Abstract
Previous studies have suggested that the level of residual disease at the end of therapy predicts outcome in chronic lymphocytic leukemia (CLL). However, available methods for detecting CLL cells are either insensitive or not routinely applicable. A flow cytometric assay was developed that can differentiate CLL cells from normal B cells on the basis of their CD19/CD5/CD20/CD79b expression. The assay is rapid and can detect one CLL cell in 104 to 105leukocytes in all patients. We have compared this assay to conventional assessment in 104 patients treated with CAMPATH-1H and/or autologous transplant. During CAMPATH-1H therapy, circulating CLL cells were rapidly depleted in responding patients, but remained detectable in nonresponders. Patients with more than 0.01 × 109/L circulating CLL cells always had significant (> 5%) marrow disease, and blood monitoring could be used to time marrow assessments. In 25 out of 104 patients achieving complete remission by National Cancer Institute (NCI) criteria, the detection of residual bone marrow disease at more than 0.05% of leukocytes in 6 out of 25 patients predicted significantly poorer event-free (P = .0001) and overall survival (P = .007). CLL cells are detectable at a median of 15.8 months (range, 5.5-41.8) posttreatment in 9 out of 18 evaluable patients with less than 0.05% CLL cells at end of treatment. All patients with detectable disease have progressively increasing disease levels on follow-up. The use of sensitive techniques, such as the flow assay described here, allow accurate quantitation of disease levels and provide an accurate method for guiding therapy and predicting outcome. These results suggest that the eradication of detectable disease may lead to improved survival and should be tested in future studies.
Introduction
The goal of conventional therapy for chronic lymphocytic leukemia (CLL) is to control the disease and rarely results in the complete eradication of detectable tumor cells.1,2Even purine analogue therapy, which is the most effective conventional therapy for inducing complete remission,3 results in a return of normal polyclonal B lymphocytes in only 40% of patients.4 This is the principle reason why the assessment of minimal residual disease (MRD) in CLL has not been considered to be important and why the criteria for response to therapy have defined “complete” remissions when there is likely to be a significant level of disease detectable by modern techniques.5 The application of novel therapies, such as allogeneic or autologous hematopoietic stem cell transplantation6 and monoclonal antibodies,7,8 have resulted in a significant proportion of patients attaining much more profound responses. There is evidence to suggest that such responses may be associated with improved outcomes.9 10 The current development of newer agents, the potential of immunomodulatory therapies, and the possible combination of 2 or more of these therapies promises to make the goal of eradicating detectable disease by the most sensitive techniques a realistic one.
CAMPATH-1H (alemtuzumab) is a humanized monoclonal antibody specific for the CD52 antigen. The antigen is expressed on all lymphocytes as well as monocytes, macrophages, and spermatozoa.11,12CAMPATH-1H is extremely effective at killing lymphocytes in vitro and in vivo.11,12 The humanization of the antibody has abrogated potential human antimurine antibody (HAMA) responses13-15 and resulted in an agent that is extremely effective at inducing remission in patients with CLL.8,16Similarly, treatment with autologous transplantation has been shown to induce complete remission in a large proportion of patients.6
Because such therapeutic strategies result in a profound depletion of CLL cells, effective monitoring of both response and outcome requires more sensitive methods for the detection of low-level disease. The 2 most effective methods currently used for the detection of MRD in CLL utilize either flow cytometry or the polymerase chain reaction (PCR). The majority of flow cytometric analyses depend on the detection of an excess of CLL cells (> 25% of CD19+ cells being CD5+) and monoclonality of light chain expression.10,17-19 Although these techniques are more sensitive than a morphologic assessment, they are hampered by the presence of normal B cells. Upper limits for detection of residual CLL cells have been set according to the normal range for CD5 expression; however, up to 90% of normal B lymphocytes express CD5 in the months following transplantation in patients without B-cell malignancies.20 Conventional flow cytometry is therefore only suitable when the majority of B lymphocytes are neoplastic. More recent techniques have used the differential expression of antigens on CLL cells in comparison with normal B cells, such as CD79b,21 or CD20.22 However, such flow cytometric techniques are only informative in a proportion of patients, because of the interpatient variation in antigen expression.
Although PCR techniques have generally been considered to be more sensitive than flow cytometric analysis, they can only be applied to approximately 70% to 80% of patients, as mutations in the immunoglobulin-H (IgH) gene can abrogate binding of consensus primers. The use of consensus primers alone to demonstrate clonality is limited by the presence of normal leukocytes, such that there is a maximum sensitivity of 1 in 104. In common with conventional flow cytometric analysis, the detection of CLL cells is also limited by the presence of normal B cells, such that CLL cells may be detected when they represent more than 2% of total B cells.23-25
The interference of normal polyclonal B cells can be overcome by generating primers that are specific to each individual patient (allele-specific oligonucleotide [ASO]–PCR) which are able to detect a single CLL cell in 106 normal cells.26 Unfortunately, ASO-PCR is extremely labor intensive and very expensive. In addition, standard ASO-PCR is not quantitative and the results are usually not immediately available. Thus ASO-PCR is not ideally suited for the monitoring and guiding of therapy. ASO-PCR combined with “real-time” PCR is quantitative but has a reduced sensitivity detecting a single CLL cell in up to 105 normal leukocytes.27
Major recent advances in flow cytometry, in particular the development of sequential gating strategies; the improvement in flow cytometer technology; and the use of multicolor fluorescence permits the analysis of extremely large numbers of cells and the subsequent identification of very small populations of cells. This paper describes the development of a flow cytometry technique able to detect a single CLL cell in 105 normal cells (MRD Flow). The technique is quantitative, applicable to all patients with CLL, not affected by the presence of polyclonal B cells, and can be used in either blood or bone marrow. We also describe its application in the monitoring of treatment and prediction of outcome in patients with CLL undergoing therapy with CAMPATH-1H and/or autologous stem cell transplantation.
Patients, materials, and methods
Patients
The MRD flow cytometry assay developed in this study has been applied to 298 bone marrow aspirate and 986 peripheral blood samples from 104 patients (85 male, 19 female; median age, 59; range, 33-73 years) with CLL. Informed consent was obtained from all patients. Trephine biopsies were analyzed at the Haematological Malignancy Diagnostic Service (HMDS) in parallel with the aspirate sample in 103 cases. In 37 sequential marrow aspirate samples, PCR analysis was performed in parallel with flow cytometric analysis. We report on 25 patients with CLL who achieved a complete remission by National Cancer Institute (NCI) criteria to any therapy and have been monitored by MRD flow cytometry. These comprise 2 groups: (1) 7 previously untreated patients received fludarabine followed by autologous peripheral blood stem cell transplantation (PBSCT) in first remission; (2) 18 patients previously treated with multiple therapies (median 3, range, 1-4), including fludarabine in all cases, received CAMPATH-1H to maximal response either followed by an autologous PBSCT (8 patients) or no further therapy (10 patients).
Responses are reported according to the NCI criteria for response5 except complete remission (CR), which is separated into 2 categories: MRD+ for patients achieving the criteria for CR but with CLL cells representing more than 0.05% of bone marrow leukocytes, and MRD− for CR patients with less than 0.05% CLL cells in the bone marrow.
Sample preparation
Leukocytes were prepared from marrow aspirates by ammonium chloride lysis. Trephine biopsies were fixed in formalin and processed to methyl methacrylate resin as reported previously.28
Flow cytometry
A minimum of 0.5 × 106 leukocytes (1.0 × 106 for dilution studies) was incubated with 5μL of each pretitred antibody per 500 000 cells for 20 minutes at 4°C, washed twice, and acquired using a Becton Dickinson (Oxford, United Kingdom) FACSort with CELLQuest v3.1 software. Between 50 000 and 800 000 total cells were analyzed in each test. In all cases, B cells were identified using a sequential gating strategy, setting a region on CD19 (HMDS) versus side scatter (SSC), followed by a light scatter region, and then ensuring that no CD3+ events fell within the combined gate. Dilution studies of B cells into K562 cells showed that this strategy allows detection of one B cell in up to 105 leukocytes with less than 10% coefficient of variation when 5 × 106 total cells were analyzed (data not shown). Antibodies used were CD5, CD10, CD11a, CD23, CD38, kappa and lambda (in-house conjugates), CD20, CD79b (Immunotech, Marseilles, France), FMC7 (Chemicon International, Harrow, United Kingdom), CD22 (Becton Dickinson), and CD79a (Serotec, Oxford, United Kingdom).
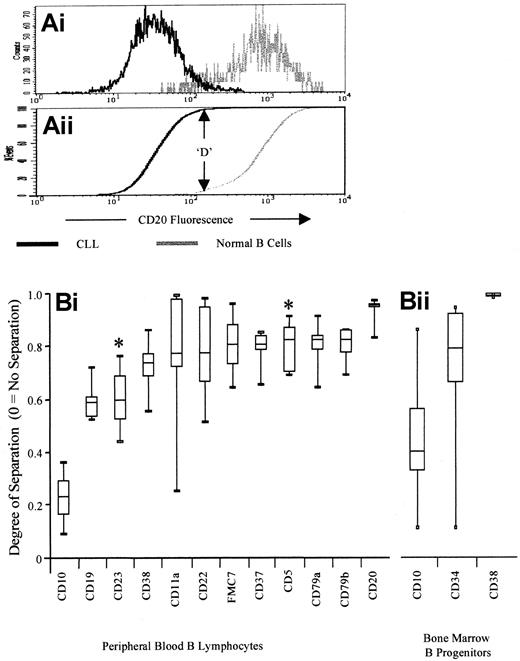
To allow measurement of the difference in antigen expression between neoplastic and normal cells while excluding the variation between antibodies, the Kolmogorov-Smirnov analysis (K-S analysis) was used.29 This calculates the cumulative proportion at which the maximum difference between 2 histograms occurs (ie, for completely separated histograms, D = 1, whereas for completely overlapping histograms, D = 0) (Figure 1). K-S analysis was applied to histograms of B cells from each CLL (n = 5) with each normal case for each antibody (ie, 25 tests per antibody). The D value was calculated for each test, and these were ranked according to median values and then by minimum value.
Kolmogorov-Smirnov analysis for identification of antibodies that differentiate between normal and neoplastic B cells.
(A) Kolmogorov-Smirnov analysis was used to identify the degree of separation provided by the panel of antigens assessed. Briefly, the cumulative fluorescence intensity of a particular antigen is plotted for both normal B cells and CLL cells. The D value is the maximum difference between these 2 distributions (1 = complete separation, 0 = complete overlap), and is used as a simple measure of the degree of difference in expression. The plot demonstrates the low level of overlap between normal and neoplastic CD20 expression, in spite of the fact that both antigens are “positive” compared with isotype control. (B) Antibodies for optimal separation of CLL cells from normal B cells. The figure shows the degree of separation (K-S D value) for a panel of antibodies assessed on normal B cells and on presentation CLL cells. Values shown are the 5th, 25th, median, 75th, and 95th percentiles, and antibodies are ranked according to median D value (worst separation on the left, best separation on the right). Antigens that are expressed at a higher level by CLL cells than by normal B cells have an asterisk above (CD5 and CD23), whereas those expressed at a lower level by CLL cells are not marked. Peripheral blood antigen expression is shown in (i)—CD10 is expressed by neither normal peripheral blood B cells nor by CLL cells, and this demonstrates the differences due to autofluorescence. Bone marrow antigen expression is shown in (ii).
Kolmogorov-Smirnov analysis for identification of antibodies that differentiate between normal and neoplastic B cells.
(A) Kolmogorov-Smirnov analysis was used to identify the degree of separation provided by the panel of antigens assessed. Briefly, the cumulative fluorescence intensity of a particular antigen is plotted for both normal B cells and CLL cells. The D value is the maximum difference between these 2 distributions (1 = complete separation, 0 = complete overlap), and is used as a simple measure of the degree of difference in expression. The plot demonstrates the low level of overlap between normal and neoplastic CD20 expression, in spite of the fact that both antigens are “positive” compared with isotype control. (B) Antibodies for optimal separation of CLL cells from normal B cells. The figure shows the degree of separation (K-S D value) for a panel of antibodies assessed on normal B cells and on presentation CLL cells. Values shown are the 5th, 25th, median, 75th, and 95th percentiles, and antibodies are ranked according to median D value (worst separation on the left, best separation on the right). Antigens that are expressed at a higher level by CLL cells than by normal B cells have an asterisk above (CD5 and CD23), whereas those expressed at a lower level by CLL cells are not marked. Peripheral blood antigen expression is shown in (i)—CD10 is expressed by neither normal peripheral blood B cells nor by CLL cells, and this demonstrates the differences due to autofluorescence. Bone marrow antigen expression is shown in (ii).
Dilution studies
Calibrite beads (Becton Dickinson) were used to allow accurate determination of dilution for sensitivity assessment. Target cells were mixed with an approximately tenfold excess of fluorescein isothiocyanate (FITC) beads, and diluent cells mixed with an approximately equivalent number of phycoerythrin (PE) beads, and the cell-to-bead ratio was calculated for both prior to dilution. Target cells were then diluted into diluent cells, and acquired as above. The excess of FITC beads allows an accurate calculation of actual target to diluent cell ratio, even at high dilutions, and independent of pipetting errors.
IgH PCR analysis
DNA was extracted and amplified as reported previously23 with a 5′ FITC-labeled JH consensus primer and a FR3 consensus primer. Products were then electrophoresed and analyzed using an ABI automated DNA sequencer model 373A (Applied Biosystems, Cheshire, United Kingdom).
Immunohistochemistry
Sections (3 μm) were stained with MGG, H&E, or CD20. For CD20 staining, 3 μm sections underwent antigen retrieval by microwaving in citrate buffer, (pH 6.0, 8 minutes irradiation, 5 minutes standing, 3 minutes irradiation, then 20 minutes standing). Sections were incubated in CD20 (Dako, Ely, Cambridgeshire, United Kingdom) for more than 1hour, and visualized using a Streptavidin Biotin technique (Dako-Duet K492), with 3,3′ diaminobenzidine (Sigma), and counterstained with Harris Haematoxylin.
Results
Identifying antibodies that can distinguish CLL cells from normal B cells
In order to detect CLL cells in a minimal disease setting, it is necessary to identify the antibodies that provide complete separation of CLL cells from normal B cells in all patients. Therefore, the expression of a range of antibodies (CD5, CD10, CD11a, CD20, CD22, CD23, CD38, CD79a, CD79b, FMC7, kappa, and lambda) was assessed on presentation CLL cells from 10 patients, and on peripheral blood B lymphocytes from 5 healthy individuals. To allow measurement of the difference between neoplastic and normal cells while excluding the variation between antibodies, Kolmogorov-Smirnov analysis was used. Each antibody showed some degree of overlap between the levels of expression by normal and by neoplastic cells. However, the greatest separation (D value closest to 1) was found for CD20, CD79a/CD79b, and CD5 respectively (Figure 1). These antibodies were unsuitable for bone marrow assessment as B-cell progenitors, which comprise up to 90% of B cells in posttransplant or post-CAMPATH-1H patients, have weak or no expression of CD20 and CD79. We therefore studied 3 antigens—CD34, CD10, and CD38—that are expressed during the early stages of B-cell differentiation, but are weak or negative on presentation CLL cells. Separation of CLL cells from normal B progenitors was again assessed using Kolmogorov-Smirnov analysis, and CD38 provided significantly better separation than CD34 or CD10 (n = 35, P < .0001, Wilcoxon signed rank test).
Determination of antibody combinations that provide resolution of CLL cells from B cells in peripheral blood and bone marrow
Because single antigens were not sufficient to allow discrimination of normal B cells from CLL cells, it was necessary to identify combinations of antibodies that would reproducibly separate these populations. The ability of antibody combinations to resolve CLL cells from normal B cells was assessed by setting regions around the CLL cells identified by 2 markers (eg, CD5 vs CD79b, CD38 vs CD79b, etc) such that the regions contained more than 95% neoplastic cells. The regions were then applied to normal samples, and the percentage of B cells that fell within each region was calculated. If less than 2% of normal B lymphocytes fell within a particular region, that region was considered adequate to resolve CLL cells from normal cells for that patient. This was performed for 17 sets of CLL cells against peripheral blood B cells from 5 healthy individuals, and bone marrow B cells from 3 healthy individuals.
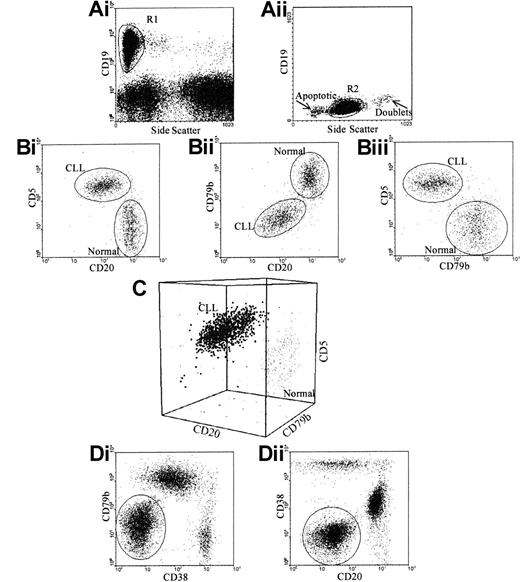
In peripheral blood, a combination of CD19, CD5, CD20, and CD79b was sufficient and necessary to discriminate CLL cells from normal B cells in all the cases tested. In bone marrow, a combination of CD19/5/20/79b was effective in 14 out of 17 cases, CD19/5/38/79 in 14 out of 17 cases and CD19/5/38/20 in 11 out of 17 cases. Of these 17 patients, 8 showed CD38 expression according to the criteria reported previously.30 In all of these cases, there was no overlap between CLL cells and B progenitors, as the latter express very high levels of CD38. Using all 3 combinations allowed discrimination of CLL cells from normal B cells in all cases (Figure2).
Gating strategy for specific identification of CLL cells.
(A) Total B cells were identified using 2 regions: (i) CD19 and side scatter, to exclude granular cells showing nonspecific CD19 binding; and (ii) forward and side scatter, to exclude apoptotic cells and doublets. (B) CLL cells were separated from normal B cells according to their CD5, CD20, and CD79b. CLL cells could be separated from normal B cells using (i) CD5 versus CD20 in 82% of cases; (ii) CD79b versus CD20 in 35% of cases; and (iii) CD5 versus CD79b in 88% of cases. (C) To separate CLL cells from normal B cells, all 3 antigens must be assessed on gated B cells. (D) To separate CLL cells from normal B cells and normal B progenitors in the bone marrow in all cases, CD38 is included; this requires 2 tests: (i) CD19 versus CD5 versus CD38 versus CD20; and (ii) CD19 versus CD5 versus CD38 versus CD79b.
Gating strategy for specific identification of CLL cells.
(A) Total B cells were identified using 2 regions: (i) CD19 and side scatter, to exclude granular cells showing nonspecific CD19 binding; and (ii) forward and side scatter, to exclude apoptotic cells and doublets. (B) CLL cells were separated from normal B cells according to their CD5, CD20, and CD79b. CLL cells could be separated from normal B cells using (i) CD5 versus CD20 in 82% of cases; (ii) CD79b versus CD20 in 35% of cases; and (iii) CD5 versus CD79b in 88% of cases. (C) To separate CLL cells from normal B cells, all 3 antigens must be assessed on gated B cells. (D) To separate CLL cells from normal B cells and normal B progenitors in the bone marrow in all cases, CD38 is included; this requires 2 tests: (i) CD19 versus CD5 versus CD38 versus CD20; and (ii) CD19 versus CD5 versus CD38 versus CD79b.
Specificity and sensitivity of MRD Flow compared with conventional flow cytometry and IgH-PCR
Serial dilution studies were performed to determine the specificity and sensitivity of the MRD Flow assay. CLL cells from 3 patients were mixed with normal leukocytes in serial 4-fold dilutions from 1:1 to 1:16 384. Three aliquots, each of one million cells, were prepared: one was stained with CD19/CD5/CD20/CD79b, one with CD19/CD5/kappa/lambda, and IgH-PCR was performed on DNA extracted from the remaining aliquot.
In addition to the PCR results, the detection of CLL cells was compared using 4 different flow cytometric gating strategies. These were (1) 2-color analysis of CD19 and CD5 coexpression, in which CLL cells are considered to be present if more than 25% of CD19+ B cells coexpress CD517-19; (2) 3-color analysis of light chain restriction by CD19+ cells, in which CLL cells are considered to be present if more than 75% of B cells express kappa or lambda; (3) 4-color analysis of light chain restriction within the CD19+CD5+ fraction, in which CLL cells are considered to be present if more than 75% of CD5+ B cells express kappa or lambda; and (4) 4-color MRD Flow analysis as described above.
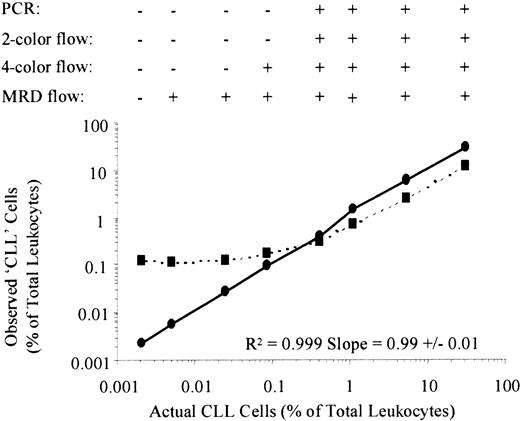
The results are shown in Table 1, with the dilution curves shown in Figure 3. Analyzing light chain expression simultaneously with CD19 and CD5 coexpression results in a modest improvement in sensitivity over analysis of CD19 and CD5 alone. However, the use of the 4-color MRD Flow technique results in a 2-log increase in sensitivity in comparison with conventional 4-color analysis, despite identical methods for identification of total B cells. This was because the MRD Flow assay has a much higher specificity for identification of CLL cells than conventional flow methods. The conventional assay could detect an abnormal kappa:lambda ratio in the CD5+ B cells when CLL cells represented approximately 30% of B cells, whereas MRD Flow could consistently detect CLL cells when they represented more than 1% of B cells. Enumeration of CLL cells by conventional techniques became increasingly inaccurate at the limits of detection because of the number of normal CD19+5+ B cells present. Thus the numbers of CLL cells observed using conventional flow were between 2- and 60-fold greater than the actual numbers (ie, 100%-6000% error). In contrast, the error between observed and actual CLL cell numbers identified by MRD Flow was on average less than 25%, and always less than 50% even at the limits of detection.
MRD Flow provides at least one log greater sensitivity and specificity of detection of CLL cells than conventional flow cytometry and consensus-primer PCR
| Detection of CLL cells . | Conventional flow cytometry . | PCR . | MRD Flow . | |
|---|---|---|---|---|
| 2-Color CD19CD5 . | 4-Color CD19/CD5 κ/λ . | Fluorescent consensus Fr3 primer . | 4-Color CD19/CD5 CD20/CD79b . | |
| Percentage of leukocytes (range) | 1.2 (0.4-2.6) | 0.4 (0.3-0.7) | 0.6 (0.4-0.7) | 0.005 (0.003-0.008) |
| Percentage of total B cells (range) | 35 (33-71) | 33 (12-71) | 52 (33-71) | 0.65 (0.47-1.62) |
| Detection of CLL cells . | Conventional flow cytometry . | PCR . | MRD Flow . | |
|---|---|---|---|---|
| 2-Color CD19CD5 . | 4-Color CD19/CD5 κ/λ . | Fluorescent consensus Fr3 primer . | 4-Color CD19/CD5 CD20/CD79b . | |
| Percentage of leukocytes (range) | 1.2 (0.4-2.6) | 0.4 (0.3-0.7) | 0.6 (0.4-0.7) | 0.005 (0.003-0.008) |
| Percentage of total B cells (range) | 35 (33-71) | 33 (12-71) | 52 (33-71) | 0.65 (0.47-1.62) |
Date shown is the median, maximum, and minimum number of CLL cells as a percentage of total leukocytes (sensitivity) or of total B lymphocytes (specificity) at which each of the assays showed a “positive” result (ie, could identify the presence of CLL cells in the polyclonal background). Criteria for detection of CLL cells are described in the Figure 3 legend.
Dilution studies demonstrating increased sensitivity of MRD Flow compared with conventional flow cytometry.
Peripheral blood leukocytes from 3 patients were serially diluted (4-fold dilutions from 1:1 to 1:16 348) into normal peripheral blood leukocytes pooled from 3 healthy individuals. Figure 3 is a representative plot from one experiment, showing the percentage of CLL cells identified by conventional 4-color flow cytometry (CD19 + 5+kappa+ or CD19 + 5+lambda+) in dotted lines, and percentage of CLL cells identified by MRD Flow (CD19/CD5/CD20/CD79b) in solid lines, against actual CLL cell percentage. At the top of the graph, results are shown as positive (+) or negative (−) for the presence of CLL cells at each dilution for the different methods of assessing residual disease. Criteria for detection of CLL cells were as follows: (1) IgH-PCR: there is at least 2-fold greater PCR product with the same size as the CLL cell rearrangement product than would be expected for a normal distribution23,31; (2) conventional 2-color flow: over 25% of the B cells coexpress CD517-19; (3) conventional 4-color flow: light chain restriction within the CD5+ B-cell compartment; and (4) MRD Flow: the number of gated B cells with an aberrant phenotype is at least 2-fold greater than background (ie, the number generated by the same gating strategy applied to a sample containing normal leukocytes only). R2values from linear regression analysis of MRD Flow results for observed versus actual percentages of CLL cells are shown at the bottom right of the graph, demonstrating linearity over the series.
Dilution studies demonstrating increased sensitivity of MRD Flow compared with conventional flow cytometry.
Peripheral blood leukocytes from 3 patients were serially diluted (4-fold dilutions from 1:1 to 1:16 348) into normal peripheral blood leukocytes pooled from 3 healthy individuals. Figure 3 is a representative plot from one experiment, showing the percentage of CLL cells identified by conventional 4-color flow cytometry (CD19 + 5+kappa+ or CD19 + 5+lambda+) in dotted lines, and percentage of CLL cells identified by MRD Flow (CD19/CD5/CD20/CD79b) in solid lines, against actual CLL cell percentage. At the top of the graph, results are shown as positive (+) or negative (−) for the presence of CLL cells at each dilution for the different methods of assessing residual disease. Criteria for detection of CLL cells were as follows: (1) IgH-PCR: there is at least 2-fold greater PCR product with the same size as the CLL cell rearrangement product than would be expected for a normal distribution23,31; (2) conventional 2-color flow: over 25% of the B cells coexpress CD517-19; (3) conventional 4-color flow: light chain restriction within the CD5+ B-cell compartment; and (4) MRD Flow: the number of gated B cells with an aberrant phenotype is at least 2-fold greater than background (ie, the number generated by the same gating strategy applied to a sample containing normal leukocytes only). R2values from linear regression analysis of MRD Flow results for observed versus actual percentages of CLL cells are shown at the bottom right of the graph, demonstrating linearity over the series.
MRD Flow was also at least 2 logs more sensitive for the detection of CLL cells than Fr3-primer IgH-PCR. Although we have previously demonstrated that this test can detect CLL cells when they represent as few as 2% of B cells, the primers also bind germline sequences in non-B cells. Although these sequences do not undergo logarithmic amplification during the PCR reaction, their presence limits the sensitivity of detection. Thus the sensitivity of consensus-primer IgH-PCR was equivalent to that of conventional flow in the presence of excess leukocytes, and the test could not detect CLL cells when they represented less than 0.4% of leukocytes. In bone marrow aspirate samples from 37 patients, CLL cells were detected by MRD Flow cytometry in 22% (8/37) PCR-negative samples, whereas all 29 PCR-positive samples also had detectable CLL cells by MRD Flow. There were no samples in which CLL cells were detected by IgH-PCR but not by MRD Flow. In keeping with the dilution study results, PCR-negative samples contained less than 0.2% CLL cells, which were readily identified by MRD Flow. Thus MRD Flow is more sensitive than previously identified flow cytometry assays as well as consensus Fr3 primer IgH-PCR. CLL cells were reproducibly detected when they represented 0.01% of leukocytes or 2% of B cells.
Detection of CLL cells in patient material: comparison with trephine biopsies
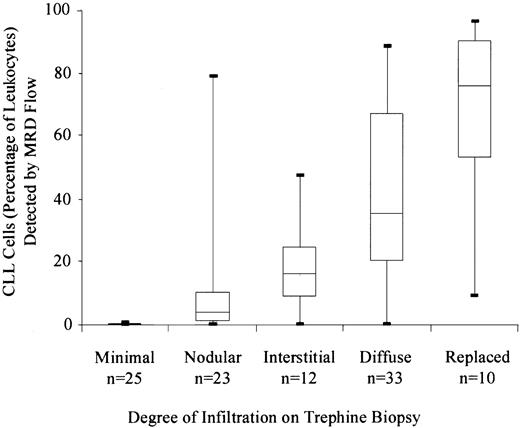
Detection of CLL cells by MRD Flow was compared with morphologic assessment of trephine biopsy in 103 samples. In general, there was good correlation between the extent of infiltration on the trephine biopsy and the percentage of CLL cells detected by MRD Flow (Figure4). CLL cells were detected by MRD Flow (ie, > 0.05% of leukocytes) in 32% (8/25) of samples with no morphologic evidence of tumor.
Correlation between the detection of CLL cells by flow cytometry and the degree of infiltration assessed on CD20-stained trephine biopsy.
The figure shows the CLL cell number (as percentage of total leukocytes), categorized according to the degree of infiltration on the trephine biopsy.
Correlation between the detection of CLL cells by flow cytometry and the degree of infiltration assessed on CD20-stained trephine biopsy.
The figure shows the CLL cell number (as percentage of total leukocytes), categorized according to the degree of infiltration on the trephine biopsy.
The potential for sampling error in aspiration—usually by contamination with peripheral blood—is a particular concern for all techniques assessing residual disease levels. Of 77 samples with morphologically detectable disease on the trephine biopsy, 5 out of 77 (4.9% of all 102 paired samples assessed) were negative by MRD Flow. In 4 of the 5 cases, B cells were detected by flow cytometry. Three of these cases had very low level disease morphologically, but one had focal areas of diffuse infiltration and one had an extensive diffuse infiltration. In these cases the majority of B cells detected by flow cytometry had a progenitor cell phenotype (strong CD38 expression, sIg−). This indicates that the aspirated samples were not heavily blood contaminated, suggesting that the differences were due to sampling error. There were no samples for which the PCR identified CLL cells but MRD Flow did not, presumably because the same sample was used to generate results. In rare samples with discrepant trephine and flow results, it is necessary to confirm that the B cells detectable on the trephine are CLL cells, and not regenerating normal B cells; a further bone marrow assessment may resolve the discrepancy.
Monitoring disease levels: comparison between peripheral blood and bone marrow during and after therapy
Patients undergoing CAMPATH-1H therapy show clearance of peripheral blood disease even if bone marrow infiltration or lymph node size are not altered.16 More sensitive analysis of the peripheral blood demonstrates that in the majority of patients, circulating CLL cells levels fall to below 0.01 × 109/L. This includes patients who show little or no alteration in the level of bone marrow disease: 82% (36/44) of samples with over 30% marrow infiltration had less than 0.01 × 109/L circulating CLL cells. Therefore, for guiding therapy, a bone marrow assessment may not be indicated until the circulating CLL count drops below 0.01 × 109/L.
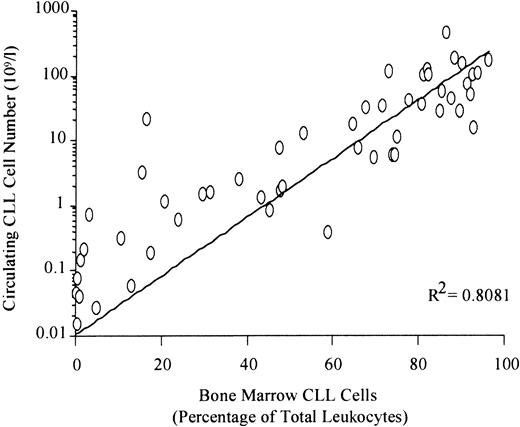
In contrast, there was a direct correlation between circulating and bone marrow disease levels in patients more than 3 months after therapy (Figure 5). If the peripheral count was more than 1.0 × 109/L, virtually all patients (33/35) had more than 30% bone marrow CLL cells. This suggests that a peripheral count above 1.0 × 109/L may provide an optimal time point to consider bone marrow examination and possible retreatment. In 14 patients with fewer than 0.01 × 109/L circulating CLL cells at least 3 months after treatment, only 2 patients had more than 0.05% bone marrow CLL cells (0.11% and 0.12% of total leukocytes respectively). Thus, the level of peripheral disease in patients more than 3 months after treatment can provide a good indication of the degree of bone marrow involvement.
Direct relationship between the absolute number of circulating CLL cells and the degree of bone marrow infiltration in patients more than 3 months after treatment.
Paired peripheral blood and bone marrow samples were analyzed by the MRD Flow assay in 66 samples from patients who had not received treatment for at least 3 months. Linear regression analysis demonstrated a direct relationship between blood and marrow levels in this setting (P < .0001).
Direct relationship between the absolute number of circulating CLL cells and the degree of bone marrow infiltration in patients more than 3 months after treatment.
Paired peripheral blood and bone marrow samples were analyzed by the MRD Flow assay in 66 samples from patients who had not received treatment for at least 3 months. Linear regression analysis demonstrated a direct relationship between blood and marrow levels in this setting (P < .0001).
Predicting outcome according to residual disease levels
The MRD Flow assay provides one of the most sensitive methods of assessing disease levels in patients with CLL. However, it is not clear whether the application of a sensitive residual disease assay can provide a more accurate prediction of outcome than the current NCI criteria. In order to assess this, we have compared the event-free and overall survival for patients achieving a complete remission by NCI criteria after CAMPATH-1H and/or autologous transplantation with or without detectable residual disease. Patients were defined as MRD+ if CLL cells represented more than 0.05% of bone marrow leukocytes at 3 months after treatment, or MRD− if there were less than 0.05% CLL cells present. Of 7 previously untreated patients receiving fludarabine and autologous PBSCT, all became MRD− after receiving a transplant. Of 10 pretreated patients receiving CAMPATH-1H alone, 8 out of 10 became MRD− whereas 2 out of 10 had detectable disease at the end of therapy. Of 8 pretreated patients receiving CAMPATH-1H followed by autologous PBSCT, 4 out of 8 became MRD− whereas the remaining 4 out of 8 had detectable disease at end of therapy.
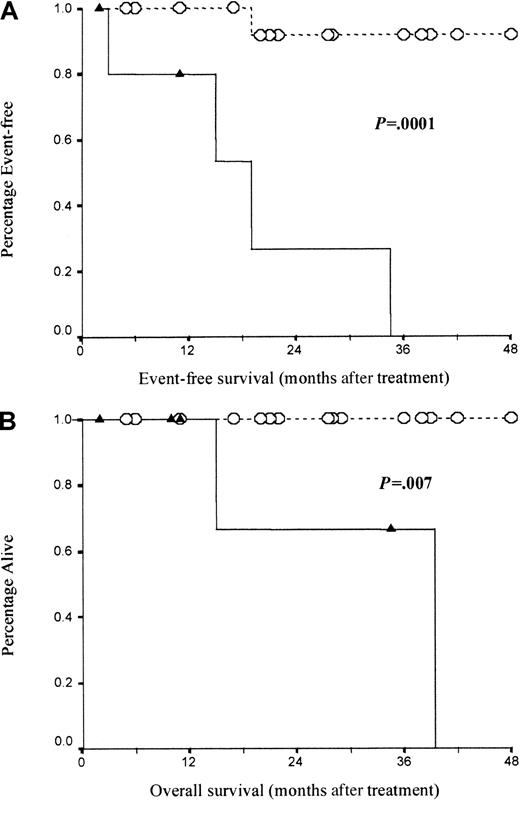
Overall, 19 (76%) out of 25 were MRD−, whereas the remaining 6 (24%) out of 25 were MRD+. Survival curves are shown in Figure 6. Event-free survival was significantly poorer (median 19 months) for the 6 MRD+patients than for those with less than 0.05% marrow CLL cells: only 1 out of 21 MRD− patients required further therapy with a median follow-up of 22 months (log rank P = .0001). Similarly, overall survival was significantly shorter for MRD+ patients (median, 39 months) than for MRD− patients who all remained alive with a median follow-up of 22 months (range, 5 to 48 months) (log rankP = .007).
Patients in complete remission but with residual disease detectable by MRD Flow have significantly poorer event-free and overall survival than those with no detectable CLL cells.
(A) Event-free survival for MRD− patients (n = 19, dotted line with open circles) compared with MRD+ patients (n = 6, solid lines with solid triangles). (B) Overall survival for the same patient group. Survival was compared using log-rank analysis.
Patients in complete remission but with residual disease detectable by MRD Flow have significantly poorer event-free and overall survival than those with no detectable CLL cells.
(A) Event-free survival for MRD− patients (n = 19, dotted line with open circles) compared with MRD+ patients (n = 6, solid lines with solid triangles). (B) Overall survival for the same patient group. Survival was compared using log-rank analysis.
Application of MRD Flow to monitoring patients after therapy
Patients achieving MRD− status after treatment have a prolonged event-free survival, but it is not clear whether any of these patients are being ‘cured.’ In order to test this, patients were monitored sequentially to determine whether early signs of relapse could be identified, and if so whether the kinetics of disease progression could be determined.
Return of CLL cells has been detected in 9 out of 18 evaluable patients initially identified as MRD− immediately after treatment. Cells were detected at a median of 15.8 months (range, 5.5-41.8) from end of treatment. The other patients remain disease-free with a median follow up of 11.7 months (range, 7.0-48.1).
All patients with detectable CLL cells show a steadily increasing disease levels with time, even in patients with low initial levels of disease. The median doubling time was 121 days (range, 54-244 days) in 6 patients assessed. As the doubling time is highly variable, prediction of time to clinical progression requires knowledge of both proliferation rate and initial disease levels. With close monitoring, it is possible that MRD Flow could be used in patients with subclinical levels of CLL to allow prediction of time to disease progression.
Discussion
In this study, we report the development of a flow cytometric assay (MRD Flow) based on the differential expression of antigens by CLL cells compared with normal B cells. The assay has sensitivity comparable with the most sensitive techniques currently available (eg, ASO-PCR) but is readily quantitative and rapid (results may be generated within 1 hour). As such, the technique may be used to guide therapy. In contrast to IgH-PCR techniques, MRD Flow is applicable to all patients assessed so far, and at all stages of disease as no prior knowledge of the presentation phenotype is necessary. There are clear advantages to MDR Flow over previous flow-cytometric assays: those that consider only CD5/CD79b21 or CD5/CD2022cannot be used to monitor all patients, whereas those that use light-chain restriction are insensitive in the presence of polyclonal B cells. We have used this assay, as well as standard criteria, to monitor response and follow-up of patients with CLL treated with CAMPATH-1H and/or autologous PBSCT.
The dynamics of response to CAMPATH-1H were varied with an immediate clearing of CLL cells from the peripheral blood in almost all of the patients but with a slower clearance of the bone marrow. Analysis of peripheral blood levels during CAMPATH-1H therapy could not predict the level of bone marrow disease, as circulating disease is rapidly depleted. As such, the peripheral blood CLL cell levels could be used to predict the most appropriate time to perform bone marrow analysis, which is not necessary until circulating disease is below 0.01 × 109/L. The dynamics of blood and bone marrow response to other agents is not clear, and requires further study. Once off treatment, the levels in the peripheral blood did provide a more direct indication of bone marrow involvement. Patients with less than 0.01 × 109/L circulating disease always had less than 1% CLL cells in the bone marrow. In contrast, patients with more than 1 × 109/L blood CLL cells almost always had more than 30% bone marrow disease.
The use of MRD Flow demonstrates that approximately a quarter of the patients who achieve a complete remission by NCI criteria after CAMPATH-1H and/or autologous transplantation had detectable disease (MRD+) whereas the remainder had no detectable disease (MRD−). MRD+ patients have a shorter overall survival and treatment-free survival than those without. Previous studies using ASO-PCR analysis,9 and even using 2-color flow cytometry10 have also demonstrated that detection of persistent disease is associated with a higher risk of relapse. Thus the level of residual disease predicts time to relapse, and attaining an MRD− status by the most sensitive technique available is an appropriate therapeutic goal. Clearly, the level of residual disease at the end of therapy could be used as a surrogate marker to allow better comparison between different treatments. The correlation between disease levels at end of therapy and event-free survival further supports the use of monitoring during therapy to allow treatment to maximal response.
The durability of response to CAMPATH-1H is varied, but patients who achieve an MRD− status at the end of therapy maintain the response for a prolonged period (up to 48 months). It is clear that these patients are not “cured,” as half develop detectable but subclinical disease over the subsequent months. However, all the patients had been previously treated and the majority were refractory to conventional therapies, indicating that “cure” was probably an unreasonable expectation. MRD flow cytometry can be used sequentially after CAMPATH-1H therapy to monitor the presence or absence of disease and to monitor the level of disease when it is detectable. Regular follow-up allows the calculation of a disease doubling time, which can be used to predict hematologic and clinical relapse. This may be particularly important for identification of patients with low levels of disease but a rapid doubling time. Such patients may well relapse rapidly with aggressive disease, and require further therapy at an early stage. It is clearly important to determine whether the doubling time alters during the course of therapy, as this parameter defines how frequently patients need to be monitored.
In summary, overall and progression-free survival are dependent on both the depth of remission and the CLL doubling time, but calculation of these values requires highly sensitive and quantitative techniques. MRD+ patients show inevitable disease progression, whereas MRD− patients attain durable remissions. Therefore, in order to obtain durable remissions, a maximal response defined by the most sensitive techniques, such as MRD Flow or ASO-PCR, is required. The advent of novel therapeutic approaches in CLL has, for the first time, allowed the legitimate goal of achieving a complete remission (MRD−) to become a possibility for a significant proportion of patients. The most pertinent question that should now be addressed in future clinical trials is not whether one therapeutic agent is superior to another, but whether a “disease eradication” approach can improve survival and ultimately lead to a cure of the disease.
We thank Millenium and Ilex Partners and the Therapeutic Antibody Centre, Oxford, United Kingdom, for provision of the CAMPATH-1H used in this study.
Supported by the Leukaemia Research Fund, London, United Kingdom and Yorkshire Cancer Research, Harrogate, United Kingdom.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Andy Rawstron, HMDS, Algernon Firth Bldg, Leeds General Infirmary, Leeds LS1 3EX, United Kingdom; e-mail:andy.rawstron@newscientist.net.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal