Abstract
Prostratin is a unique phorbol ester that stimulates protein kinase C activity but is nontumor promoting. Remarkably, prostratin is also able to inhibit de novo human immunodeficiency virus type 1 (HIV-1) infection yet up-regulate viral expression from latent proviruses. Prostratin's lack of tumor promotion, coupled with its ability to block viral spread yet induce latent proviral expression, prompted studies to determine whether this compound could serve as an inductive adjuvant therapy for patients treated with highly active antiretroviral therapy (HAART). The current experiments indicate that prostratin is a potent mitogen for mononuclear phagocytes possessing many of the activities of phorbol myristate acetate (PMA) with notable functional differences. Prostratin, like PMA, accelerates differentiation of the myeloid cell-lines, HL-60 and THP-1, as well as mononuclear phagocytes from bone marrow and peripheral blood. Enzyme-linked immunosorbent assay and gene array analyses indicate significant changes in the expression of proteins and messenger RNA after treatment of cells with prostratin, consistent with phagocyte activation and differentiation. Prostratin blocks HIV-1 infection relating to down-regulation of CD4 receptor expression. The array analysis indicates a similar down-regulation of the HIV-1 coreceptors, CXCR4 and CCR5, and this may also reduce viral infectivity of treated host cells. Finally, prostratin is capable of up-regulating HIV-1 expression from CD8+ T lymphocyte–depleted peripheral blood mononuclear cells of patients undergoing HAART. This novel observation suggests the agent may be an excellent candidate to augment HAART by inducing expression of latent HIV-1 with the ultimate goal of eliminating persistent viral reservoirs in certain individuals infected with HIV-1.
Introduction
Prostratin (12-deoxyphorbol 13-acetate) is a unique phorbol ester that lacks tumor-promoting ability even at doses 100 times higher than those sufficient for oncogenesis induced by phorbol myristate acetate (PMA).1 Nonetheless, prostratin stimulates protein kinase C (PKC) enzymatic activity in vitro,similar to PMA, as well as other bioactive phorbol esters and remarkably also exhibits additional important activities including its ability to inhibit the replication of human immunodeficiency virus type 1 (HIV-1).1,2 This inhibition of HIV-1 replication is partially related to prostratin's down-regulation of CD4 receptor expression on target host cells suggesting that prostratin is capable of inhibiting viral spread. Conversely, prostratin up-regulates expression of viral gene products from latent proviral templates, such as those that are harbored in UI and ACH-2 cell lines.2This is a property that prostratin shares with PMA and other mitogenic agents. Prostratin's lack of oncogenic potential coupled with its ability to block HIV-1 viral spread yet up-regulate latent HIV-1 provirus expression are important features that could perhaps be exploited as an effective adjuvant inductive therapy for patients treated with virally suppressive highly active antiretroviral therapy (HAART).
Many individuals infected with HIV-1 treated with HAART generally manifest a sharp decrease in plasma-borne virus, and their prognosis is very good with regard to a rebound in immune system functions. Viral reservoirs, refractory to HAART, nonetheless persist.3 A withdrawal of HAART from patients can result in re-emergence of circulating virus; therefore, current anti–HIV-1 research efforts are increasingly focused on strategies for eliminating latent viral reservoirs that persist despite HAART. Studies were initiated to determine whether prostratin could be an effective agent to either specifically inhibit HIV-1 spread or induce latent proviral expression from patients on HIV-1 suppressive HAART. Initially, a striking activity of prostratin was noted. A rapid increase in the number of adherent phagocytes from peripheral blood mononuclear cell (PBMC) cultures was observed within hours after a single exposure to prostratin in micromolar concentrations or less. This effect occurred following treatment of both human and murine in vitro bone marrow cultures. PMA shares this property with prostratin and is as effective at equivalent concentrations.
Prostratin, like PMA, also induces differentiation of the promonocytic cell lines, HL-60 and THP-1, as noted by their rapid adherence to plastic and subsequent morphologic changes indicative of maturing macrophages. Our studies further indicate a coincidence between the increase in the induction of adherent phagocytes with an up-regulation of tumor necrosis factor-alpha (TNF-α) production, whereas interleukin (IL)-3 levels were unaffected in prostratin-treated PBMC cultures. Gene array analyses were used to define the mechanistic basis for prostratin action. Changes in expression for 375 genes were monitored within THP-1 cells after treatment with prostratin. Several classes of messenger RNAs (mRNAs) including those for cytokines, chemokines, adhesion molecules, receptors, and housekeeping functions were up-regulated. Significantly, many genes required for HIV-1 infection were down-regulated for mRNA expression including the primary HIV-1 receptor, CD4, as well as the coreceptors, CXCR-4 and CCR-5. Notably, the coreceptor-binding chemokines, RANTES and MIP1-β, mRNAs were also down-regulated. Down-regulation of surface protein CD4 display was confirmed on purified CD4+ T cells, and CD4+HLA-DR+ resting T cells, which occurred in a dose-dependent manner after exposure to prostratin.
We further establish that prostratin has an antireplicative effect on HIV-1 in PBMC cultures extending previous studies2 by demonstrating this effect for 3 viral strains with distinctive tropisms—T lymphocyte cell line–tropic (CXCR4), NL4-3; macrophage-tropic (CCR5), BAL; and dual-tropic, 89.6. The final studies demonstrate prostratin induction of HIV-1 expression within cells of patients undergoing virally suppressive HAART. This important observation suggests that the drug may be a reasonable candidate to augment HAART through up-regulation of virus expression with the goal of eliminating persistent reservoirs in select individuals infected with HIV-1.
Materials and methods
Cell cultures and proliferation
Nucleated cells from peripheral blood were isolated on Histopaque-1077 (Sigma, St Louis, MO) and washed twice in serum-free media, then resuspended in complete RPMI 1640 medium containing 10% fetal bovine serum (FBS), 0.05 U penicillin-0.05 U streptomycin, and 2 mM l-glutamine. All cells cultures were maintained at 37°C and 5% CO2. Cell number was determined by visual counting using a hemocytometer. Cytokine levels were determined using commercial enzyme-linked immunosorbent assay (ELISA) kits (Biosource, Cammarillo, CA) specific for human IL-3 or TNF-α. HL-60 cells were maintained in Iscoves media supplemented with 20% FBS; THP-1 cells were grown in RPMI media with 10% FBS. Nonspecific esterase activity was detected using the Sigma diagnostic kit 91-A. Whole bone marrow was harvested from the long bones of mice and plated at a density of 2 × 106 cells/mL.
Prostratin and HIV-1 infection
The PBMCs isolated from healthy donors were treated with prostratin to a final concentration of 0.263 μM, then infected 24 hours after treatment with 3 HIV-1 strains, NL4-3, BAL, and 89.6, with an input of 25 pg/mL HIV-1 p24 antigen equivalent virus. Uninfected PBMCs served as a control. Duplicate plates were run as controls with virus addition but no prostratin treatment. HIV-1 p24 antigen content was determined by high-range ELISA using a kit from Zeptometrix (Buffalo, NY).
Gene array analysis
The THP-1 cells (5 × 106) were grown in individual wells of a 6-well plate. One population was treated with a single dose 0.3 μM prostratin. Total RNA was then extracted 24 hours later from treated and untreated cells using the RNAgents kit from Promega (Madison, WI). Synthesis of complementary DNA (cDNA) from 2 μg total RNA from each of the conditions was performed using an array-specific primer set provided by Sigma-Genosys (Woodlands, TX) and radiolabeled by incorporation of α-32P-CTP (NEN) according to kit instructions except for use of 2 U Superscript enzyme and RNAse OUT, both obtained from Gibco BRL (Rockville, MD). Labeled cDNAs were hybridized to the filters overnight at 68°C and then washed as recommended by Sigma-Genosys. Image data were captured using a Molecular Dynamics phosphoimager. Analysis of the data to quantitate gene expression level differences was performed by Sigma-Genosys. The 2 samples compared were standardized one to the other using total genomic DNA controls at the edges of the filters (not shown), as well as standardizing against selected housekeeping genes visually apparent in the last lane on the right side of the array image.
Dose response of CD4 down-regulation by prostratin on CD4+ T-cell populations
The PBMCs were isolated from an HIV-1–seronegative donor by Histopaque density centrifugation and cultured overnight in complete RPMI 1640 to partially deplete macrophages. The next day helper T cells were isolated using the MACS CD4 Positive T Cell isolation kit from Miltenyi Biotec (Auburn, CA) as per the manufacturer's recommended protocol. Briefly, 3 × 108 PBMCs were incubated with saturating hapten-conjugated antibodies against CD8, CD11b, CD16, CD19, CD36, and CD56 antibodies. The non–T helper cells were magnetically labeled by using MACS microbeads that are coupled to an antihapten antibody. More than 99% of the resultant cells were CD4+ as measured by fluorescence-activated cell sorting (FACS). PBMCs (1 × 108) were then depleted of HLA-DR+ cells using the MACS HLA-DR microbeads from Miltenyi Biotec as per the manufacturer's recommendation again with the protocol yielding a greater than 99% CD4+HLA-DR–resting T-cell population. Then, 5 × 106 of the isolated CD4+ T cells and CD4+ HLA-DR–resting T cells were each plated in 10 wells of a 24-well tissue culture plate. Each of the cell populations were exposed in duplicate to doses of 0, 0.1, 0.3, 1, and 1.5 μM prostratin and incubated for 48 hours. Cells (1 × 106) from each of the cell populations were then double-stained with saturating titers of monoclonal antibodies, anti–CD4-quantum red and anti–CD3-fluorescein isothiocyanate (FITC; Sigma). The cells were analyzed by flow cytometry gating for CD3 and measuring the mean fluorescence of the anti-CD4 antibody above an unstained control.
Stimulation of latent HIV-1 by prostratin or PMA
Duplicate populations of 1 × 106 U1 cells were treated with doses of 0.1, 0.3, 0.9, or 2.7 μM of either prostratin or PMA, as well as a population treated with 10 μg/mL phytohemagglutinin (PHA). Supernatant samples were drawn daily for 4 days, stored at −80°C, and analyzed for p24 concentration using commercial high-range ELISA (Zeptometrix).
Induction of HIV-1 expression from patient PBMCs of infected individuals on HAART with prostratin
Blood samples were collected via peripheral phlebotomy from 6 HIV-1–seropositive patients receiving HAART consistently with fewer than 50 copies of viral RNA per milliliter in the peripheral blood plasma as determined by standard clinical reverse transcriptase–polymerase chain reaction (Quest Diagnostics, Teterboro, NJ).4 Patients signed institutional review board–approved consent forms. PBMCs, depleted of CD8+ T cells, were isolated as described previously,5 suspended in complete RPMI 1640 medium supplemented with 0.1% gentamicin (Gibco BRL), then equally divided within a 6-well tissue culture plate. Cells were treated with PHA from Sigma, IL-2 from Gibco BRL,5prostratin/PHA/IL-2, prostratin/IL-2, prostratin alone, and prostratin continuously added with IL-2. On day 0, PHA was added to one well to a final concentration of 10 μg/mL, and prostratin was added to a final concentration of 0.263 μM to the remaining wells. Forty-eight hours after treatment, the PBMCs were resuspended in fresh culture medium and the supernatants were retained at −80°C as the initial time point. A second dose to 0.263 μM prostratin was added to the initial prostratin wells and wells requiring IL-2 were maintained with 50 IU/mL IL-2 for the remainder of the experiment. Cultures were maintained for 6 to 8 weeks. A coculture of PHA and IL-2–stimulated CD8+T cell–depleted PBMCs from HIV-1–seronegative donors, collected as described above, was added to each of the wells at this point as well.4 Supernatant aliquots were obtained and frozen once or twice per week, then assayed subsequently for p24 antigen via ELISA (NEN or Zeptometrix). Once a week the samples were replenished with 1 × 106 CD8+ T cell–depleted PHA/IL-2–stimulated PBMCs from HIV-1–seronegative individuals. The continuous prostratin/IL-2 regimen was reconstituted to 0.263 μM prostratin weekly. Samples from 2 initial patients were treated with a truncated protocol. The first sample, designated “patient 5,” lacked the PHA/prostratin with IL-2 and continuous prostratin/IL-2 condition but had a no mitogen addition. The second patient, designated “patient 1,” had continuous prostratin with IL-2 but lacked the PHA/prostratin/IL-2 addition. All remaining samples were treated with regimens as described above.
Results
Effect of prostratin on human PBMCs
Human PBMCs were cultured in the presence of 0.263 μM prostratin or other mitogens and cytokines. As shown in Figure1B, numerous cells began to adhere to the bottom surface of the culture dish within a few hours after exposure to prostratin, indicating rapid differentiation of mononuclear phagocytes within the cell cultures. This effect was not observed for treatment of the same population of cells with dimethylsulfoxide (DMSO), absent prostratin, nor observed with PHA, IL-2, or a combination of PHA and IL-2 (Figure 1A). Pronounced phagocyte adherence also occurred after treatment of human (not shown) and murine bone marrow cells cultured in vitro as illustrated in Figure 1D, as compared to a duplicate marrow culture treated with PHA and IL-2 in Figure 1C. The adherent cells expressed high levels of nonspecific esterase activity indicative of differentiated and maturing macrophages (data not shown).
Prostratin induces differentiation of peripheral blood and marrow mononuclear phagocytes.
Equivalent numbers (2 × 106 cells/mL) of human PBMCs or murine bone marrow cultures were plated and treated with PHA/IL-2 (A,C) or 0.263 μM prostratin (B,D). Numerous adherent mononuclear phagocytes of darkened and irregular flattened shape were apparent in both of the prostratin-treated cultures within hours following exposure to the compound (magnified inset, bottom right corner with select monocytes denoted by arrows). Cells were photographed 24 hours after treatment. These observations are representative of multiple experiments and microscopic fields.
Prostratin induces differentiation of peripheral blood and marrow mononuclear phagocytes.
Equivalent numbers (2 × 106 cells/mL) of human PBMCs or murine bone marrow cultures were plated and treated with PHA/IL-2 (A,C) or 0.263 μM prostratin (B,D). Numerous adherent mononuclear phagocytes of darkened and irregular flattened shape were apparent in both of the prostratin-treated cultures within hours following exposure to the compound (magnified inset, bottom right corner with select monocytes denoted by arrows). Cells were photographed 24 hours after treatment. These observations are representative of multiple experiments and microscopic fields.
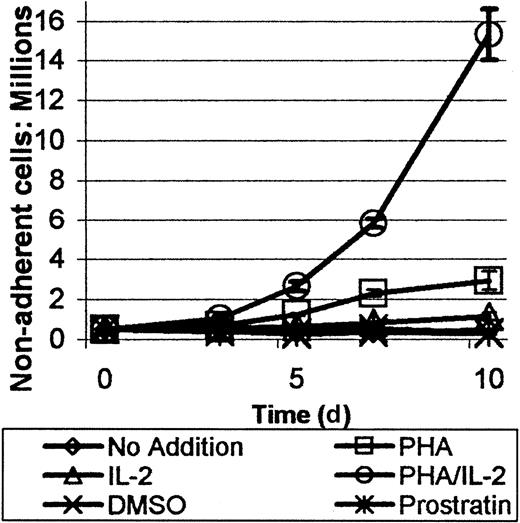
The effect of prostratin on the proliferation of nonadherent cells was also of interest because the compound appeared to be a reasonably potent mitogen for phagocytes. The proliferation of the nonadherent cells was gauged by removing aliquots of cells from the treated cultures consisting of either no addition of compound, DMSO, prostratin, PHA/IL-2, or PHA alone. PHA/IL-2 treatment induced a large increase in the number of cells. When PHA or IL-2 was added individually, an intermediary effect on cellular proliferation was observed. Cell numbers for DMSO and no compound addition cultures remained relatively constant over the course of measurement. As shown in Figure 2, no significant increase in nonadherent cell number occurred after treatment of PBMCs with a single dose of prostratin at 0.263 μM. It should be noted that some cells remained tightly attached to the adherent phagocytes on the dish surface after prostratin treatment, which could have led to a slight underestimate of nonadherent cell number.
Prostratin induces proliferation of the nonadherent cellular fraction of human PBMCs.
Human PBMCs, 5 × 105 per well, were treated with a variety of mitogenic or proliferative agents including 10 μg/mL PHA, 50 IU/mL IL-2, PHA/IL-2, or 0.263 μM prostratin as indicated. No addition and DMSO served as control cultures. Proliferation of cells in all cultures peaked on day 6 after treatment. PHA/IL-2 treatment was most effective followed by PHA and IL-2, as single agents. Prostratin did not exhibit a significant proliferative effect. Each point represents the mean of 2 trials ± 1 SD. Cases where error bars are not clearly visible result from low SD, typically less than 1% in deviance.
Prostratin induces proliferation of the nonadherent cellular fraction of human PBMCs.
Human PBMCs, 5 × 105 per well, were treated with a variety of mitogenic or proliferative agents including 10 μg/mL PHA, 50 IU/mL IL-2, PHA/IL-2, or 0.263 μM prostratin as indicated. No addition and DMSO served as control cultures. Proliferation of cells in all cultures peaked on day 6 after treatment. PHA/IL-2 treatment was most effective followed by PHA and IL-2, as single agents. Prostratin did not exhibit a significant proliferative effect. Each point represents the mean of 2 trials ± 1 SD. Cases where error bars are not clearly visible result from low SD, typically less than 1% in deviance.
Differentiation of HL-60 and THP-1 promonocytic cell lines
The HL-60 and THP-1 cell lines were established from patients with acute promonocytic leukemia and acute monocytic leukemias, respectively.6,7 HL-60 cells are capable of differentiating into granulocytes, monocytes, or eosinophils, or they become macrophagelike depending on the inducer. However, HL-60 and THP-1 cells differentiate along the macrophage lineage on exposure to phorbol esters such as 12-O-tetradecanoylphorbol-13-acetate (TPA) or PMA.8 Differentiation of these suspension cell lines occurs rapidly with even a single exposure to such inducers marked by rapid adherence to plastic surfaces or each other. Over time, the treated cells developed a broad, flat, rounded cytoplasm. These classical morphologic criteria for differentiation were monitored for both cell lines in a dose response to PMA and prostratin.
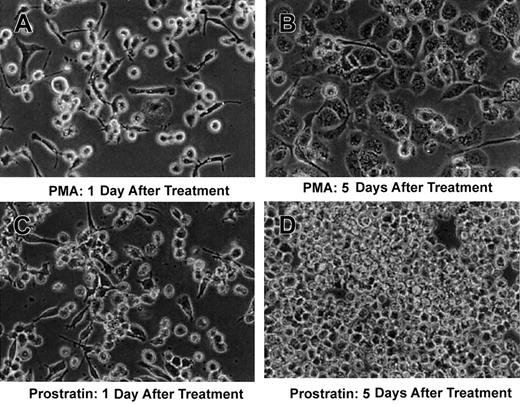
The cell lines were exposed to concentrations of 0.1, 0.3, 0.9, and 2.7 μM PMA or prostratin. Adherence occurred most prominently at 0.3 and 0.9 μM; a reduction in cell number and cellular fragmentation, indicative of cell death, occurred for both compounds at 2.7 μM. A modest increase in the number of adherent HL-60 cells with prostratin versus PMA occurred (not shown). THP-1 cells behaved differently with more adherent cells evident after PMA treatment versus prostratin at both 0.3- and 0.9-μM concentrations. As shown in Figure3, the THP-1 cells reversed phenotype, rounded up, and detached from the plastic surface and resumed proliferation by day 5 after prostratin treatment, whereas PMA-treated cells retained a uniform morphology typical of mature macrophage. The cells in panel D of Figure 3 appear morphologically identical to untreated THP-1 suspension cells.
Prostratin induces differentiation of the monocytic cell-line, THP-1.
THP-1, a suspension myeloid cell line, was treated with 0.3 μM PMA (A,B) or 0.3 μM prostratin (C,D). PMA-treated cells assumed the morphology typical of maturing macrophage 5 days after treatment as shown in panel B; prostratin-treated cells appeared to revert to a nonadherent phenotype as indicated in panel D. These observations are representative of 3 independent experiments.
Prostratin induces differentiation of the monocytic cell-line, THP-1.
THP-1, a suspension myeloid cell line, was treated with 0.3 μM PMA (A,B) or 0.3 μM prostratin (C,D). PMA-treated cells assumed the morphology typical of maturing macrophage 5 days after treatment as shown in panel B; prostratin-treated cells appeared to revert to a nonadherent phenotype as indicated in panel D. These observations are representative of 3 independent experiments.
Mechanism of action of prostratin based on changes in expression of multiple genes
Three approaches were used to detect changes in the levels of gene expression—ELISA, gene array, and FACS analysis—to determine the mechanisms of action of this unique phorbol ester. Gene array analysis was used to assess changes in the expression of multiple genes within several functional classes. This seemed warranted because prostratin is a unique nontumor-promoting phorbol ester, yet exhibits appealing characteristics relative to the expression and replication of HIV-1, which are likely influenced by changes in multiple pathways rather than by the modulation in expression of a few individual host genes.
Initially, PBMCs isolated from HIV-1–seronegative donors were treated with a variety of stimulatory compounds and controls, including prostratin, as well as PHA/IL-2, PHA alone, IL-2 alone, DMSO, or no addition. ELISA indicated no significant change in IL-3 levels (data not shown). Increased numbers of macrophages were observed in prostratin-treated cultures, as noted previously. This suggested that TNF-α would be a suitable candidate for detection because this cytokine is known to be up-regulated during macrophage activation and differentiation and can function in an autocrine fashion.9As shown in Figure 4, prostratin induced the highest level of TNF-α release into the cell supernatant. PHA and PHA/IL-2 appeared to have less of an effect, whereas IL-2 and the controls, DMSO or no addition, had little to no effect on TNF-α production. In one experiment, TNF-α production peaked rapidly after several days in culture, likely due to cytolysis of the phagocytes (data not shown). Nonetheless, prostratin can have a reasonably potent stimulatory effect on the release of this cytokine from PBMCs cultured in vitro.
Prostratin up-regulates TNF-α expression in treated PBMCs.
Human PBMCs were treated with mitogenic or proliferative agents as indicated. TNF-α release into the culture media was measured by ELISA and peaked by day 2 after treatment for cultures exposed to prostratin, PHA/IL-2, or PHA alone. IL-2 and DMSO control cultures elicited no significantly greater effect in TNF-α production than no addition. Each point represents the mean of 2 trials ± 1 SD.
Prostratin up-regulates TNF-α expression in treated PBMCs.
Human PBMCs were treated with mitogenic or proliferative agents as indicated. TNF-α release into the culture media was measured by ELISA and peaked by day 2 after treatment for cultures exposed to prostratin, PHA/IL-2, or PHA alone. IL-2 and DMSO control cultures elicited no significantly greater effect in TNF-α production than no addition. Each point represents the mean of 2 trials ± 1 SD.
Next, gene array analysis was used to obtain a comprehensive display of changes in mRNA expression related to prostratin induction. The effects of prostratin on the promonocytic cell line, THP-1, were monitored because, unlike PBMCs, they represent a single cell type and would be more likely to respond uniformly relative to primary cells isolated from various donors. THP-1 cells are also partially differentiated toward the macrophage phenotype and therefore it was hypothesized the analysis would be less complex than treated cells of a more naı̈ve state.
Total RNA was isolated from untreated THP-1 cells or the same cells exposed to 0.3 μM prostratin for 24 hours. Radiolabeled cDNA was synthesized from the 2 sets of total RNAs and then hybridized to the array filter set. Figure 5 illustrates the primary data captured by the phosphoimager for each gene position. The gene pixel value for each position was expressed as a fraction of the total pixels in the array (filter A, no treatment; filter B, prostratin treatment) after subtracting the pixels contributed by the genomic DNA pixel values. This was performed to normalize pixel values to the untreated control sample, because the overall level of radiolabeled cDNAs hybridized to the filter for the prostratin-treated THP-1 cells was somewhat greater. After this equilibration, the increases in gene expression were determined as B/A pixel data for filter B values greater than filter A values. The fold decrease induced by prostratin was determined as a fraction of A/B in those instances where B pixel values were less than the A pixel values. A 2-fold or greater increase in expression was noted for 80 genes, and a 2-fold or greater decrease was identified for 34 genes comparing treated versus untreated cells. Table 1 lists the gene name and fold increase or decrease for the first 35 genes of each category.
Gene array analysis of prostratin-treated THP-1 cells.
Total RNA was isolated from untreated THP-1 cells or THP-1 cells exposed to 0.3 μM prostratin for 24 hours. Radiolabeled cDNA was synthesized for each RNA sample and hybridized to duplicate array filters. Images were captured by a phosphoimager analysis after overnight exposure and array values normalized as indicated in “Materials and methods.” Each horizontal doublet represents the positions of genes analyzed for expression level differences. Long arrows denote positions of IL-1β and short arrows denote positions for MMP-9 and are scribed to illustrate differences of the primary data as specific examples.
Gene array analysis of prostratin-treated THP-1 cells.
Total RNA was isolated from untreated THP-1 cells or THP-1 cells exposed to 0.3 μM prostratin for 24 hours. Radiolabeled cDNA was synthesized for each RNA sample and hybridized to duplicate array filters. Images were captured by a phosphoimager analysis after overnight exposure and array values normalized as indicated in “Materials and methods.” Each horizontal doublet represents the positions of genes analyzed for expression level differences. Long arrows denote positions of IL-1β and short arrows denote positions for MMP-9 and are scribed to illustrate differences of the primary data as specific examples.
Effect of prostratin on multiple gene expression as determined by gene array
| Gene name . | Fold increase . | Gene name . | Fold decrease . |
|---|---|---|---|
| IL-1β | 35.53 | PARC | 7.34 |
| CKRL-1 | 30.38 | TIMP-4 | 5.27 |
| Osteopontin | 27.54 | erbB1 | 4.92 |
| IL-8 | 24.99 | CD6 | 4.75 |
| MMP-9 | 15.25 | EMMPRIN | 4.57 |
| OSM Rβ | 9.98 | β-NGF | 4.06 |
| MPIF-1 | 8.90 | Tpo | 3.24 |
| IFN-α/β Rα | 8.06 | IGF binding protein 1 | 3.21 |
| Integrin-α5 | 6.59 | IGF binding protein 4 | 3.06 |
| Ephrin-A1 | 6.42 | Inhibin (α subunit) | 3.01 |
| LIGHT | 5.42 | BMP-8 | 2.98 |
| TACE | 5.32 | CD64 | 2.79 |
| Activin RIIA | 4.56 | Endothelin-2 | 2.76 |
| TIMP-1 | 4.52 | CD34 | 2.66 |
| SARP-1 | 4.18 | RANTES | 2.58 |
| MIP-1α | 4.14 | CCR-5 | 2.56 |
| PBEF | 3.87 | IL-12 p40 | 2.53 |
| Integrin-β2 | 3.82 | FGF R2 | 2.50 |
| Bob | 3.62 | NT-4 | 2.49 |
| GAPDH | 3.14 | IGF-I R | 2.46 |
| Integrin-αL | 3.11 | MIP-1β | 2.42 |
| MCP-1 | 3.09 | CXCR-4 | 2.40 |
| PDGF-A chain | 2.95 | CD4 | 2.39 |
| FGF R3 | 2.85 | CD38 | 2.36 |
| IFN-α/β Rβ | 2.80 | ICAM-3 | 2.36 |
| MMP-7 | 2.77 | PDGF Rα | 2.27 |
| GRO-γ | 2.75 | CXCR-2 | 2.22 |
| IL-1α | 2.74 | HCC-4 | 2.19 |
| PIN | 2.72 | CCR-6 | 2.16 |
| HLA-A 0201 heavy chain | 2.70 | N-Cadherin | 2.15 |
| Integrin-α3 | 2.65 | FGF-11 | 2.12 |
| Integrin-α2 | 2.64 | CD27L | 2.12 |
| Urokinase R | 2.64 | GFRα2 | 2.09 |
| Oncostatin M | 2.62 | MMP-15 | 2.03 |
| IL-3 Rα | 2.61 | ICAM-2 | 1.96 |
| Gene name . | Fold increase . | Gene name . | Fold decrease . |
|---|---|---|---|
| IL-1β | 35.53 | PARC | 7.34 |
| CKRL-1 | 30.38 | TIMP-4 | 5.27 |
| Osteopontin | 27.54 | erbB1 | 4.92 |
| IL-8 | 24.99 | CD6 | 4.75 |
| MMP-9 | 15.25 | EMMPRIN | 4.57 |
| OSM Rβ | 9.98 | β-NGF | 4.06 |
| MPIF-1 | 8.90 | Tpo | 3.24 |
| IFN-α/β Rα | 8.06 | IGF binding protein 1 | 3.21 |
| Integrin-α5 | 6.59 | IGF binding protein 4 | 3.06 |
| Ephrin-A1 | 6.42 | Inhibin (α subunit) | 3.01 |
| LIGHT | 5.42 | BMP-8 | 2.98 |
| TACE | 5.32 | CD64 | 2.79 |
| Activin RIIA | 4.56 | Endothelin-2 | 2.76 |
| TIMP-1 | 4.52 | CD34 | 2.66 |
| SARP-1 | 4.18 | RANTES | 2.58 |
| MIP-1α | 4.14 | CCR-5 | 2.56 |
| PBEF | 3.87 | IL-12 p40 | 2.53 |
| Integrin-β2 | 3.82 | FGF R2 | 2.50 |
| Bob | 3.62 | NT-4 | 2.49 |
| GAPDH | 3.14 | IGF-I R | 2.46 |
| Integrin-αL | 3.11 | MIP-1β | 2.42 |
| MCP-1 | 3.09 | CXCR-4 | 2.40 |
| PDGF-A chain | 2.95 | CD4 | 2.39 |
| FGF R3 | 2.85 | CD38 | 2.36 |
| IFN-α/β Rβ | 2.80 | ICAM-3 | 2.36 |
| MMP-7 | 2.77 | PDGF Rα | 2.27 |
| GRO-γ | 2.75 | CXCR-2 | 2.22 |
| IL-1α | 2.74 | HCC-4 | 2.19 |
| PIN | 2.72 | CCR-6 | 2.16 |
| HLA-A 0201 heavy chain | 2.70 | N-Cadherin | 2.15 |
| Integrin-α3 | 2.65 | FGF-11 | 2.12 |
| Integrin-α2 | 2.64 | CD27L | 2.12 |
| Urokinase R | 2.64 | GFRα2 | 2.09 |
| Oncostatin M | 2.62 | MMP-15 | 2.03 |
| IL-3 Rα | 2.61 | ICAM-2 | 1.96 |
The mRNA expression levels increased in THP-1 cells after prostratin treatment relative to untreated cells are indicated as fold differences on the left side; mRNA expression levels decreased by fold after prostratin treatment are listed on the right side. The table lists the first 35 genes in each category.
Differences in mRNA expression levels were consistent with differentiation and activation of THP-1 cells toward a macrophagelike phenotype demonstrated clearly in Figure 3. For example, phagocyte attachment to plastic, involving deposition of an extracellular matrix, would include up-regulation of integrin genes and matrix metalloproteinases (MMP-7, MMP-8, and MMP-9).10 Cytokines and, in particular, many of the interleukin family of genes showed increased expression levels, including their receptors as indicated in Table 1. The same was evident for the interferon family of genes and their receptors. Remarkably, TNF-α mRNA exhibited just under a 2-fold level of increased expression (data not shown). However, because the RNA from the treated cells had been extracted within a day after prostratin treatment, it is possible that the levels of this cytokine would subsequently rise at later times after treatment, as was demonstrated for prostratin treatment of PBMCs, which peaked at day 2.
There was a 4-fold up-regulation of the β-chemokine MIP-1α mRNA. This chemokine binds to the HIV-1 coreceptor, CCR5, and this binding event blocks HIV-1 entry.11 Fewer genes exhibited down-regulation after prostratin treatment relative to those up-regulated; however, some are significant given the ability of prostratin to inhibit de novo viral infection. First, as shown in Table1, the primary HIV-1 receptor, CD4, was decreased over 2-fold, somewhat less than the coreceptors, CXCR4 and CCR5. Although MIP-1α expression was increased, the related interactive coreceptor β-chemokines, MIP-1β and RANTES, were down-regulated approximately 2.5-fold.
Down-regulation of CD4 surface receptor also occurred after prostratin treatment in a dose-dependent manner on CD4+ or CD4+HLA-DR− T cells, which correlates with reduced CD4 mRNA levels observed by the gene array analysis. T cells were chosen in this study for ease of cell manipulation and analysis rather than THP-1 cells because prostratin treatment induces adherence of these cells to vessel surfaces. Furthermore, the response of CD4+ T lymphocytes extends and corroborates the previous receptor down-regulation to a primary cell type.
CD4+ and CD4+HLA-DR− T cells were isolated from an HIV-1–seronegative donor via Miltenyi-negative selection and the cells were treated with 0, 0.1, 0.3, 1.0, and 1.5 μM prostratin and incubated for 48 hours. The cell populations were more than 99% pure as determined by FACS analysis. The cells were then analyzed by FACS for CD4 expression on CD3 gated cells. Figure6 demonstrates several-fold CD4 down-regulation for both CD4+ and CD4+HLA-DR− T cells in a dose-dependent manner. Interestingly, there was no significant variation in the down-regulation of the CD4 receptor between the CD4+ and CD4+HLA-DR− T cells.
Prostratin induced down-regulation of CD4 on isolated total T-helper (Th) CD4+ and CD4+ resting Th cells.
CD4+ and CD4+HLA-DR− T cells were isolated from HIV-1–seronegative donors via negative selection and exposed in duplicate to doses of 0, 0.1, 0.3, 1.0, and 1.5 μM prostratin for 48 hours. The cells were then double-stained for CD3 and CD4. The cells were gated for CD3 and analyzed for mean CD4 expression above background. Each point reflects the mean of 2 trials ± 1 SD.
Prostratin induced down-regulation of CD4 on isolated total T-helper (Th) CD4+ and CD4+ resting Th cells.
CD4+ and CD4+HLA-DR− T cells were isolated from HIV-1–seronegative donors via negative selection and exposed in duplicate to doses of 0, 0.1, 0.3, 1.0, and 1.5 μM prostratin for 48 hours. The cells were then double-stained for CD3 and CD4. The cells were gated for CD3 and analyzed for mean CD4 expression above background. Each point reflects the mean of 2 trials ± 1 SD.
Prostratin inhibition of HIV-1 infection
It was previously reported that prostratin possessed a dual role in affecting HIV-1 infection and replication.1,2Prostratin not only stimulated HIV-1 expression in latently infected U1 and ACH-2 cells, but it was also able to inhibit de novo infection of treated T-lymphotropic cell lines.1,2 To extend these observations, human PBMCs from uninfected donors were treated with prostratin 48 hours before infection with BAL, an M-CCR5–tropic strain, or NL4-3, a T-cell line (CXCR4)–tropic strain of HIV-1, or finally 89.6, a dual-tropic viral strain. The concentration of cell-free p24 antigen was determined by ELISA and served as a means for monitoring the effects of prostratin on spreading HIV-1 infection. As shown in Figure 7, cultures treated with prostratin prior to infection with all 3 HIV-1 strains generated less p24 antigen than untreated controls, supporting the inhibitory role of prostratin on de novo infection of host cells by HIV-1.2
Dynamics of HIV-1 infection of human PBMCs after prostratin treatment.
PBMCs were exposed to 0.263 μM prostratin, then infected with HIV-1 virion-containing supernatants derived from 293T cells transfected with HIV-1 expression plasmids for the 3 strains as indicated with equivalent p24 antigen input of virus. Supernatant samples were withdrawn on the days after infection, as indicated, and assessed for HIV-1 p24 antigen content by ELISA.
Dynamics of HIV-1 infection of human PBMCs after prostratin treatment.
PBMCs were exposed to 0.263 μM prostratin, then infected with HIV-1 virion-containing supernatants derived from 293T cells transfected with HIV-1 expression plasmids for the 3 strains as indicated with equivalent p24 antigen input of virus. Supernatant samples were withdrawn on the days after infection, as indicated, and assessed for HIV-1 p24 antigen content by ELISA.
Induction of HIV-1 expression in U1 cells with prostratin versus PMA
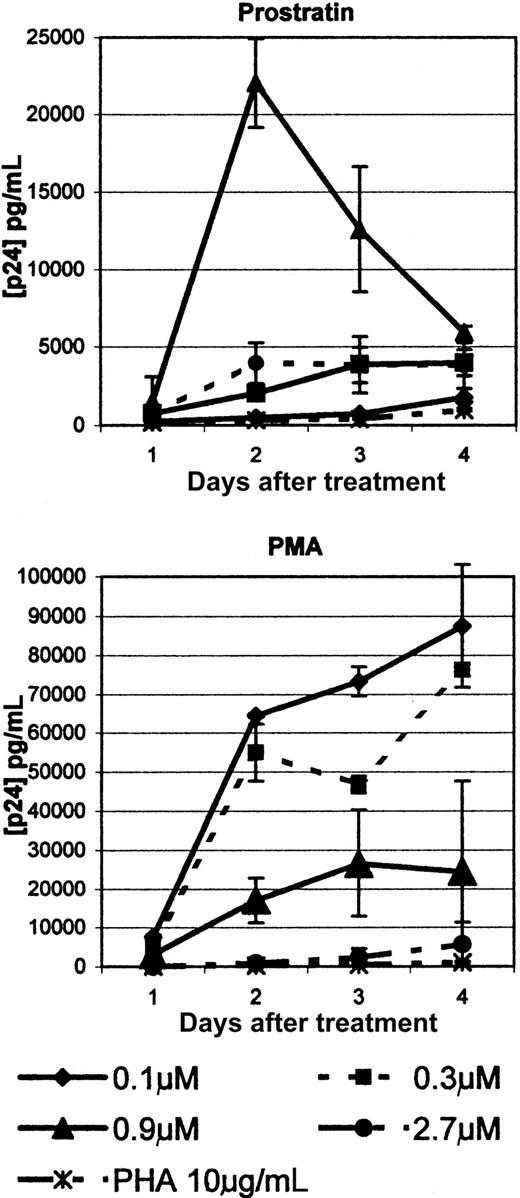
We extended previous observations of prostratin-induced proviral DNA expression by comparing PMA and prostratin up-regulation expression of latent HIV-1 proviral DNA within U1 cells in a dose-dependent manner. Because prostratin and PMA are functionally distinct, it was of interest to determine whether differences between the activities of these compounds might correlate with their potency to up-regulate viral expression.
Therefore, U1 cells were used to compare the responsiveness of viral up-regulation following exposure to these related phorbol esters. Duplicate U1 cell populations were treated with 0.1, 0.3, 0.9, and 2.7 μM PMA or prostratin. PMA induced several-fold higher levels of viral expression for all concentrations except the highest dose, as shown in Figure 8. PMA, at 2.7 μM, was cytotoxic and may account for low viral production at that concentration. PMA induced a steady increase in viral expression at the 2 lowest concentrations. Prostratin behaved differently than PMA in that the optimal dose for viral expression was 0.9 μM. The lower concentrations and the highest at 2.7 μM had little effect on viral induction. Like PMA, prostratin appeared to be cytotoxic at the highest concentration with reduced numbers of surviving cells and visible cellular fragmentation.
Prostratin induction of HIV-1 expression from U1 cells.
U1 cells were treated with 0.1, 0.3, 0.9, and 2.7 μM PMA or prostratin. Culture media was removed from days 1 through 4 after treatment and assessed for the levels of p24 antigen by ELISA. Each point represents the mean of 2 trials ± 1 SD.
Prostratin induction of HIV-1 expression from U1 cells.
U1 cells were treated with 0.1, 0.3, 0.9, and 2.7 μM PMA or prostratin. Culture media was removed from days 1 through 4 after treatment and assessed for the levels of p24 antigen by ELISA. Each point represents the mean of 2 trials ± 1 SD.
Prostratin clearly induces virus expression at an optimal dose but, unlike PMA, the effect appears transient with a steady decline of virus release beginning 2 days after treatment. This transient character of prostratin's effects, relative to PMA, is reminiscent of observations shown in Figure 3 and likely reflects the restricted action of this unique phorbol. Nonetheless, both compounds were significantly more effective at stimulating latent virus than PHA, as indicated in Figure 8.
Induction of HIV-1 expression from the PBMCs of HIV-1–infected individuals on virally suppressive HAART
In most patients, HAART reduces circulating HIV-1 to undetectable levels; however, sanctuary sites harboring replication-competent proviral DNA persist.3 A number of mitogens and cytokines have been shown to up-regulate expression of latent proviral DNAs in HIV-1–infected cells; however, they are generally lethally proinflammatory or tumor promoting.9 These features limit their usefulness to induce expression of HIV-1 from reservoir sites within patients on HAART to promote viral clearance. Given this, the potentially advantageous properties of prostratin were explored with experiments focused on determining whether prostratin could up-regulate HIV-1 expression from latent virus within cultured PBMCs of HIV-1–infected patients on suppressive HAART.
Although prostratin exhibited a heterogeneous effect on HIV-1 production from PBMCs of a cohort of individuals infected with HIV-1 on suppressive HAART, the compound was nonetheless able to up-regulate viral expression in cells isolated from 4 of 6 patients. As illustrated in Figure 9, cells from patient 1 elicited the most robust response for viral expression after PHA/IL-2 treatment with high levels of viral production within the first week. Prostratin/IL-2 exposure and even prostratin alone also subsequently induced considerable virus expression. Weekly redosing of the cells with prostratin elicited a weaker then abortive response from patient 1 late in the course of treatment. The abortive response may relate to prostratin's ability to block spreading infection to the HIV-1–seronegative donor cells. In contrast, cells from patient 2 failed to produce virus with PHA/IL-2. Patient 2 cells underwent p24 antigen release after the second week with prostratin/IL-2. In the third week, both the prostratin/IL-2 and prostratin continuous/IL-2 conditions showed low peaks of p24 antigen production. Interestingly, continuous administration of prostratin quickly attenuated its stimulatory affect on viral production in contrast to single administration of prostratin/IL-2. The PHA/prostratin/IL-2 regimen failed to stimulate p24 antigen production, suggesting an interference effect with combined use of these mitogens because prostratin/IL-2, without the PHA, was able to stimulate p24 antigen production.
Prostratin induction of HIV-1 expression from HAART patient PBMCs.
CD8+ T cell–depleted PBMCs from HAART patients were treated with a variety of mitogenic or proliferative agents either alone or in combination (10 μg/mL PHA with 50 IU/mL IL-2, or 0.263 μM prostratin) as indicated and cocultured with HIV-1–seronegative donor CD8+ T cell–depleted PBMCs as described in “Materials and methods.” Media was withdrawn to determine p24 antigen content by ELISA once or twice a week as indicated. Boxed insets represent an amplified view of graph region below.
Prostratin induction of HIV-1 expression from HAART patient PBMCs.
CD8+ T cell–depleted PBMCs from HAART patients were treated with a variety of mitogenic or proliferative agents either alone or in combination (10 μg/mL PHA with 50 IU/mL IL-2, or 0.263 μM prostratin) as indicated and cocultured with HIV-1–seronegative donor CD8+ T cell–depleted PBMCs as described in “Materials and methods.” Media was withdrawn to determine p24 antigen content by ELISA once or twice a week as indicated. Boxed insets represent an amplified view of graph region below.
Like cells from patient 2, cells from patient 4 responded to prostratin/IL-2, and also responded to PHA/prostratin/IL-2, though they did not produce virus with PHA/IL-2, indicating that prostratin was likely the common effector for up-regulation of virus production, as well as in the PHA/prostratin/IL-2 condition. This is in contrast to cells from patient 2, in which PHA appeared to inhibit viral production in conjunction with prostratin/IL-2. Cells from patients 3 and 4 produced only low levels of virus.
Cells from patient 3 expressed the most virus after PHA/IL-2 treatment, albeit at quite low levels, peaking at about 500 pg/mL and aborting during the experimental course. PHA/IL-2 treatment began to produce p24 antigen early in the second week; prostratin and continuous prostratin/IL-2 demonstrated a very low level of p24 antigen production that was rapidly aborted. In the third week as with the PHA/IL-2 conditions p24 antigen levels were falling from a plateau; there was a transient low level peak of p24 antigen from the PHA/prostratin/IL-2 condition, giving evidence of a potential antagonistic effect between these compounds. The cells from patients 5 and 6 responded vigorously only to PHA/IL-2 and not to any of the prostratin-containing conditions. Significantly, cells from patient 6 gave no p24 production in the PHA/prostratin/IL-2 condition, indicating that the prostratin was able to inhibit either viral expression or spread despite the scale of viral production after treatment with the PHA/IL-2 combination alone.
Discussion
The initial discovery of anti–HIV-1 properties of prostratin was based on an empirical anti–HIV-1 bioassay-guided fractionation and purification of individual chemical constituents from solvent extracts of the tropical plant, Homalanthus nutans.1 The plant has an interesting history of usage in traditional herbal medicine, primarily for the treatment of jaundice, and is particularly prominent in Samoan ethnapharmacology.12 Prostratin is 12-deoxyphorbol-13-acetate and a member of a general structural class of compounds well known for tumor-promoting properties. A classical example of a tumor-promoting phorbol ester is PMA.13 Like the tumor-promoting phorbols, prostratin potently activates PKC in vitro. However, prostratin conspicuously lacks an oxygen functionality associated with a lengthy hydrophobic membrane targeting chain at position C-12, in contrast to typical tumor-promoting phorbol esters. This feature may permit prostratin to actually act in vivo as a physiologic antagonist for certain PKC-mediated processes, especially those underlying tumor promotion. Thus, prostratin substantially lacks chemical and biologic properties that have previously discouraged consideration of phorbols for specific PKC-targeted drug development. The detailed molecular interactions of prostratin with particular PKC isoforms that convey its unique attributes remain to be elucidated. Nevertheless, the apparent lack of oncogenic potential of prostratin, combined with its ability to inhibit HIV-1 infection, as well as stimulate latent lentivirus expression, presently qualify it for further consideration as a novel candidate for therapeutic development.
At the outset, it was evident that prostratin, like PMA, is a potent mitogen. The gross effects of prostratin on primary cells were consistent with effects of related phorbol esters inducing rapid attachment of phagocytes to the surface of the culture dishes after treatment of PBMCs or marrow cultures. In the absence of prostratin treatment, this process would ordinarily occur over a period of days.14 In each case, the adherent cells were strongly positive for nonspecific esterase activity indicative of the macrophage phenotype. Gross effects induced by prostratin on the nonadherent population of cells derived from PBMCs were not observed, thereby focusing our analyses of the effect of prostratin treatment of the myeloid lineage.
Additional studies were initiated using 2 well-characterized cell-lines, HL-60 and THP-1, to define the activities of this unique phorbol ester on promonocytes and monocytes directly, because primary PBMCs represent a heterogeneous mixture of cell types. HL-60 cells were derived from a 36-year-old woman with acute promyelocytic leukemia.6 These cells have properties consistent with leukemic promyelocytes and are capable of terminal differentiation suggesting a dissociation of genetic control elements for replication and commitment to differentiation.6
In contrast, the THP-1 cell line was derived from blood cells of a 1-year-old boy with acute monocytic leukemia.7 The cell line is considered to be monocytic in nature on the basis that cells bear α-naphthyl esterase and phagocytic activities as well as produce lysozyme and restore T-lymphocyte responsiveness to concanavalin A.7
THP-1 cells are likely further advanced toward the mature macrophage phenotype than HL-60 cells, although both cell lines respond similarly to treatment with either PMA or prostratin. Both phorbol esters induce rapid attachment of both cell types to plastic surfaces within an hour or 2 after exposure. Initially, the attached cells exhibit a variety of shapes but then appear to mature based on the subsequent formation of a large, thin, rounded cytoplasm indicative of maturing macrophage. A striking difference was noted with regard to the response of THP-1 cells to a single dose of prostratin versus PMA. The ability of prostratin to differentiate THP-1 cells appeared reversible 4 to 5 days after treatment. The molecular basis for this apparent reversibility is unclear; however, our current efforts are directed toward discerning comparative differences in gene expression that occur for both HL-60 and THP-1 cells treated with PMA versus prostratin using gene array technology discussed in detail below. It is also of great interest to identify whether differences exist in the expression of specific genes induced with PMA versus prostratin that may relate to the lack of oncogenic potential for the latter nontumor-promoting phorbol ester.
Expanding the knowledge base of the mechanism of action for prostratin is of critical importance if the compound is to be of clinical utility. Studies have determined that prostratin is not a tumor promoter and further may possess a potent antitumorigenic effect based on its ability to inhibit PMA induction of ornithine decarboxylase, edema, and hyperplasia in CD-1 mouse skin.15-17
Our experiments had 2 divergent objectives for treatment of human PBMCs and the monocytic cell line, THP-1, with prostratin. We first sought to define assays that could monitor, quantitatively, the effect of prostratin on readily obtainable primary human cells such as PBMCs. The visually apparent differentiation and maturation of phagocytes within the PBMC population, which rapidly occurs after prostratin exposure, suggested that assessing changes in TNF-α levels appeared most reasonable. To monitor an additional parameter, differences in IL-3 levels were also quantitated to crudely gauge T-lymphocyte activation. ELISA indicated reproducibly that TNF-α levels rose and IL-3 levels remained unchanged after treatment of PBMCs with prostratin. ELISA analysis of TNF-α levels could provide a suitable means to assay patient response to prostratin if use of the compound is clinically warranted.
Our second objective was to define the biochemical mode of action of this unique compound. Cytokine gene array analysis was chosen because changes in the level of gene expression for hundreds of loci could be measured simultaneously and quantitatively. Many of the genes that were up-regulated are consistent with phagocyte activation and differentiation. These include IL-1α, IL-1β, IL-8, interferon-α/β Ra, MIP-1α, interferon (INF)-α/β Rb, IL-2, IL-6 receptor, IL-1 receptor, IL-5 receptor, IL-10, IL-10 receptor-α, TNF-α, INF-γ, IL-7, IL-2 receptor β, and IL-7 receptor.9 Furthermore, hints at mechanism of action may be provided by noting that many of the up-regulated genes are under the transcriptional activation control of nuclear factor-κB (NF-κB) promoters such as IL-1β,18 osteoprotogerin, and perhaps osteopontin,19 IL-8,20 and MMP-9.21 On this basis, it is possible that prostratin can function through the zeta isoform of PKC (PKC-ζ), which activates NF-κB.22 Significantly, many of the mRNAs that were down-regulated by prostratin such as CD4,23 INF-binding protein 1 and 4,24 and thrombopoietin (Tpo),25 bear promoters with the Ets recognition site.
The down-regulation of surface CD4 expression was also observed on CD4+HLA-DR− T cells using FACS analysis in a dose response to prostratin treatment. Prostratin induced a 5-fold down-regulation of CD4 on CD4+ T cells from a mean of 24.37-fold above background without prostratin treatment to a mean of 2.56-fold above background for 1.5 μM prostratin. The initial activation state of the T cells had no effect on the down-regulation. These results affirm the more modest down-regulation of CD4 mRNA for prostratin-treated THP-1 cells but extend them to primary T-lymphocytic cells expressing the cell surface receptor protein.
Our final studies sought to determine the potential clinical applicability of the compound. The effects of prostratin on HIV-1 expression and replication were studied to confirm and extend previously reported activities of the compound. First, infection and replication of M-tropic, T-cell line–tropic, and dual-tropic strains were monitored in PBMCs treated with prostratin relative to an untreated control. As has been demonstrated previously, prostratin significantly reduced viral infectivity. This was observed for 3 HIV-1 strains with varying tropism. This effect is consistent with the down-regulation of CD4 receptor expression. The gene array analysis suggests that the block in viral infection may not be restricted to simple down-regulation of this receptor alone. Indeed, both CXCR-4 and CCR-5 coreceptor mRNAs undergo a similar reduction in expression. This may more reasonably account for the general potency of phorbol esters to truncate de novo infection of treated host cells.
The gene array analysis also indicated a more than 4-fold increase in MIP-1α expression, which may also contribute to a reduction in viral spread via coreceptor blockade.11 However, there appears to be a down-regulation of MIP-1β and RANTES. It is unclear how these seemingly converse effects would ultimately affect the dynamics of viral spread.
Secondly, we confirmed the ability of prostratin to stimulate latent virus in U1 cells as has been previously reported2 and extended these studies by comparing this property to PMA and PHA. Prostratin was more potent than PHA, although it is difficult to assess whether PHA was equivalently dosed. Nonetheless, prostratin was clearly less potent for inducing viral expression in U1 cells than PMA. This is an additional difference in the pharmokinetic activities of these 2 related phorbol esters, and it is of interest to define whether this is mechanistically related to the lack of oncogenic potential of prostratin.
The major chemical difference between prostratin and the various oncogenic phorbol esters, including PMA, is prostratin's lack of ester-bound hydrophobic moities at the C-12 position, thought to serve as membrane insertion points to mimic diacyl glycerol in PKC activation. Szallasi and Blumberg demonstrated that 12-deoxyphorbol 13-phenylacetate, -isobutyrate, or -angelate was inflammatory but either not tumor promoting or weakly tumor promoting; indeed, prostratin appears to be 144-fold less inflammatory than PMA.15
Finally, the ability of prostratin to induce viral expression from primary cells isolated from HIV-1–seropositive patients treated with HAART with consistent viral loads below 50 copies of RNA per milliliter plasma was examined. Each patient was treated with virally suppressive HAART for over a year with completely stable suppression of plasma viral RNA. A somewhat heterogeneous affect of prostratin on viral levels was observed in the coculture supernatants. This heterogeneity was anticipated because p24 antigen release in the supernatant of in vitro cultures requires amplification of the viral infection through de novo infection of HIV-1–seronegative donor cells to accumulate at a detectable level. As a result of prostratin's ability to reduce viral spread, it is not surprising our analyses of primary patient cells exhibit some nonuniformity in the action of the compound because they bear differences in inducible viral levels with other treatment regimens. Despite this limitation, it seems apparent that prostratin, either alone or in conjunction with other activators, stimulates HIV-1 production from HIV-1–infected patients' PBMCs, which was demonstrated in 4 of 6 individuals on suppressive HAART. It was further noted that prostratin was able to down-regulate de novo infection of primary cells either by reducing the efficacy of PHA/IL-2 treatment to propagate an infection, or by abortive viral production in the case of continuous administration in some patient cell cultures.
Our studies, as well as previous data, indicate that prostratin might find clinical utility in potential HIV-1 eradication protocols for patients on suppressive HAART. It has been recently demonstrated that latently infected cells, harboring the HIV-1 provirus, persist. Furthermore, low-level cryptic or residual HIV-1 replication is found in patients on suppressive HAART with clinical assays revealing “undetectable” plasma viral RNA levels.3,4,26-30 A series of small clinical trials have attempted to stimulate HIV-1 provirus out of latency from these patients using IL-2 alone31 and IL-2 combined with the monoclonal antibody, OKT3.32 Other approaches have used granulocyte-macrophage colony-stimulating factor and macrophage colony-stimulating factor (M-CSF) in an attempt to stimulate HIV-1 out of monocytic reservoirs.33 Our current clinical eradication protocols using IL-2 and OKT3 are not based on repeated administration of proinflammatory compounds and we anticipate clinical use of prostratin would be similar.
Prostratin is potentially a more useful agent than OKT3 and IL-2, for instance, because it appears to have stimulatory effects on expression of latent HIV-1 from T lymphocytes (Jerome Zack et al, personal written communication, May 2001), as well as myeloid cells. It has been suggested for stimulation of HIV-1 out of latency, tissue-bound and blood-borne monocyte/macrophage must be approached, because they may be a significant reservoir for HIV-1 within patients on suppressive HAART.34 M-CSF has been hypothesized as a potential phagocyte stimulatory agent in this regard.35Nevertheless, prostratin would likely stimulate both potential compartments for HIV-1 in vivo, and unlike other phorbol esters, is nontumorgenic. Preliminary pharmacokinetic studies are encouraging; 5 of 5 mice survived, with no obvious ill effects at a 100 μmol/kg intragastric dose of prostratin with plasma concentrations reaching up to 1.42 μmol/L. Mice also survived intravenous administration, with one reaching a peak plasma concentration of 3.62 μmol/L. These studies suggest that the use of prostratin in stimulatory therapeutic protocols for HIV-1–infected individuals on suppressive HAART, both with and without antiretroviral drug intensification,36 holds considerable promise for future clinical trials.
The authors wish to thank Carol Coates, RN, and Colleen Discenzo for obtaining blood samples in the HIV clinics, Michelle Vanella for figure formats, and Rita M. Victor and Brenda O. Gordon for excellent secretarial assistance.
Supported in part by US Public Health Service grant R01AI46289 (R.J.P.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Joseph Kulkosky, Dorrance H. Hamilton Laboratories, Center for Human Virology, Division of Infectious Diseases, Department of Medicine, Jefferson Medical College, Thomas Jefferson University, Philadelphia, PA 19107; e-mail:joseph.kulkosky@mail.tju.edu.







This feature is available to Subscribers Only
Sign In or Create an Account Close Modal