α–induced CgR was not always easy to show and evaluate. The variability of the degree of response, the time needed to achieve a CCgR, its stability and duration, and the confounding effects of disease-related risk21-23 were difficult to evaluate considering the small sample size, because CCgRs are comparatively rare events. In addition the follow-up time is short, most having been generated in the last decade. Also, though in all reports the numbers of CCgRs are counted, the characteristics and the fate of the patients who achieve a CCgR are frequently reported together with those of other responders, partial or even less than partial, rather than separately. It is widely recognized that the specificity of a conventional cytogenetic evaluation is relatively low and it has been reported that the leukemia-specific BCR/ABL transcript can be found in almost all the cases of CCgR.24-26 However, those having a CCgR have less residual disease than other responders24-26and there is little doubt that CCgRs are a fascinating élite of patients who have the highest sensitivity to IFN-α and are the most likely candidates for prolonged survival and possibly cure.5,17,23 27 For these reasons, better knowledge of more CCgRs is required and this is possible only through an international cooperative effort. In this report, we describe the characteristics and the long-term outcome of the largest series of CCgRs that has ever been collected, thanks to the cooperation of the investigators of 9 European Union countries.
Patients, materials, and methods
Patients
This study is based on 317 cases of complete cytogenetic responders (CCgRs) who have been identified by a group of European investigators on CML (EICML). The group was established in Bologna after an international meeting on CML, in 1992, and since then has regularly held an annual meeting to exchange and discuss data and programs on the treatment of CML with IFN-α. Group member cooperation led to several collaborative studies.11,22,28 In 1998, the investigators agreed to establish a registry of the CCgRs to IFN-α. The data were collected in 1999 and were checked and analyzed during 2000. The method that was used to collect the cases was to look at the files of the national study groups and to sort out all the available information on CCgRs. The invitation to contribute to the registry was extended to 2 institutions (Bordeaux and London/Hammersmith) that were represented at the 1998 meeting and to all the centers contributing to the Italian group. The eligibility criteria for the registry were Ph+ CML and the achievement of a CCgR at least once after any regimen containing IFN-α. Those patients who had achieved the first CCgR only after intensive chemotherapy or a procedure including any bone marrow or peripheral blood stem cell transplantation were excluded. A total of 507 cases were submitted; 19 were rejected and 488 were registered. These 488 patients were divided into 2 groups, one group of 159 patients who had been prospectively assigned to combination treatment with IFN-α and low-dose arabinosyl cytosine (LDAC) and one group of 329 patients who had been assigned to treatment with IFN-α and did not receive LDAC. Because the response to IFN-α and LDAC may be different from the response to IFN-α alone29 and because the follow-up of the IFN-α plus LDAC patients is still short, the IFN-α plus LDAC group has not yet been analyzed and we report here only on the patients who were treated with IFN-α without LDAC. Twelve of these cases have been submitted to alloBMT in chronic phase (CP), after achieving a CCgR. They are not included in this analysis, which concerns the remaining 317 cases. A total of 214 cases were collected from the database of 9 national study groups in Austria, Belgium and the Netherlands, France, Germany, Italy, Spain, Sweden, and the United Kingdom; 103 cases were collected from single institutions in Italy, France, and the United Kingdom (Table1). The first patient started treatment in 1983 and the last patient in 1997, with the median 1992. The observation time of living patients ranges between 12 and 171 months (median, 66 months).
Cases collected from European countries, from the database of national study groups or from single institutions
| Country . | No. cases from national study groups . | No. cases from single institutions . | Total . |
|---|---|---|---|
| Austria | 4 | 0 | 4 |
| Belgium and the Netherlands | 10 | 0 | 10 |
| France | 72 | 39 | 111 |
| Germany | 25 | 0 | 25 |
| Italy | 59 | 60 | 119 |
| Spain | 19 | 0 | 19 |
| Sweden | 3 | 0 | 3 |
| United Kingdom | 22 | 4 | 26 |
| Total | 214 | 103 | 317 |
| Country . | No. cases from national study groups . | No. cases from single institutions . | Total . |
|---|---|---|---|
| Austria | 4 | 0 | 4 |
| Belgium and the Netherlands | 10 | 0 | 10 |
| France | 72 | 39 | 111 |
| Germany | 25 | 0 | 25 |
| Italy | 59 | 60 | 119 |
| Spain | 19 | 0 | 19 |
| Sweden | 3 | 0 | 3 |
| United Kingdom | 22 | 4 | 26 |
| Total | 214 | 103 | 317 |
Definitions and methods
A CCgR was defined as the absence of any Ph+metaphase by conventional cytogenetics in at least one evaluation if the number of analyzed metaphases was 20 or more and in at least 2 consecutive evaluations if the number of analyzed metaphases was less than 20 (range, 8-19). CCgR loss was defined by the reappearance of more than 10% Ph+ metaphases in one evaluation or more than 1% Ph+ metaphases in 2 consecutive evaluations. Other cytogenetic responses or status were qualified partial (PCgR) or less than partial when the percentage of Ph+ metaphases was less than 33% or more than 33%, respectively. In 5 cases, and on some occasions, fluorescence in situ hybridization substituted for conventional cytogenetics. Molecular biology data, including occasionally quantitative evaluation of the BCR/ABL transcripts, were collected in many cases, but were not analyzed because several different nonstandardized techniques were used.
Hematologic response was qualified complete (CHR) if the white blood cell (WBC) count was less than 10 × 109/L, the differential blood count was normal, the platelet count was less than 500 × 109/L, and the spleen could not be palpated. The risk profile of the patients at diagnosis was calculated using the Sokal formulation30 as well as the new European prognostic formulation (Euro)28 (Table2).
Formulations for the definition of the risk profile at diagnosis
| . | Sokal . | Euro . |
|---|---|---|
| Age (y) | 0.0116 (age, 43.4) | 0.6666 when age 50 or older |
| *Spleen (cm) | 0.0345 (spleen − 7.51) | 0.042 × spleen |
| Platelet (× 109/L) | 1.0956 when 1500 or higher | |
| †Myeloblasts (%) | 0.0887 (myeloblasts − 2.10) | 0.0584 × myeloblasts |
| †Eosinophils (%) | — | 0.0413 × eosinophils |
| †Basophils (%) | — | 0.2039 when basophils at least 3% |
| Relative risk (RR) | Exponential of the total | Total × 1000 |
| . | Sokal . | Euro . |
|---|---|---|
| Age (y) | 0.0116 (age, 43.4) | 0.6666 when age 50 or older |
| *Spleen (cm) | 0.0345 (spleen − 7.51) | 0.042 × spleen |
| Platelet (× 109/L) | 1.0956 when 1500 or higher | |
| †Myeloblasts (%) | 0.0887 (myeloblasts − 2.10) | 0.0584 × myeloblasts |
| †Eosinophils (%) | — | 0.0413 × eosinophils |
| †Basophils (%) | — | 0.2039 when basophils at least 3% |
| Relative risk (RR) | Exponential of the total | Total × 1000 |
In the Sokal30 formulation, all four variables are continuous. In the Euro formulation,28 spleen and myeloblasts are continuous, whereas age and platelet count enter into the calculation only when age is at least 50 years and platelet count is at least 1500 × 109/L. Low-risk patients have an RR less than 0.8 with the Sokal and at most 780 with Euro formulation. Intermediate-risk patients have an RR between 0.8 and 1.2 with the Sokal and between 781 and 1479 with the Euro formulation. High-risk patients have an RR greater than 1.2 with the Sokal and at least 1480 with the Euro method.
Maximum distance from costal margin.
Percent in peripheral blood.
All the patients were reported to have received the first dose of IFN-α while in first CP. However, the criteria distinguishing CP from more advanced phases, accelerated or blastic (AB), were not predetermined. Therefore the identification of the progression as well as the date of the progression were accepted as they were originally recorded in each national or institutional database. Whilst taking into account that the criteria for the definition of AB phase were not exactly the same in all the cases, it may be useful to note that the strictest requirements for the definition of AB phase were those of the Italian group.6 For the Italian group AB phase was indicated by at least 2 of the following criteria: a peripheral blood sample containing more than 10% blast cells or more than 30% blast cells and promyelocytes; a bone marrow aspirate containing more than 15% blast cells or more than 50% blast cells and promyelocytes; a spleen that could be palpated more than 10 cm below the left costal margin, with a WBC count of less than 25 × 109/L; involvement of the central nervous system, bone, lymph nodes, or other extrahematologic sites; and cytogenetic evaluation revealing double Ph, trisomy 8, or isochromosome 17.
All time calculations were made with the method of Kaplan and Meier,31 from the date of first IFN-α dose, or first CCgR, or IFN-α discontinuation, or CCgR loss, to last contact or to the relevant event. The relevant event was either the date of the last cytogenetic examination or the date of death, as appropriate. The curves were always truncated at 10 years, when the number of living patients was 40 or less. No events occurred in these 40 patients during the subsequent years. Because this study is descriptive and the nature of the data, as well as the way the data were collected, do not allow comparisons, no statistical tests were applied apart from the log-rank test,32 which was used to evaluate if patients with a different risk profile had a different survival and CCgR duration.
Results
Patient characteristics
The main clinical and hematologic characteristics of the 317 patients, at diagnosis and before any treatment, are shown in Table3. Median age was 49 years, with 138 patients (43%) more than 50 years old and 56 patients (18%) more than 60 years old. Sex (57% men), platelet count (median, 325 × 109/L), and the percentage of eosinophils and basophils in peripheral blood (median, 2.0% and 2.2%, respectively) were within the expected range of a CML patient population eligible for IFN-α treatment.4-10 The spleen was palpable only in 43% of patients, and in these cases was relatively small (median, 4 cm below the costal margin). The hemoglobin level was normal in many cases (median, 127 g/L). The WBC count was low in many patients (median, 66 × 109/L). The number of patients with a very high platelet count (> 1500 × 109/L) was small (1%). The percentage of myeloblasts in peripheral blood was also low (median 0, mean 1.0% ± 2.2%). Case distribution according to the Sokal and Euro risk score was unbalanced, with an excess of low-risk cases (62% Sokal and 58% Euro) and a low frequency of high-risk cases (12% Sokal and 6% Euro).
Main clinical and hematologic characteristics of the 317 cases of CCgR, at diagnosis and before any treatment
| Age (y) | Mean ± SD | 47.4 ± 13.0 |
| Median (range) | 49.0 (9-73) | |
| Gender | male, no. of cases | 180 (57%) |
| Spleen | Palpable, no. of cases | 133 (43%) |
| Spleen (cm from costal margin) | Mean ± SD | 6.2 ± 5.1 |
| Median (range) | 4.0 (1-24) | |
| Hemoglobin (g/L) | Mean ± SD | 126 ± 19 |
| Median (range) | 127 (55-166) | |
| WBC count (× 109/L) | Mean ± SD | 98 ± 95 |
| Median (range) | 66 (12-471) | |
| Platelet count (× 109/L) | Median (range) | 325 (81-3264) |
| Platelet count (greater than 1500 × 109/L) | No. of cases | 4 (1%) |
| Myeloblasts (%) | Mean ± SD | 1.0 ± 2.2 |
| Median (range) | 0 (0-25.0) | |
| Eosinophils (%) | Mean ± SD | 2.1 ± 2.2 |
| Median (range) | 2.0 (0-15.0) | |
| Basophils (%) | Mean ± SD | 3.3 ± 3.4 |
| Median (range) | 2.2 (0-23.0) | |
| Basophils (at least 3%) | No. of cases | 148 (47%) |
| Transcript | b2a2 | 51 (16%) |
| b3a2 ± b2a2 | 110 (35%) | |
| Not known | 156 (49%) | |
| Sokal score (no. cases) | Less than 0.8 (low risk) | 179 (62%) |
| 0.8-1.2 (intermediate risk) | 76 (26%) | |
| Greater than 1.2 (high risk) | 35 (12%) | |
| Euro score (no. of cases) | At most 780 (low risk) | 164 (58%) |
| 781-1479 (intermediate risk) | 101 (36%) | |
| At least 1480 (high risk) | 16 (6%) | |
| Age (y) | Mean ± SD | 47.4 ± 13.0 |
| Median (range) | 49.0 (9-73) | |
| Gender | male, no. of cases | 180 (57%) |
| Spleen | Palpable, no. of cases | 133 (43%) |
| Spleen (cm from costal margin) | Mean ± SD | 6.2 ± 5.1 |
| Median (range) | 4.0 (1-24) | |
| Hemoglobin (g/L) | Mean ± SD | 126 ± 19 |
| Median (range) | 127 (55-166) | |
| WBC count (× 109/L) | Mean ± SD | 98 ± 95 |
| Median (range) | 66 (12-471) | |
| Platelet count (× 109/L) | Median (range) | 325 (81-3264) |
| Platelet count (greater than 1500 × 109/L) | No. of cases | 4 (1%) |
| Myeloblasts (%) | Mean ± SD | 1.0 ± 2.2 |
| Median (range) | 0 (0-25.0) | |
| Eosinophils (%) | Mean ± SD | 2.1 ± 2.2 |
| Median (range) | 2.0 (0-15.0) | |
| Basophils (%) | Mean ± SD | 3.3 ± 3.4 |
| Median (range) | 2.2 (0-23.0) | |
| Basophils (at least 3%) | No. of cases | 148 (47%) |
| Transcript | b2a2 | 51 (16%) |
| b3a2 ± b2a2 | 110 (35%) | |
| Not known | 156 (49%) | |
| Sokal score (no. cases) | Less than 0.8 (low risk) | 179 (62%) |
| 0.8-1.2 (intermediate risk) | 76 (26%) | |
| Greater than 1.2 (high risk) | 35 (12%) | |
| Euro score (no. of cases) | At most 780 (low risk) | 164 (58%) |
| 781-1479 (intermediate risk) | 101 (36%) | |
| At least 1480 (high risk) | 16 (6%) | |
Spleen size is given in centimeters as the maximum distance from costal margin, in the cases where the spleen was palpable (zero values are excluded from the calculation of the mean and the median). Myeloblasts, eosinophils, and basophils are in peripheral blood. The Sokal and Euro risk groups are identified as described in “Patients, materials, and methods” and Table 2. In some cases, the data were not avaiable (spleen, 7 cases; myeloblasts, 13 cases; eosinophils, 22 cases; basophils, 17 cases; Sokal, 27 cases; Euro, 36 cases).
Treatment
The type of IFN-α was human recombinant α2b in 149 cases (47%), α2a in 101 cases (32%), α2c in 4 cases (1%), and lymphoblastoid in 20 cases (6%). In 43 cases the type was not reported or more than one IFN-α type was used. The dose of IFN-α that was actually administered before the first CCgR could be estimated in 235 of 317 cases (74%). The average weekly dose ranged between 3 and 74 MIU (median, 37 MIU). The minimum weekly dose ranged between 0 (because the treatment was sometimes interrupted) and 69 MIU (median, 21 MIU). The maximum weekly dose ranged between 9 and 88 MIU (median, 49 MIU). The total dose of IFN-α that was given before detecting the first CCgR ranged between 284 and 16 027 MIU (median, 2912 MIU). We looked for any possible relationships between IFN-α type, IFN-α dose, and time to first CCgR and CCgR duration or survival, but no difference was detected. The doses and schedules of IFN-α after the first CCgR could be retrieved only in a minority of cases and were not analyzed.
The administration of other antileukemic drugs prior or concurrently to IFN-α could be retrieved in 248 of 317 cases (78%). Seventy-four patients (30%) never received any other antileukemic drugs. Fifteen patients (6%) received busulfan (Bus) prior to IFN-α. Ninety-four patients (38%) received hydroxyurea (HU) before IFN-α; they behaved the same as the patients who had never received HU. Sixty-five patients (26%) were given HU concurrently with and sometimes after IFN-α; for these patients the time to first CCgR was slightly longer (median, 22 months versus 18 months), suggesting that they could be less sensitive to IFN-α than those who did not require HU. Twelve patients who were already in CCgR underwent autografting subsequently. They were not censored at the time of autografting.
Hematologic response
A CHR was obtained in all 317 cases, prior to achieving a CCgR. The median time from the first IFN-α dose to CHR was 2.7 months, with 56% of patients in CHR at 3 months, 83% at 6 months, 93% at 9 months, and 100% at 12 months. Response was slightly slower in high-risk patients (median, 3.6 months for Sokal high risk, and 5 months for Euro high risk). It should not be overlooked that these are likely to be maximum estimates because the calculation of the time from the first IFN-α dose to CHR may be biased by some delay in capturing the response.
CgR
In a similar manner, the kinetics of CgR may be biased by a delay in capturing response. With that in mind, the calculated median time from the first IFN-α dose to the first CCgR was 19 months (95% CI, 17-21 months), with 25% of patients in CCgR after 1 year, 61% after 2 years, and 82% after 3 years (Figure 1). To arrive at 100% took 4 more years, because one patient achieved the first CCgR only after 7 years of treatment with IFN-α. In some patients the first CgR was already complete but in the great majority of patients the first CgR was not yet complete, either less than partial or partial. The time to achieve these responses was shorter (Figure 1), with a median of 7 months to the first response, irrespective of the degree, and of 11 months to the first PCgR. Almost all the patients had some response within 1 year of treatment.
Time from first IFN-α dose to first CgR.
The curve a shows the time to the first CgR, whatever the degree (from less than partial to complete, whichever came first); the median is 7 months, and almost all the patients had achieved a CgR within 1 year. The curve b shows the time to the first partial or complete CgR, whichever came first; the median is 11 months, and almost all the patients had achieved a CgR within 2 years. The curve c shows the time to the first CCgR; the median is 19 months (95% CI, 17-21), but the latest complete response was recorded after 7 years.
Time from first IFN-α dose to first CgR.
The curve a shows the time to the first CgR, whatever the degree (from less than partial to complete, whichever came first); the median is 7 months, and almost all the patients had achieved a CgR within 1 year. The curve b shows the time to the first partial or complete CgR, whichever came first; the median is 11 months, and almost all the patients had achieved a CgR within 2 years. The curve c shows the time to the first CCgR; the median is 19 months (95% CI, 17-21), but the latest complete response was recorded after 7 years.
Survival
Survival was calculated from the first IFN-α dose and from the first CCgR (Figure 2). Calculating survival from the first IFN-α dose may provide a biased estimate because to become a CCgR a patient has to survive until the response is achieved. Calculating survival from first CCgR provides a true estimate of the life expectancy after the achievement of a CCgR. The survival from first CCgR is 86% (95% CI, 80%-91%) at 5 years and 72% (95% CI, 61%-82%) at 10 years. These survival estimates are likely to be different according to the risk profile, either Sokal (Figure3) or Euro score (Figure4). In fact, for low-risk patients 5-year survival from the first CCgR was 93% (Sokal) or 89% (Euro), whereas for high-risk patients it was 54% (Sokal) or 51% (Euro). After 10 years, the survival probability of low-risk patients was 89% Sokal (95% CI, 81%-98%) or 81% Euro (95% CI, 67%-94%), but could not be estimated for high-risk patients, because no high-risk patient was followed up for as long as this. The survival of intermediate-risk patients was significantly shorter than the survival of low-risk patients using the Sokal classification.30 With the Euro classification,28 the difference was not significant.
Overall survival.
Overall survival is shown from the first IFN-α dose (10-year survival 75%; 95% CI, 66%-84%) or from the date of the first CCgR (10-year survival 72%; 95% CI, 62%-82%). No events have occurred after 120 months, as yet. (a) Number of cases at risk from the first IFN-α dose. (b) Number of cases at risk from the first CCgR.
Overall survival.
Overall survival is shown from the first IFN-α dose (10-year survival 75%; 95% CI, 66%-84%) or from the date of the first CCgR (10-year survival 72%; 95% CI, 62%-82%). No events have occurred after 120 months, as yet. (a) Number of cases at risk from the first IFN-α dose. (b) Number of cases at risk from the first CCgR.
Survival from the first CCgR, according to Sokal risk.30
The overall log-rank test is significant (P < .0001). The 10-year survival is 89% (95% CI, 81%-98%) for low-risk patients versus 70% (95% CI, 49%-91%) for intermediate-risk patients (P = .04, log-rank test). The survival of high-risk patients is much shorter (P < .0001 versus low risk,P = .003 versus intermediate risk, log-rank test).
Survival from the first CCgR, according to Sokal risk.30
The overall log-rank test is significant (P < .0001). The 10-year survival is 89% (95% CI, 81%-98%) for low-risk patients versus 70% (95% CI, 49%-91%) for intermediate-risk patients (P = .04, log-rank test). The survival of high-risk patients is much shorter (P < .0001 versus low risk,P = .003 versus intermediate risk, log-rank test).
Survival from the first CCgR according to Euro risk.28
The overall log-rank test is significant (P < .0001). The 10-year survival is 81% (95% CI, 67%-94%) for low-risk patients versus 78% (95% CI, 64%-92%) for intermediate-risk patients (P = .15, log-rank test). The survival of high-risk patients is much shorter (P < .0001 versus low risk,P = .001 versus intermediate risk, log-rank test).
Survival from the first CCgR according to Euro risk.28
The overall log-rank test is significant (P < .0001). The 10-year survival is 81% (95% CI, 67%-94%) for low-risk patients versus 78% (95% CI, 64%-92%) for intermediate-risk patients (P = .15, log-rank test). The survival of high-risk patients is much shorter (P < .0001 versus low risk,P = .001 versus intermediate risk, log-rank test).
CCgR duration
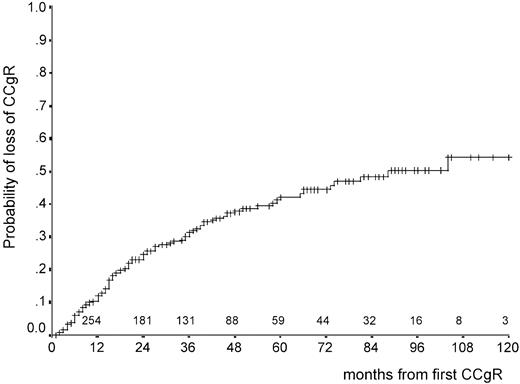
At last contact, 212 patients were still in first continuous CCgR and 105 patients had lost CCgR. CCgR was lost at a rate of 12% per year during the first 2 years and at a slower rate thereafter (Figure5). After 5 years the cumulative proportion of cases in continuous CCgR was 58% (95% CI, 51%-65%). After 10 years, it was 46% but only 3 cases were still at risk. The duration of the response showed an association with the prognostic score, either Sokal or Euro, with high-risk patients losing the response more rapidly than the others (Figure6).
Time from first CCgR to CCgR loss.
The probability of CCgR loss is 42% (95% CI, 35%-49%) at 5 years and 50% (95% CI, 40%-60%) at 8 years. The numbers above the abscissa are the number of cases at risk.
Time from first CCgR to CCgR loss.
The probability of CCgR loss is 42% (95% CI, 35%-49%) at 5 years and 50% (95% CI, 40%-60%) at 8 years. The numbers above the abscissa are the number of cases at risk.
Time from first CCgR to CCgR loss, according to Sokal30 and Euro28 risk.
High-risk patients lose the response more rapidly than low-risk and intermediate-risk patients (P ≤ .01, log-rank test).
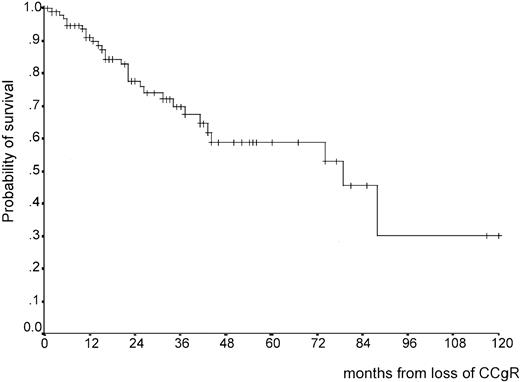
The outcome of the patients who lost CCgR is described in Table4 and Figure7. At last contact, many of these patients were alive and in CP, but 32 had progressed to AB phase (Table4). The survival curve after CCgR loss shows a steady rate of events during the first 3 years (Figure 7), but the number of observations is not yet sufficient to allow estimation of the median, which might range anywhere between 4 and 8 years, or the extent of long-term survival.
Current status of the 105 patients who have lost CCgR
| . | Case no. . | Case proportion . |
|---|---|---|
| Still in CP, partial CgR | 36 | 34% |
| Still in CP, less than partial CgR | 20 | 19% |
| Still in CP, no CgR | 11 | 10% |
| Dead in CP | 54-150 | 6% |
| Progressed to AB phase | ||
| Alive | 104-151 | 10% |
| Dead | 22‡ | 21% |
| Total | 104 |
| . | Case no. . | Case proportion . |
|---|---|---|
| Still in CP, partial CgR | 36 | 34% |
| Still in CP, less than partial CgR | 20 | 19% |
| Still in CP, no CgR | 11 | 10% |
| Dead in CP | 54-150 | 6% |
| Progressed to AB phase | ||
| Alive | 104-151 | 10% |
| Dead | 22‡ | 21% |
| Total | 104 |
The status is not known in one patient.
One of 5 after autologous BMT.
One of 10 after alloBMT.
Three of 22 after alloBMT.
Survival from CCgR loss.
After 4 years the number of patients at risk is 15 and does not allow to calculate the median.
Survival from CCgR loss.
After 4 years the number of patients at risk is 15 and does not allow to calculate the median.
It was impossible to evaluate if the continuation or the discontinuation of IFN-α treatment after CCgR loss had any effects on the subsequent course of the disease, because many of the patients who lost the response from complete to partial were continued on IFN-α, whereas many of the patients who lost the response from complete to less than partial or none discontinued IFN-α permanently. Obviously, the former survived much longer than the latter, 90% versus 30% at 5 years after CCgR loss (data not shown).
Discontinuation of IFN-α
Treatment with IFN-α was permanently discontinued in 75 patients (24% of the total; Table 5). In 8 patients the cause of discontinuation was not known or could not be identified. In 23 patients IFN-α discontinuation was motivated by response loss either by CgR loss (only 3 patients) or by CHR loss (20 patients), indicating very clearly that the majority of the patients who had lost CCgR were maintained in IFN-α until the CHR was also lost. Almost all these 23 patients progressed and died of leukemia or as the result of the transplant. In 36 cases discontinuation was motivated by side effects, chronic toxicity, or loss of compliance to a chronic treatment. In this group of patients the median duration of IFN-α treatment was 43 months (range, 7-91 months) and the median time from the first CCgR to discontinuation was 14 months (range, 1-77 months). Of these 36 patients, 15 are still in continuous CCgR and only 4 have progressed to AB phase. In only 8 patients was the decision to discontinue based on the consideration that a stable CCgR had been achieved and that treatment might no longer be necessary. Seven of these 8 patients had been given IFN-α for a long time, 57 to 86 months, and the median time from the first CCgR to discontinuation was 62 months (range, 14-89 months). Seven of these 8 patients were still in continuous CCgR at last contact. Although the median survival of the 23 patients who had discontinued IFN-α for response loss was only 14 months, the median survival of the 44 patients who went off treatment for other reasons was not yet reached, and 78% of them were projected to be alive 6 years after discontinuation. The survival and the kinetics of the cytogenetic relapse of these 44 patients are shown in Figure 8.
Current status of the 75 patients who discontinued IFN-α forever, according to the reasons for treatment discontinuation
| Current status . | Treatment no longer necessary . | Chronic toxicity, loss of compliance . | Response loss . | Not known . | Total . |
|---|---|---|---|---|---|
| Alive in CCgR | 7 | 15 | 15-150 | 2 | 25 |
| Dead in CCgR | 15-150 | 4 | — | 1 | 6 |
| Alive in PCgR | — | 6 | — | — | 6 |
| Dead in PCgR | — | 1 | — | 1 | 2 |
| Alive in less than partial CgR | — | 5 | — | 2 | 7 |
| Dead in less than partial CgR | — | 1 | — | 1 | 2 |
| Alive in AB phase | — | 3 | 3 | — | 6 |
| Dead in AB phase | — | 1 | 195-151 | 1 | 21 |
| Total | 8 | 36 | 23 | 8 | 75 |
| Current status . | Treatment no longer necessary . | Chronic toxicity, loss of compliance . | Response loss . | Not known . | Total . |
|---|---|---|---|---|---|
| Alive in CCgR | 7 | 15 | 15-150 | 2 | 25 |
| Dead in CCgR | 15-150 | 4 | — | 1 | 6 |
| Alive in PCgR | — | 6 | — | — | 6 |
| Dead in PCgR | — | 1 | — | 1 | 2 |
| Alive in less than partial CgR | — | 5 | — | 2 | 7 |
| Dead in less than partial CgR | — | 1 | — | 1 | 2 |
| Alive in AB phase | — | 3 | 3 | — | 6 |
| Dead in AB phase | — | 1 | 195-151 | 1 | 21 |
| Total | 8 | 36 | 23 | 8 | 75 |
In 8 cases (first column) the reason was that it was believed that treatment was no longer necessary. In the other cases, treatment discontinuation was decided either because the treatment was no longer tolerated (second column) or because the response had been lost (third column).
alloBMT.
alloBMT in 3 cases.
Discontinuation of IFN-α.
The curves show the fate of the 44 patients who discontinued IFNα forever while being in CCgR. The upper curve shows the survival and the lower curve shows the time to CCgR loss. Both curves are calculated from the date of IFN-α discontinuation.
Discontinuation of IFN-α.
The curves show the fate of the 44 patients who discontinued IFNα forever while being in CCgR. The upper curve shows the survival and the lower curve shows the time to CCgR loss. Both curves are calculated from the date of IFN-α discontinuation.
Deaths in CP
Nine patients died in CP, 21 to 88 months after the first IFN-α dose. They are listed in Table 6. Two of these patients died rather early, one of acute liver failure associated with paracetamol and one with progressive dementia. The other 7 patients died after a longer treatment period, from 34 to 88 months, one of a bronchogenic carcinoma and 6 of a cardiac or other vascular accident. Five of these 9 patients were still in CCgR at the last cytogenetic examination before death.
Data on patients who died
| Case no. . | Gender . | Age at death (y) . | Cause of death . | Months in IFN-α . | Months off IFN-α . | Cause of IFN-α discontinuation . | Last cytogenetics before death . |
|---|---|---|---|---|---|---|---|
| 185 | M | 46 | Liver failure | 21 | Less than 1 | Liver failure | PCgR |
| 515 | M | 60 | Dementia | (∼ 30) | (At most 12) | Dementia | CCgR |
| 411 | F | 63 | Cardiac arrest | 86 | 6 | Liver toxicity | CCgR |
| 123 | M | 66 | Myocardial infarction | 42 | — | (death) | CCgR |
| 495 | M | 67 | Not identified, sudden death at home | 56 | — | (death) | CCgR |
| 238 | M | 69 | Bronchogenic carcinoma | 71 | 4 | Cancer | PCgR |
| 237 | M | 71 | Pulmonary thromboembolism | 88 | 3 | Not identified | Less than PCgR |
| 253 | M | 73 | Myocardial infarction | 70 | 19 | Loss of compliance | CCgR |
| 519 | M | 76 | Ictus cerebri | 34 | 25 | Loss of compliance | Less than PGgR |
| Case no. . | Gender . | Age at death (y) . | Cause of death . | Months in IFN-α . | Months off IFN-α . | Cause of IFN-α discontinuation . | Last cytogenetics before death . |
|---|---|---|---|---|---|---|---|
| 185 | M | 46 | Liver failure | 21 | Less than 1 | Liver failure | PCgR |
| 515 | M | 60 | Dementia | (∼ 30) | (At most 12) | Dementia | CCgR |
| 411 | F | 63 | Cardiac arrest | 86 | 6 | Liver toxicity | CCgR |
| 123 | M | 66 | Myocardial infarction | 42 | — | (death) | CCgR |
| 495 | M | 67 | Not identified, sudden death at home | 56 | — | (death) | CCgR |
| 238 | M | 69 | Bronchogenic carcinoma | 71 | 4 | Cancer | PCgR |
| 237 | M | 71 | Pulmonary thromboembolism | 88 | 3 | Not identified | Less than PCgR |
| 253 | M | 73 | Myocardial infarction | 70 | 19 | Loss of compliance | CCgR |
| 519 | M | 76 | Ictus cerebri | 34 | 25 | Loss of compliance | Less than PGgR |
Nine patients died in chronic phase; they are listed by age. In case no. 185 liver failure was attributed to acute paracetamol toxicity. In case no. 515 the exact duration of IFN-α treatment could not be retrieved, but was estimated to be about 30 months. In addition to these 9 patients, 2 patients died because of BMT, one allogeneic and one autologous. Both were submitted to BMT in CCgR.
Discussion
In this paper we report the characteristics and the outcome of 317 patients with Ph+ CML who achieved a CCgR with IFN-α alone or in combination with HU (159 cases) and with Bus (15 cases). These patients were treated in 9 European countries over a 15-year period from 1983 to 1997. The interest, biologic and clinical, in these rare patients who are so sensitive to IFN-α is obvious. The necessity of establishing a multinational registry to satisfy this interest is illustrated by the data reported in Table7, where the main studies of IFN-α treatment in CML are listed by the year of the first relevant report. These studies combine 2227 cases of CML but only 284 cases of CCgR, for an overall CCgR rate of 13%. The total number and the dispersion of these 284 cases of CCgR do not allow a meaningful specific analysis. Moreover, although all these studies showed and discussed a positive relationship between survival and CgR, either complete or more frequently complete plus partial, no study provided a specific and detailed description of the characteristics and the outcome of CCgRs. In particular their long-term fate remains vague because apart from the Houston series,5 the Italian studies,22,23and the small Kloke series,19 the observation time of the reported patients was shorter than 5 years. Because the data on CCgR are rare and dispersed and the available evidence is small, it was not surprising that the issue of the CCgR was discussed only in one of 4 recent major reviews of the treatment of CML.20,27,39 40
Main reports of treatment of CML with IFN-α alone or in combination with HU
| Reference . | No. of cases . | No. of CCgR . | Study and dose . |
|---|---|---|---|
| Talpaz et al4 1991 and Kantarjian et al51995 | 274 | 72 (26%) | Single center, standard dose |
| Ozer et al33 1993 | 107 | 14 (13%) | Multicenter, variable dose |
| ICSG on CML6 171994 and 1998 | 218 | 23 (10%) | Multicenter, randomized, standard dose |
| Hehlmann et al71994 | 133 | 6 (5%) | Multicenter, randomized, standard dose |
| Schofield et al34 1994 | 41 | 3 (7%) | Single center, low dose |
| Allan et al8 1995 | 293 | 17 (6%) | Multicenter, randomized, standard dose |
| Ohnishi et al9 1995 | 85 | 7 (8%) | Multicenter, randomized, standard dose |
| Montastruc et al35 1995 and Mahon et al37 1998 | 113 | 37 (33%) | Single center, standard dose |
| Thaler et al36 1996 | 74 | 6 (8%) | Multicenter, low dose |
| Guilhot et al291997 | 314 | 28 (9%) | Multicenter, randomized, standard dose |
| BENELUX CML Study Group10 1998 | 100 | 9 (8%) | Multicenter, randomized, low dose |
| ICSG on CML18 1999 | 272 | 28 (10%) | Multicenter, standard dose |
| Steegmann et al38 1999 | 132 | 25 (19%) | Multicenter, standard dose |
| Kloke et al192000 | 71 | 9 (13%) | Single center, standard dose |
| Total | 2227 | 284 (13%) |
| Reference . | No. of cases . | No. of CCgR . | Study and dose . |
|---|---|---|---|
| Talpaz et al4 1991 and Kantarjian et al51995 | 274 | 72 (26%) | Single center, standard dose |
| Ozer et al33 1993 | 107 | 14 (13%) | Multicenter, variable dose |
| ICSG on CML6 171994 and 1998 | 218 | 23 (10%) | Multicenter, randomized, standard dose |
| Hehlmann et al71994 | 133 | 6 (5%) | Multicenter, randomized, standard dose |
| Schofield et al34 1994 | 41 | 3 (7%) | Single center, low dose |
| Allan et al8 1995 | 293 | 17 (6%) | Multicenter, randomized, standard dose |
| Ohnishi et al9 1995 | 85 | 7 (8%) | Multicenter, randomized, standard dose |
| Montastruc et al35 1995 and Mahon et al37 1998 | 113 | 37 (33%) | Single center, standard dose |
| Thaler et al36 1996 | 74 | 6 (8%) | Multicenter, low dose |
| Guilhot et al291997 | 314 | 28 (9%) | Multicenter, randomized, standard dose |
| BENELUX CML Study Group10 1998 | 100 | 9 (8%) | Multicenter, randomized, low dose |
| ICSG on CML18 1999 | 272 | 28 (10%) | Multicenter, standard dose |
| Steegmann et al38 1999 | 132 | 25 (19%) | Multicenter, standard dose |
| Kloke et al192000 | 71 | 9 (13%) | Single center, standard dose |
| Total | 2227 | 284 (13%) |
The table lists, ordered by the year of the first relevant publication, the main reports of the treatment of CML with IFN-α (alone or in combination with HU), for a total of 2227 cases from 10 multicenter prospective studies and 4 single center studies. The total number of CCgR is 284 (13%). Standard dose means a scheduled IFN-α dose of 5 MIU m2/d, but many patients received a dose lower than scheduled, especially in the British study.8 Low dose means a scheduled IFN-α dose at most 3 MIU/d.
A number of questions on CCgR need answering. Major clinical questions are whether it is possible to predict the CCgR, how much IFN-α is required and for how long, which treatment to use after the response has been achieved, what happens if or when IFN-α is discontinued, and what are the expected duration of the response, length of survival, and late adverse events of any type. Major biologic questions concern the relationship between IFN-α, the molecular characteristics of leukemia, and the immune system of the host. Only some of these questions can be discussed using the data of this registry because of the limitations that are intrinsic to the retrospective nature of the registry itself.
The first question concerns the characteristics of the CCgRs, whether a CCgR can be predicted, and how long it may take to recognize a CCgR. Predicting a CCgR with clinically useful accuracy may be difficult simply because CCgRs occur rarely (13% on the average; range, 5%-33%). The data of the registry provide a solid confirmation to prior suggestions that a CCgR is obtained more frequently in low-risk cases, the risk being assessed either with the old Sokal formulation30 or with the new formulation that was devised specifically by the European study group for patients treated with IFN-α.28 It is useful to remember that low risk means a small spleen, a normal platelet count, and no or few myeloblasts, eosinophils, and basophils in peripheral blood. In addition to these characteristics, the registry data suggest that CCgRs also have a low WBC count and a normal hemoglobin level at diagnosis. It is possible that CCgRs have a different or specific molecular profile, but the difference does not seem to concern the site of the breakpoint in the major BCR region of chromosome 22 and the transcript type, because the proportion of cases with b2a2 and with b3a2 was exactly the same as in an unselected patient population.24,41 42
The time that is required to detect a CCgR is substantial. In this registry the median time to first CCgR was 19 months (95% CI, 17-21 months) and only 61% of cases were in CCgR after 2 years. However, almost 80% of patients had achieved a CHR in 6 to 9 months and some degree of CgR in 12 to 18 months. These are upper estimates because the criteria for the definition of CHR were very strict and because many patients were evaluated at long intervals, so that many CgRs were captured with a substantial delay. In summary, achieving a CCgR is more likely to occur in low-risk patients who have a CHR within 6 months and any degree of CgR within 12 months, improving with time.
Approximately one third of all CCgRs received only IFN-α, whereas one third were pretreated with HU. One third received HU together with IFN-α, but this was probably a mere reflection of different treatment policies. It is impossible to understand if there was any benefit from the combination of IFN-α with HU and in these patients the time to first CCgR was slightly longer, suggesting that they could be less sensitive to IFN-α than the patients who never required HU. Also the doses of IFN-α that were required to achieve a CCgR reflected different predetermined treatment policies. The average weekly dose could be as low as 3 MIU and as high as 74 MIU (total dose) with a median value of 37 MIU, which would correspond to a schedule of about 5 MIU daily. These data cannot help answer the question of whether the response to IFN-α is dose-related, but point out that many CCgRs were obtained at doses that are consistently lower than the “standard” 5 MIU/m2 daily dose.
A second major clinical issue is how much treatment to give after CCgR and for how long. The registry has no data on the amount of IFN-α that was prescribed after the first CCgR, but provides a clear picture of the policy that was applied in Europe during the last 15 years. The policy was to keep almost all patients on treatment, whether in complete or incomplete CgR, until the loss of hematologic response (23 patients) or until not tolerated (36 patients). The fate of these 36 patients is of interest because only 4 of them have progressed to AB phase and 15 of them are still alive and in continuous CCgR. It is even more interesting to look at the outcome of the small group of patients who were put off treatment because it was felt that treatment might be no longer necessary, a stable CCgR having been achieved. Seven of these 8 patients are still in continuous CCgR; one received an allograft in CCgR and died as a result of the transplant. The history of these patients may support the suggestion that was made at the M. D. Anderson Hospital, based on a remarkable institutional experience, that “… IFNα therapy should continue for 2 or 3 years after achievement of CCgR.”27(p213)
A third important issue is the duration of CCgR and overall survival of CCgRs, over a long period. This issue was not completely covered by any prior reports because of the small numbers and insufficient follow-up times. The registry shows that the cumulative probability of remaining in continuous CCgR after 8 years is about 50% (95% CI, 40%-60%; Figure 5). The probability of remaining in CCgR is significantly less for high-risk patients (Figure 6). The fate of the patients who lose the CCgR is not yet completely clear (Table 4 and Figure 7). At last contact only 32 of 105 patients had progressed to AB phase, whereas 56 of 105 patients had still some degree of CgR, either partial or less than partial (Table 4). However, the observation time after CCgR loss is not yet sufficient for a reliable long-term evaluation and the median survival after CCgR loss may lie anywhere between 4 and 8 years.
In any case, achieving and maintaining a stable CCgR is the main requirement for a long survival. The long-term survival of CCgRs is remarkable, up to 72% (95% CI, 62%-82%) after more than 8 years from first CCgR. However, even in this selected group of CCgRs, the initial risk group was found to be important. Figures 3 and 4 show very clearly that high-risk CCgRs have a shorter survival than all other cases. The difference between low-risk and intermediate-risk patients is not significant using the new Euro risk (Figure 4) but is significant using the old Sokal risk (Figure 3). The implications of these data are important because they help to clarify the benefit that a high-risk patient can expect from IFN-α. A high-risk patient who achieves a CCgR has a substantial survival prolongation over those who do not achieve a CCgR, the 5-year survival of the former being 50% to 60% versus 35% to 40% for those who do not achieve a CCgR.6,10 18 However, the absolute benefit, that is, the probability of becoming a very long survivor, is small, because high-risk patients die of leukemia even when they respond completely to IFN-α. In contrast, low-risk patients have the maximum absolute benefit from a complete response to IFN-α because they tend to remain alive for a yet undefined length of time. The fate of intermediate-risk patients lies in between, but is not yet completely clear and requires an independent validation.
Prolonging the survival may allow the observation of late adverse events and an increasing proportion of patients will be expected to die without progressing to AB phase. In these cases it is very difficult to identify a specific relationship with the treatment or to establish how much the disease itself contributed to death, despite no progression. In this registry, only 2 deaths may be related directly or indirectly with IFN-α, namely, a patient with acute liver failure and a patient with dementia. Apart from a patient with bronchogenic carcinoma, all other deaths without progression were due to cardiac or vascular accidents (Table 6). Because the age at death ranged from 60 to 76 years, this was not unexpected. However, because some late cardiovascular deaths were already reported in the Italian study,17 we believe that elderly patients should be monitored very carefully while receiving long-term IFN-α treatment.
The registry has collected a number of data concerning the molecular status of CCgRs. In many cases the BCR/ABL transcript was always detectable, in some cases it was occasionally undetectable, and in a few cases it was always undetectable, as has been reported by several investigators over the last 10 years.24-26 These registry data are not presented in more detail because they have been produced over a long period of time, in many different laboratories, and with different techniques. These data cannot contribute more to the debate on the concept of the cure of CML.43-46 It is our purpose to collect and to distribute the cells of these CCgRs to an international system of referenced laboratories, not only to determine if there are cases of true complete molecular remission but also to investigate if the molecular and biologic patterns of the residual disease can help improve the knowledge and the treatment of CML.
The case contribution from J. Goldman (London); M. Risso (Genova); S. Tringali (Palermo); D. Russo (Udine); A. M. Liberati (Perugia); L. Cavanna (Piacenza); A. Ambrosetti (Verona); D. Ferrero, G. Rege Cambrin, A. Capaldi, and M. Bertini (Torino); D. Dini (Modena); and S. Pardini (Sassari) is kindly acknowledged. The technical assistance of Katia Vecchi and Sandra Cescutti is greatly appreciated.
Supported by COFIN 1999 Italy, contract 9906317115-003.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Michele Baccarani, Institute of Hematology and Medical Oncology “L. and A. Seràgnoli,” S. Orsola Hospital, 40138 Bologna, Italy; e-mail: baccarani@med.unibo.it.







This feature is available to Subscribers Only
Sign In or Create an Account Close Modal