Abstract
The role of ferritin expression on the labile iron pool of cells and its implications for the control of cell proliferation were assessed. Antisense oligodeoxynucleotides were used as tools for modulating the expression of heavy and light ferritin subunits of K562 cells. mRNA and protein levels of each subunit were markedly reduced by 2-day treatment with antisense probes against the respective subunit. Although the combined action of antisense probes against both subunits reduced their protein expression, antisense repression of one subunit led to an increased protein expression of the other. Antisense treatment led to a rise in the steady-state labile iron pool, a rise in the production of reactive oxygen species after pro-oxidative challenges and in protein oxidation, and the down-regulation of transferrin receptors. When compared to the repression of individual subunits, co-repression of each subunit evoked a more than additive increase in the labile iron pool and the extent of protein oxidation. These treatments had no detectable effects on the long-term growth of cells. However, repression of ferritin synthesis facilitated the renewal of growth and the proliferation of cells pre-arrested at the G1/S phase. Renewed cell growth was significantly less dependent on external iron supply when ferritin synthesis was repressed and its degradation inhibited by lysosomal antiproteases. This study provides experimental evidence that links the effect of ferritin repression on growth stimulation to the expansion of the labile iron pool.
Introduction
The labile iron pool (LIP)1,2 of mammalian cells has been implicated in cell iron regulation and in the production of intracellular reactive oxygen species (ROS).3,4 A major role in the intracellular modulation of that pool has been attributed to ferritin (FT), particularly to its heavy subunits (H-FT).5,6 This view is supported by results obtained with cells overexpressing H-FT subunits in an iron-independent manner.3,4 In accordance with the role of FT as an iron scavenger, those cells displayed reduced steady-state levels of LIP,4 reduced ROS production, and increased resistance to ROS toxicity.3,4 Similar results were obtained with HeLa cells transfected with the wild-type H-FT cDNA linked to an inducible promoter.7
Although H-FT evoked a reduction in LIP and thereby affected related cellular properties, growth and proliferation were modified only at very high levels of H-FT expression.3,7 These and other studies based on H-FT–transfected cells raise some questions about the significance of strategies based on FT overexpression for understanding FT involvement in the control of cell growth. On the one hand, the transcribed FT message might be only partially translated, and most of the resultant polypeptides might even lack the requisite functional properties.8,9 On the other hand, cellular compensatory properties might mask putative FT functions.3 Moreover, short-term effects of FT expression might not necessarily be reflected in the long term, and vice versa. In that respect, recent studies10-13 suggested a link between the repression of H-FT expression and the promotion of cell proliferation by oncogenes such as c-myc14,15 and E1A.10-13,16 However, earlier studies with c-myc showed the opposite, namely that stimulation of growth correlated with H-FT expression.17
To resolve the cellular roles of FT in iron metabolism and growth through the modulation of LIP, we opted for a complementary approach to that of gene overexpression. Antisense (AS) technology, which is based on Watson-Crick hybridization with unique target mRNAs, was used as a means to selectively suppress FT expression. AS oligodeoxynucleotides (ODNs) were the tools used because they have already proven effective in repressing gene expression in various erythroid and myeloid cells18,19 and because, in iron-overexposed cells, they were effective in repressing H-FT expression.20 The FT-AS-ODNs were used here to specifically manipulate either (or both) FT subunits in a gradual manner and independently of the iron status of the cells. The AS-mediated repression of FT was analyzed at both the message and the protein levels, and the resultant changes in LIP, ROS production, and protein oxidation were assessed in cells by fluorescence detection of metals,2 oxidation of carboxydihydrofluorescein (CDCF),21 and carbonyl protein oxidation assay,22 respectively. Our results provide experimental evidence for the concept that FT repression induces an expansion of LIP and of LIP-related properties. Those properties allow a more expeditious resumption of cell growth after its arrest, in support of the proposed role of FT repression in the modulation of short-term cell growth.
Materials and methods
Materials and cells
Calcein, calcein-am, and CDCF-DA (2′-7′-CDCF-diacetate) were procured from Molecular Probes (Eugene, OR), apotransferrin from Kamada (Beth Kama, Israel), AS-ODNs from Microsynth (Balgach, Switzerland). Unless specified otherwise, all other chemicals were from Sigma Chemical (St Louis, MO) or best available grade. Salicylaldehyde hydrazone (SIH) was kindly provided by Prof Prem Ponka (McGill University, Montreal, Canada), and recombinant L-FT and H-FT and antibodies against them were kindly provided by Dr Paolo Santambroggio (DIBIT, Milan, Italy). Human erythroleukemia K562 cells were grown and maintained as previously described.1
FT quantitation by ELISA
For FT determination, samples of approximately 400 000 cells in 400 μL were centrifuged, and the pellet was extracted at 4°C for 15′ with 200 μL Tris-Cl 10 mM, 150 mM NaCl, 0.3% Triton X-100 (Boehringer Mannheim, Germany), 0.25% NaN3, pH 7.4, containing an antiprotease l (P-8340; Sigma), and 250 μM phenylmethylsulfonyl fluoride. The extract was centrifuged at 8500g for 2 minutes at 4°C, and the supernatant was sampled for protein determination (BCA method; Sigma). Samples of 2 μg protein were added to each anti-FT precoated well of 96-well immunosorbent plates (Nunc, Roskilde, Denmark). The latter were prepared by 1-hour incubation at 37°C with mouse antihuman H-FT or mouse antihuman L-FT monoclonal antibodies (20 μg/mL) in carbonate buffer (pH 9.6). The plates were subsequently treated with 3% bovine serum albumin (BSA) in phosphate-buffered saline (PBS), pH 7.4, for 2 hours at room temperature (blocking step). As FT calibration standards, we used 0 to 1 ng/well recombinant H-FT or L-FT. Those standards and the extracted cytosolic samples were added to the precoated wells in 3% BSA and incubated for 1 hour at 37°C. For H-FT determination, we used rabbit serum raised against human placental FT (containing approximately equal amounts of anti–H and anti–L-FT antibodies). For L-FT determination, we used a rabbit serum raised against human spleen FT (with 95% anti–L-FT and 5% anti–H-FT activities) (courtesy of Prof Konijn, Jerusalem, Israel). Sera were added to the wells and incubated for 3 hours at room temperature. Goat–antirabbit IgG conjugated to β-galactosidase (Amersham, United Kingdom) served as secondary antibodies in conjunction with fluorescein–β-galactoside as fluorogenic substrate. These secondary antibodies were incubated for 1 hour at 37°C and then for 1 hour at room temperature. The intensity of fluorescence (488-525 nm) was followed with time in a Fluostar II fluorescence plate reader (BMG GmbH, Offenburg, Germany) and calibrated with normal rabbit IgG as standard.
Assessment of RNA levels of H-FT, L-FT, and L7 by reverse transcription–polymerase chain reaction
Total RNA from K562 cells was isolated using Ultraspec RNA isolation reagent (Biotec Laboratories, Houston, TX) after cells were subjected to different FT AS-ODN treatments for 48 hours. This RNA (1 μg) was reverse-transcribed and polymerase chain reaction (PCR)–amplified using the Titan One Step reverse transcription (RT)-PCR system (Roche Diagnostics, Mannheim, Germany) according to the manufacturer's guidelines. In brief, total RNA was added to the PCR mix (which included the AMV reverse transcriptase, the Taq and Pwo DNA polymerases, and 40 U/μL RNase Inhibitor), together with the following primer pair for human H-FT (accession L20941): forward, 5′-GCCAAATACTTTCTTCACC-3′; reverse, 5′-TTCATTATCACTGTCTCCC-3′. These primers spanned a 390-bp sequence between residues 365 and 754. For human L-FT (accession M10119), the primer pair was: forward, 5′-AGGACATCAAGAAGCCAG-3′; reverse, 5′-AGGAAGTCACAGAGATGGG-3′. These primers spanned a 151-bp sequence between residues 318 and 468. For human L7 (an internal control, accession #X57958), the primer pair was: forward, 5′-GAAGAGAAGAAGAAGGAGG-3′; reverse, 5′-GGTACATAGAAGTTGCCAG-3′. These primers spanned a 239-bp sequence between residues 50 and 288. After reverse transcription at 50°C for 30 minutes (which was terminated by heating for 2 minutes at 94°C), touch down (TD) PCR was performed for 10 cycles using the following temperatures: denaturation (1 minute) at 94°C, annealing (2 minutes) at 65°C with a lowering increment of 2°C per cycle, and elongation (3 minutes) at 68°C. Then thermocycling was continued for up to 21 cycles under the following conditions: denaturation for 30 seconds at 94°C, annealing for 30 seconds at 45°C, and elongation for 45 seconds (+5 seconds per cycle) at 68°C. Samples were collected at cycles 16 to 21 after the TD-PCR. All RT-PCR reactions were performed using the Mastercycler thermocycler (Eppendorf, Hamburg, Germany). The samples were separated on a 1.6% agarose gel containing the SYBR-gold nucleic acid stain (Molecular Probes, Eugene, OR) and photographed under ultraviolet illumination. Optical densities of samples taken from cycles 16 to 21 were within a linear range.
Immunofluorescence
Cells fixed in methanol and treated with 3% BSA were incubated first with mouse monoclonal antihuman H-FT and subsequently with fluorescein isothiocyanate (FITC)–conjugated antimouse IgG and 1 μg/mL 4′-6′-diamidino-2-phenylindole (DAPI). Samples of 10 μL were loaded on a glass slide and examined in the Olympus IX70 (Tokyo, Japan) fluorescence microscope (exc 490 nm, em 520 nm). Images were processed using the EPIX (Buffalo Grove, IL) XCAP Image Acquisition, Display, Processing and Analysis software.
LIP measurement and cell ROS production
LIP was measured by the calcein-based fluorescence method, as described in detail previously.2 ROS was determined in cells (106/mL) loaded with 10 μM CDCDHF-am(6′-carboxy-2′,7′-dichlorodihydrofluorescein-acetomethoxy) for 20′ at 37°C.21 The loading medium consisted of Dulbecco minimum essential medium (DMEM) supplemented with 20 mM Na-HEPES and 1% BSA. The reaction was stopped by rapid centrifugation and resuspension in HBS supplemented with hydroxyethyl-starch-DFO (Biomedical Frontiers, MN) to prevent extracellular metal catalyzed oxidation of the substrate, which might leak from the cells. The initial rate of H2O2(5 μM)-induced conversion of the nonfluorescent 2′-7′ CDCF to the 2′-7′ oCDCF was measured fluorometrically (PTI, South Brunswick, NJ) (exc 488-517 nm). Maximum fluorescence (Fmax) was attained by permeabilizing cells with 0.5% Triton X-100 and exposing them to fresh 1 mM ferrous ammonium sulfate and 1 mM H2O2. ROS formation rate was assessed by the amount of oCDCF produced per second. oCDCF concentration in the cuvette at any given time was given by (oCDCF)c = (F/Fmax) × (oCDCF)cmax, where F and Fmax were the fluorescence at any given time and the maximal fluorescence, respectively, (both in relative units) and (oCDCF)cmax was the factor obtained by calibration with standard oCDCF. The cellular concentration of oCDCF, (oCDCF)in, was calculated by (oCDCF)in = (oCDCF)c/(NC*VC), where NC was the cell concentration in the cuvette (cells/mL) and VC was the volume of a single cell (ml/cell). Cells were counted in a cell counter, and the cell volume was obtained as described elsewhere.23
Cell iron content
Total cell iron was measured in triplicate samples of 5 × 106 cells suspended in 1 mL HBS buffer (150 mM NaCl, 20 mM HEPES). The cells were mixed with 1 mL acid mixture (3 N HCl, 10% trichloroacetic acid, and 3% thioglycolic acid) and incubated for 2 hours at 37°C, cooled, centrifuged at 3000 rpm for 30′, and mixed with 0.5 mL bathophenanthroline sulfonate (0.045% in 4.5 N Na-acetate, 0.2% thioglycolic acid). Light absorbance of the solution was then read at 535 nm in a Spectronic 3000 UV-VIS Photodiode Spectrophotometer (Milton Roy, Oostende, Belgium).
Tf-55Fe uptake and TfR expression
K562 cells treated with FT–AS-ODN and resuspended (106/mL) in DMEM supplemented with 20 mM HEPES and 1 mg/mL BSA were exposed to an oversaturating concentration (15 μg/mL) of Tf-55Fe alone (total) or with 1 mg/mL Tf-Fe (nonspecific). Cells were subsequently incubated at 37°C in 5% CO2 and after 0, 30, 60, and 120 minutes were placed on ice for stopping the uptake. After 2 washes with cold PBS, 20-minute stripping with 1 mg/mL pronase E, 2 more washes with cold PBS, and resuspension in 200 μL PBS, triplicate 50 μL samples from each time point were counted for radioactivity in an aqueous Quicksafe A (Zinsser, Maidenhead, United Kingdom) scintillation fluid in a Beta counter (Beckman, Irvine, CA). Samples were also assayed for protein by the BCA method. Specific Tf-55Fe uptake was determined by subtracting the nonspecific counts from the total counts for each time point. The time 0 point, obtained by incubation at 4°C (no stripping), represented the saturating cell surface Tf binding, or TfR level.
Protein oxidation
Oxidative modification of proteins was assessed in cytosolic extracts by the carbonyl assay.22 Cells were extracted with 50 mM phosphate buffer, pH 7.4, containing 0.1% digitonin, antiprotease cocktail (see above), and 1 mM EDTA. Cells were subsequently centrifuged at 8500g for 2 minutes at 4°C to remove the nuclei. This was confirmed by obtaining a 260/280 absorption ratio of less than 1, and allowed omission of the streptomycin step for the removal of contaminating nucleic acids.
Cell growth
Viable cells were counted in a Coulter Cell Counter (Coulter Electronics, Harpended, Herts, United Kingdom), and their metabolic activity was measured by the Alamar Blue method according to the manufacturer's instructions. In brief, cells were plated in quadruplicate in 96-well culture plates at a density of 50 000/mL. Alamar Blue (5% final) was added at 24-hour intervals, and fluorescence (540-590 nm) was read in a BMG Fluostar plate reader after 4 hours of incubation in culture conditions. For the subtraction of background fluorescence, we used metabolically inhibited cells (ie, cells grown in the presence of 5 μM cycloheximide). Subtracted fluorescence values were normalized to those of untreated cells. Cell death index was determined on 10 000 cells by flow cytometry (Facscalibur; BDIS, San Jose, CA) with and without 5 μg/mL propidium iodide. The percentage of propidium iodide–positive (dead) cells was calculated from the 2 distribution curves.
Cell-cycle progression after growth arrest
K562 cells (initially at 200 000/mL), treated with different FT AS-ODNs for 24 hours, were supplemented with hydroxyurea (2 mM) for an additional 24-hour incubation. Cells were released from growth arrest after 2 washes with PBS and resuspension in growth medium. The distribution of 10 000 cells in the different cell-cycle phases (at 3, 6, and 9 hours) was obtained by flow cytometry as described above. For this analysis, cells were fixed in 75% methanol (1 hour at −20°C), washed, and treated with propidium iodide (5 μg/mL). For assessing the dependence of growth renewal on external iron, K562 cells treated with AS-ODN for 24 hours were supplemented with 20 μM lovastatin for 24 hours, washed, and transferred to serum-free media containing different concentrations (0-50 μg/mL) of Tf-Fe. The medium optionally contained 4 mM mevalonic acid (a lovastatin inhibitor) and 20 μg/mL leupeptin (a lysosomal protease inhibitor), which were renewed every 3 hours. At 3, 6, and 10 hours, they were analyzed for cell-cycle distribution. The percentage of cells in the S phase at each time point was obtained by ModFit LT Cell Cycle Analysis software (Verity, Topsham, ME).
Results
Repression of FT subunits
Sequences of the human FT mRNA (H and L subunits), approximately 20 bp long, were screened by the software package GCG to trace for potential AS-ODNs. The main criteria for sequence exclusion were nonspecific homology to mRNA sequences other than hours and L-FT, self-annealing, repeat annealing, and high GC content. We selected 3 evenly spaced AS-ODNs in which the phosphodiester bonds of the 3 3′ terminal bases were replaced with phosphorothioate bonds to minimize degradation by intracellular nucleases. Of these, a 20-bp long sequence complementary to a region in the center of the H-FT transcript was found empirically to maximally repress H-FT expression, and it was chosen for subsequent assays. This sequence (ASH-ODN) was 5′-CCAAATGTAATGCACACTCC-3′. The AS sequence for L-FT (ASL-ODN), 5′-GAGAGAGGTAGGTGTAGGAG-3′, was obtained in a similar manner. One of the H-FT AS-ODN (5′-GAGATGCGGAGGATGCAAAT-3′) was built in reverse order (IN-ODN) and used as a negative control in all experiments. Desferrioxamine treatment (DFO; 50 μM for 24 hours) was used as a positive control for the repression of expression of both FT subunits.24 AS-ODNs (1 nM-1 μM) were applied to cells (2 × 105/mL) in full medium with or without daily replenishment for 24, 48, and 72 hours. The effect of AS-ODNs on hours and L-FT levels in K562 cells is shown in Table1. The most effective AS-ODN treatments against either H-FT or L-FT were found empirically to be after 48-hour incubation with 1 nM AS-ODN of the respective probes. Under these conditions, H-FT and L-FT levels were lowered to approximately 25% to 50% of the levels found in cells incubated with the reversed or inverted sequence (IN-ODN) of the AS-ODN, comparable to those attained with 50 μM DFO for 24 hours.
Individual and combined effects of antisense oligodeoxynucleotides to heavy and light ferritin subunits on the labile iron pool, pro-oxidant-induced reactive oxygen species production, and iron content of cells
| AS added (48-h incubation) . | H-FT* . | L-FT* . | Normalized LIP* . | ROS production* . | Protein oxidation* . | Iron content (μM) . |
|---|---|---|---|---|---|---|
| None | 100 ± 25 | 100 ± 18 | 100 ± 0.36 | 100 ± 46 | 100 ± 4.47 | 727 ± 63 |
| Inversed H-FT | 94 ± 32 | 151 ± 22 | 94 ± 0.54 | 108 ± 0.38 | 112 ± 1.23 | 955 ± 347 |
| H-FT | 24 ± 7† | 270 ± 32‡ | 150 ± 18‡ | 231 ± 0.77† | 99 ± 4.8 | 615 ± 17 |
| L-FT | 169 ± 17‡ | 45 ± 14‡ | 121 ± 9‡ | 192 ± 19‡ | 112 ± 2.3 | 735 ± 22 |
| H- and L-FT | 18 ± 4† | 21 ± 14‡ | 259 ± 2.68† | 270 ± 38‡ | 152 ± 1.9† | 795 ± 30 |
| AS added (48-h incubation) . | H-FT* . | L-FT* . | Normalized LIP* . | ROS production* . | Protein oxidation* . | Iron content (μM) . |
|---|---|---|---|---|---|---|
| None | 100 ± 25 | 100 ± 18 | 100 ± 0.36 | 100 ± 46 | 100 ± 4.47 | 727 ± 63 |
| Inversed H-FT | 94 ± 32 | 151 ± 22 | 94 ± 0.54 | 108 ± 0.38 | 112 ± 1.23 | 955 ± 347 |
| H-FT | 24 ± 7† | 270 ± 32‡ | 150 ± 18‡ | 231 ± 0.77† | 99 ± 4.8 | 615 ± 17 |
| L-FT | 169 ± 17‡ | 45 ± 14‡ | 121 ± 9‡ | 192 ± 19‡ | 112 ± 2.3 | 735 ± 22 |
| H- and L-FT | 18 ± 4† | 21 ± 14‡ | 259 ± 2.68† | 270 ± 38‡ | 152 ± 1.9† | 795 ± 30 |
All AS-ODN concentrations were 1 nM. FT levels were measured by enzyme-linked immunosorbent assay, described in “Materials and methods.” Levels of FT in the untreated cells were 308 ± 78 and 76 ± 14 ng/mg protein for H-FT and L-FT, respectively. Those control levels varied among experiments within the ranges of 150 to 400 and 50 to 150 ng/mg protein for H-FT and L-FT, respectively. DFO treatment (50 μM for 24 hours) was used as a positive control for repression of expression of both FT subunits.24 LIP levels were measured by the calcein-based method2 in cells taken from exponentially growing cultures, and their values were normalized to intracellular calcein. LIP level of the untreated cells here was 1.12 ± 0.004 μM and varied among experiments within the range of 0.08 to 0.14. ROS production was determined 100 seconds after 5 μM H2O2 challenge by the CDCF method, as described in “Materials and methods.” Its level in the untreated cells here was 2.6 ± 1.20 nM CDCF oxidized/second and varied among experiments within the range of 1 to 3 nM CDCF oxidized/second. Protein oxidation was measured by the carbonyl assay,22 as described in “Materials and methods.” Its level in the untreated cells was 10.2 ± 0.45 nmol CO/mg protein and varied among experiments within the range of 10 to 20 nmol CO/mg protein. Ferric ammonium citrate treatment (50 μM for 48 hours) resulted in levels of protein oxidation similar to those attained by the combined AS treatment against hours and L-FT.
AS-ODN indicates antisense oligodeoxynucleotides; H-FT and L-FT, heavy and light ferritin subunits; LIP, labile iron pool; ROS, reactive oxygen species.
Values are in percentage of untreated cells. Values for the AS-ODN–treated cells were different from the values in the untreated cells. Values were determined by independent t test. Data are from 1 of 3 representative experiments, each performed in triplicate.
P = .01.
P = .05.
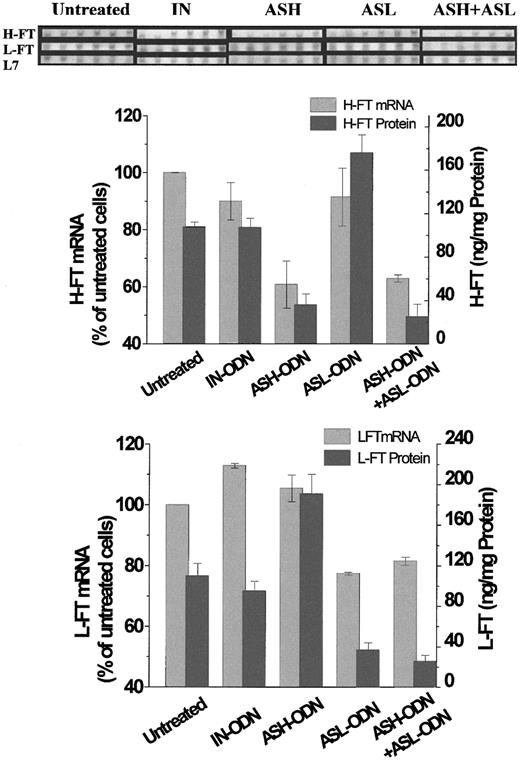
Blockage of FT expression by AS-ODN was also assessed at the message level. As shown in Figure 1, the FT-AS-ODN treatments reduced the L7-normalized mRNA level of H-FT (ASH-ODN) to 60% of untreated cells and that of L-FT (ASL-ODN) to 77% of untreated cells. The combined AS-ODN treatments (ASH-ODN + ASL-ODN) lowered the mRNA levels of both subunits, whereas the AS-ODN treatments against one FT subunit did not affect the message level of the other. The IN-ODN treatment exerted no significant effect on the FT mRNA levels compared to the untreated cells. After these results, we tentatively attributed the mode of repression to the reduction in the respective mRNA levels.
RT-PCR analysis and expression profiles of the mRNA of H-FT and L-FT and RT-PCR analysis of L7 rRNA, all extracted from cells treated with AS-ODN against hours and L-FT.
SYBR-GOLD stains are of cycles 16 to 21 after a touchdown PCR step of 10 cycles, as described in “Materials and methods.” Below the stains are depicted the relative changes in L7-normalized H-FT and L-FT mRNA levels, together with their respective protein levels (measured by ELISA). Those changes were calculated using densitometric analysis of bands of cycle 18, which were within the linear range of increase in luminosity against cycle number. Means and standard deviations of 3 independent experiments are shown here. Cell incubations: untreated, 48 hours, no AS-ODN added; AS-ODN, 48 hours with 1 nM AS-ODN against H-FT (ASH-ODN), L-FT (ASL-ODN), or both (ASH-ODN+ASL-ODN); IN-ODN, 48 hours with 1 nM one ASH-ODN sequence in reverse order. See “Results” for ODN sequences. ASH-ODN and ASH-ODN+ASL-ODN treatments produced H-FT mRNA band densities that were significantly lower than those of the untreated cells (P ≤ .013 and P ≤ 2.8 × 10−4, respectively). ASL-ODN and ASH-ODN+ASL-ODN also significantly reduced the L-FT mRNA band densities (P≤ 9.87 × 10−5 and P ≤ .001, respectively). Statistics for the protein expression levels are compiled in Table 1.
RT-PCR analysis and expression profiles of the mRNA of H-FT and L-FT and RT-PCR analysis of L7 rRNA, all extracted from cells treated with AS-ODN against hours and L-FT.
SYBR-GOLD stains are of cycles 16 to 21 after a touchdown PCR step of 10 cycles, as described in “Materials and methods.” Below the stains are depicted the relative changes in L7-normalized H-FT and L-FT mRNA levels, together with their respective protein levels (measured by ELISA). Those changes were calculated using densitometric analysis of bands of cycle 18, which were within the linear range of increase in luminosity against cycle number. Means and standard deviations of 3 independent experiments are shown here. Cell incubations: untreated, 48 hours, no AS-ODN added; AS-ODN, 48 hours with 1 nM AS-ODN against H-FT (ASH-ODN), L-FT (ASL-ODN), or both (ASH-ODN+ASL-ODN); IN-ODN, 48 hours with 1 nM one ASH-ODN sequence in reverse order. See “Results” for ODN sequences. ASH-ODN and ASH-ODN+ASL-ODN treatments produced H-FT mRNA band densities that were significantly lower than those of the untreated cells (P ≤ .013 and P ≤ 2.8 × 10−4, respectively). ASL-ODN and ASH-ODN+ASL-ODN also significantly reduced the L-FT mRNA band densities (P≤ 9.87 × 10−5 and P ≤ .001, respectively). Statistics for the protein expression levels are compiled in Table 1.
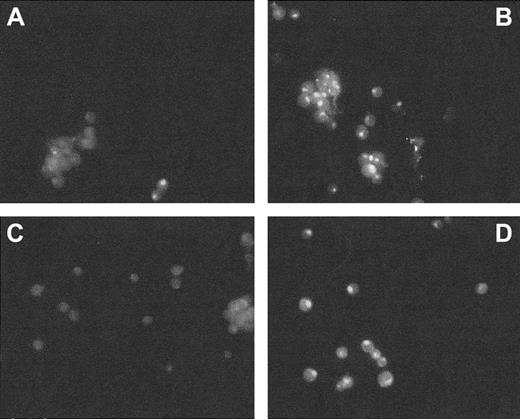
Although repression of FT was most pronounced at the protein level (Table 1), it was incomplete. To address whether this fact was associated with cells being affected differentially by the AS-ODNs, we analyzed them by immunofluorescence staining of FT with FITC-labeled antibodies and of the cell nuclei with DAPI. We observed that the expression of both FT subunits was abolished only in approximately two thirds of the population of AS-ODN–treated cells (Figure2). It remains to be established whether the pattern of the AS-ODN effect reflects heterogeneity of transport of the ODN into cells at different stages of growth or properties associated with AS-ODN interaction with mRNA.
Immunofluorescence assessment of H-FT levels in K562 cells.
Cells were incubated for 48 hours with 1 nM ASH-ODN (A), nothing (untreated cells, B), preimmune rabbit serum (C), and 1 nM IN-ODN sequence (D). Immunofluorescence was assayed by incubating the cells with mouse monoclonal antibodies against human H-FT. FITC-conjugated antirabbit IgG served as a secondary antibody.
Immunofluorescence assessment of H-FT levels in K562 cells.
Cells were incubated for 48 hours with 1 nM ASH-ODN (A), nothing (untreated cells, B), preimmune rabbit serum (C), and 1 nM IN-ODN sequence (D). Immunofluorescence was assayed by incubating the cells with mouse monoclonal antibodies against human H-FT. FITC-conjugated antirabbit IgG served as a secondary antibody.
LIP, ROS, and TfR modulation by FT repression
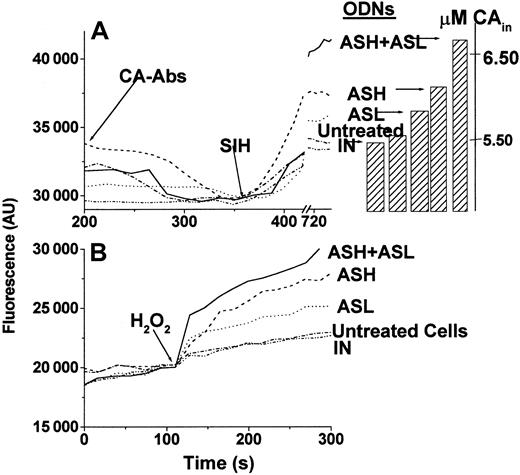
The effects of time and L-FT repression by AS-ODN on LIP and ROS formation were assessed in parallel. We postulated that by specifically decreasing hours and L-FT levels, the AS-ODN should evoke a sustained increase in steady-state LIP and ensuing metal-catalyzed ROS formation. This action of the AS-ODN is essentially different from a direct action on the LIP by chelating probes that is transient because of the activation of iron regulatory proteins (IRPs). Steady-state LIP, measured 48 hours after AS-ODN administration by a fluorescence method,2 was indeed significantly higher in the AS-ODN–treated cells than in untreated cells or cells treated with IN-ODN (Table 1, Figure 3A). An analogous rise in ROS production was observed when the AS-ODN–treated cells were challenged with H2O2 (5 μM) (Table 1, Figure3B). This result indicates that the up-modulation of LIP by H-FT and L-FT AS-ODN treatment is associated with a catalytic (labile) reservoir. AS-ODN effects on all cellular parameters (including the LIP) were maximal within 48 hours. Thereafter the effects gradually disappeared even if the AS-ODNs were replenished (data not shown), thus indicating the occurrence of possible intracellular compensatory changes. Although the LIP levels in cells with various FT levels were different, their total iron content was apparently similar (Table 1). It is important to state, in that context, that the LIP is only a minor fraction (less than 1%) of the total cell iron (data not shown and3).
LIP and ROS in K562 cells.
(A) Determination of LIP in K562 cells. After quenching of non–cell-associated CA fluorescence by anti-CA antibodies, the permeable chelator SIH was added (arrow), and LIP was determined as specified in 2 from the stabilized signal attained after its addition. Cell incubations before LIP determination: (-·-·-), 48 hours, no ODN added; (–··–··–), 48 hours with 1 nM IN-ODN; (-----), 48 hours with 1 nM ASH-ODN; (·····), 48 hours with 1 nM ASL-ODN; (———), 48 hours with 1 nM both ASH-ODN and ASL-ODN. Bars represent the μM concentrations of calcein in the cells after the addition of the chelator SIH. (B) Determination of pro-oxidant prompted ROS formation in K562 cells. ROS production was determined based on the rise in fluorescence in CDCF-DA-am–loaded cells, as described in detail in “Materials and methods.” H2O2 (5 μM) was used as the pro-oxidant and was added to the cells where indicated. Treatment annotations are the same as in panel A.
LIP and ROS in K562 cells.
(A) Determination of LIP in K562 cells. After quenching of non–cell-associated CA fluorescence by anti-CA antibodies, the permeable chelator SIH was added (arrow), and LIP was determined as specified in 2 from the stabilized signal attained after its addition. Cell incubations before LIP determination: (-·-·-), 48 hours, no ODN added; (–··–··–), 48 hours with 1 nM IN-ODN; (-----), 48 hours with 1 nM ASH-ODN; (·····), 48 hours with 1 nM ASL-ODN; (———), 48 hours with 1 nM both ASH-ODN and ASL-ODN. Bars represent the μM concentrations of calcein in the cells after the addition of the chelator SIH. (B) Determination of pro-oxidant prompted ROS formation in K562 cells. ROS production was determined based on the rise in fluorescence in CDCF-DA-am–loaded cells, as described in detail in “Materials and methods.” H2O2 (5 μM) was used as the pro-oxidant and was added to the cells where indicated. Treatment annotations are the same as in panel A.
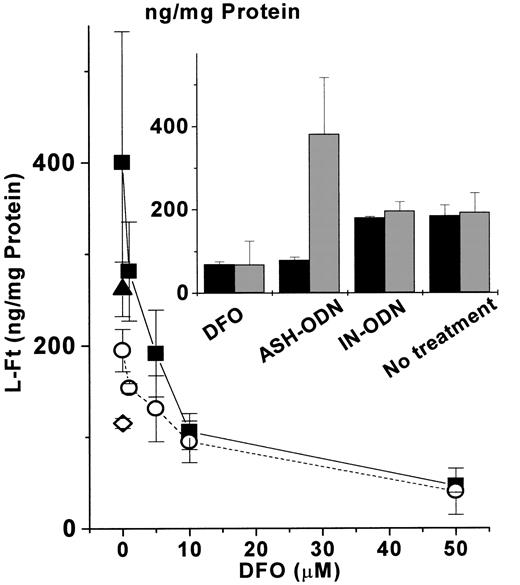
Earlier studies showed that overexpression of an IRE-mutated H-FT transgene repressed the levels of L-FT, possibly through a reduction of the cellular LIP.25 In this work we applied the inverse approach, based on AS-ODN tools, for a specific repression of each FT subunit. It was therefore interesting to assess whether the repression of one subunit might lead to an increased expression of its counterpart by an expansion of the LIP. The results shown in Table 1 indicate a compensatory increase in each FT subunit when its counterpart is repressed. We tested whether this increase was mediated by the increased LIP (Table 1). The results presented in Figure4 indicate that the compensatory increase in L-FT, observed when ASH-ODN was administered to the cells, was reversible with the addition of the iron chelator DFO. This effect of DFO was concentration dependent. DFO reversed the ASH-ODN–mediated up-regulation of L-FT by scavenging iron because the addition of iron-bound DFO could not revert L-FT up-regulation in H-FT–repressed cells. These results support an active role of each FT subunit in modulating LIP, with each balancing the another when one of them was repressed. To overcome this compensation, we used AS-ODN treatment against both FT subunits. The double AS-ODN treatment down-regulated the expression of both FT subunits (Table 1), suggesting it overcame the compensatory increase is observed in one FT subunit when the other was repressed (Table 1).
Influence of iron chelation on L-FT up-modulation by H-FT repression.
DFO (0-50 μM) was added for the last 24 hours to cells incubated for 48 hours with 1 nM of either ASH-ODN (▪) or IN-ODN (○). In both the ASH-ODN (▴) and the IN-ODN (⋄), 50 μM iron-bound DFO was also supplemented. (Inset) Influence of 48-hour incubation with 1 nM ASH-ODN on the expression of both H-FT (▪) and L-FT (░) compared with the influences of DFO (50 μM, 24 hours), IN-ODN (1 nM, 48 hours), and none (48 hours). Protein levels were quantified by ELISA, as described in “Materials and methods.” Data are from a representative experiment performed in triplicate.
Influence of iron chelation on L-FT up-modulation by H-FT repression.
DFO (0-50 μM) was added for the last 24 hours to cells incubated for 48 hours with 1 nM of either ASH-ODN (▪) or IN-ODN (○). In both the ASH-ODN (▴) and the IN-ODN (⋄), 50 μM iron-bound DFO was also supplemented. (Inset) Influence of 48-hour incubation with 1 nM ASH-ODN on the expression of both H-FT (▪) and L-FT (░) compared with the influences of DFO (50 μM, 24 hours), IN-ODN (1 nM, 48 hours), and none (48 hours). Protein levels were quantified by ELISA, as described in “Materials and methods.” Data are from a representative experiment performed in triplicate.
The double AS-ODN treatment brought forth an apparently synergistic increase in LIP compared to the sum of the AS effects on each FT subunit separately (Table 1). This can probably be attributed to the double AS treatment overcoming the compensatory increase in the nonrepressed subunit, which may restrain LIP up-regulation when only one FT subunit is repressed. However, the effect of the double AS-ODN treatment on ROS production was only partially additive, possibly indicating a saturating property.
We have established that the repression of FT expression by AS-ODN probes can lead to an increased steady-state LIP, and we have ascertained that this iron pool is indeed reactive (Table 1, Figure 4). To corroborate the latter, we also examined the effects of metal-stimulated oxidation of proteins, which is assumed to proceed through free radical attack. We found that only FT double AS-ODN treatment led to a significant rise in protein oxidation, comparable to the rise obtained on incubating the cells with 50 μM ferric ammonium citrate (data not shown), which is known to substantially increase the LIP26 (Table 1).
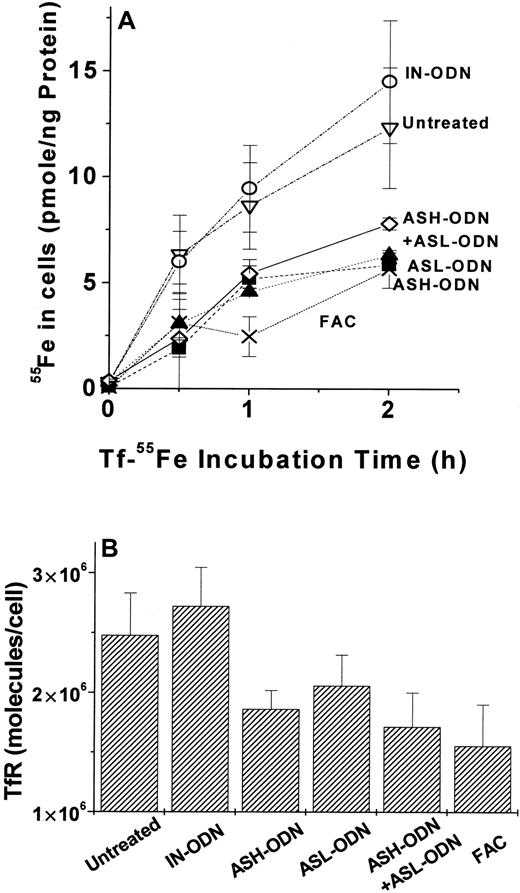
The up-modulation of LIP by repression of either or of both FT subunits was concomitant with a decrease in both TfR-mediated iron uptake (Figure 5A) and surface expression (Figure 5B). This observation indicates that the increased LIP formed by FT repression is also reactive inasmuch as the reduced TfR expression is probably related to IRP inactivation.27 28Despite the large differences in the steady-state LIP levels evoked by the double FT–AS-ODN and the single FT–AS-ODN treatments (Table 1), their effects on Tf-Fe uptake and TfR expression were similar (Figure5). This and the fact that addition of iron salts (20 μM for 24 hours) had similar effects on Tf-Fe uptake and TfR expression because of the different FT–AS-ODN treatments (Figure 5) point to a ceiling effect of LIP on TfR modulation.
Tf-55Fe uptake and transferrin receptor (TfR) surface expression in cells treated with AS-ODN against FT.
(A) Tf-55Fe uptake. (B)Transferrin receptor (TfR) surface expression. After incubation with various AS-ODNs, uptake of Tf-55Fe and surface expression of TfR were assayed, as described in “Materials and methods.” Treatments in panel A were none (▿), IN-ODN (○), ASH-ODN (▪), ASL-ODN (▴), ASH-ODN + ASL-ODN (⋄), and ferric ammonium citrate (20 μM, 24 hours) (X). TfR surface expression in all treatments besides IN-ODN were significantly different (P ≤ .05) in the untreated cells, as determined by independent t test. Data are from a representative experiment performed in triplicate.
Tf-55Fe uptake and transferrin receptor (TfR) surface expression in cells treated with AS-ODN against FT.
(A) Tf-55Fe uptake. (B)Transferrin receptor (TfR) surface expression. After incubation with various AS-ODNs, uptake of Tf-55Fe and surface expression of TfR were assayed, as described in “Materials and methods.” Treatments in panel A were none (▿), IN-ODN (○), ASH-ODN (▪), ASL-ODN (▴), ASH-ODN + ASL-ODN (⋄), and ferric ammonium citrate (20 μM, 24 hours) (X). TfR surface expression in all treatments besides IN-ODN were significantly different (P ≤ .05) in the untreated cells, as determined by independent t test. Data are from a representative experiment performed in triplicate.
Growth modulation by FT repression and iron chelation
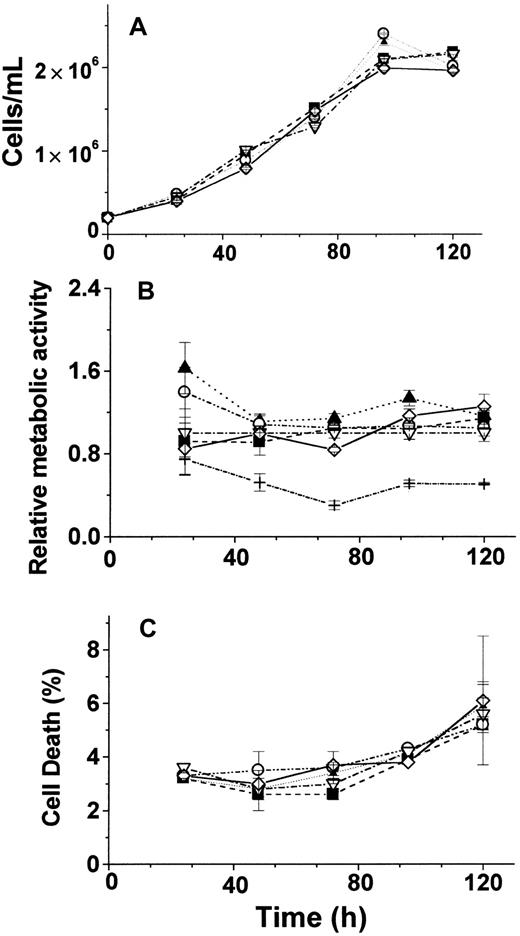
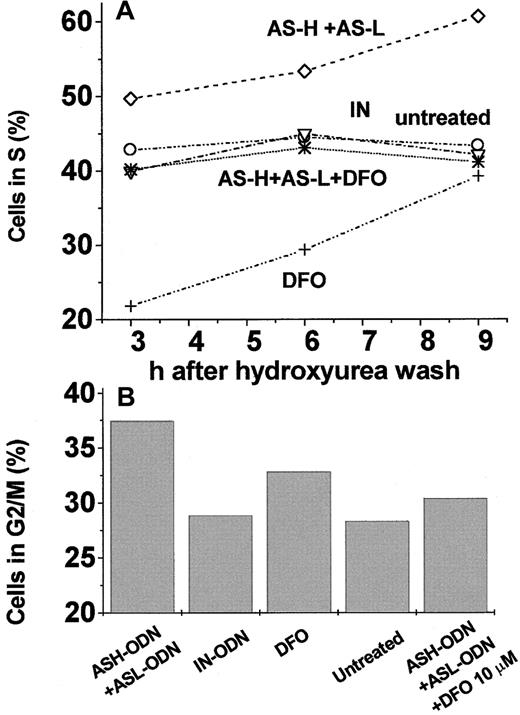
The effects of FT repression by AS-ODN treatment of cells were assessed in cells grown in normal culture conditions and following stimulation after growth arrest. Viable cell count (Figure6A), gross metabolic activity (Figure6B), and cell death index (Figure 6C) were found to be similar in AS-ODN–treated cells and in control cells when observed for a period of 6 days. The same results were obtained regardless of whether the AS-ODN probes were replenished daily (data not shown). On the other hand, when the cells were growth arrested at G1/S, FT AS-ODN treatment had a prominent effect on recovery from arrest. This was manifested in the DNA content of cells released from hydroxyurea-mediated cell-cycle arrest at G1/S (Figure7): 3 to 9 hours after the release from the blockage, 50% to 60% of the cells entered the S phase when treated with AS-ODN against H-FT+L-FT compared with 40% to 45% when treated with none or with IN-ODN (Figure 7A). To corroborate that the above results truly and significantly reflected entry of cells into the S phase, we also assessed the cell DNA content 24 hours after lifting the cell-cycle blockage. A positive correlation was found between the percentage of cells in G2/M and the percentge of cells in the S phase 15 to 21 hours earlier (Figure 7A-B).
Effects of long-term treatment with AS-ODN.
Cell proliferation (A), metabolic activity (B), and death index (C) after long-term incubation with various AS-ODN treatments. Cells were exposed to 1 nM of the following treatments: (▪), ASH-ODN; (▴), ASL-ODN; (○), IN-ODN; (▵), untreated cells; (⋄), ASH-ODN + ASL-ODN; +, DFO 50 μM for 24 hours. Relative metabolic activity was calculated using the fluorescence (Fl) of Alamar Blue–treated cells (see “Materials and methods”) and according to the formula (Fl − FlCHX)/(FlUntr − FlCHX), where CHX is 5 μM cycloheximide and Untr is untreated cells. Data are from a representative experiment performed in triplicate.
Effects of long-term treatment with AS-ODN.
Cell proliferation (A), metabolic activity (B), and death index (C) after long-term incubation with various AS-ODN treatments. Cells were exposed to 1 nM of the following treatments: (▪), ASH-ODN; (▴), ASL-ODN; (○), IN-ODN; (▵), untreated cells; (⋄), ASH-ODN + ASL-ODN; +, DFO 50 μM for 24 hours. Relative metabolic activity was calculated using the fluorescence (Fl) of Alamar Blue–treated cells (see “Materials and methods”) and according to the formula (Fl − FlCHX)/(FlUntr − FlCHX), where CHX is 5 μM cycloheximide and Untr is untreated cells. Data are from a representative experiment performed in triplicate.
Percentage of cells in the S and G2/M phases after release from hydroxyurea cell-cycle arrest of K562 cells subjected to different AS-ODN treatments.
(A) Cells were treated as follows: ⋄, 1 nM ASH-ODN +ASL-ODN for 48 hours; ○, 1 nM IN-ODN for 48 hours; +, 50 μM DFO for the last 24 hours of incubation (added with hydroxyurea); ▿, untreated cells; ∗, 1 nM ASH-ODN+ASL-ODN for 48 hours plus 10 μM DFO added for the last 24 hours of incubation. After 24 hours, hydroxyurea (2 mM) was added for the last 24 hours of incubation, and cells were harvested and analyzed by flow cytometry as described in “Materials and methods.” (B) The same cells were analyzed by flow cytometry 24 hours after the release from hydroxyurea. Data are from a representative experiment.
Percentage of cells in the S and G2/M phases after release from hydroxyurea cell-cycle arrest of K562 cells subjected to different AS-ODN treatments.
(A) Cells were treated as follows: ⋄, 1 nM ASH-ODN +ASL-ODN for 48 hours; ○, 1 nM IN-ODN for 48 hours; +, 50 μM DFO for the last 24 hours of incubation (added with hydroxyurea); ▿, untreated cells; ∗, 1 nM ASH-ODN+ASL-ODN for 48 hours plus 10 μM DFO added for the last 24 hours of incubation. After 24 hours, hydroxyurea (2 mM) was added for the last 24 hours of incubation, and cells were harvested and analyzed by flow cytometry as described in “Materials and methods.” (B) The same cells were analyzed by flow cytometry 24 hours after the release from hydroxyurea. Data are from a representative experiment.
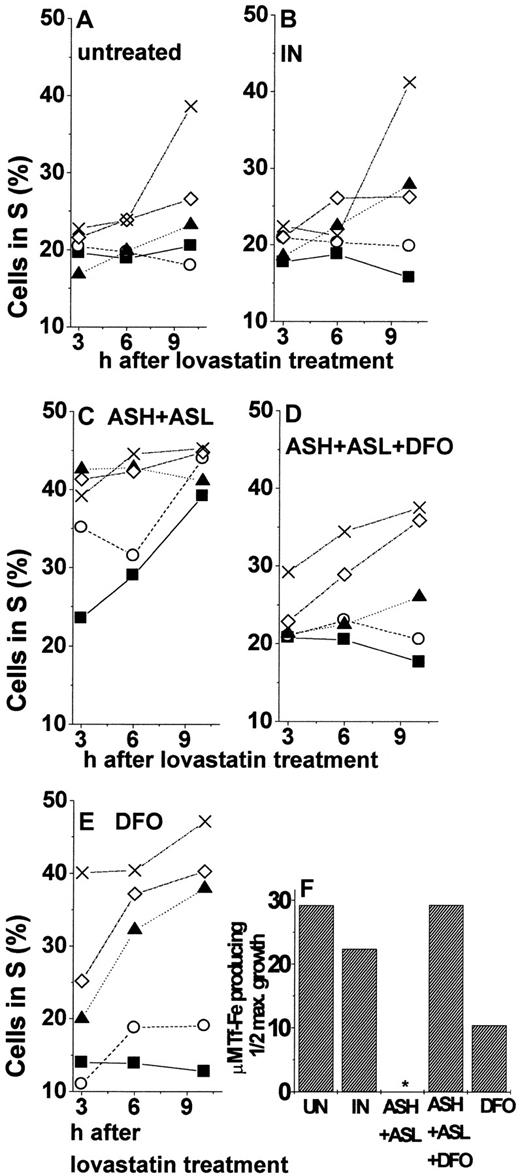
To assess the role of iron in the cell cycle properties affected by FT repression, we examined the effects of DFO on the entry of cells into S phase after their release from growth arrest. Iron chelator treatment led to a general repression of growth as reflected in the lower percentage of cells in the S phase. (Although the percentage of DFO-treated cells at the S stage remained below control levels up to 9 hours after the blockage was lifted [Figure 7A], growth resumption was relatively faster. We attributed the latter to increased expression of TfR induced by iron deprivation.52-54) This was also found when the initial treatment of cells included FT–AS-ODN (Figure 7A). Based on the specificity of DFO for iron chelation, we can infer that the increase in cell iron availability evoked by AS repression of FT (Table 1, Figure 3A) contributed to a faster resumption of DNA synthesis. Two major physiological mechanisms furnish iron to the LIP, membrane iron uptake systems,1 and iron release from FT, primarily by protein degradation.29-31 To set up conditions whereby cells will be dependent only on external iron, we used leupeptin as a blocker of lysosomal proteases and the ensuing release of iron from FT.30 For arresting cells at the G1/G0 phase, we used the cell-cycle blocker lovastatin. (We chose lovastatin rather than hydroxyurea for its ability to confine cell arrest to the G1 and not the G1/S phase.55,56 Nonetheless, a small fraction of the cell population is also arrested at the G2 phase [Muller et al57 and data not shown]. The latter probably introduced some variability to the cell-stage distribution 24 hours after cell-cycle arrest was lifted, thus preventing us from tracing further cell-cycle progression, as shown earlier [Figure 7B].) We surmised that because FT–AS-ODN (both hours and L) treatment up-modulates LIP, growth resumption should be less dependent on external iron in the FT–AS-ODN–treated cells than in the untreated ones. K562 cells were incubated in full medium in the presence of FT–AS-ODN probes, and 24 hours later they were growth arrested at G1 by 20 μM lovastatin. Release from lovastatin blockage was achieved by adding 4 mM mevalonic acid. The cells were suspended in an iron-free medium supplemented with Tf-Fe (various concentrations), and leupeptin was added periodically (every 3 hours) to maintain a constant proteolytic blockage. As shown in Figure8, cells arrested at G1resumed growth relatively faster and at much lower Tf-Fe concentrations when they were pretreated with hours and L-FT–AS-ODN. Inspection of Figure 8C shows that, even without the addition of Tf-Fe, up to 39% of the cells were already in the S phase by 10 hours after release from lovastatin. Such an effect was equivalent to the one attained by the addition of 50 μg/mL Tf-Fe to cells treated either with nothing (Figure 8A) or with IN-ODN (Figure 8B). Moreover, exposing the cells for 24 hours to relatively low concentrations of DFO (10 μM) in the presence of FT–AS-ODN or to DFO alone at higher concentrations (50 μM) rendered their progression through the cell cycle more dependent on Tf-Fe (Figure 8D-E, respectively). This was reflected in the percentage of cells that reached the S phase after lovastatin release, as shown in the time dependence of growth (Figure 8E) or in the Tf-Fe concentrations required for half-maximal growth (Figure 8F). As shown in Figure 8F, cells treated with AS-ODN against both hours and L-FT subunits passed half-maximal percentage in S even without Tf-Fe supplementation. We tentatively ascribe the increased dependence of the growth rate on Tf-Fe concentration (Figure 8E) to increased TfR expression induced by DFO depletion of the LIP.32 Taken together, the results presented in Figure 8 strongly suggest that the pool of iron affected by FT is rate limiting for the renewal of DNA synthesis. FT–AS-ODN treatment, which expanded the LIP, allowed intracellular-iron–depleted cells to bypass the need for importing iron when growth arrest was lifted.
Percentage of cells in S phase at different times after release from lovastatin arrest.
K562 cells were treated for 24 hours with (A) none, (B) 1 nM IN-ODN, (C) 1 nM ASH-ODN+ASL-ODN, (D) ASH-ODN+ASL-ODN+10 μM DFO (added for the last 24 hours of incubation), (E) 50 μM DFO (added for the last 24 hours of incubation). Then the cells were growth arrested by 24-hour incubation with 10 μM lovastatin, washed, and resuspended in media containing 2 mM mevalonic acid, 20 μM leupeptin, and 0 (▪), 5 (○), 10 (▴), 25 (⋄), or 50 (X) μg/mL Tf-Fe. Percentage of cells in the S phase was analyzed 3, 6, and 10 hours later by flow cytometry, as described in “Materials and methods.” (F) Tf-Fe concentrations (μg/mL) producing half-maximal growth after 10-hour release from lovastatin arrest. Half-maximal concentrations were calculated using first-order dependence of cell cycle progression on Tf-Fe concentrations, as verified by analysis of variance for linear regression with the following levels of significance: untreated,P = .01; IN-ODN, P = .025; *ASH-ODN+ASL-ODN, half-maximal growth not obtained because without Tf-Fe, already 85% of the cells (out of the maximal) were in S phase. Tf-Fe concentration for 90% growth was 10 μg/mL, P = .07; ASH-ODN+ASL-ODN+DFO (10 μM), P = .025; DFO (50 μM), P = .01. Data are from a representative experiment.
Percentage of cells in S phase at different times after release from lovastatin arrest.
K562 cells were treated for 24 hours with (A) none, (B) 1 nM IN-ODN, (C) 1 nM ASH-ODN+ASL-ODN, (D) ASH-ODN+ASL-ODN+10 μM DFO (added for the last 24 hours of incubation), (E) 50 μM DFO (added for the last 24 hours of incubation). Then the cells were growth arrested by 24-hour incubation with 10 μM lovastatin, washed, and resuspended in media containing 2 mM mevalonic acid, 20 μM leupeptin, and 0 (▪), 5 (○), 10 (▴), 25 (⋄), or 50 (X) μg/mL Tf-Fe. Percentage of cells in the S phase was analyzed 3, 6, and 10 hours later by flow cytometry, as described in “Materials and methods.” (F) Tf-Fe concentrations (μg/mL) producing half-maximal growth after 10-hour release from lovastatin arrest. Half-maximal concentrations were calculated using first-order dependence of cell cycle progression on Tf-Fe concentrations, as verified by analysis of variance for linear regression with the following levels of significance: untreated,P = .01; IN-ODN, P = .025; *ASH-ODN+ASL-ODN, half-maximal growth not obtained because without Tf-Fe, already 85% of the cells (out of the maximal) were in S phase. Tf-Fe concentration for 90% growth was 10 μg/mL, P = .07; ASH-ODN+ASL-ODN+DFO (10 μM), P = .025; DFO (50 μM), P = .01. Data are from a representative experiment.
Discussion
FT has been implicated in cell iron detoxification,33,34 protection against oxidative stress,5,6,35 and various inflammatory conditions.36,37 The major regulatory mechanism of FT expression has been associated with the inactivation of IRPs by an alleged rise in LIP. However, it has also been assumed that FT actively modulates the LIP by IRP-independent mechanisms acting at the transcriptional level.13,38,39 That form of FT gene repression has been thought to support oncogene-induced proliferation10-16 by purportedly increasing iron availability.
In the current study we examined the hypothesis that attenuation of FT expression has implications for cell growth because of the alleged capacity of FT to down-modulate LIP levels.4,6 To ascertain that FT can actively reduce the LIP, FT expression was manipulated independently of the cellular LIP by using AS technology. Repression of FT gene expression involves AS-ODN hybridization with the target,18 leading either to blockage of translation or to target degradation by RNase H. The main drawbacks of this approach are nonspecific actions and potential toxicity of the AS-ODN probes40 41 and a possible feedback up-regulation of the target of suppression. The most effective and selective AS probes were obtained by screening the potency of AS-ODN against various sites along the sequences of either H-FT or L-FT, using the specific inhibition of only one FT subunit as the selection criterion. The effects obtained with nM concentrations of probes were optimal after 48-hour treatment (approximately 70% mean inhibition) (Table 1, Figure 1) but were apparently heterogeneous in the cell population (Figure 2). Qualitatively and quantitatively similar results were obtained by transient transfection with an FT-AS fragment (O.K. et al, unpublished observations, 2000).
AS-ODN repression of FT synthesis shifted the cells into a state of relatively higher LIP (Table 1, Figure 3A), indicating that FT repression can override the LIP-mediated control of FT levels through IRPs.27 LIP increase attained by AS-ODN repression of each FT subunit was specific inasmuch as it was accompanied by an increased synthesis of the counterpart subunit (Table 1) and a decreased activity of TfR (Figure 5). The proposed involvement of LIP in the mode in which one FT subunit affects expression of the other25 was also supported by the fact that the inter-subunit effect was prevented by iron chelators (Figure 4). The possibility that the AS-ODN–mediated repression of FT was evoked by a potential chelating activity of the ODN42 can be rejected because (1) an ODN containing an identical composition of bases in the reverse order (IN-ODN) produced no repression of FT, (2) FT repression by the AS-ODN was subunit-specific, and (3) LIP was increased, rather than reduced, by the FT–AS-ODN action. Changes in LIP evoked by active down-modulation of FT were also reflected in increased ROS formation and in oxidative modification of cell proteins. This complements studies of up-modulation of FT expression that resulted in reduced LIP and ROS formation.3 4 Taken in toto, all these studies provide experimental support for the alleged “active” role of FT in regulating LIP and attenuating ROS generation.
This work assessed the hypothesized role of FT as a cell growth modulator based on its capacity to modulate LIP levels. Although we found AS treatments against H-FT, L-FT, or both to substantially increase LIP levels, they failed to affect long-term cell growth, viability, and metabolic activity (Figure 6). In contrast, growth resumption of cells arrested at G1/S (Figure 7) or at G1 (Figure 8) was significantly faster when FT expression was inhibited and LIP was increased. Two lines of evidence support the hypothesis that FT contribution to the short-term cell growth was through increased LIP. First, AS effects were reversed with the addition of an iron chelator (Figures 7, 8). Second, growth was less dependent on iron import when cells were FT-repressed (Figure 8). Nonetheless, the differential effects of FT repression on stimulated growth vis-a-vis normal growth remain unexplained. In both forms of growth, iron can be a rate-limiting factor (Hann et al,43Kovar and Franek,44 Kovar,45 Seligman et al,46 and O.K., unpublished observations, 2000), and FT might control cell growth by restricting cell iron availability.7 It is possible that a substantial expansion of LIP occurred only in a fraction of cells, those most affected by AS-ODNs (Figure 2), and that in the long term these effects are diluted out in the population. In the short term, however, such as in growth resumption after arrest or at a particular stage in the cell cycle, the demands for iron might be more acute and, thus, the AS effects more pronounced. This applies also to the G1 cyclins E/cdk2, D/cdk4,47,48 and D149 and the S cyclin A/cdk2,48 which are iron-dependent factors that contribute to cell cycle progression. In nonsynchronized cultures, LIP iron might not be rate limiting because those factors are maintained at steady levels and no accumulation of stage-specific, iron-dependent factors is anticipated. On the other hand, in synchronized cultures, an accumulation of such factors is apparently needed for cell cycle progression; thus, iron from the LIP might be essential.
Clearly, asynchronous or unstimulated cell growth might differ from accelerated growth after the release from cycle arrest or from oncogene activation in their demands for labile iron. This is reflected by both oncogene down-modulation in iron deprivation50 and potentiation in iron supplementation.51 Our work is in support of the paradigm that stimulated cell growth is sustained by FT repression-mediated up-regulation of the LIP.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Z. Ioav Cabantchik, Department of Biological Chemistry, Institute of Life Sciences, Hebrew University, Jerusalem 91904, Israel; e-mail: ioav@cc.huji.ac.il.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal