Abstract
Previously, it was reported that patients with multiple myeloma (MM) who have higher baseline levels of blood CD4+or CD19+ cells have longer survival. This article extends the analysis of immune cell levels and survival in a large cohort (N = 504) of patients with MM entered on Eastern Cooperative Oncology Group (ECOG) phase 3 trial (9486). Newly diagnosed patients with MM received 2 cycles of vincristine, bischloroethylnitrosourea, melphalan, cytoxan, prednisone (VBMCP) and were treated on one of 3 randomized arms: VBMCP with either interferon or high-dose cyclophosphamide, or VBMCP alone. Blood immune cell levels were studied at trial entry (baseline), after 2 cycles of chemotherapy, after 2 years of therapy, and at relapse. Baseline CD3+, CD4+, CD8+, CD19+, and CD4+ subset cell levels were all positively associated with survival (P = .0087 toP < .0001). A multivariate analysis incorporating CD4+ and CD19+ cell levels defined 3 separate groups of patients with MM to survival outcome. Higher CD19+ blood levels were positively associated with MM-patient survival at entry to the study, at year 2, and at relapse (P < .0001 at all 3 timepoints). Patients with MM had evidence of immune cell reconstitution after 2 years of therapy, but the rate and extent of recovery was greater for CD8+, which was greater than CD4+, which was greater than CD19+. This latter data affirms the positive relationship between the quantitative status of the blood immune system in MM and survival. In addition, the importance of the CD19+ blood cells to survival is evident throughout the course of MM. Therapeutic efforts to maintain an intact immune system may be crucial in maximizing chemotherapeutic and/or immunotherapy efforts in this disease.
Introduction
Multiple myeloma (MM) is a malignancy characterized as a clonal B-cell malignancy that is not curable by current treatment. An interesting feature of this disease is the extensive and complex set of immunodeficiencies that accompany this plasma cell dyscrasia. We and others have characterized these immune abnormalities in myeloma.1 2 Associated immune abnormalities include hypogammaglobulinemia, T-cell subset quantitative and qualitative abnormalities, and alterations in natural killer and/or lymphokine-activated killer cell numbers and function. Although the delineation of these immune abnormalities in myeloma is important, there is no clear definition of the precise relationship of these immune cells to the malignant plasma cell process. Our strategy to test the latter possibility was to determine if these immune cell aberrations are associated with the clinical outcome of patients with MM.
Evidence that there is a direct, positive association with immune cell quantitative and/or qualitative parameters and response to therapy or survival would strengthen the hypothesis that the nonmalignant cell populations can directly or indirectly control the myeloma disease process. Most prior in vitro work used to determine what role the immune system has in modulating the plasma cell malignancy has highlighted the T cell. Thus, T-cell–mediated anti-idiotype and T-suppressor (ie, CD8+ cells) function have been shown to influence the plasma cell clone.3-5 Indeed, considerable recent work has focused on the development of effective cellular immunity in vivo that could suppress the malignant plasma cells. This includes the generation of anti-idiotypic T-cell function and the use of donor lymphocyte infusions to treat myeloma patients.3-7
We have previously acquired relevant information about the immune system in MM and its relationship to the clinical disease and/or disease progression by obtaining blood immunophenotyping for patients with MM treated on phase 3 ECOG chemotherapy trials. Thus, we have the opportunity to evaluate patients with MM prior to treatment and then as they undergo induction and maintenance therapy. In addition, we have had the opportunity to study the immune system when patients with MM are observed with discontinuation of the induction therapy.
In earlier reports, we documented the relationship of blood CD19+ and CD4+ blood cells when sampled at diagnosis to survival in patients with MM.8 9 Those studies found initial CD19+ and CD4+ blood levels independently were strongly and positively associated with MM survival. This report now extends the data for CD19+ cell levels in relationship to patient survival over the length of the ECOG phase 3 trial (9486) and at relapse. In addition, other immune cell (ie, CD3+, CD4+, CD4+ subsets, CD16+, and CD8+cells) levels in patients with MM on this trial were also analyzed for possible relationships of these immune cell levels to clinical outcome. These data strongly support our original hypothesis that the status of the MM patient's immune system is critical to his or her clinical outcome.
Patients, materials, and methods
Myeloma clinical trial (9486) and patient study time points
ECOG trial 9486, a multicenter trial conducted as both a clinical and correlative laboratory protocol, has been previously described.10 Briefly, previously untreated patients with MM with active disease were randomized to one of 3 protocols: VBMCP (vincristine 1-2 mg/M2 intravenous [IV] day 1, bischloroethylnitrosourea 20 mg/M2 IV day 1, melphalan 8 mg/M2 per ora days 1-4, cyclophosphamide 400 mg/M2 IV day 1, and prednisone 40 mg/M2 po days 1-7 in 5-week cycles) or to VBMCP alternating with interferon-alpha2 with the latter being given at 5 mU/M2 3 times per week starting on day 22 of each 6-week cycle after the 2 initial cycles of VBMCP. The high-dose cyclophosphamide arm consisted of a treatment schedule of cyclophosphamide (600 mg/M2) IV for days 1 through 4 and prednisone (100 mg/M2) for cycles 3 and 5 of VBMCP. Patients older than 70 years of age were ineligible for the high-dose cyclophosphamide arm. In all patients, the therapy protocol was continued until disease progression or a maximum of 2 years.
The sequential time points of study during the trial were pretreatment, after cycle 2 (prior to treatment on the randomized arms), at 2 years, and at relapse. The 2-year time point represented the end of the induction and maintenance phase.
Purification of blood lymphocytes and flow cytometry determination of the blood immune cells in myeloma
Venous blood drawn from patients with MM was separated into peripheral blood mononuclear cells (PBMCs) by Ficoll-Hypaque centrifugation. The PBMCs were then placed in RPMI 1640 with 10% fetal bovine serum under sterile conditions and analyzed by one-color flow cytometry. In brief, the PBMCs at 1 × 106/mL were incubated with phycoerythrin (PE) and/or fluorescein-conjugated (FITC) anti-CD19 (pan B cell), anti-CD16 (natural killer cell), anti-CD3 (pan T cell), anti-CD4 (T helper cell), and anti-CD8 (T suppressor) monoclonal antibodies for 30 minutes at 4°C in the dark. To assess naive and activated T helper cells, we performed 2-color flow cytometry on PBMCs. Thus, FITC anti-CD4 was combined with PE-conjugated anti-CD45RA or PE-conjugated anti-CD45R0 to discriminate between naive or activated T helper cells respectively. All monoclonal antibodies were obtained from Becton Dickinson, San Jose, CA. These cells were washed with 2 mL of phosphate buffered saline (PBS) containing bovine serum albumin and azide buffer. The cells were next analyzed by a FACstar flow cytometer (Becton Dickinson) for fluorescent levels of monoclonal antibody binding by analysis of at least 10 000 cells using multiparameter flow cytometry. The acquired data were analyzed by a simulset version 2.3.3 and Lysis version consort 1.0.2 consort 40 system.
Statistical methods
Survival was computed from the time of study registration to the date of death or date last known alive. For 2-year survival we used a landmark analysis where survival was computed from the time of the year 2 sample to the date of death or date last known alive. Survival curves were calculated using the Kaplan-Meier11 method and compared by the log rank test. The proportional hazards model12 was used to evaluate the effects of immune parameters and prognostic factors on survival. To dichotomize immune cell measurements like CD19, a split was chosen by relative risk trees to minimize within group variances of the martingale residuals.13 To test whether phenotype differences between time points are equal to 0, the t test was used. Postrelapse survival (PRS) by CD19 at relapse is estimated conditional on relapse within 5 years of entry (N = 135), because the full marginal distribution of PRS is not identifiable. Simple linear regression was used to analyze the degree of reconstitution of relapse values from baseline counts.
Results
Relationship of blood immune cells at baseline to clinical outcome
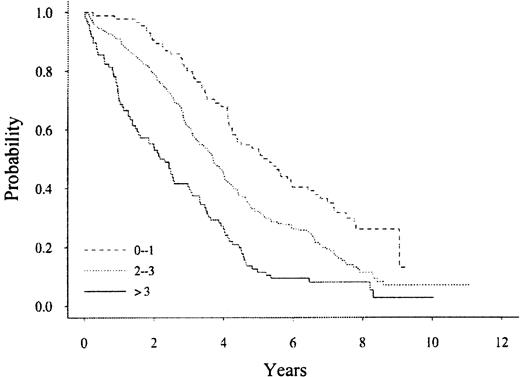
Table 1 summarizes the levels of blood immune cells at baseline and their association with survival using a univariate analysis. All immune blood cells except for CD16+ cells and activated T cells (CD2+/HLADR+) were positively associated with survival. In a multivariate model of these immune cells, CD4+ and CD19+ cells were the significant immune-based parameters (P = .0025 and .0192, respectively). We have previously published a multivariate model that can be used to segregate patients with myeloma at different risks of survival.14 15 We reanalyzed the multivariate model of known prognostic factors (ie, serum β2 microglobulin, plasma cell labeling index, soluble IL-6 receptor, plasmablastic morphology, hemoglobin, creatinine, C-reactive protein) with the baseline immune cell data. The Cox proportional hazards model listing the significant prognostic parameters is shown in Table2. In this multivariate model, 2 of the immune blood cells, naive CD4+ and CD19+, are components. The patients with MM can be subdivided into one of 3 categories for survival (Figure 1) based on the numbers of prognostic factors we describe in this new multivariate model. In this model, each risk factor was weighed equally for placement of each patient with MM into a specific risk group.
The levels of blood immune cells at baseline and their association with survival using a univariate analysis
| Immune blood cell . | Median values* . | P† . |
|---|---|---|
| CD2 | 970 | .0046 |
| CD3 | 866 | .0087 |
| CD4 | 395 | < .0001 |
| CD8 | 143 | .0074 |
| CD16 | 102 | .1858 |
| CD19 | 125 | < .0001 |
| CD2+/HLADR+ | 92 | .2275 |
| Naive CD4 | 147 | .0007 |
| Activated CD4 | 142 | .0049 |
| Immune blood cell . | Median values* . | P† . |
|---|---|---|
| CD2 | 970 | .0046 |
| CD3 | 866 | .0087 |
| CD4 | 395 | < .0001 |
| CD8 | 143 | .0074 |
| CD16 | 102 | .1858 |
| CD19 | 125 | < .0001 |
| CD2+/HLADR+ | 92 | .2275 |
| Naive CD4 | 147 | .0007 |
| Activated CD4 | 142 | .0049 |
Median levels of blood immune cells in cells per μL.
P value indicating relationship to survival.
The Cox proportional hazards model listing the significant prognostic parameters
| Covariate . | Unfavorable category . | Hazard ratio . | P . |
|---|---|---|---|
| Morphology | Plasmablastic | 2.211 | < .0001 |
| PCLI | ≥ 1% | 1.631 | < .0001 |
| β2M | ≥ 2.7 | 1.551 | .0006 |
| Naive CD4 | < 147 | 1.395 | .0026 |
| sIL-6R | ≥ 270 | 1.429 | .0034 |
| Plasma cell % | ≥ 30% | 1.371 | .0039 |
| CD19 | < 125 | 1.277 | .0381 |
| Covariate . | Unfavorable category . | Hazard ratio . | P . |
|---|---|---|---|
| Morphology | Plasmablastic | 2.211 | < .0001 |
| PCLI | ≥ 1% | 1.631 | < .0001 |
| β2M | ≥ 2.7 | 1.551 | .0006 |
| Naive CD4 | < 147 | 1.395 | .0026 |
| sIL-6R | ≥ 270 | 1.429 | .0034 |
| Plasma cell % | ≥ 30% | 1.371 | .0039 |
| CD19 | < 125 | 1.277 | .0381 |
PCLI indicates plasma cell labeling index; β2M, β2 microglobulin; sIL-6R, soluble interleukin-6 receptor.
A multivariate analysis of patients with multiple myeloma subdivided into 1 of 3 categories for survival based on the numbers of prognostic factors.
P < .0001.
A multivariate analysis of patients with multiple myeloma subdivided into 1 of 3 categories for survival based on the numbers of prognostic factors.
P < .0001.
Thus, patients with low risk (0-1 risk factor, N = 84) have a median survival of 5.2 years, whereas high-risk patients (> 3 risk factors, N = 96) have a median survival of 2.2 years (P < .0001).
CD19+ levels in relationship to survival for patients over the course of their disease
Because baseline CD19+ levels (≥ 125 cells/μL) were most strongly associated with MM survival,8 we studied any additional associations of the CD19+ cells with clinical outcome during the clinical trial. We dichotomized CD19 at particular cutoff points selected by a method described in the statistical section above to select cutoff points for CD19+ cells that divide the patients with MM into subgroups with the greatest differences in survival. Patients above the cutoff values were designated as “high,” and patients below the cutoff value were called “low.” The cutoff points separating CD19+ cell levels into “high” or “low” categories decreased to 50 per μL at the 2-year time point, which is more than 50% lower than the baseline cut point level. The cutoff point increased to a value close to the baseline median value of 110 per μL at relapse.
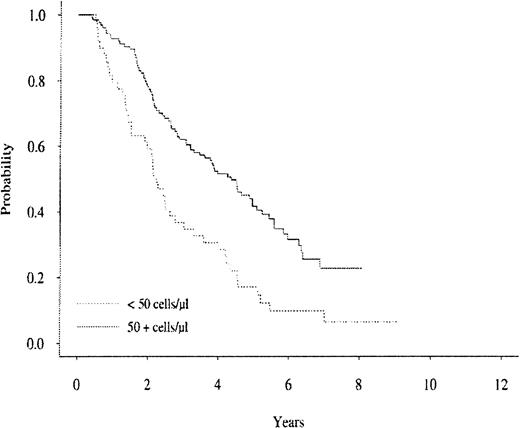
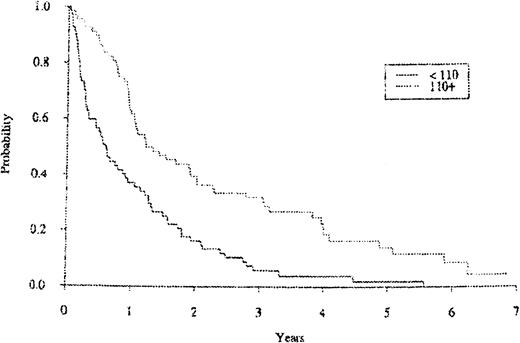
In a univariate analysis, CD19+ blood levels predicted for subsequent survival for patients with MM when measured at the 2-year time point and at the relapse time point. Figure2 shows the subsequent survival curve for patients with MM in relation to their blood CD19+ cell levels sampled at the 2-year time point. The 2-year CD19+values were positively associated with a better subsequent survival using a selected cutoff value of 50 cells per μL (P < .0001). In addition, the 2-year median CD19+ levels yielded similar significance in subsequent survival as a cutoff value for a MM subgroup with better subsequent survival (data not shown). The blood CD19+ cell levels for the patients who relapsed were also strongly and positively associated with subsequent survival. Figure 3 shows the subsequent survival curve for patients with MM in relation to their blood CD19+ cell level cutoff values when sampled at the relapse time point. Interestingly, when considering patients with MM randomized to the interferon arm (N = 32), those patients had significantly higher CD19+ counts at relapse (after year 2) than those patients randomized to the VBMCP alone arm (N = 40,P = .0071) with medians of 206.5 and 111, respectively. There was no statistically significant difference in median CD19+ cell counts at relapse (after year 2) for patients in the VBMCP arm versus the patients randomized to the VBMCP arm plus high-dose cytoxan (data not shown).
The survival curve for the MM cohort in relation to CD19+ cell levels at the 2-year study time.
P < .0001.
The survival curve for the MM cohort in relation to CD19+ cell levels at the 2-year study time.
P < .0001.
The survival curve for the MM cohort in relation to CD19+ cell levels at relapse times.
P < .0001.
The survival curve for the MM cohort in relation to CD19+ cell levels at relapse times.
P < .0001.
Table 3 summarizes the survivorship in years based on the selected cutoff values that were used to designate patients with MM as high or low CD19+ patients at baseline and the 2-year time point. If a patient with MM had blood CD19+-positive cell levels that placed him or her in a high CD19+ group, the survivorship of that patient was significantly longer than a patient with MM who had low CD19+ cells at those 2 time points during his or her disease course.
Survivorship based on the classification and regression tree–selected cutoff values used to designate patients with multiple myeloma as high or low CD19+ patients at baseline and the 2-year time point
| CD19 levels3-150 . | Baseline3-151 . | 2 year3-151 . |
|---|---|---|
| Low CD19 | 2.8 | 2.2 |
| High CD19 | 4.0 | 4.4 |
| P | < .0001 | < .0001 |
| CD19 levels3-150 . | Baseline3-151 . | 2 year3-151 . |
|---|---|---|
| Low CD19 | 2.8 | 2.2 |
| High CD19 | 4.0 | 4.4 |
| P | < .0001 | < .0001 |
MM indicates multiple myeloma.
Patients with MM were subdivided into low or high CD19 subgroups based on their level of CD19+ cells being below or above the CART-detemined levels. The levels were determined separately for each patient with MM at baseline (N = 504) and at 2-year time point (N = 173).
Data is presented as survival in years from the time of CD19 cell.
Forty-six patients with MM who had low CD19+ values at baseline (ie, < 125 cells/μL) submitted a 2-year sample for CD19+ estimation. Interestingly, the survival for these patients after the 2-year time point was also positively associated with the 2-year CD19+ cell level. The median subsequent survival was 3.1 years versus 1.8 years for patients with MM who had high (≥ 50 cells/μL) versus low (< 50 cells/μL) CD19+cells, respectively. Thus despite low baseline CD19+ values for this subgroup, the overall median survival for patients with MM able to keep their CD19+ levels at greater than or equal to 50 cells/μL at the 2-year time point versus patients with MM who did not, was 5.2 and 3.8 years, respectively.
Immune cell values in patients with MM at baseline and at the 2-year time point
Because of the positive relationship between blood immune cell levels and clinical outcome to therapy, we wanted to determine if chemotherapy could significantly reduce blood immune cell levels. Table4 summarizes the analysis of differences among immune blood cell values for patients with MM at presentation and over the 2-year treatment course of the ECOG protocol. Almost all blood immune cells fell significantly from baseline with the exception of activated T cells (CD2+/HLADR+). Analysis of blood CD19+ cells is used to illustrate the impact of chemotherapy on the blood immune cells over the 3 time points used to measure these changes (Table 5). The levels of CD19+ blood cells in patients with MM fell by 58% from the median from the time patients entered the study until the 2-year time point. This reduction in cell levels was comparable to the decreases seen in the other immune cell types (data not shown). The greatest decrease for CD19+ cells was noted between the baseline study and the 2-month time point and was similar for the other immune cell types (data not shown). This decrement occurred after only 2 cycles of chemotherapy and before the patients with MM were treated on one of the randomized arms.
Summary of the analysis of differences between immune blood cell values for patients with multiple myeloma at presentation and over the 2-year treatment course of the Eastern Cooperative Oncology Group protocol
| Cell type . | Median* . | P value . |
|---|---|---|
| Total white cell count | − 1750 | < .0001 |
| Total lymphocytes | − 845 | < .0001 |
| CD2 | − 305 | < .0001 |
| CD4 | − 274 | < .0001 |
| CD8 | − 75 | < .0001 |
| CD16 | − 34 | .0042 |
| CD19 | − 103 | < .0001 |
| CD2+/HLADR+ | 19 | .4163 |
| Naive CD4 | − 78 | < .0001 |
| Activated CD4 | − 103 | < .0001 |
| CD3 | − 414 | < .0001 |
| Cell type . | Median* . | P value . |
|---|---|---|
| Total white cell count | − 1750 | < .0001 |
| Total lymphocytes | − 845 | < .0001 |
| CD2 | − 305 | < .0001 |
| CD4 | − 274 | < .0001 |
| CD8 | − 75 | < .0001 |
| CD16 | − 34 | .0042 |
| CD19 | − 103 | < .0001 |
| CD2+/HLADR+ | 19 | .4163 |
| Naive CD4 | − 78 | < .0001 |
| Activated CD4 | − 103 | < .0001 |
| CD3 | − 414 | < .0001 |
Summary of the change in CD19+ cell absolute levels during therapy
| Time point . | Mean . | Median . | Range . |
|---|---|---|---|
| Baseline (N = 504) | 319 ± 14.96 | 233 | 0-3196 |
| 2 months (N = 405) | 168 ± 9.62 | 108 | 0-1366 |
| 2 years (N = 173) | 165 ± 15.3 | 98 | 7-1107 |
| Relapse (N = 96) | 185 ± 21.74 | 117 | 0-881 |
| Time point . | Mean . | Median . | Range . |
|---|---|---|---|
| Baseline (N = 504) | 319 ± 14.96 | 233 | 0-3196 |
| 2 months (N = 405) | 168 ± 9.62 | 108 | 0-1366 |
| 2 years (N = 173) | 165 ± 15.3 | 98 | 7-1107 |
| Relapse (N = 96) | 185 ± 21.74 | 117 | 0-881 |
The ratio of CD4+ to CD8+ cells calculated for the patients with MM from baseline to their relapse times showed a decline over this study. The median ratio decreased from 1.0 at baseline to 0.8 at the 2-month time point, 0.7 at the 2-year time point, and 0.6 at relapse. The range in ratios was very large for the early time points (baseline and 2-month time points, data not shown) and reflect a few patients with MM with very large ratios. For example, at baseline there were only 7 patients with MM with ratios greater than 10. However, there was no clinical or biologic characteristic that was distinctive for these latter patients except that all 7 had high β2 microglobulin levels (median 5.6 and range 3.0-11.2 μg/mL). The alteration in the ratio over time reflected a greater decrease in CD4+ levels than the CD8+ cells.
We also assessed the ratio of naive to activated CD4+ cells over time for this MM cohort (Table 6). The median ratio at baseline was 0.8, at the 2-month study, 1.3, and at the 2-year time point, 1.8. However, by the time a relapse occurred, the naive-to-activated CD4+ cell ratio had fallen back to 1.0. Healthy, age-matched controls tested in our laboratory had a ratio of 1.4 for CD4-to-CD8 and a ratio of 1.3 for naive-to-activated CD4 cells.
Ratio of naive to activated CD4 T cells at different time points of the Eastern Cooperative Oncology Group trial
| Time points . | N6-150 . | Median6-151 . |
|---|---|---|
| Baseline | 444 | 0.80 |
| 2 months | 370 | 1.33 |
| 2 years | 162 | 1.82 |
| Relapse | 96 | 1.12 |
| Time points . | N6-150 . | Median6-151 . |
|---|---|---|
| Baseline | 444 | 0.80 |
| 2 months | 370 | 1.33 |
| 2 years | 162 | 1.82 |
| Relapse | 96 | 1.12 |
Numbers of myeloma patients studied at each time point.
Median value of the ratio of naive to activated CD4+ T cells.
Reconstitution of immune blood cell values after therapy
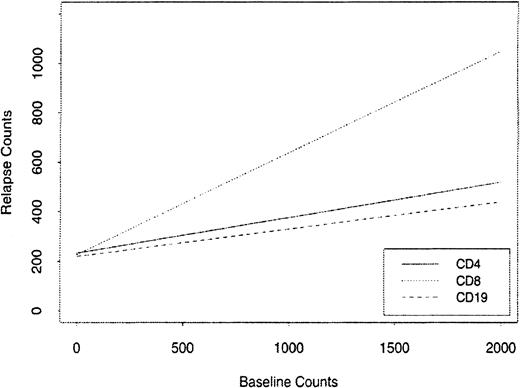
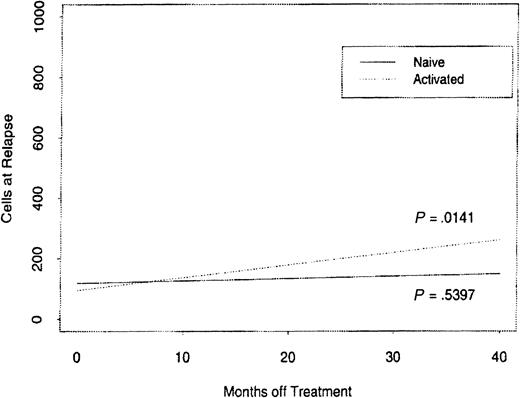
We studied the rates of recovery for the major immune cell subsets CD4+, CD8+, and CD19+. A return toward higher levels of CD19+ cells was found in the 96 patients with MM who relapsed after the 2-year time point (Table 5). But the values were still significantly lower than the baseline values despite being off therapy from 4 to 41 months. Figure4 shows the relationship of the absolute relapse counts of blood CD4+, CD8+, and CD19+ cells in these patients to their baseline counts using a linear regression plot. The associated slopes of the regression lines are 0.4109, 0.1430, and 0.1072, respectively, but only CD8+ and CD4+ cells have a significant linear association with relapse and baseline counts. The magnitude of the CD8 slope indicates that CD8+ cells reconstitute much faster than CD4+and CD19+ cells. The median recovery at relapse compared with baseline counts was 93% for CD8+T cells but only 63% for CD4+ T cells. To further assess the recovery potential of the CD4+ T cells we compared the time off therapy to the levels of naive and activated CD4+T cells (Figure 5). The naive cell increase in blood level was much less compared with the recovery of activated CD4+ T cells (P = .5394).
The relationship of the absolute relapse counts for CD19, CD4, and CD8 cells in patients with MM to their baseline counts using a linear regression plot.
The relationship of the absolute relapse counts for CD19, CD4, and CD8 cells in patients with MM to their baseline counts using a linear regression plot.
The reconstitution rates of CD4 naive and activated T-cell subsets when comparing lymphocyte cell counts to time off therapy.
The reconstitution rates of CD4 naive and activated T-cell subsets when comparing lymphocyte cell counts to time off therapy.
Discussion
This report confirms the strong association between the status of the blood immune system in patients with MM and their clinical outcome to therapy. We have previously reported that high initial levels of CD19+ cells and CD4+ cells in newly diagnosed MM predicted for longer survival.8 9 Our prior publications on CD19+ and CD4+ cells were based on an analysis of baseline values. That is, we investigated whether CD19 and CD4 cell levels prior to treatment impact on MM patient outcome. This current report represents a longitudinal analysis where the emphasis is on the changing CD4 and CD19 cell levels over time and in relation to disease course. Thus we are now able to describe the impact of treatment and the course of MM on immune cell levels. Our specific objectives in this study were twofold: (1) further quantitative analysis of immune blood cells during therapy to see if that would provide more information on survival; and (2) detect if immune blood cells in patients with MM could be reconstituted after therapy. This analysis revealed that levels of both CD19+and naive CD4+ cells could be placed into a multivariate model that clearly distinguishes between 3 subgroups of patients with MM based on survival. This latter model should be helpful in the development of prognostic information of patients with MM entered on clinical treatment trials. The immune blood cell with the most consistent impact on survival over the 2 years of therapy and at relapse is the CD19+ cell. We were also able to show that immune blood cells in patients with MM have the capacity to be reconstituted, albeit at differential rates.
The multivariate model we developed using a variety of morphologic, immune, and serum-based assays, provides for a relatively simple means of segregating patients with MM based on their survival outcome. We have previously published on the efficacy of the plasmablastic type, plasma cell labeling index (PCLI) and the β2 microglobulin level as prognostic features of clinical outcome in MM.14-17 This earlier model14 has now been expanded based on this trial to include a biologic factor that may be involved in the pathogenesis of the disease (ie, soluble IL-6R), aggressive biologic parameters of the myeloma clone (plasmablastic, % plasma cells), and blood immune parameters (CD19+, naive CD4+) in patients with MM. The relationship of blood immune cells to disease outcome corroborates earlier studies that indicated potential asssociations between the immune status and disease. For example, analysis of marrow lymphocyte subpopulations in MM revealed progressive decreases in the percentage of CD4+ cells with advancing stage.18 Another study showed evidence for the presence of idiotype-specific and major histocompatibility complex (MHC)–restricted CD4+ and CD8+ T cells in patients with monoclonal gammopathies,19 implying that T cells may have antimyeloma function.
We have found that blood levels of circulating B cells defined by the presence of reactivity to the monoclonal antibody (Leu12) were positively and strongly associated with favorable clinical outcome in MM8. In this report, we show that CD19+ blood cells have significant positive associations with clinical outcome for patients with MM when measured later in their disease course. Thus, for patients with MM after 2 years of induction and/or maintenance therapy the cohort of patients with higher CD19+ cell levels are more likely to have longer survival. A similar positive relationship for longer survival in association with higher CD19+ cell levels was seen for patients with MM who relapsed after the 2 years of therapy. Importantly, we also detected that some patients with MM with low CD19+ levels at baseline who were able to maintain the level of CD19+ cells at 2 years at greater than or equal to 50 cells per μL had enhanced survival time compared with patients with MM who had lower CD19 levels. It therefore appears that the B cell has important biologic and therapeutic consequences for the patient with MM. Efforts to determine methods for maintaining B-cell levels could be of benefit. Because we have found that interferon-exposed patients with MM had higher CD19+ cell levels at relapse even after 2 years of therapy, we intend to continue to explore the impact of this cytokine on the levels of human B-cell populations in this disease.
CD19 is a cell-surface antigen present on the membrane of all circulating mature B cells, B-cell precursors, and some dendritic cells.20,21 It is a member of the immunoglobulin (Ig) superfamily and can act as an accessory molecule for signal transduction.22 The latter function suggests that this surface molecule may be of critical importance to the ability of a B cell to respond to antigenic challenges. Indeed, when antigen binds to B cells, a series of events occur that should ultimately result in B-cell proliferation and differentiation into either memory B cells or antibody-producing plasma cells. In our initial report of blood immune cells in patients with MM,8 we found that patients with higher levels of CD19+ cells had a decreased incidence, severity, and mortality from infections. It is conceivable that the consistent positive association of favorable outcome with higher CD19+ cells is related to the ability of the patient with MM to deal with infections. However, the most likely time for infections in patients with MM to impact on mortality is in the first 2 months of their clinical course23 and the association with CD19+ cells with survival is seen after that as well. Importantly, this study was not designed to test if blood CD19+ B cells or even naive CD4+ T cells can serve as independent markers compared with the less available or perhaps more expensive tests we now use, such as plasma cell labeling indices.
An additional important finding in this study is the impact of therapy on the blood immune cells of patients with MM. We have observed that patients with MM who complete 2 years of therapy have a lowered overall level of immune blood cells. The greatest rate of decrease in immune cells is seen after the first 2 cycles of therapy. This finding suggests that after initial therapy, patients with myeloma are able to generate compensatory rates of lymphopoiesis which blunt more dramatic changes in blood immune cell production. After discontinuation of all therapy, these patients can reconstitute the levels of immune blood cells, but the mechanism and rates of reconstitution clearly favor CD8+ cells in comparison with CD4+ or CD19+ cells. These observations are particularly important because the initial levels of both CD4+ and CD19+ cells have positive associations with survival in MM. The latter cell is potentially more critical because the levels of the CD19+ cell are positively associated with enhanced survival outcomes at 2 years after therapy and at relapse. The decrease in immune T cells with chemotherapy resulted in the imbalance of CD4+-to-CD8+ T cells and in naive-to-activated CD4+ cells. These latter alterations reflect that two major impacts of therapy are the CD4+ cells and the naive pool within the CD4+ T cells. These latter results may reflect the decreased thymic pool of naive CD4+ T cells in aging humans.24 Information about the immune blood cell reconstitution potential in aging and/or disease will be relevant to both chemotherapeutic and immunotherapeutic strategies in this disease.
These findings clearly demonstrate that repeated sampling of blood immune cells in MM, in particular the CD19+ cell, confers additional survival prediction and biological information. It is important to emphasize that the absolute level of CD19+cells that predict for better survival change as the patient population is treated. Nevertheless, the positive relationship of CD19+ cell levels with survival extends to the outcome of the patient with MM over the course of several years. Finally, this cooperative group study has generated a revised multivariate model that can be used to subdivide patients with MM based on survival outcome. This should be a valuable resource in the design of future clinical trials for this disease.
Conducted by the Eastern Cooperative Oncology Group and supported in part by Public Service Grants CA13650, CA23318, CA15947, CA21115, and CA66636 from the National Cancer Institute, National Institutes of Health, and the Department of Health and Human Services.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Neil E. Kay, Mayo Medical Center, 200 First St SW, Rochester, MN 55905; e-mail: kay.neil@mayo.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal