Abstract
A potential therapeutic option for patients with Fanconi anemia is collection of peripheral blood stem cells prior to the development of severe pancytopenia. These hematopoietic cells potentially could be infused when symptomatic bone marrow failure develops, as autologous rescue after chemotherapy in the event of leukemic transformation, or as targets for gene therapy. Eight patients with Fanconi anemia were mobilized with 10 μg/kg per day of granulocyte colony-stimulating factor (median, 10 ± 4 days) to determine the feasibility of collecting peripheral blood stem cells for future use. Six patients achieved a peripheral blood CD34+ count of ≥ 6/μL and underwent apheresis. The collection goal was 2 × 106 CD34+ cells/kg based on a predicted weight 5 years from the date of collection. A mean of 2.6 ± 0.9 × 106 CD34+ cells/kg of the weight at the time of collection were collected, which corresponded to 1.9 ± 0.4 × 106 CD34+cells/kg of the target weight. The collections required a mean of 4 ± 3 days (range, 2-8 days) of apheresis. Six of the 8 subjects had ≥ 1 × 106 CD34+ cells/kg cryopreserved based on both actual and target weights, and 4 subjects had ≥ 2 × 106 CD34+ cells/kg cryopreserved based on the target weight. These results suggest that some patients with Fanconi anemia can have adequate numbers of CD34+ cells mobilized and collected from the peripheral blood prior to the onset of severe bone marrow failure, but they may require an extended mobilization and multiple days of collection.
Introduction
Fanconi anemia is a rare genetic syndrome comprising a constellation of congenital anomalies, bone marrow failure, and a predisposition to cancer.1-4 In vitro analysis of cells from patients with Fanconi anemia demonstrates increased chromosomal breakage,5 increased cytotoxicity to bifunctional alkylating agents, and poor growth of hematopoietic progenitors.6,7 Complementation analysis using somatic cell hybrids has suggested that mutations in up to 7 distinct genes may independently be responsible for the Fanconi anemia phenotype.8 The recent cloning of several of the genes responsible for Fanconi anemia has provided the first insights into how the molecular defects may be responsible for the clinical syndrome.9-18 Most patients experience progressive pancytopenia accompanied by severe bone marrow hypocellularity. Based on experience with bone marrow transplantation, the progressive aplasia appears to represent cumulative loss of hematopoietic stem cells within the medullary cavity. In addition, recent studies in mice defective in the FANCC or FANCA gene demonstrate that the hematopoietic stem cells display a significant reconstitution defect when compared to wild-type hematopoietic stem cells.19 20 Thus the bone marrow failure of Fanconi anemia appears to reside, at least in some part, in the hematopoietic stem cell compartment.
The optimal treatment for Fanconi anemia remains to be defined. Therapeutic interventions have focused on amelioration of the symptoms of bone marrow failure.1 Supportive care with red blood cell and platelet transfusions, antibiotic therapy, androgens,21,22 and hematopoietic cytokines23-25 has helped to increase the median survival to approximately 20-25 years.1,26 Bone marrow transplantation can correct the hematopoietic failure27-30; however, patients with Fanconi anemia have increased toxicity from the preparative regimens31,32 and a high rate of malignancies after transplantation.33
Many children with Fanconi anemia are diagnosed prior to the onset of severe panycytopenia.34 A potential therapeutic option that has not been investigated is the collection of hematopoietic stem cells prior to, or early in the course of, the quantitative and qualitative stem cell damage that occurs during bone marrow failure. The hematopoietic stem cells could be cryopreserved and subsequently reinfused at a time when the bone marrow failure has progressed to the point of requiring supportive therapy. The cryopreserved cells could be reinfused with the hope of delaying the need for a bone marrow transplant or of allowing additional time to identify a suitable allogeneic donor. Cryopreserved hematopoietic cells could alternatively be used as autologous rescue after chemotherapy in the event of leukemic transformation. Finally, cryopreserved cells could be used as targets for gene therapy protocols with a corrective Fanconi anemia gene should this technology become feasible. In this report, we describe our experience collecting granulocyte colony-stimulating factor (G-CSF)– mobilized peripheral blood stem cells from 8 patients with Fanconi anemia prior to the onset of severe bone marrow failure.
Patients, materials, and methods
Study patients
Peripheral blood stem cells were mobilized and collected from subjects with Fanconi anemia who had been diagnosed by increased sensitivity to chromosomal breakage in response to mitomycin C or diepoxybutane. All subjects had normal bone marrow cytogenetics within 6 months of collection. The study was approved by the Indiana University–Purdue University Indianapolis Institutional Review Board and all participants or their parents provided written informed consent.
Peripheral blood stem cell mobilization and collection
Subjects were mobilized with 10 μg/kg of G-CSF (Neupogen, Amgen, Thousand Oaks, CA) administered subcutaneously once a day. A target of 12 CD34+ cells/μL in the peripheral blood was originally proposed as the criteria to initiate apheresis. However, it became evident that this goal was unrealistic, and the criterion to undergo collection was set at 6 CD34+cells/μL. Four of the subjects did not start apheresis until after the CD34+ cell count had already reached 6 cells/μL while attempting to reach 12 cells/μL. Subjects who did not achieve 6 CD34+ cells/μL by 15 days of G-CSF administration did not undergo apheresis. Mobilized peripheral blood stem cells were collected on the Cobe Spectra (Cobe-BCT, Lakewood, CO). Approximately 3-6 blood volumes were processed during each daily collection, which lasted up to 11 hours. ACD-A was the only anticoagulant used. Subjects underwent daily apheresis in an attempt to collect a total of 2 × 106 CD34+ cells/kg based on the projected fifth-percentile weight 5 years from the time of collection. This goal was chosen based on traditional cell targets used for bone marrow transplantation.35 In addition, if CD34+ cells were to be isolated, we estimated approximately a 50% loss, which is expected during the purification process.36 We felt that 1 × 106CD34+ cells/kg was a reasonable number of cells to be used for reinfusion or potential gene therapy trials. Five years in the future was chosen as a time when the bone marrow failure would be anticipated to progress to the point of requiring symptomatic treatment. Adverse reactions during mobilization and apheresis were scored on a scale of 1-4, from low to high severity. Symptoms related to hypocalcemia were treated with intravenous and oral calcium (Tums, SmithKline Beecham, Philadelphia, PA). Autologous platelets from the collection were routinely reinfused for postapheresis counts of < 50 000/μL.
Immunophenotyping
All antibodies were purchased from Becton Dickinson (San Jose, CA) and used at concentrations recommended by the manufacturer. Initial characterization of cells expressing CD34 in the collected product was performed on a fluorescein isothiocyanate CD45 white blood cell gate using the HPCA-2 anti-CD34 antibody. Approximately 500 000 cells were stained with 10 μL of directly conjugated monoclonal antibody for 20 minutes at 4°C. Cells were washed once with RBC Lysis buffer (Becton Dickinson, Mountain View, CA), then with phosphate-buffered saline containing 1% human serum albumin and 0.1% sodium azide. All centrifugations were at 1800 rpm for 5 minutes at room temperature. Cells were either analyzed immediately or fixed with 1% paraformaldehyde and stored at 4°C until analysis could be performed. A Becton Dickinson FACScan flow cytometer was used to analyze 10 000 to 50 000 events using Cellquest software (Becton Dickinson, San Jose, CA). In order to eliminate granulocytes with nonspecific antibody binding from the analysis, a mononuclear gate was created based on CD45 and CD14 expression (Simultest Leucogate, Becton Dickinson) and forward and side light scatter. The total number of CD34+ cells was calculated based on the relative percentage of CD34+ cells in the total number of nucleated cells.
Statistical analysis
Correlations (R2 values) between study parameters were determined using the curve-fitting function on graphical analyses generated in Cricket Graph version 1.3.1 (Cricket Software, Malvern, PA) and analyzed using the t test. All tests were conducted at 0.05 level of significance.
Results
Subjects
Eight patients with Fanconi anemia were enrolled between 1996 and 1998 in a clinical trial to collect and cyropreserve G-CSF–mobilized peripheral blood stem cells. There were 5 female and 3 male participants, with a mean age of 13 ± 6 years of age (Table1). The subjects had a mean weight of 46 ± 21 kg and a mean target weight of 58 ± 14 kg. All 8 subjects had evidence of bone marrow failure (Table 1). None of the subjects, however, had 2 or more cell lines greatly diminished as defined by a hemoglobin level < 8 g/dL, absolute neutrophil count < 0.5 × 109/L, or platelets < 20 000 × 109/L. Subject 5 was being treated with G-CSF (2 μg/kg every other day) prior to enrollment on the protocol. The mean hemoglobin level for the subjects was 11.6 ± 1.8 g/dL, mean corpuscular volume (MCV) 106 ± 9.8 fL, white blood count (WBC) 4.4 ± 2.9 × 109/L, absolute neutrophil count 2.3 ± 3.0 × 109/L, and platelet count 64 ± 52 × 109/L (Table 1).
Subject characteristics at study entry
| Subject . | Sex . | Age (y) . | Actual Weight . | Target Weight . | Hb . | MCV . | WBC . | AGC . | Platelets . |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Female | 10 | 22 | 42 | 12.1 | 108 | 3.6 | 1.0 | 60 |
| 2 | Female | 25 | 59 | 59 | 10.9 | 110 | 2.9 | 1.7 | 20 |
| 3 | Female | 5 | 19 | 43 | 11.0 | 100 | 5.0 | 1.5 | 34 |
| 4 | Male | 9 | 25 | 45 | 10.2 | 111 | 2.4 | 0.8 | 44 |
| 5 | Male | 12 | 47 | 75 | 12.6 | 100 | 11.2 | 9.6 | 81 |
| 6 | Female | 17 | 58 | 60 | 14.6 | 90 | 3.9 | 2.1 | 184 |
| 7 | Female | 12 | 68 | 70 | 8.5 | 108 | 3.4 | 1.2 | 49 |
| 8 | Male | 15 | 70 | 73 | 12.7 | 123 | 2.7 | 0.4 | 38 |
| Mean | 13 | 46 | 58 | 11.6 | 106 | 4.4 | 2.3 | 64 |
| Subject . | Sex . | Age (y) . | Actual Weight . | Target Weight . | Hb . | MCV . | WBC . | AGC . | Platelets . |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Female | 10 | 22 | 42 | 12.1 | 108 | 3.6 | 1.0 | 60 |
| 2 | Female | 25 | 59 | 59 | 10.9 | 110 | 2.9 | 1.7 | 20 |
| 3 | Female | 5 | 19 | 43 | 11.0 | 100 | 5.0 | 1.5 | 34 |
| 4 | Male | 9 | 25 | 45 | 10.2 | 111 | 2.4 | 0.8 | 44 |
| 5 | Male | 12 | 47 | 75 | 12.6 | 100 | 11.2 | 9.6 | 81 |
| 6 | Female | 17 | 58 | 60 | 14.6 | 90 | 3.9 | 2.1 | 184 |
| 7 | Female | 12 | 68 | 70 | 8.5 | 108 | 3.4 | 1.2 | 49 |
| 8 | Male | 15 | 70 | 73 | 12.7 | 123 | 2.7 | 0.4 | 38 |
| Mean | 13 | 46 | 58 | 11.6 | 106 | 4.4 | 2.3 | 64 |
Weight is given in kg; Hb level, g/dL; MCV, fL; WBC × 103/mm3; AGC × 103/mm3; platelet count × 103/mm3.
Hb indicates hemoglobin; AGC, absolute granulocyte count.
Peripheral blood stem cell mobilization
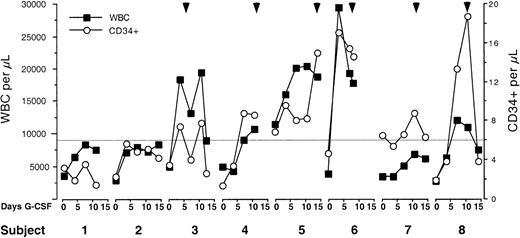
All 8 subjects were mobilized with 10 μg/kg per day of G-CSF. A target of 6 CD34+ cells/μL in the peripheral blood was used as criterion to undergo apheresis for collection of peripheral stem cells. All of the subjects responded to G-CSF with an increase in their white blood count (Figure1 and Table2). However, only 6 subjects reached the CD34+ target to initiate apheresis (Figure 1 and Table 2). There was a mean of 10 ± 4 days of G-CSF mobilization (range, 5-14 days) prior to apheresis. Not unexpectedly, there was a correlation between the maximal WBC achieved during G-CSF administration and the initial hemoglobin level (R2 = 0.541, P = .04) and platelet count (R2 = 0.643,P = .02). There was similarly an inverse relationship between the maximal WBC and the initial MCV (R2 = 0.598, P = .02). The initial CD34+ count correlated with the initial WBC (R2 = 0.76, P = .005). Maximal peripheral blood CD34+ count tended to correlate with the initial hemoglobin level (R2 = 0.370,P = .11) and the maximal WBC response to G-CSF (R2 = 0.384, P = .10).
Mobilization of leukocytes and CD34+ cells during G-CSF administration.
Daily WBC and CD34+ count during G-CSF mobilization. Arrowheads indicate the day apheresis started. Solid horizontal line represents target CD34+ cell count of 6 CD34+cells/μL required to undergo apheresis. Two patients did not achieve the target CD34+ cell count and did not undergo apheresis.
Mobilization of leukocytes and CD34+ cells during G-CSF administration.
Daily WBC and CD34+ count during G-CSF mobilization. Arrowheads indicate the day apheresis started. Solid horizontal line represents target CD34+ cell count of 6 CD34+cells/μL required to undergo apheresis. Two patients did not achieve the target CD34+ cell count and did not undergo apheresis.
Mobilization and collection of peripheral blood stem cells
| Subject . | Initial WBC . | Maximal WBC . | Initial CD34+/μL . | Maximal CD34+/μL . | Total days G-CSF . | Days of apheresis . | Blood volumes . | Liters processed . | Nucleated cells . | Total CD34+ . | Actual CD34+/kg . | Target CD34+/kg . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 3.6 | 8.3 | 3.2 | 3.6 | 10 | — | — | — | — | — | — | — |
| 2 | 2.9 | 8.4 | 2.3 | 5.7 | 14 | — | — | — | — | — | — | — |
| 3 | 5 | 19.4 | 3.5 | 7.8 | 12 | 7 | 40 | 60 | 14.2 × 1010 | 8.5 × 107 | 4.4 × 106 | 2.0 × 106 |
| 4 | 2.4 | 10.7 | 1.4 | 8.8 | 21 | 8 | 47 | 93 | 7.9 × 1010 | 6.7 × 107 | 2.7 × 106 | 1.5 × 106 |
| 5 | 11.2 | 20.4 | 10.3 | 15.0 | 18 | 2 | 11 | 41 | 10.0 × 1010 | 9.2 × 107 | 2.0 × 106 | 1.2 × 106 |
| 6 | 3.9 | 29.5 | 4.7 | 17.7 | 6 | 2 | 12 | 56 | 18.7 × 1010 | 13.7 × 107 | 2.4 × 106 | 2.3 × 106 |
| 7 | 3.4 | 7.0 | 6.5 | 8.8 | 14 | 3 | 18 | 96 | 12.0 × 1010 | 13.9 × 107 | 2.0 × 106 | 2.0 × 106 |
| 8 | 2.7 | 12.1 | 1.9 | 18.7 | 13 | 3 | 12 | 68 | 8.6 × 1010 | 16.0 × 107 | 2.3 × 106 | 2.2 × 106 |
| Mean | 4.4 | 14.5 | 4.2 | 10.8 | 14 | 4 | 23 | 69 | 11.9 × 1010 | 11.3 × 107 | 2.6 × 106 | 1.9 × 106 |
| Subject . | Initial WBC . | Maximal WBC . | Initial CD34+/μL . | Maximal CD34+/μL . | Total days G-CSF . | Days of apheresis . | Blood volumes . | Liters processed . | Nucleated cells . | Total CD34+ . | Actual CD34+/kg . | Target CD34+/kg . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 3.6 | 8.3 | 3.2 | 3.6 | 10 | — | — | — | — | — | — | — |
| 2 | 2.9 | 8.4 | 2.3 | 5.7 | 14 | — | — | — | — | — | — | — |
| 3 | 5 | 19.4 | 3.5 | 7.8 | 12 | 7 | 40 | 60 | 14.2 × 1010 | 8.5 × 107 | 4.4 × 106 | 2.0 × 106 |
| 4 | 2.4 | 10.7 | 1.4 | 8.8 | 21 | 8 | 47 | 93 | 7.9 × 1010 | 6.7 × 107 | 2.7 × 106 | 1.5 × 106 |
| 5 | 11.2 | 20.4 | 10.3 | 15.0 | 18 | 2 | 11 | 41 | 10.0 × 1010 | 9.2 × 107 | 2.0 × 106 | 1.2 × 106 |
| 6 | 3.9 | 29.5 | 4.7 | 17.7 | 6 | 2 | 12 | 56 | 18.7 × 1010 | 13.7 × 107 | 2.4 × 106 | 2.3 × 106 |
| 7 | 3.4 | 7.0 | 6.5 | 8.8 | 14 | 3 | 18 | 96 | 12.0 × 1010 | 13.9 × 107 | 2.0 × 106 | 2.0 × 106 |
| 8 | 2.7 | 12.1 | 1.9 | 18.7 | 13 | 3 | 12 | 68 | 8.6 × 1010 | 16.0 × 107 | 2.3 × 106 | 2.2 × 106 |
| Mean | 4.4 | 14.5 | 4.2 | 10.8 | 14 | 4 | 23 | 69 | 11.9 × 1010 | 11.3 × 107 | 2.6 × 106 | 1.9 × 106 |
WBC × 109/L; G-CSF administered as 10 μg/kg per day; total blood volumes and liters of blood processed during apheresis; number of CD34+ cells collected relative to actual and target weight; target weight based on fifth-percentile weight 5 years in the future.
Peripheral blood stem cell collection
Six of the subjects underwent apheresis to collect peripheral blood stem cells. Means of 11.9 ± 4.1 × 1010nucleated cells and 11.3 ± 3.7 × 107CD34+ cells were collected from the subjects (Table 2). This corresponded to a mean of 2.6 ± 0.9 × 106CD34+ cells/kg of the subject's weight at the time of collection and 1.9 ± 0.4 × 106 CD34+cells/kg of the subject's target weight (Table 2). Apheresis was required for a mean of 4 ± 3 days (range, 2-8 days) to achieve the target CD34+ cell yields (Table 2). The 2 subjects who required the most days of apheresis (7 and 8 days) to reach their target cell number based on future weight achieved 2.3 and 1.9 × 106 CD34 cells/kg actual weight by day 4 of the collection. The subjects had a mean of 23 ± 16 total blood volumes (range, 12-40) processed (Table 2). The mean total number of days of G-CSF administration for all of the subjects, including days during apheresis, was 14 ± 5 days (range, 6-21 days, Table 2).
The maximal number of CD34+ cells/μL in the peripheral blood correlated with the total number of CD34+ cells collected (R2 = 0.739, P = .03) but not the number of CD34+ cells/kg (R2 = 0.134, P = .47). There was an inverse correlation between number of apheresis days and subject age (R2 = 0.656, P = .05) and weight (R2 = 0.695, P = .04). This corresponded to the younger subjects having more blood volumes processed (R2 = 0.660, P = .05) but not to an increase in the total volume of blood processed (R2 = 0.03, P = .74). There was similarly no correlation between total quantity of blood processed and total number of CD34+ cells collected, the number of CD34+ cells/kg, age, or weight (P = .74-.93). Generally, however, the subjects with the highest hemoglobin level, WBC, and maximal WBC response to G-CSF had the least total blood processed (R2 = 0.599, P = .07;R2 = 0.549, P = .09; andR2 = 0.622, P = .06 respectively). Older and heavier subjects also tended to have the highest CD34+ cell collections (R2 = 0.600, P = .07 andR2 = 0.814, P = .01, respectively), but the younger and smaller subjects had higher numbers of CD34+ cells/kg (R2 = 0.581,P = .08 and R2 = 0.575,P = .08, respectively).
Adverse events
Four of 8 subjects complained of mild bone pain and 4 complained of a flulike syndrome during mobilization with G-CSF. Five subjects required a temporary central venous catheter for the apheresis procedure and 3 complained of pain at the insertion site. Two received acetaminophen with codeine and one received acetaminophen alone. Five of the subjects complained of mild tingling during apheresis, which was treated with oral and/or intravenous calcium. Two subjects complained of headache during the apheresis, one requiring acetaminophen with codeine. One subject complained of facial flushing and one complained of abdominal discomfort during apheresis. Three of the subjects required platelet transfusions after apheresis (2, 3, and 5 single-donor units), and red blood cell products were required to prime the apheresis machine for 3 subjects (1, 3, and 7 days). Two of the subjects undergoing apheresis were not exposed to blood products.
Discussion
We originally pursued collection of mobilized peripheral blood from patients with Fanconi anemia prior to the onset of severe bone marrow failure to provide a potential therapeutic option when hematologic support became necessary. The cryopreserved cells potentially could be used to provide stem cell support for hematopoiesis, for reconstitution after chemotherapy, or as targets for gene therapy. The feasibility and efficacy of each of these approaches remains to be determined. One concern in the collection of large numbers of CD34+ cells was that patients with Fanconi anemia could not be mobilized due to the presence of bone marrow failure, just as heavily treated cancer patients often have compromised ability to mobilize CD34+ cells. However, our results indicate that mobilization and collection of peripheral blood stem cells is feasible for some patients with Fanconi anemia. We were able to collect ≥ 1 × 106 CD34+ cells/kg measured at both the actual and target weights from 6 of 8 subjects who participated in the trial and ≥2 × 106CD34+ cells/kg from 6 subjects at their collection weight. However, we were only able to collect ≥ 2 × 106CD34+ cells/kg at the target weight from 4 of the subjects. The majority of the collections required prolonged administration of G-CSF (6-21 days), and 2 subjects required 7-8 days of apheresis. Nevertheless, the collected numbers of cells would appear to be sufficient to attempt autologous reconstitution after progressive bone marrow failure or after high-dose chemotherapy. Several questions remain, however, including whether hematopoietic stem cells from patients with Fanconi anemia have similar stability when cyropreserved compared to stem cells from patients with other disorders and whether similar numbers of CD34+ cells are required for stem cell reconstitution compared to healthy donors. A fundamental question is whether cryopreserved stem cells from patients with Fanconi anemia can contribute to hematopoiesis once infused in an autologous bone marrow environment without immunosuppressive conditioning.
Previous studies have demonstrated that a variety of cytokines can stimulate hematopoietic cells to improve peripheral blood counts in patients with Fanconi anemia.25,37,38 G-CSF has previously been used with success to increase neutrophil counts in children with Fanconi anemia for prolonged durations.23 Interestingly, some of these patients also experienced an increase in hemoglobin level or platelet count. Eight of 12 patients increased the CD34+count in their bone marrow at some point during the administration of G-CSF. The peripheral blood CD34+ cell count increased in 5 of 12 patients by 3- to 18-fold after 4 weeks. The analysis of the subjects in the current study indicated that there was a 2-fold increase in CD34+ cells in the peripheral blood by days 4-5 of G-CSF (data not shown) and a mean individual 3.6-fold increase during G-CSF administration (Table 2 and data not shown). However, there were no clear predictors of who would mobilize sufficiently to reach the collection goals. One of the 2 subjects who did not undergo apheresis increased the peripheral CD34+ count by 2.5-fold, but 2 of those who underwent apheresis did not increase the peripheral CD34 count by more than 2-fold. Although those who met the criteria for apheresis had a higher initial CD34+ count, there was considerable overlap in this small group of patients.
It is noteworthy that the 2 subjects who required the most days of apheresis (7 and 8 days) were the youngest (ages 5 and 9 years). There are several possible explanations for this observation. Although it may simply be related to the quantity of blood that was processed each day, it could also indicate that the older patients represented a subpopulation in which bone marrow failure had not progressed as rapidly; hence they had more hematopoietic reserve. The total volume of blood processed did not correlate with the total number of CD34+ cells collected, the number of CD34+cells/kg, age, or weight. However, although older subjects had higher total numbers of CD34+ cells, with the prolonged number of apheresis days the younger patients tended to achieve the highest CD34+ counts/kg. The evaluation is complicated by the fact that the younger subjects' target CD34+ cell/kg collection was relatively higher than that of the older subjects since the younger subjects would be expected to gain more weight over the ensuing 5 years. This most likely accounts for the higher CD34+ count/kg at the time of collection, which corresponds to both the youngest subjects achieving approximately 2 × 106/kg for their actual weight by 4 days of apheresis.
Peripheral blood mobilization of CD34+ cells for use as targets for gene therapy has previously been described for 3 patients with Fanconi anemia.39 The patients received 3-4 cycles of G-CSF at 10 μg/kg for 5 days with 1 to 3 days of apheresis every 3-4 months. Patient 1 mobilized poorly, achieving 0-3.4 CD34+ cells/μL peripheral blood on successive collections, resulting in infusion of 0.003-0.011 × 106/kg modified cells. The second patient's peripheral CD34+ cell count varied from 0.9-4.1 to 33.6-116 CD34+ cells/μL during successive mobilizations. The number of infused modified cells similarly varied from 0.003 to 0.85 × 106/kg. Patient 3 consistently mobilized the most successfully, achieving 19-92 CD34+ cells/μL with infusion of 1.1-1.9 × 106/kg modified cells after 3 successive collections. There appeared to be a correlation between the number of circulating CD34+ cells and the number of CD34+cells collected in these patients. Infusion of gene therapy–modified cells consistently produced increased bone marrow cellularity and increased peripheral blood counts for a short time. This observation is important since it suggests that after genetic modification autologous cells can migrate and repopulate the bone marrow in patients with Fanconi anemia. One subject in the gene therapy study, however, did appear to have a substantial increase in gene therapy–marked bone marrow cells after receiving radiation for squamous cell carcinoma of the vulva, which developed while on the study. Whether this indicates that immunosuppression will be required for optimal autologous reconstitution is not clear.
We recently evaluated our experience in mobilizing healthy, young adult peripheral blood stem cell donors for research collections.36 Donors were mobilized with 4 daily G-CSF doses of 480 μg (3.5-9.4 μg/kg per day) and a single 2–blood volume apheresis. The mean number of nucleated cells collected was 4.6 × 1010, which included 1.9 × 108CD34+ cells corresponding to 2.7 × 106CD34+ cells/kg. Similarly, many transplantation centers aim for peripheral counts of 20 CD34+ cells/μL prior to starting apheresis. These results contrast with the difficulty in obtaining comparable numbers of CD34+ cells from the patients with Fanconi anemia.
The improved ability to collect and store hematopoietic stem cells coupled with the potential for gene therapy has led investigators and families of children with Fanconi anemia to attempt to store hematopoietic stem cells from the patients prior to the development of severe bone marrow failure. Although collection of peripheral blood stem cells from some patients with Fanconi anemia is feasible, it is difficult to identify in advance the patients in whom it will be successful. It is similarly difficult to predict which patients will require prolonged G-CSF administration or many days of apheresis. The alternative approach to collecting hematopoietic stem cells is by bone marrow harvest. Unfortunately, there is little published data available on the numbers of CD34+ cells that can be collected with a bone marrow harvest from patients with Fanconi anemia. The efficacy as well as the risks of these 2 approaches remains to be better ascertained in this patient population. Only with more information can a definitive recommendation be made of which approach should be undertaken for collection of hematopoietic stem cells for therapeutic uses in patients with Fanconi anemia.
Acknowledgment
We would like to thank Amgen for providing the G-CSF.
Supported by the Fanconi Anemia Research Fund, the Centers of Excellence in Molecular Hematology (National Institute of Diabetes and Digestive and Kidney Diseases P30DK49218), and the Jonathan and Jennifer Simmons Charitable Trust.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
James M. Croop, Section of Pediatric Hematology/Oncology, Rm 2720, James Whitcomb Riley Hospital for Children, 702 Barnhill Dr, Indianapolis, IN 46202; e-mail:jcroop@iupui.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal