Abstract
Band 3 and glycophorin A (GPA) are the 2 most abundant integral proteins in the human erythrocyte membrane. Earlier studies suggested that the 2 proteins may associate not only in the mature erythrocyte membrane, but also during their posttranslational processing and intracellular trafficking. The purpose of this study was to directly examine the GPA–band 3 interaction in vivo and determine the nature of this association during erythroid membrane biogenesis. Transgenic mice were generated expressing the human glycophorin A gene and were used to examine how the induction of human GPA expression affected the levels of murine GPA and band 3 expression in the red cell membrane. Murine GPA expression was reduced in erythrocytes expressing human GPA, whereas the level of band 3 expression remained constant, implying a tight coupling of band 3 and GPA expression in the membrane of mature red cells. In vivo GPA dimerization was not modulated solely by the GPA transmembrane motif, but the distance between this motif and the basic residues on the cytoplasmic side of the transmembrane domain may also be important. In addition, GPA monomers with varying degrees of glycosylation dimerized, providing clear evidence that carbohydrate structures on the extracellular domain do not affect dimerization. The association between the multiple transmembrane-spanning protein, band 3, and the single transmembrane-spanning sialoglycoprotein, GPA, may serve as a model for interactions of other multi-pass and single-pass polypeptides during membrane biogenesis.
Introduction
The 2 most abundant integral proteins in the human erythrocyte membrane are the sialoglycoprotein glycophorin A (GPA) and the anion exchanger band 3. GPA, present at 800 000 copies per cell, is a class I transmembrane protein assembled into dimers in the mature erythrocyte membrane.1-3 Human GPA undergoes homodimerization by associations between hydrophobic membrane-spanning domains.4,5 With its high sialic acid content, GPA is the main contributor to the net negative cell-surface charge and is thus critical for minimizing cell–cell interactions and preventing red cell aggregation. In contrast, erythrocyte band 3 is a multifunctional protein with its N- and C-terminal domains on the cytoplasmic face of the lipid bilayer and approximately 14 membrane spans.6-8 The C-terminal 52-kd membrane domain (residues 360-919) of band 3 is responsible for anion transport across the membrane, whereas the 40-kd N-terminal cytoplasmic domain plays a crucial structural role in linking the bilayer with the spectrin-based skeletal network. There are 1 × 106 copies of band 3 per cell, which are present as a mixture of dimers and tetramers. Approximately 40% to 60% of band 3 appears to be associated with the spectrin-based skeleton.
Although evidence to support a direct interaction between GPA and band 3 has remained equivocal, intriguing data suggest that the 2 proteins may associate not only in the mature erythrocyte membrane, but also during their posttranslational processing and/or intracellular trafficking during erythropoiesis. Evidence supporting an interaction in the plasma membrane includes the finding that binding of antibody to the extracellular domain of GPA decreases both the rotation9 and lateral mobility10 of band 3. In addition, the expression of blood group antigen Wrbrequires the surface expression of both GPA and band 311,12and appears to involve an interaction between E658 (single-letter amino acid code) of band 3 and amino acid residues within the extracellular α-helical domain and transmembrane junction of GPA.13,14 Indications that the 2 integral proteins interact intracellularly with one another during synthesis, processing, or transport to the membrane include evidence that bothXenopus oocytes15,16 and yeast17cotransfected with GPA and band 3 cDNAs show an increased level of cell-surface band 3 compared with those transfected with band 3 cDNA alone. Also striking is the fact that, although GPA is synthesized in erythroblasts of band 3 null mice, it fails to assemble on the plasma membrane, resulting in red cells that completely lack not only band 3 but also GPA.18 Considered together, these data strongly suggest an interdependence of band 3 and GPA during normal membrane biogenesis.
To directly study the GPA–band 3 interaction in vivo and determine the nature of this association during erythroid membrane biogenesis, we generated mice transgenic for the human glycophorin (GYPA)gene and examined how the induction of human GPA expression affected the levels of murine GPA and band 3 expression in the red cell membrane. We document that there indeed exists a tight coupling between band 3 and GPA expression in the membrane of mature red cells. Furthermore, our studies provide interesting insights into the nature of GPA dimerization.
Materials and methods
Generation of GPA transgenic mouse lines
A bacterial artificial chromosome (BAC) clone containing the promoter region and the entire genomic GYPAgene was isolated from a human BAC library (Research Genetics, Huntsville, AL; catalog number 96010) by a polymerase chain reaction (PCR)-based method with a pair of primers (5′-TATTAGCTCAGAGCCTCACACATT-3′ and 5′-GAAATTTCTGAAACTTCATGAGCTC-3′) that amplified a 291-bp fragment of human genomic DNA (Figure1A; sequence-tagged site [STS] marker 2). The 159F4 BAC clone was purified according to a standard protocol (Incyte Genomics, St Louis, MO) and used to produce transgenic mice by microinjection into fertilized FVB mouse eggs. The presence of the transgene in the offspring was tested by PCR using the oligonucleotide pair described above on DNA extracted from tail biopsy specimens. The amplification reaction contained 2.5 μL of tail DNA preparation mixed with 0.5 μM of each oligonucleotide, 1.5 mM MgCl2 (Perkin Elmer, Branchburg, NJ), 0.2 mM dNTP (Gibco BRL, Rockville, MD), and 0.05 U AmpliTaq DNA polymerase (Perkin Elmer) in 25 μL of PCR buffer II (Perkin Elmer). The thermal cycle conditions were: 94°C for 4 minutes followed by 35 cycles at 94°C for 30 seconds, 60°C for 30 seconds, and extension at 72°C for 1 minute, followed by 1 cycle at 72°C for 4 minutes. The PCR products were analyzed by gel electrophoresis in a 2% agarose gel. Among the 12 founder mice positive for the transgene as tested by PCR, 4 founder mice expressed the glycophorin A protein in red blood cell (RBC) membranes at readily detectable levels. The founder mouse expressing the highest level of protein was used to generate a GYPA transgenic line on the FVB background. Transgenic mice from these breedings were used for the experiments described herein. Animal care was in accordance with the Lawrence Berkeley National Laboratory Animal Welfare and Research Committee guidelines as stated in protocol AWRC 6803.
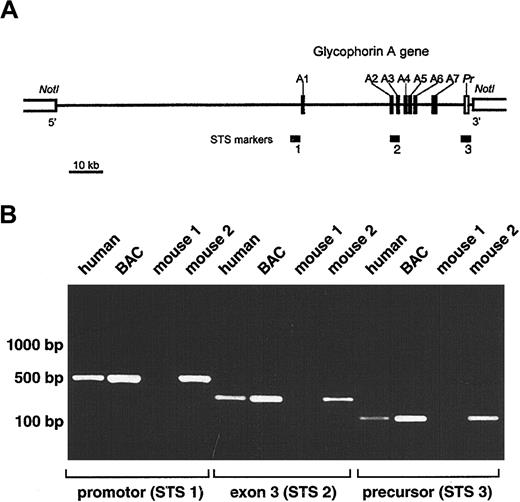
Development of transgenic mice expressing human GPA.
(A) Schematic representation of selected BAC clone 159F4 containing the entire glycophorin A gene (GYPA). The insert (121 kb) is shown by a horizontal line between NotI sites in the BAC vector. Closed boxes along the line represent the 7 exons of theGYPA gene (A1-A7), and the open box denotes the 3′-flanking precursor (Pr) genomic segment. STS markers used to screen the human genomic BAC library (STS 2; intron2/exon3) and to confirm the presence of the promoter (STS 1) and precursor (STS 3) are designated by horizontal bars. (B) PCR analysis of offspring of transgenic mice. Genomic DNA isolated from tails of mice 3 weeks of age was tested by PCR for the presence of the GYPA transgene using the following sets of primers: STS 1, STS 2, and STS 3. Human genomic DNA and BAC clone 159F4 DNA were used as positive controls.
Development of transgenic mice expressing human GPA.
(A) Schematic representation of selected BAC clone 159F4 containing the entire glycophorin A gene (GYPA). The insert (121 kb) is shown by a horizontal line between NotI sites in the BAC vector. Closed boxes along the line represent the 7 exons of theGYPA gene (A1-A7), and the open box denotes the 3′-flanking precursor (Pr) genomic segment. STS markers used to screen the human genomic BAC library (STS 2; intron2/exon3) and to confirm the presence of the promoter (STS 1) and precursor (STS 3) are designated by horizontal bars. (B) PCR analysis of offspring of transgenic mice. Genomic DNA isolated from tails of mice 3 weeks of age was tested by PCR for the presence of the GYPA transgene using the following sets of primers: STS 1, STS 2, and STS 3. Human genomic DNA and BAC clone 159F4 DNA were used as positive controls.
Western blot analysis
Human blood was obtained from consenting donors. Mouse blood was collected from mouse tails in potassium EDTA-treated microtubes. RBC membrane ghosts were prepared by washing RBCs 3 times in 15 volumes of 10 mM phosphate-buffered saline (PBS), pH 7.4, followed by 3 washes in 15 volumes of lysis buffer (Na2HPO4 6.8 mM, NaH2PO4 2.25 mM, pH 8.0). In some experiments, 20 μg of the ghost protein preparations was digested by the enzyme PNGase F, which hydrolyzes N-glycan chains from glycopeptides (New England Biolabs, Beverly, MA). After mixing with loading buffer and boiling (or not), 1 to 4 μg of proteins was separated on 10% gels or 10% to 20% sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) gradient gels (Novex, San Diego, CA). Either the gels were stained by a solution of Coomassie brilliant blue or the proteins contained in the gels were transferred onto nitrocellulose membrane. After blocking overnight at 4°C with 10 mM PBS, pH 7.4, 0.05% Tween-20, and 5% nonfat dry milk, the blots were washed in PBS, pH 7.4, 0.05% Tween-20 for 15 minutes, then probed for 1 hour with either 1:1000 dilution of monoclonal anti-GPA antibody BRIC 163 (kindly provided by Dr David Anstee, Bristol Institute for Transfusion Services, Bristol, United Kingdom) or 10 ng/mL purified rat antimouse TER-119 monoclonal antibody (BD Biosciences-Pharmingen, San Diego, CA) or 1 μg/mL antispectrin antibody (kindly provided by Dr Stanley Schrier, Stanford University, Stanford, CA) in PBS, pH 7.4, 0.05% Tween-20, and 0.5% nonfat dry milk. After 3 washes of 15 minutes each in PBS, pH 7.4, 0.05% Tween-20, and 0.5% nonfat dry milk, the blots were incubated at room temperature for 1 hour with 1:2000 dilution of antimouse or 1:3000 dilution of antirat Ig linked to horseradish peroxidase (Amersham Pharmacia Biotech, Piscataway, NJ). The blots were then washed 3 times for 15 minutes in PBS, pH 7.4, 0.05% Tween-20, and 0.5% nonfat dry milk and finally developed using the Renaissance chemiluminescence detection kit (New England Biolabs, Beverly, MA).
Flow cytometric analysis
Flow cytometric analysis was performed as described previously, with a number of modifications.19 Human RBCs were washed 3 times and resuspended in HEPES-buffered saline (10 mM HEPES, 145 mM NaCl, pH 7.4), and murine RBCs were washed 3 times and resuspended in HEPES-buffered saline for mouse (10 mM HEPES, 165 mM NaCl, pH 7.4), at a final concentration of 2 × 106 cells/mL. Samples were assayed with varying concentrations of R10 monoclonal antibody (kindly provided by Dr David Anstee) labeled with fluorescein isothiocyanate (FITC), Ter-119–phycoerythrin (PE) antibody (Pharmingen, San Diego, CA), or with eosin-5-maleimide (EMA; Molecular Probes, Eugene, OR). Flow cytometric analysis was performed with a FACScan flow cytometer (Becton Dickinson, San Diego, CA) equipped with Cellquest software (Becton Dickinson). Light-scatter signals were used to discriminate the population of intact single RBCs, and the mean fluorescence signal associated with cells for each labeled component was quantified using the geo mean fluorescence parameter provided with the software.
Results
Transgenic mice expressing human GPA protein
A human genomic BAC library was screened by a PCR-based method using a set of primers specific for intron 2 to exon 3 of the humanGYPA gene (Figure 1A; STS marker 2). Positive BAC clones were then tested for the presence of the promoter (STS marker 1) and precursor (STS marker 3). Because STS marker 1 amplified a fragment present in both the GYPA and glycophorin B (GYPB)promoters, the specificity for GYPA was confirmed by restriction digests using NheI, which cuts only theGYPA PCR product, and MspI and NciI, which cut only the GYPB PCR product. Fluorescence in situ hybridization was used to mark the location of the 5′-flanking region of exon 7 in the BAC clone (Figure 1A). Using these strategies, we selected BAC clone 159F4 (121 kb) containing the entire humanGYPA gene to generate transgenic mice.
Purified BAC clone 159F4 DNA was microinjected into the pronuclei of fertilized mouse eggs. The offspring were then tested for the presence of the GYPA transgene by PCR on DNA extracted from tail biopsy specimens using oligonucleotide pairs STS 1, STS 2, and STS 3 (Figure 1B). Among the 19 mice born from microinjected eggs, 12 were positive for GYPA transgene integration.
To determine whether human GPA was synthesized and assembled onto the erythrocyte membranes of the transgenic founder animals, we analyzed mouse red cells by flow cytometry. Using R10 monoclonal antibody specific for the extracellular domain of human GPA,20 we observed that GPA was indeed expressed on the cell surface and that founder mice expressed human GPA at different levels. Analysis of the mouse erythrocytes with the highest level of GPA expression is depicted in Figure 2. In this experiment, the mean fluorescence was 2000 (arbitrary units) for human red cells, 200 for transgenic mouse cells, and 20 for wild-type mouse cells. Hence, the highest level of human GPA expression in the founder mice was approximately 20% of its expression on human erythrocytes because human cells are about twice the size of mouse erythrocytes. The founder expressing the highest level of protein was selected to generate the transgenic line.
Flow cytometric analysis of GPA expression.
Human, wild-type mouse, and GYPA transgenic mouse RBCs were labeled with FITC-conjugated monoclonal antibody specific for human GPA (R10-FITC) and analyzed by flow cytometry.
Flow cytometric analysis of GPA expression.
Human, wild-type mouse, and GYPA transgenic mouse RBCs were labeled with FITC-conjugated monoclonal antibody specific for human GPA (R10-FITC) and analyzed by flow cytometry.
Human GPA does not dimerize with murine GPA
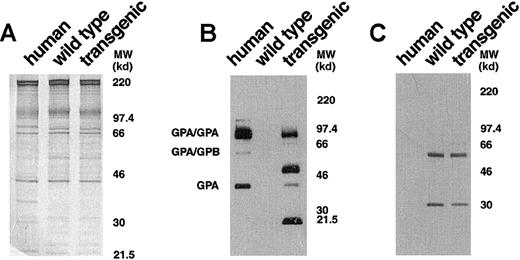
To obtain a more detailed characterization of the expressed human GPA in the transgenic mice, we analyzed erythrocyte membranes by Western blotting with monoclonal antibody BRIC 163, which is specific for the cytoplasmic domain of human GPA.21 In the control human red cell membranes, this antibody highlighted GPA homodimers, GPA/GPB heterodimers, and GPA monomers (Figure3B). In GYPA transgenic mouse membranes, immunoreactive bands corresponding to the monomeric and homodimeric forms of human GPA were identified (Figure 3B). This established that the membrane-assembled forms of human GPA in the transgenic animals were the same as those present in the human erythrocyte and included the cytoplasmic tail.
Western blot analysis of human and murine GPA expression.
(A) Equivalent loads (4 μg/lane) of human, wild-type mouse, andGYPA transgenic mouse red cell proteins were subjected to electrophoresis in a 10% SDS-PAGE gel and stained with Coomassie brilliant blue. No differences were noted in the major red cell membrane proteins in the transgenic sample when compared with the normal mouse sample. (B) Human, wild-type mouse, and GYPAtransgenic mouse red cell proteins (4 μg/lane), separated on an 8% SDS-PAGE gel, were immunoblotted using monoclonal antibody BRIC 163 against the cytoplasmic domain of human GPA. (C) Human, wild-type mouse, and GYPA transgenic mouse red cell proteins were immunoblotted using monoclonal antibody TER-119 against mouse GPA. MW indicates molecular weight.
Western blot analysis of human and murine GPA expression.
(A) Equivalent loads (4 μg/lane) of human, wild-type mouse, andGYPA transgenic mouse red cell proteins were subjected to electrophoresis in a 10% SDS-PAGE gel and stained with Coomassie brilliant blue. No differences were noted in the major red cell membrane proteins in the transgenic sample when compared with the normal mouse sample. (B) Human, wild-type mouse, and GYPAtransgenic mouse red cell proteins (4 μg/lane), separated on an 8% SDS-PAGE gel, were immunoblotted using monoclonal antibody BRIC 163 against the cytoplasmic domain of human GPA. (C) Human, wild-type mouse, and GYPA transgenic mouse red cell proteins were immunoblotted using monoclonal antibody TER-119 against mouse GPA. MW indicates molecular weight.
Surprisingly, additional bands migrating at apparent molecular weights of approximately 51 and 21.5 kd were also detected by BRIC 163 in the GYPA transgenic mouse RBC membranes (Figure 3B). We hypothesized that these bands might result from heterodimer formation between human GPA and mouse GPA, given that the 2 GPA homologues share the highest sequence identity22 in their dimerization motifs, differing by only a single amino acid.23-26 To address the origin of the 2 bands, we needed to identify a readily available probe specific for murine GPA. We chose monoclonal antibody TER-119 as a potential candidate because it binds to cells in the erythroid lineage in a pattern similar to the expression of GPA during erythroid differentiation.27,28 To define the specificity of TER-119 more precisely, we applied several strategies. First, we examined whether increasing the concentration of SDS would dissociate all of the molecular-weight species that were immunoreactive with TER-119 into a single band of monomer, which is characteristic of the behavior of GPA.5 As depicted in Figure4A, the TER-119 immunoreactive bands all dissociated to the monomer. Second, we showed that this monomer was immunoreactive with a rare antibody specific for murine GPA18 (Figure 4B). Finally, as a complementary and independent approach, we investigated whether TER-119 bound to erythrocyte membranes from band 3 knockout mice, which are known to lack GPA.18 No immunoreactive bands were visualized by Western blot analysis of band 3 null membranes treated with TER-119 (Figure 4C) in samples that contained as much spectrin as wild-type controls. Together, these data provide convincing evidence that TER-119 recognizes murine GPA.
Western blot analysis of TER-119 antibody specificity.
(A) Wild-type (lane 1) and transgenic mouse (lane 2) red cell proteins were solubilized with increased concentrations of SDS, separated on a 10% SDS-PAGE gel, and immunoblotted using monoclonal antibody TER-119. (B) Wild-type mouse red cell proteins, separated on a 10% SDS-PAGE gel, were immunoblotted using rabbit polyclonal antibody against the cytoplasmic domain of human GPA (lane 1) or monoclonal antibody TER-119 (lane 2). (C) Wild-type mouse and band 3 null mouse red cell proteins were immunoblotted using monoclonal antibody TER-119. MW indicates molecular weight.
Western blot analysis of TER-119 antibody specificity.
(A) Wild-type (lane 1) and transgenic mouse (lane 2) red cell proteins were solubilized with increased concentrations of SDS, separated on a 10% SDS-PAGE gel, and immunoblotted using monoclonal antibody TER-119. (B) Wild-type mouse red cell proteins, separated on a 10% SDS-PAGE gel, were immunoblotted using rabbit polyclonal antibody against the cytoplasmic domain of human GPA (lane 1) or monoclonal antibody TER-119 (lane 2). (C) Wild-type mouse and band 3 null mouse red cell proteins were immunoblotted using monoclonal antibody TER-119. MW indicates molecular weight.
Having shown that TER-119 can be used as a probe for mouse GPA, we tested for the presence of GPA mouse/human heterodimers in the transgenic mouse erythrocyte membrane by reprobing with TER-119 the Western blot that had been initially reacted with the antihuman GPA, BRIC 163. As shown in Figure 3C, TER-119 recognized bands of different molecular weights than those recognized by BRIC 163. These results clearly show that the human molecules encoded by the transgene do not dimerize with endogenous murine GPA to a significant degree in the mouse membrane.
Glycosylated and nonglycosylated forms of human GPA are expressed in the mouse membrane
We next explored the possibility that the additional molecular-weight forms of human GPA in the transgenic mouse membrane resulted from heterogeneous glycosylation. Human GPA is highly glycosylated in human erythrocytes, containing 1 N-linked glycosylation site provided by the consensus sequence N-D-T (residues 26-28)29,30 and 15 O-linked oligosaccharide side chains. To test the N-glycosylation status of human GPA expressed in the mouse, we treated ghost preparations with PNGase F, which specifically hydrolyzes N-glycan chains from glycopeptides. Western blot analysis of the banding pattern of normal control and enzyme-treated membranes, after electrophoresis on a 10% to 20% tricine gradient gel, revealed a similar decrease in molecular weight of the GPA dimer and monomer in human and mouse membranes after N-glycan hydrolysis (Figure5A). Of the species of approximately 51 kd and 21.5 kd present in the mouse, only the 51-kd form shifted to lower molecular weight after enzymatic digestion. Interestingly, a band of approximately 11 kd was also observed in the mouse membranes, which could be distinguished using electrophoresis conditions capable of detecting this low-molecular-weight protein species. Like the 21-kd species, the 11-kd form did not change in size after enzymatic digestion. This small form of GPA has an apparent molecular weight similar to the calculated molecular weight of the nonglycosylated GPA polypeptide backbone. We speculated that the species of approximately 21.5 kd was a dimer of 11-kd monomers and that the species of approximately 51 kd was actually a dimer containing 11-kd and 40-kd monomers. To explore this possibility, we took advantage of previous data showing that the equilibrium between human GPA dimer and monomer is dependent upon the concentrations of SDS and protein and the duration and temperature of incubation.5 We therefore compared the pattern of bands on PNGase F–treated membranes incubated at 25°C with those incubated at 100°C (Figure 5B). Interestingly, the 11-kd band increased dramatically in the samples incubated at 100°C. This increase in the 11-kd form was coupled with a decrease in the 21.5-kd band, strongly suggesting that the 21.5-kd form was a homodimer composed of 2 11-kd monomers. In addition, the 100°C sample contained less of the 51-kd species than the 25°C sample, consistent with a temperature-induced dissociation of this protein to the nonglycosylated 11-kd monomer and the glycosylated 40-kd monomer. From this series of experiments, we conclude that human GPA synthesized in the transgenic mouse undergoes variable posttranslational processing, resulting in glycosylated and nonglycosylated polypeptides. We also conclude that dimerization can occur between 2 glycosylated monomers, 2 nonglycosylated monomers, and a glycosylated and nonglycosylated monomer.
Western blot analysis of GPA after hydrolysis of N-glycan chains.
(A) Following hydrolysis of N-glycan chains by PNGase F, red cell membrane proteins (2 μg/lane) were separated on a 10% to 20% tricine gradient gel and then immunoblotted with BRIC 163 antibody. (B)GYPA transgenic mouse red cell proteins treated with PNGase F were solubilized with increased concentrations of SDS, incubated for 3 minutes at either 25°C or 100°C, separated on a 10% to 20% tricine gradient gel, and then immunoblotted with BRIC 163 antibody. (−) and (+) refer to proteins before and after enzyme treatment, respectively. MW indicates molecular weight.
Western blot analysis of GPA after hydrolysis of N-glycan chains.
(A) Following hydrolysis of N-glycan chains by PNGase F, red cell membrane proteins (2 μg/lane) were separated on a 10% to 20% tricine gradient gel and then immunoblotted with BRIC 163 antibody. (B)GYPA transgenic mouse red cell proteins treated with PNGase F were solubilized with increased concentrations of SDS, incubated for 3 minutes at either 25°C or 100°C, separated on a 10% to 20% tricine gradient gel, and then immunoblotted with BRIC 163 antibody. (−) and (+) refer to proteins before and after enzyme treatment, respectively. MW indicates molecular weight.
Murine GPA expression is decreased in erythrocytes expressing human GPA, whereas the level of band 3 expression remains constant
To study further the postulated GPA–band 3 interaction, we examined how the induction of human GPA expression affected the levels of murine GPA and band 3 stably assembled on the red cell membrane. We hypothesized that if an interaction between integral proteins band 3 and GPA were important for their intracellular trafficking, then human GPA might compete with mouse GPA for band 3 binding, resulting in a decreased surface expression of mouse GPA. To test this hypothesis, we compared the levels of expression of mouse GPA in wild-type and transgenic red cells at various concentrations of TER-119. Flow cytometric analysis showed that the level of expression of murine GPA in transgenic erythrocytes was decreased compared with that in the wild type at each of the antibody concentrations tested (Figure6A). In sharp contrast, no change in band 3 expression was noted between transgenic and wild-type erythrocytes when band 3 molecules were labeled with various concentrations of EMA (Figure 6B). These data strongly imply a competition between mouse GPA and human GPA for band 3 and add strength to the postulate of a direct interaction between the sialoglycoprotein and the anion exchanger.
Bar histogram of surface expression of murine GPA and band 3.
(A) Wild-type (░) and GYPA transgenic (■) mouse red blood cells were labeled with varying concentrations of TER-119–PE and analyzed by flow cytometry. (Wild type: n = 4; transgenic: n = 3.) (B) Band 3 in wild-type and GYPA transgenic mouse red blood cells was labeled with varying concentrations of EMA and analyzed by flow cytometry. (Wild type: n = 4; transgenic: n = 3.) The error bars represent SDs.
Bar histogram of surface expression of murine GPA and band 3.
(A) Wild-type (░) and GYPA transgenic (■) mouse red blood cells were labeled with varying concentrations of TER-119–PE and analyzed by flow cytometry. (Wild type: n = 4; transgenic: n = 3.) (B) Band 3 in wild-type and GYPA transgenic mouse red blood cells was labeled with varying concentrations of EMA and analyzed by flow cytometry. (Wild type: n = 4; transgenic: n = 3.) The error bars represent SDs.
Discussion
We have successfully produced transgenic mice with red cells expressing human GPA protein and have begun to use them as a model to study GPA dimerization and the interaction of band 3 and GPA in vivo. A major finding of the current investigations was that murine GPA expression was decreased in erythrocytes expressing human GPA. These results strongly suggest the existence of a molecular mechanism regulating the total amount of GPA stably assembled on the erythrocyte membrane. One potential mechanism for this regulation could be that the intracellular trafficking or membrane insertion of GPA is dependent upon an interaction with band 3. We found that the level of band 3 expression remained constant in the wild-type and transgenic mouse membranes whereas the murine GPA expression decreased incrementally as the human GPA expression increased; this implies a competition between mouse GPA and human GPA for endogenous band 3 during their intracellular trafficking.
Our data, therefore, are consistent with a number of earlier observations and lend strength to the argument that band 3 and GPA interact. That this interaction exists during transit through the Golgi apparatus is suggested by studies of naturally occurring red cell mutants showing that band 3 glycosylation is altered when GPA expression is abnormal. In En(a-) and MkMk red cells, which completely lack GPA, the average length of the N-glycan chain attached to band 3 is increased.31-33 In contrast, glycosylation of band 3 is reduced in Dantu red cells, which contain an increased amount of glycophorin-associated carbohydrate because of the presence of normal and mutant GPA.34 Interestingly, these changes in glycosylation have a net result of maintaining the red cell negative surface charge, which is critical in preventing red cell aggregation in the circulation.
Also striking was our finding that human GPA molecules encoded by the transgene did not dimerize with endogenous murine GPA. This observation was surprising in light of the high degree of homology between mouse and human sequences in the transmembrane span, known to be critical for human GPA dimer formation. Characterization of the region of GPA involved in dimerization began with pioneering studies by Furthmayr and Marchesi, suggesting that interaction occurred through the hydrophobic transmembrane domains.5 Further investigations with synthetic peptides of the transmembrane domains confirmed the specificity of this interaction both in detergent and in plasma membrane vesicles.4 A number of studies have now identified the sequence motif required for GPA dimerization under various in vitro conditions. Site-directed mutagenesis of GPA in SDS micelles defined 7 critical amino acids: L75I76XXG79V80XXG83V84XXT87.23-25By nuclear magnetic resonance spectroscopy of GPA peptide in aqueous detergent micelles, MacKenzie et al showed that I,76V,80V84on the α helix of one monomer interface with L,75G,79G,83T87 on the second monomer.26 Studies to pinpoint the dimerization motif have also been done in a membrane environment using the transmembrane domain of GPA fused to the cytoplasmic domain of ToxR, which in the dimer state activates transcription.35 In this system, the sequence L75I76XXG79XXXG83XXXT87showed the same degree of association as wild type and, interestingly, the motif G79XXXG83 by itself demonstrated strong assembly.
The human transmembrane domain sequence of GPA differs from the mouse at 3 sites: T74, F78, and V84(Figure 7). The mouse sequence therefore contains the L75I76XXG79XXXG83XXXT87motif critical for dimer formation in ToxR chimeric proteins, as well as 6 of the 7 amino acids critical for association in detergent. The single amino acid difference within the dimerization motif, as defined in detergent, is that V84 is altered to I in mouse GPA. However, the identical alteration occurs in human GPB, and human GPB and GPA form heterodimers that are stable under the conditions of SDS-PAGE.36,37 It has also been shown that mutagenesis of V84 has little effect on the dimerization of GPA,38,39 further suggesting that the presence of the I should not by itself inhibit human/mouse GPA heterodimer formation. There are, however, 2 other differences between the transmembrane regions that may contribute to the lack of dimerization. Recent work has shown that the distance between the dimerization motif and the basic residues that flank the cytoplasmic side of the transmembrane domain is important in determining the dimerization of GPA in SDS-PAGE.40 These clustered basic residues (R96R97 and K100K101) probably localize one end of the transmembrane segment by binding the acidic head groups of the SDS in the micelles. Mouse GPA differs from human GPA and GPB in the replacement of the residue equivalent to R96 of human GPA by S. This has the effect of increasing the length of the hydrophobic sequence between the dimerization motif and the boundary defined by the basic cluster by 1.5 Å compared with this distance in human GPA and GPB. The increased distance may change both the axial and rotational registration between the dimerization interfaces of human and mouse GPA monomers so that they cannot interact readily. A further difference between human and mouse GPA is in the overall length of the hydrophobic helical transmembrane segments (Figure 7). As well as being extended by one amino acid at the C-terminal end, the mouse GPA helix is extended by 2 amino acids at the N-terminal end, where the helices are probably initiated by the P residues. The greater length of the mouse transmembrane helix would require the transmembrane regions of mouse and human GPA monomers to take up different orientations when located in the same SDS micelle.
Alignment of human GPA, mouse GPA, and human GPB sequences in region of transmembrane domain.
Residues that form the dimer interface in human GPA and corresponding residues in mouse GPA and human GPB are in bold. The portions of the sequences that form the hydrophobic helical transmembrane domains are underlined.
Alignment of human GPA, mouse GPA, and human GPB sequences in region of transmembrane domain.
Residues that form the dimer interface in human GPA and corresponding residues in mouse GPA and human GPB are in bold. The portions of the sequences that form the hydrophobic helical transmembrane domains are underlined.
We also observed that in the transgenic red cell membranes, dimerization occurred between 2 glycosylated monomers, 2 nonglycosylated monomers, and a glycosylated and nonglycosylated monomer. These results clearly show that glycosylation of the extracellular domain of human GPA molecules does not affect the ability of the transmembrane domains to dimerize in vivo.
Although monoclonal antibody TER-119 has been widely used as a specific marker for the erythroid lineage in studies of mouse hematopoiesis,27,28 its antigenic specificity had not been precisely defined. We discovered that TER-119 binds to the same protein bands as a well-characterized antibody to the cytoplasmic domain of mouse GPA.18 We also found that TER-119 fails to react with mouse red cells lacking GPA. From these data, we conclude that TER-119 recognizes an epitope on murine GPA. This epitope is exposed on the cell surface and hence must localize to the extracellular domain of GPA. As such, TER-119 is the only antibody probe currently available that is specific for the extracellular domain of murine GPA.
In summary, mice transgenic for the human GYPA gene have provided a number of valuable insights into the interrelationship between band 3 and GPA during erythrocyte membrane biogenesis and into the regulation of GPA dimerization. We have learned that in vivo GPA dimerization is not regulated solely by the transmembrane spanning domain, but that the distance between the dimerization motif and the basic residues that flank the cytoplasmic side of the transmembrane domain is important. In addition, the carbohydrate structures on the GPA extracellular domain do not affect dimerization because we observed that monomers with varying degrees of glycosylation dimerized. Finally, we have discovered that murine GPA expression is reduced in erythrocytes expressing human GPA and the level of band 3 expression remains constant, implying a tight coupling of band 3 and GPA expression in the membrane of mature red cells. These data underscore the added power of studying full-length proteins in their native environment. Moreover, a number of protein interactions in the red cell membrane have been described, such as Rh50-LW41 and Kell-KX,42 in which one binding partner has multiple transmembrane spans and the other has a single span. We speculate that the association between band 3 and GPA may serve as a model for interactions of other multipass and single-pass polypeptides during membrane biogenesis.
We would like to thank Dr David Anstee for providing us with monoclonal antibodies BRIC 163 and R10 and Dr Stanley Schrier for providing us with antispectrin antibody. We are very grateful to Drs Uli Weier and Robert Lersch for their invaluable help in FISH analysis and Ms Alicia Sheppard for expert assistance in preparation of this manuscript.
Supported in part by National Institutes of Health grants DK26263, DK32094, DK56267, and HL31579; and by the Director, Office of Health and Environment Research Division, U.S. Department of Energy, under contract DE-AC03-76SF00098.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Joel Anne Chasis, Lawrence Berkeley National Laboratory, Bldg 74, 1 Cyclotron Rd, Berkeley, CA 94720; e-mail:jachasis@lbl.gov.






This feature is available to Subscribers Only
Sign In or Create an Account Close Modal