Abstract
There is controversy as to whether murine definitive hematopoiesis originates from yolk sac (YS) or the intraembryonic region. This study reports the generation of definitive hematopoietic stem cells (HSCs) from both early YS and intraembryonic paraaortic splanchnopleures (P-Sp) on AGM-S3 stromal cells derived from the aorta-gonad-mesonephros (AGM) region at 10.5 days post coitum (dpc). YS and P-Sp cells at 8.5 dpc generated no definitive hematopoiesis-derived colony-forming cells in cocultures with AGM-S3 cells, but spleen colony-forming cells and HSCs capable of reconstituting definitive hematopoiesis in adult mice simultaneously appeared on day 4 of coculture. Precursors for definitive HSCs were present in YS and P-Sp at 8.0 dpc, a time when YS and embryo were not connected by blood vessels. It is proposed that precursors with the potential to generate definitive HSCs appear independently in YS and intraembryonic P-Sp and that the P-Sp or AGM region affords the microenvironment that facilitates generation of definitive hematopoiesis from precursors.
Introduction
The hematopoietic system of vertebrates, derived from the mesodermal germ layer in early embryogenesis, occurs in 2 waves. At early stages, the first wave, primitive hematopoiesis, takes place in the visceral yolk sac (YS) or its analog. The primitive hematopoiesis is followed by a second wave, known as definitive hematopoiesis and which is observed in intraembryonic areas. Experiments using chimeric embryos in nonmammalian vertebrates demonstrated that mesodermally derived ventral compartments (YS or its analog) and dorsal compartments (intraembryonic region) contribute to hematopoiesis in a different manner. In the avian species, the intraembryonic region containing the dorsal aorta is responsible for definitive hematopoiesis, while the YS produces only transient embryonic hematopoiesis.1,2 In amphibians, the intraembryonic region containing the pronephros is the major source of definitive hematopoiesis.3,4 The ontogenic source of mammalian definitive hematopoiesis has remained controversial because the in utero development of mammals excludes embryo grafting experiments. Results of earlier mouse studies led to the general acceptance of a model that murine definitive hematopoiesis begins in YS, shifts to fetal liver (FL), and finally resides in bone marrow (BM), in contrast to the conclusion derived from nonmammal vertebrates.5 6 However, recent studies have shown that early development of murine hematopoiesis is more complex than heretofore considered.
During mouse embryogenesis, primitive hematopoiesis appears at 7.5 days post coitum (dpc), a time when primitive nucleated erythrocytes are visible in YS blood islands.5 Primitive erythrocytes are distinguishable from definitive ones, which are first detected in FL at 9 dpc, as based on the specific expression of embryonic-type globins.7 Colony-forming cells (CFCs) are also detectable as early as 7.5 dpc in YS and later at 9 dpc in FL.5,8However, spleen colony-forming cells (CFU-Ss) and long-term repopulating hematopoietic stem cells (LTR-HSCs) are absent in YS before the circulation has been established.9,10 Recently, it has been shown that intraembryonic paraaortic splanchnopleures (P-Sp), which goes on to differentiate into the dorsal aorta, genital ridge/gonad, and pro/mesonephros (aorta-gonad-mesonephros, or AGM) region, harbors lymphohematopoietic progenitors, CFU-Ss, and LTR-HSCs prior to liver colonization.11,12 Lymphohematopoietic progenitors are simultaneously identifiable in P-Sp and YS at 8.5 dpc.13 CFU-Ss appear at the YS and AGM region at late 9 dpc, but the number and frequency of CFU-Ss in the AGM region greatly exceed those in the YS.9 HSCs capable of long-term multilineage repopulation in irradiated adult recipients are first noted in the AGM region at 10 dpc, before such activity can be observed in YS and FL.10 In contrast, other studies have shown that HSCs in 9 dpc YS as well as P-Sp can contribute to definitive hematopoiesis when conditioned newborn mice instead of irradiated adult mice serve as recipients for hematopoietic transplantation.14 15
While the developmental relationship between primitive and definitive hematopoiesis in murine embryogenesis remains unanswered, localization and developmental stage are critical factors for embryonic hematopoiesis; hence the importance of the microenvironment surrounding embryonic hematopoietic cells or their precursors. To better understand the roles of the microenvironment in the development of murine embryonic hematopoiesis, several stromal cell lines were isolated from various tissues at different stages of development, such as YS at 9.5, 10, and 10.5 dpc and FL at 14 dpc, and their supportive activities on hematopoiesis were analyzed.16-19 We and another group recently established endothelial cell lines, AGM-S3 and DAS 104-4, from the AGM region at 10.5 and 11.0 dpc, respectively.20 21 These cell lines support the development of murine and human immature hematopoietic cells including LTR-HSCs.
In the present work, we asked if AGM-S3 cells have the potential to support the generation of LTR-HSCs capable of reconstituting adult definitive hematopoiesis from early YS and intraembryonic P-Sp, since it has been shown that LTR-HSCs initiate autonomously within the 10 dpc AGM region in an in vitro organ culture system.11When cocultured with AGM-S3, cells isolated from 8.0 and 8.5 dpc P-Sp generate CFU-Ss and LTR-HSCs. Surprisingly, definitive LTR-HSCs were also generated from YS even at 8.0 dpc, a time when YS and the embryo were not connected by blood vessels. We propose that precursors capable of generating definitive hematopoiesis are already present at early YS and are independent of simultaneously existing P-Sp and that the P-Sp or AGM region provides the microenvironment for generation of definitive hematopoiesis from precursors.
Materials and methods
Mice
C57BL/6 Ly-5.1 mice were kindly provided by Dr K. Ikuta (Kyoto University, Japan). and C57BL/6 Ly-5.2 mice were obtained from Shizuoka Laboratory Animal Center (Shizuoka, Japan). These mice were bred and maintained in a specific pathogen-free microisolator environment. In the transplantation experiments, recipient mice were given neomycin (1.1 g/1000 mL) in their tap water to drink during the first month after irradiation and transplantation.
Cell preparation
In this study, each embryo was staged according to the reported criteria.22 YS and P-Sp were removed from the embryo of C57BL/6 Ly-5.2 mice as described.12,23 24 YS and P-Sp were first incubated with 0.1% collagenase (Sigma Chemical, St Louis, MO) for 1 hour at 37°C in phosphate-buffered saline supplemented with 10% fetal bovine serum (FBS; Hyclone, Logan, UT), washed once in washing solution, and subsequently incubated with cell dissociation buffer containing ethylenediaminetetraacetic acid (EDTA)/ethyleneglycotetraacetic acid. BM cells were from femurs and tibiae of adult mice. Cells were dissociated by gentle pipetting and passed through a nylon mesh.
Antibodies
The antibodies used for immunofluorescence staining included 104 (anti–Ly-5.2), RB6-8C5 (anti–Gr-1), M1/70 (anti–Mac-1), RA3-6B2 (anti-CD45R/B220), and 30-H12 (anti–Thy-1.2), all purchased from PharMingen (San Diego, CA). All antibody incubations were carried out for 30 minutes on ice.
Culture with stromal cell lines
YS and P-Sp cells were cultured with stromal cell lines in α–minimal essential medium (α-MEM; Flow Laboratories, Rockville, MD) containing 10% FBS. Cells including stromal ones were harvested using 0.05% trypsin containing 0.53 mM EDTA (Gibco, Grand Island, NY) on the designated day of culture and passed through a nylon mesh. AGM-S3 stromal cell line was established from 10.5 dpc AGM region–derived cells as described previously.21 In brief, AGM tissues were removed from 10.5 dpc embryos of C3H/HeN mice, dissected to some pieces, and cultured in 24-well plates overnight with a drop of α-MEM containing 10% FBS at 37°C in a humidified atmosphere flushed with 5% CO2 in air, and 1 mL culture medium was added to the well the next day. When the adherent cells appeared around the tissues 1 week later, the AGM tissues were removed. After 1 additional week of incubation, the adherent cells were harvested from the well using 0.05% trypsin plus 0.53 mM EDTA and were plated in 6-well plates. After 2 weeks of incubation, the cultured cells were irradiated with 900-rad γ-ray to deplete hematopoietic cells. The medium was replaced with fresh culture medium once weekly. Adherent cells were harvested 2 weeks later using trypsin, and 50 to 100 cells were seeded into 24-well plates. After 3 weeks of incubation, cells expanded in a well were harvested and used for cell cloning by the limiting dilution technique.
Cell sorting
BM mononuclear cell suspensions were separated using a density gradient centrifugation method and stained with fluorescein isothiocyanate (FITC)–anti-Ly-5.2. The stained cells were sorted on a FACSVantage (Becton Dickinson, Mountain View, CA) as reported.25
Transplantation
YS and P-Sp cells from Ly-5.2 mice were transferred together with 1 × 105 unfractionated BM mononuclear cells from Ly-5.1 mice into sublethally irradiated Ly-5.1 mice. Peripheral blood (PB) was collected from the tail vein of the recipient mice for up to 6 months after transplantation. Red blood cells were removed, and the nucleated PB cells were stained with FITC–anti-Ly-5.2 and phycoerythrin–antimyeloid cells (Mac-1 and Gr-1), anti–B lymphocytes (B220), or anti–T lymphocytes (Thy-1) and analyzed on a FACScan (Becton Dickinson). The mice in which donor-derived (Ly-5.2+) cells made up more than 1% of all B220+, Thy-1+, and Mac-1+/Gr-1+ cells in PB were scored as being positive for successful reconstitution.
CFC assay
Clonal cell culture was done in triplicate as described.26,27 Briefly, 1 mL culture mixture containing cells from YS or P-Sp cells of Ly-5.2 mice or their progenies cultured with AGM-S3 cells, α-MEM, 1.2% methylcellulose (Shinetsu Chemical, Tokyo, Japan), 30% FBS, 1% deionized fraction V bovine serum albumin (Sigma), 10−4 M mercaptoethanol (Eastman Organic Chemicals, Rochester, NY), 100 ng/mL rat stem cell factor (SCF; Amgen, Thousand Oaks, CA) and human interleukin (IL)-6 (Tosoh, Kanagawa, Japan), 20 ng/mL mouse IL-3 (Kirin Brewery, Tokyo, Japan) and human thrombopoietin (Kirin), 2 U/mL human erythropoietin (Kirin) and 10 ng/mL human granulocyte colony-stimulating factor (G-CSF) (Kirin) was plated in each of 35-mm suspension culture dishes and incubated at 37°C in a humidified atmosphere flushed with 5% CO2 in air. Colony types were determined on days 7 to 14 of incubation by in situ observation using an inverted microscope and according to the criteria described.26 28
CFU-S assay
Eight-week-old C57BL/6 recipient mice were irradiated (9.5 Gy) with a 60Co source and intravenously given donor cells. On day 8 after injection, the number of colonies in the recipient spleen were counted.
RT-PCR analysis for hemoglobin
Reverse transcription–polymerase chain reaction (RT-PCR) analysis was performed using a previously described method with some modification.21,24 Messenger RNA (mRNA) was prepared from individual erythroid bursts or erythrocyte-containing hematopoietic mixed colonies using QuickPrep Micro mRNA Purification kit (Pharmacia, Uppsala, Sweden), and reverse-transcribed using T-primed First-Strand Kit (Pharmacia). Hemoglobin-specific complementary DNA was amplified with Taq DNA polymerase using pairs of oligonucleotide primers as follows29: α-globin-5′, CTCTCTGGGGAAGACAAAAGCAAC-3′; α-globin-3′, GGTGGCTAGCCAAGGTCACCAGAC-3′. βmajor-globin-5′, CTGACAGATGCTCTCTTGGG-3′; βmajor-globin-3′, CACAACCCCAGAAACAGACA-3′. βH1-globin-5′, AGTCCCCATGGAGTCAAAGA-3′; βH1-globin-3′, CTCAAGGAGACCTTTGCTCA-3′. ε-globin-5′, GGAGAGTCCATTAAGAACCTAGACAA-3′; ε-globin-3′, CTGTGAATTCATTGCCGAAGTGAC-3′. ζ-globin-5′, GCTCAGGCCGAGCCCATTGG-3′; ζ-globin-3′, TAGCGGTACTTCTCAGTCAG-3′. β-actin-5′, GTGGGCCGCTCTAGGCACCAA-3′; β-actin-3′, CTCTTTGATGTCACGCACGATTTC-3′. Samples were denatured at 94°C for 5 minutes, followed by amplification rounds consisting of 94°C for 30 seconds (denaturing), 55°C to 60°C for 1 minute (annealing), and 72°C for 2 minutes (extension) for 40 cycles. Products were separated on a 2.0% agarose gel, stained with ethidium bromide, and photographed. Messenger RNA derived from granulocyte/macrophage colonies, adult BM cells, and 8.0 dpc YS cells were used as negative controls and controls for definitive and primitive erythropoiesis, respectively.
Results
Generation of blood cells from 8.5 dpc P-Sp and YS cocultured with AGM-S3 cells
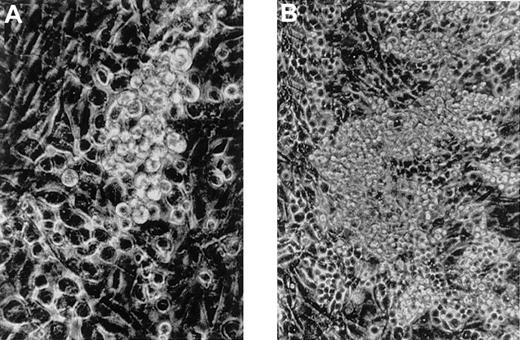
When the cells isolated from 8.5 dpc P-Sp were cocultured with AGM-S3 cells, small round cells were first evident on day 2 or 3 of culture (Figure 1A) and gradually increased up to day 7 (Figure 1B). Cytospin preparation on day 7 of culture showed that most of the nonadherent cells were macrophages and some immature cells were present. The cells isolated from 8.5 dpc YS also produced blood cells, mainly macrophages, with a similar time course.
Generation of hematopoietic cells from 8.5 P-Sp cells cocultured with AGM-S3 cells.
Small round cells were first detected on day 2 or 3 of culture (A, original magnification, × 200) and gradually increased by day 7 (B, original magnification, × 100).
Generation of hematopoietic cells from 8.5 P-Sp cells cocultured with AGM-S3 cells.
Small round cells were first detected on day 2 or 3 of culture (A, original magnification, × 200) and gradually increased by day 7 (B, original magnification, × 100).
Generation of CFCs from 8.5 dpc P-Sp and YS cocultured with AGM-S3 cells
To determine if the blood cells generated from early YS and P-Sp contain CFCs, cells isolated from YS and P-Sp at 8.5 dpc (0.5 embryo equivalents) were cultured with SCF, IL-3, IL-6, G-CSF, erythropoietin, and thrombopoietin. As shown in Table 1, YS at 8.5 dpc contained various types of CFCs. When the YS cells were cocultured with AGM-S3 cells, CFCs slightly increased until day 2 of culture and then decreased, and CFCs were not detected after day 6 of culture. P-Sp at 8.5 dpc contained only a small number of CFCs, and these disappeared by day 4.
Colony formation from 8.5 dpc YS and P-Sp cells (0.5 embryo equivalents) or their progenies cocultured with AGM-S3 cells
| Cell source . | Culture period, d . | No. of colonies . | ||||
|---|---|---|---|---|---|---|
| Granulocyte/ macrophage colonies . | Erythroid bursts . | Megakaryocyte colonies . | Hematopoietic mixed colonies . | Total . | ||
| 8.5 dpc YS | 0 | 7 ± 1 | 1 ± 1 | 1 ± 1 | 7 ± 3 | 16 ± 4 |
| 2 | 10 ± 2 | 16 ± 3 | 1 ± 1 | 9 ± 4 | 36 ± 9 | |
| 4 | 4 ± 3 | 2 ± 1 | 1 ± 1 | 5 ± 2 | 12 ± 4 | |
| 6 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | |
| 10 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | |
| 8.5 dpc P-Sp | 0 | 4 ± 2 | 0 ± 0 | 0 ± 1 | 2 ± 1 | 6 ± 3 |
| 2 | 0 ± 1 | 1 ± 1 | 0 ± 0 | 0 ± 0 | 1 ± 0 | |
| 4 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | |
| 6 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | |
| 10 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | |
| Cell source . | Culture period, d . | No. of colonies . | ||||
|---|---|---|---|---|---|---|
| Granulocyte/ macrophage colonies . | Erythroid bursts . | Megakaryocyte colonies . | Hematopoietic mixed colonies . | Total . | ||
| 8.5 dpc YS | 0 | 7 ± 1 | 1 ± 1 | 1 ± 1 | 7 ± 3 | 16 ± 4 |
| 2 | 10 ± 2 | 16 ± 3 | 1 ± 1 | 9 ± 4 | 36 ± 9 | |
| 4 | 4 ± 3 | 2 ± 1 | 1 ± 1 | 5 ± 2 | 12 ± 4 | |
| 6 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | |
| 10 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | |
| 8.5 dpc P-Sp | 0 | 4 ± 2 | 0 ± 0 | 0 ± 1 | 2 ± 1 | 6 ± 3 |
| 2 | 0 ± 1 | 1 ± 1 | 0 ± 0 | 0 ± 0 | 1 ± 0 | |
| 4 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | |
| 6 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | |
| 10 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | |
The number of colonies indicates the mean ± SD of triplicate cultures.
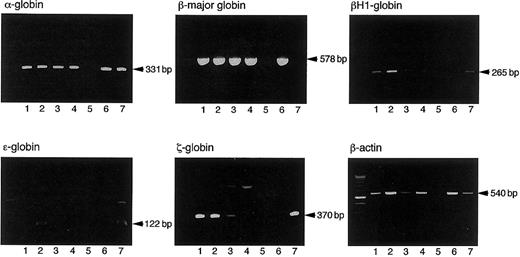
The hemoglobin types of erythrocytes in erythroid bursts or erythrocyte-containing mixed colonies were analyzed by RT-PCR, the objective being to determine if the CFCs derived from primitive or definitive hematopoiesis. In the culture of cells isolated from 8.5 dpc YS, all 7 erythroid bursts and 7 mixed colonies examined expressed both embryonic hemoglobins, such as βH1, ε, and ζ globins, and adult hemoglobins, such as α and β-major globins (Figure2, lanes 1 and 2), indicating that they were derived from primitive hematopoiesis. Since Palis et al reported on the presence of a small number of definitive erythroid progenitors in 8.5 dpc YS,30 the number of colonies we analyzed might be insufficient to detect them. When colonies derived from CFCs in the coculture on day 4 were analyzed, all 5 erythroid bursts and 7 mixed colonies also expressed both embryonic and adult hemoglobins (Figure 2, lanes 3 and 4). All 2 erythroid bursts and 4 mixed colonies derived from 8.5 dpc P-Sp cells or their progenies cocultured with AGM-S3 cells for 2 days also expressed both embryonic and adult hemoglobins (data not shown). Thus, AGM-S3 cells supported primitive CFCs but did not induce the generation of definitive CFCs from early YS and P-Sp.
Expression of embryonic and adult hemoglobin by erythroid bursts and erythrocyte-containing mixed colonies derived from 8.5 dpc YS cells and their progenies cocultured with AGM-S3 cells for 4 days.
Lane 1: an erythroid burst derived from 8.5 dpc YS cells. Lane 2: a hematopoietic mixed colony derived from 8.5 dpc YS cells. Lane 3: an erythroid burst derived from the cells cultured from 8.5 dpc YS cells on AGM-3 cells for 4 days. Lane 4: a hematopoietic mixed colony derived from the cells cultured from 8.5 dpc YS cells on AGM-3 cells for 4 days. Lane 5: a granulocyte/macrophage colony derived from 8.5 dpc YS cells. Lane 6: adult BM cells. Lane 7: 8.0 dpc YS cells.
Expression of embryonic and adult hemoglobin by erythroid bursts and erythrocyte-containing mixed colonies derived from 8.5 dpc YS cells and their progenies cocultured with AGM-S3 cells for 4 days.
Lane 1: an erythroid burst derived from 8.5 dpc YS cells. Lane 2: a hematopoietic mixed colony derived from 8.5 dpc YS cells. Lane 3: an erythroid burst derived from the cells cultured from 8.5 dpc YS cells on AGM-3 cells for 4 days. Lane 4: a hematopoietic mixed colony derived from the cells cultured from 8.5 dpc YS cells on AGM-3 cells for 4 days. Lane 5: a granulocyte/macrophage colony derived from 8.5 dpc YS cells. Lane 6: adult BM cells. Lane 7: 8.0 dpc YS cells.
Generation of CFU-Ss from 8.5 dpc P-Sp and YS cocultured with AGM-S3 cells
We next examined whether cells generated from 8.5 dpc P-Sp and YS contain CFU-Ss. As shown in Table 2, 8.5 dpc P-Sp and YS (1 embryo equivalent) contained no CFU-Ss. Cells isolated from 8.5 dpc P-Sp and YS were cocultured with AGM-S3 cells for 4 days and then assayed daily for CFU-Ss. No CFU-S activity was found in the cells cultured from P-Sp and YS for 1 to 3 days. However, both P-Sp and YS generated a substantial number of CFU-Ss in the coculture with AGM-S3 cells at day 4. Therefore, AGM-S3 cells can induce generation of CFU-Ss from early YS and P-Sp.
Generation of CFU-S from 8.5 dpc P-Sp and YS (one embryo equivalent) in coculture with AGM-S3 cells
| Cells injected . | Before coculture . | After coculture . | |||
|---|---|---|---|---|---|
| Day 1 . | Day 2 . | Day 3 . | Day 4 . | ||
| None | 0 | 0 | 0 | 0 | 0 |
| P-Sp | 0 | 0 | 0 | 0 | 7 ± 3 (n = 10) |
| YS | 0 | 0 | 0 | 0 | 3 ± 2 (n = 6) |
| Cells injected . | Before coculture . | After coculture . | |||
|---|---|---|---|---|---|
| Day 1 . | Day 2 . | Day 3 . | Day 4 . | ||
| None | 0 | 0 | 0 | 0 | 0 |
| P-Sp | 0 | 0 | 0 | 0 | 7 ± 3 (n = 10) |
| YS | 0 | 0 | 0 | 0 | 3 ± 2 (n = 6) |
Cells isolated from 8.5 dpc P-Sp and YS or their progenies cocultured with AGM-S3 cells were given intravenously to mice, and 8 days later the number of colonies in spleens of recipients was counted.
Generation of LTR-HSCs from 8.5 dpc P-Sp and YS cocultured with AGM-S3 cells
We then examined the generation of HSCs capable of reconstituting hematopoiesis from 8.5 dpc P-Sp and YS in the coculture with AGM-S3 cells using a competitive repopulating assay (Table3). We first transplanted only AGM-S3 cells cultured for 6 days into 8 Ly-5.1 mice to examine whether AGM-S3 cells have the hematopoietic repopulating ability. Twelve weeks after transplantation, there were no Ly-5.2 cells in PB of all recipients. When cells isolated from 8.5 dpc P-Sp and YS (2 embryo equivalents) of Ly-5.2 mice were respectively transplanted into 9 irradiated Ly-5.1 adult mice, no recipient mouse showed successful engraftment. Four recipients transplanted with 8.5 dpc P-Sp or YS cells cultured without stroma for 6 days also showed no successful engraftment. However, all 4 recipients transplanted with 8.5 dpc P-Sp or YS cells cocultured with AGM-S3 cells for 6 days had Ly-5.2+ donor-type myeloid and lymphoid cells in the PB at 12 weeks after transplantation (Table 3 and Figure 3). Figure4 shows representative PB profiles of mice transplanted with cells cocultured from 8.5 dpc P-Sp (Figure 4A) and YS (Figure 4B) with AGM-S3 cells for 6 days. Stable chimerism was maintained for 6 months in recipients engrafted with the progeny of 8.5 dpc P-Sp or YS.
Generation of LTR-HSCs from 8.5 dpc P-Sp and YS (2 embryo equivalents) in coculture with AGM-S3 cells
| Cell source . | Culture condition . | No. of mice reconstituted/ transplanted . | % donor cells in reconstituted mice expressing . | ||
|---|---|---|---|---|---|
| B220 . | Thy-1 . | Gr-1/Mac-1 . | |||
| None | AGM-S3 cells | 0/8 | NA | NA | NA |
| P-Sp | Before culture | 0/9 | NA | NA | NA |
| Without stroma | 0/4 | NA | NA | NA | |
| AGM-S3 cells | 4/4 | 66.2 | 46.0 | 32.7 | |
| YS | Before culture | 0/9 | NA | NA | NA |
| Without stroma | 0/4 | NA | NA | NA | |
| AGM-S3 cells | 4/4 | 74.1 | 55.6 | 36.3 | |
| Cell source . | Culture condition . | No. of mice reconstituted/ transplanted . | % donor cells in reconstituted mice expressing . | ||
|---|---|---|---|---|---|
| B220 . | Thy-1 . | Gr-1/Mac-1 . | |||
| None | AGM-S3 cells | 0/8 | NA | NA | NA |
| P-Sp | Before culture | 0/9 | NA | NA | NA |
| Without stroma | 0/4 | NA | NA | NA | |
| AGM-S3 cells | 4/4 | 66.2 | 46.0 | 32.7 | |
| YS | Before culture | 0/9 | NA | NA | NA |
| Without stroma | 0/4 | NA | NA | NA | |
| AGM-S3 cells | 4/4 | 74.1 | 55.6 | 36.3 | |
Cells isolated from 8.5 dpc P-Sp and YS of Ly-5.2 mice or their progenies were transplanted into Ly-5.1 mice, and 12 weeks later Ly-5.2+ B lymphocytes (B220+), T lymphocytes (Thy-1+), and myeloid cells (Gr-1+/Mac-1+) were analyzed by flow cytometry.
NA indicates not analyzed.
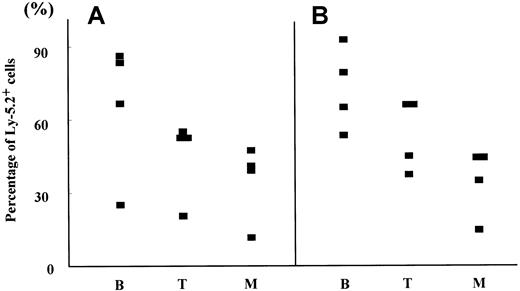
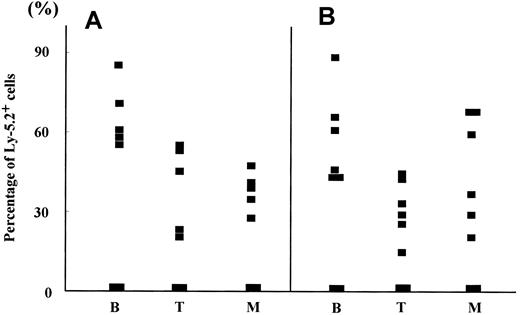
Hematopoietic reconstitution by 8.5 dpc P-Sp and YS cells cocultured with AGM-S3 cells.
The percentages of Ly-5.2+ cells in B220+ cells (B), Thy-1+ cells (T), and Gr-1/Mac-1+ cells (M) were analyzed in PB of Ly-5.1 recipients engrafted with 8.5 dpc P-Sp (A) and YS cells (B) cocultured with AGM-S3 cells (Table 3) 12 weeks after transplantation.
Hematopoietic reconstitution by 8.5 dpc P-Sp and YS cells cocultured with AGM-S3 cells.
The percentages of Ly-5.2+ cells in B220+ cells (B), Thy-1+ cells (T), and Gr-1/Mac-1+ cells (M) were analyzed in PB of Ly-5.1 recipients engrafted with 8.5 dpc P-Sp (A) and YS cells (B) cocultured with AGM-S3 cells (Table 3) 12 weeks after transplantation.
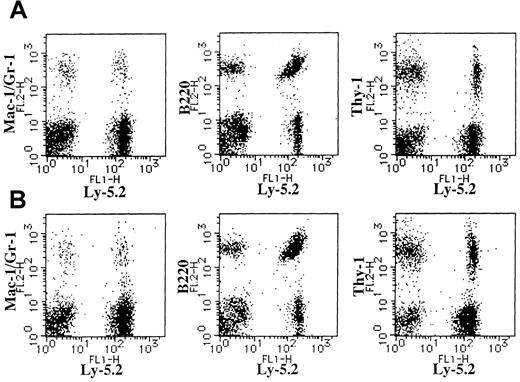
Expression of Ly-5.2 and Mac-1/Gr-1, B220, or Thy-1 on BM cells of Ly-5.1 recipients.
(A) A recipient engrafted with the cells cultured from 8.5 dpc P-Sp cells of Ly-5.2 mouse embryo on AGM-S3 cells for 6 days. (B) A recipient engrafted with the cells cultured from 8.5 dpc YS cells of Ly-5.2 mouse embryo on AGM-S3 cells for 6 days.
Expression of Ly-5.2 and Mac-1/Gr-1, B220, or Thy-1 on BM cells of Ly-5.1 recipients.
(A) A recipient engrafted with the cells cultured from 8.5 dpc P-Sp cells of Ly-5.2 mouse embryo on AGM-S3 cells for 6 days. (B) A recipient engrafted with the cells cultured from 8.5 dpc YS cells of Ly-5.2 mouse embryo on AGM-S3 cells for 6 days.
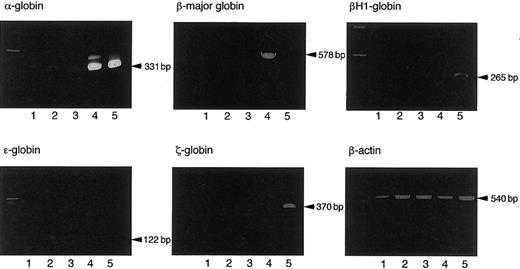
To confirm that LTR-HSCs generated in the coculture with AGM-S3 cells produce definitive erythrocytes, we cultured donor-type Ly-5.2+ cells sorted from BM cells of recipients engrafted with the cells harvested on day 6 of coculture of early YS and P-Sp 20 weeks after the transplantation, and hemoglobin types of erythrocytes were determined in case of erythroid bursts and erythrocyte-containing mixed colonies derived from the sorted cells. All of 3 and 4 erythroid bursts and 4 and 5 mixed colonies derived from donor-type BM cells of the mice transplanted with the progeny of 8.5 dpc P-Sp and YS cells, respectively, contained definitive erythrocytes expressing adult but not embryonic hemoglobins (Figure 5 and data not shown). These findings indicate that AGM-S3 cells can support the generation of definitive LTR-HSCs from early YS and P-Sp.
Expression of embryonic and adult hemoglobins by an erythroid burst and an erythrocyte-containing mixed colony from the Ly-5.2+ cells sorted from BM cells of Ly-5.1 mouse recipients successfully engrafted with the cells cultured from 8.5 dpc Ly-5.2 mouse YS cells on AGM-S3 cells for 6 days.
Lane 1: an erythroid burst. Lane 2: a hematopoietic mixed colony. Lane 3: a granulocyte/macrophage colony. Lane 4: adult BM cells. Lane 5: 8.0 dpc YS cells.
Expression of embryonic and adult hemoglobins by an erythroid burst and an erythrocyte-containing mixed colony from the Ly-5.2+ cells sorted from BM cells of Ly-5.1 mouse recipients successfully engrafted with the cells cultured from 8.5 dpc Ly-5.2 mouse YS cells on AGM-S3 cells for 6 days.
Lane 1: an erythroid burst. Lane 2: a hematopoietic mixed colony. Lane 3: a granulocyte/macrophage colony. Lane 4: adult BM cells. Lane 5: 8.0 dpc YS cells.
The time course of the generation of definitive LTR-HSCs was also examined. Progenies of 8.5 dpc P-Sp or YS (2 embryo equivalents) cocultured with AGM-S3 cells for 2 to 4 days were transplanted (Table4). None of 4 mice transplanted with the cells harvested at days 2 and 3 of coculture of P-Sp or YS showed successful engraftment. However, 9 of 11 and 5 of 10 recipients transplanted with cells harvested on day 4 of coculture of 8.5 dpc P-Sp or YS, respectively, were successfully engrafted. Thus, LTR-HSCs as well as CFU-Ss were simultaneously generated from 8.5 dpc P-Sp and YS on day 4 of coculture with AGM-S3 cells.
Time course of the generation of LTR-HSCs from 8.5 dpc P-Sp and YS (2 embryo equivalents) in cocultures with AGM-S3 cells
| Cell source . | No. of mice reconstituted/transplanted . | ||
|---|---|---|---|
| Day 2 . | Day 3 . | Day 4 . | |
| P-Sp | 0/4 | 0/4 | 9/11 |
| YS | 0/4 | 0/4 | 5/10 |
| Cell source . | No. of mice reconstituted/transplanted . | ||
|---|---|---|---|
| Day 2 . | Day 3 . | Day 4 . | |
| P-Sp | 0/4 | 0/4 | 9/11 |
| YS | 0/4 | 0/4 | 5/10 |
Cells cultured from 8.5 dpc P-Sp and YS cells of Ly-5.2+ mice were transplanted into Ly-5.1+mice, and 12 weeks later Ly-5.2+ B lymphocytes (B220+), T lymphocytes (Thy-1+), and myeloid cells (Gr-1+/Mac-1+) were analyzed by flow cytometry. The mice in which Ly-5.2+ cells made up more than 1% of all B220+, Thy-1+, and Gr-1+/Mac-1+ cells in PB were scored as reconstituted mice.
We also determined if generation of LTR-HSCs from early YS or P-Sp could be induced by other stromal cell lines, such as adult BM-derived MS-5 cells,31 OP9 cells established from newborn calvaria of the op/op mice that possess a mutation in the macrophage colony-stimulating factor (M-CSF) gene,32 or fibroblastoid stromal cells 3T3 cells. When 8.5 dpc P-Sp or YS cells (5 embryo equivalents) cocultured with these stromal cells for 6 days were respectively transplanted into 4 recipients, no mice showed successful engraftment.
Generation of LTR-HSCs from 8.0 dpc P-Sp and YS cocultured with AGM-S3 cells
The above results indicate that both 8.5 dpc P-Sp and YS have the potential to generate definitive LTR-HSCs in coculture with AGM-S3 cells. However, because the blood connection between YS and embryo has already been established at 8.5 dpc, precursors for the definitive LTR-HSCs may pass through the circulation from one site to another. We then examined the generation of LTR-HSCs from P-Sp and YS at 8.0 dpc, when YS and embryo are not yet connected by blood vessels. When 8.0 dpc P-Sp or YS cells (2 embryo equivalents) cocultured with AGM-S3 cells for 4 days were transplanted, 6 of 8 and 5 of 7 recipients had donor-derived myeloid and lymphoid cells in PB 10 weeks after transplantation (Table 5 and Figure6). Figure7 shows representative PB profiles of mice transplanted with cells cocultured from 8.0 dpc P-Sp (Figure 7A) and YS (Figure 7B) with AGM-S3 cells 12 weeks after transplantation. Both mice had donor-derived Gr-1/Mac-1+ myeloid cells, B220+ B cells, and Thy-1+ T cells, the chimerism of which was maintained for more than 6 months. Therefore, precursors capable of generating definitive LTR-HSCs appear independently in early YS and P-Sp.
Generation of LTR-HSCs from 8.0 dpc P-Sp and YS (2 embryo equivalents) in coculture with AGM-S3 cells
| Cell source . | No. of mice reconstituted/ transplanted . | % donor cells in reconstituted mice expressing . | |||
|---|---|---|---|---|---|
| B220 . | Thy-1 . | Gr-1/Mac-1 . | |||
| P-Sp | Before culture | 0/4 | NA | NA | NA |
| 4 days culture | 5/7 | 66.1 | 39.3 | 38.1 | |
| YS | Before culture | 0/4 | NA | NA | NA |
| 4 days culture | 6/8 | 58.3 | 32.3 | 47.3 | |
| Cell source . | No. of mice reconstituted/ transplanted . | % donor cells in reconstituted mice expressing . | |||
|---|---|---|---|---|---|
| B220 . | Thy-1 . | Gr-1/Mac-1 . | |||
| P-Sp | Before culture | 0/4 | NA | NA | NA |
| 4 days culture | 5/7 | 66.1 | 39.3 | 38.1 | |
| YS | Before culture | 0/4 | NA | NA | NA |
| 4 days culture | 6/8 | 58.3 | 32.3 | 47.3 | |
Cells isolated from 8.0 dpc P-Sp and YS cells of Ly-5.2 mice and their progenies were transplanted into Ly-5.1 mice, and 12 weeks later Ly-5.2+ B lymphocytes (B220+), T lymphocytes (Thy-1+), and myeloid cells (Gr-1+/Mac-1+) were analyzed by flow cytometry.
NA indicates not analyzed.
Hematopoietic reconstitution by 8.0 dpc P-Sp and YS cells cocultured with AGM-S3 cells.
The percentages of Ly-5.2+ cells in B220+ cells (B), Thy-1+ cells (T), and Gr-1/Mac-1+ cells (M) were analyzed in PB of Ly-5.1 recipients transplanted with 8.0 dpc P-Sp (A) and YS cells (B) cocultured with AGM-S3 cells (Table 5) 12 weeks after transplantation.
Hematopoietic reconstitution by 8.0 dpc P-Sp and YS cells cocultured with AGM-S3 cells.
The percentages of Ly-5.2+ cells in B220+ cells (B), Thy-1+ cells (T), and Gr-1/Mac-1+ cells (M) were analyzed in PB of Ly-5.1 recipients transplanted with 8.0 dpc P-Sp (A) and YS cells (B) cocultured with AGM-S3 cells (Table 5) 12 weeks after transplantation.
Expression of Ly-5.2 and Mac-1/Gr-1, B220, or Thy-1 on BM cells of Ly-5.1 recipients.
(A) A recipient engrafted with the cells cultured from 8.0 dpc P-Sp cells of Ly-5.2 mouse embryo on AGM-S3 cells for 6 days. (B) A recipient engrafted with the cells cultured from 8.0 dpc YS cells of Ly-5.2 mouse embryo on AGM-S3 cells for 6 days.
Expression of Ly-5.2 and Mac-1/Gr-1, B220, or Thy-1 on BM cells of Ly-5.1 recipients.
(A) A recipient engrafted with the cells cultured from 8.0 dpc P-Sp cells of Ly-5.2 mouse embryo on AGM-S3 cells for 6 days. (B) A recipient engrafted with the cells cultured from 8.0 dpc YS cells of Ly-5.2 mouse embryo on AGM-S3 cells for 6 days.
Discussion
The origin of mouse definitive HSCs
Whether mouse definitive HSCs originate from YS or intraembryonic regions has been controversial. In the present study, we found the generation of the definitive hematopoiesis from both early YS and intraembryonic P-Sp on AGM-S3 stromal cells. In the transplantation experiments using adult mice, the progeny of early YS and P-Sp had the potential of mutilineage reconstitution of definitive hematopoiesis in congeneic recipients, and the stable engraftment was evident for 6 months after transplantation. These findings indicate that both early YS and P-Sp can generate definitive HSCs with a similar repopulating potential by coculture with AGM-S3 stromal cells. In addition, the generation of definitive HSCs was noted even at 8.0 dpc, a time before blood vessels link YS and embryo. Hence, the precursors with the potential to generate definitive HSCs appear independently in the 2 sites, even if the precursors may move to another site.
However, the present result does not mean that the cells with the potential in both YS and P-Sp contribute to definitive hematopoiesis in vivo. Because the localization and ontogenetic stage are critical factors for development of embryonic hematopoiesis, it is unclear whether early YS and P-Sp can generate definitive HSCs in their circumstances in vivo. Engrafting experiments using a whole embryo culture might clarify the in vivo fate of the precursors with the potential to generate LTR-HSCs in coculture with AGM-S3 cells.
Environment inducive for generation of definitive HSCs
AGM-S3 stromal cells established from the 10.5 dpc AGM region induced definitive CFU-Ss and LTR-HSCs from 8.0 and 8.5 dpc P-Sp, but other stromal cells such as MS-5, OP9, and 3T3 cells could not do so. This finding indicates that AGM-S3 cells specifically express the molecule(s) capable of supporting the generation of CFU-Ss and LTR-HSCs. Our previous study showed that AGM-S3 cells express SCF, IL-11, and oncostatin M,21 but any combinations of these cytokines did not induce the generation of CFU-Ss or LTR-HSCs from early P-Sp (data not shown). Yet to be identified molecule(s) may be responsible for the generation of CFU-Ss or LTR-HSCs. However, 8.0 or 8.5 dpc P-Sp seems to take a longer time to generate CFU-S and LTR-HSCs in cocultures with AGM-S3 cells than is required in vivo. Molecules expressed on AGM-S3 cells may not completely reconstitute the hematopoietic microenvironment of the AGM region for generation of CFU-Ss and LTR-HSCs.
The present observation that definitive CFCs were not evident even on day 10 of culture despite the appearance of CFU-Ss and LTR-HSCs on day 4 indicates that AGM-S3 cells could not support the differentiation of CFCs from LTR-HSCs or CFU-Ss generated from P-Sp. In the developing mouse embryo, CFU-Ss appear in the AGM region and simultaneously in YS at late 9 dpc, and LTR-HSCs are detected in the AGM region at 10 dpc, while no hematopoietic clusters or blood islands indicative of the differentiation or maturation of hematopoietic cells are found in AGM region.11 Thus, the AGM region plays a specific role in the development of embryonic hematopoiesis. The hematopoietic activity of AGM-S3 cells on the generation of CFU-Ss and LTR-HSCs, but not CFCs, from P-Sp is in accord with the specific function of the AGM region as a hematopoietic tissue. In this context, it is of interest that numerous macrophages were generated in the coculture with AGM-S3 cells from early YS and P-Sp, although they do not express M-CSF.21 It may be that these macrophages originate from primitive hematopoiesis and are regulated by mechanisms differing from these related macrophages in definitive hematopoiesis.33
AGM-S3 cells also supported the generation of CFU-Ss and LTR-HSCs from early YS. In contrast to our result, Medvinsky et al11showed that when 10 dpc YS or P-Sp explants were cultured for 3 days in isolation, only P-Sp generated CFU-Ss and LTR-HSCs. Because the explants are considered to include various cells surrounding the precursors for CFU-Ss and LTR-HSCs, the discrepancy between our result and theirs reflects differences in the function of the microenvironment in YS and P-Sp. The AGM region may provide the specific microenvironment for the generation of CFU-Ss and LTR-HSCs from their precursors.
Precursors for definitive LTR-HSCs and P-Sp
When cells isolated from early YS and P-Sp were cocultured with AGM-S3 cells, they generated CFU-Ss and LTR-HSCs with a similar time course. The similar hematopoietic activity of early YS and P-Sp suggests that the precursors in both sites resemble each other, albeit being generated independently. Yoder et al14 reported the presence of the cells capable of repopulating in conditioned neonatal but not adult mice in 9.0 dpc YS and P-Sp. They showed that YS contained a larger number of neonate-repopulating cells than P-Sp at 9.0 dpc, thereby suggesting that cells in YS arise autonomously of those in P-Sp. Precursors that generated adult-repopulating cells on AGM-S3 cells may also produce the neonate-repopulating cells in vivo.
The order of appearance of hematopoietic activities observed during early mouse embryogenesis is reserved compared with the events that occur during adult hematopoiesis.34 In the developing mouse embryo, CFU-S activity is first detected at late 9 dpc, whereas stem cell activity is not found until late 10 dpc. In the present study, CFU-Ss and LTR-HSCs were generated from early YS and P-Sp on day 4 of coculture with AGM-S3 cells. One possibility is that CFU-Ss are the progeny of LTR-HSCs generated from early YS and P-Sp, although we detected no time lag of their appearance because of the difference of the sensitivity of assay systems used for CFU-Ss and LTR-HSCs. Another possibility is that CFU-Ss and LTR-HSCs are independently generated. In this case, there are 2 other possibilities: Firstly, CFU-Ss and LTR-HSCs are derived from the same progenitors and, secondly, have different ancestors. Use of single cells isolated by various markers such as c-Kit, CD34,23Sca-1,35 or VE-cadherin36 may answer these questions.
Supported by the Program for Promotion of Fundamental Studies in Health Science of the Organization for Pharmaceutical Safety and Research of Japan and, in part, by research funding from Kirin Brewery (T.N.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Kohichiro Tsuji, Dept of Clinical Oncology, Institute of Medical Science, University of Tokyo, 4-6-1 Shirokanedai, Minato-ku, Tokyo 108-8639, Japan; e-mail: tsujik@ims.u-tokyo.ac.jp.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal