Abstract
Superoxide is the most important armory on the primary defense line of monocytes against invading pathogens, and the identification of new stimuli and the characterization of the regulatory mechanism of superoxide generation are of paramount importance. In this study, we identified 3 novel peptides by screening a synthetic hexapeptide combinatorial library and modification of 1 of the peptides. The isolated peptides that can induce superoxide generation in human monocytes are His-Phe-Tyr-Leu-Pro-Met-CONH2 (HFYLPM), Met-Phe-Tyr-Leu-Pro-Met-CONH2 (MFYLPM), and His-Phe-Tyr-Leu-Pro-D-Met-CONH2 (HFYLPm). All 3 peptides also caused intracellular calcium ([Ca++]i) rise. We tested the specificities of the peptides on cells of different origin by looking at [Ca++]i rise. All 3 peptides acted specifically on leukocytes and not on nonimmune cells. Among leukocytes, HL60 and Jurkat T cells were stimulated specifically by MFYLPM or HFYLPM, respectively. As a physiologic characteristic of the peptides, we observed that all 3 peptides induced chemotactic migration of monocytes. Studying receptor specificity, we concluded that the 3 peptides might act on some shared and some distinct receptor(s) on leukocytes. Studying intracellular signaling set in motion by the peptides revealed that HFYLPM, but not MFYLPM or HFYLPm, induced chemotaxis via phospatidylinositol-3 kinase and protein kinase C. Because HFYLPM, MFYLPM, and HFYLPm not only exhibit different specificities depending on cell type and status of differentiation but also stimulate cells via distinct receptors and signaling, the 3 novel peptides might be useful tools to study leukocyte activation.
Introduction
Reactive oxygen species (ROS) such as superoxide, hydrogen peroxide, and hydroxyl radicals are generated by phagocytic cells upon activation by invading microorganisms or inflammatory debris.1,2 The production of these ROS enables the phagocytes such as monocytes to play a critical role in human immune responses. The activation of the ROS generation system, which is one of the earliest steps in the host defense against invading microorganisms, is tightly regulated in the immune systems.1-3 To perform their proper roles, monocytes in the resting state have to be activated, and this is a very critical aspect of the host defense mechanism.
Monocyte activation can be induced by various extracellular stimuli such as bacterial endotoxin (lipopolysaccharide), immunoglobulins, and several chemoattractants.4-7 Among these extracellular stimuli, chemoattractants including several chemokines that regulate the activities of monocytes have been receiving attention for a long time. Many chemoattractants stimulate leukocytes via the activation of pertussis toxin (PTX)–sensitive G protein–coupled receptor.1 Upon binding to its corresponding cell surface receptor, a chemoattractant induces intracellular Ca++mobilization, cytoskeletal rearrangements, exocytosis, histamine release, receptor induction, adhesion, the production of bioactive lipids, and the activation of the respiratory burst system via NADPH oxidase activation.1,8 9 With this important role of chemoattractants for monocyte function in mind, the identification of new chemoattractants and the characterization of their mechanisms of action are very much needed.
Several recent studies have reported the use of combinatorial peptide libraries to identify sequences involved in various biological responses. Houghten et al developed a positional-scanning synthetic-peptide combinatorial library (PS-SPCL) method that is an easy and powerful tool for identifying peptide sequences in certain biological reactions.10 This method has been adopted for various purposes, including the identification of human immunodeficiency virus protease inhibitors, interleukin-8–specific antagonists, inhibitor for nuclear factor of activated T cells, and ligands for opioid receptors.11-14 Also, we already identified one bioactive hexapeptide that stimulates phosphoinositide hydrolysis by screening hexapeptide combinatorial libraries.15
In this study we adopted the PS-SPCL method to find novel peptides that can stimulate superoxide generation in human monocytes. We found that the peptides His-Phe-Tyr-Leu-Pro-Met-CONH2(HFYLPM), Met-Phe-Tyr-Leu-Pro-Met-CONH2 (MFYLPM), and His-Phe-Tyr-Leu-Pro-D-Met-CONH2 (HFYLPm), which has been modified from HFYLPM, can stimulate human monocytes, leading to superoxide generation. All of these peptides also caused an intracellular calcium increase and induce chemotactic migration in human monocytes. Because the 3 peptides not only exhibit different specificities in their effect on leukocytic cells dependent on the cell type as well as the status of differentiation but also act via distinct receptors and signaling pathways, the peptides will prove useful agents in the study of leukocyte activation.
Materials and methods
Materials
Fmoc amino acids were obtained from Millipore (Bedford, MA). Rapidamide resin was purchased from Dupont (Boston, MA). Peripheral blood mononuclear cell (PBMC) separation medium (Histopaque-1077), cytochrome c, and N-formyl-methionyl-leucyl-phenylalanine (fMLF) were purchased from Sigma (St Louis, MO). Fura-2 pentaacetoxymethylester (fura-2/am) was purchased from Molecular Probes (Eugene, OR). RPMI 1640 was obtained from Life Technologies (Grand Island, NY). Dialyzed fetal bovine serum and supplemented bovine serum were purchased from Hyclone Laboratories (Logen, UT). PTX, GF109203X, and PD98059 were purchased from Calbiochem (San Diego, CA). LY294002 was from Biomol Research Laboratories (Plymouth Meeting, PA).
Isolation of leukocytes
Peripheral blood leukocyte concentrates were donated by the Ulsan Red Cross Blood Center (Ulsan, Korea). PBMCs were separated on a Histopaque-1077 gradient. After 2 washings with HBSS without Ca++ and Mg2+, the PBMCs were suspended in 10% fetal bovine serum containing RPMI and incubated for 60 minutes at 37°C to let the monocytes attach to the culture dish. The cells were washed 5 times with warmed RPMI medium to wash out lymphocytes, and then the attached monocytes were collected as described previously.16 Human neutrophils were isolated according to the standard procedures of dextran sedimentation, hypotonic lysis of erythrocytes, and a lymphocyte separation medium gradient as described previously.17 The isolated human leukocytes were then used promptly.
Cell culture and HL60 cell differentiation
U937 (human histiocytic lymphoma cells), HL60 (human promyelocytic leukemia cells), Jurkat (human T-cell leukemia cells), NIH3T3 (NIH Swiss mouse embryo fibroblasts), 3Y1 (rat embryonic fibroblasts), 3T3L1 (preadipocytes), and PC12 (rat adrenal pheochromocytoma cells) were obtained from the American Type Culture Collection (Rockville, MD) and maintained as recommended. The cells were maintained at about 1 × 106/mL under standard incubator conditions (humidified atmosphere, 95% air, 5% CO2, 37°C). HL60 cells were induced to differentiate into the granulocyte phenotype by adding dimethyl sulfoxide (DMSO) (final concentration 1.25%, vol/vol) for 4 days to the culture medium as has been described.18
Preparation of peptide libraries and synthesis and analysis of peptides
The hexapeptide libraries were prepared in the Peptide Library Support Facility of Pohang University of Science and Technology as described previously.15,19 Finally, 114 peptide pools (Cys was excluded in the construction of the libraries) were individually dissolved in water at a final concentration of 27 nM per peptide sequence in each pool. The peptides were synthesized by the solid-phase method described previously.15,19 Briefly, peptides were synthesized on a Rapidamide support resin and assembled following the standard Fmoc/t-butyl strategy on an acid-labile linker. The composition of the peptides was confirmed by amino acid analysis as described previously.15
Initial screening of the PS-SPCLs and measurement of superoxide generation
For initial screening of the PS-SPCLs, we measured the superoxide anion generation of each peptide pool by measuring reduction of cytochrome c using a microtiter 96-well plate enzyme-linked immunosorbent assay reader (Bio-Tekinstruments, EL312e, Winooski, VT) as described.20 The human monocytes (9 × 105 cells/100 μL RPMI 1640 medium per well of a 96-well plate) were preincubated with 50 μM cytochrome c at 37°C for 1 minute and then incubated with the indicated concentrations of peptide pools (final 0.5 nM per peptide sequence for the initial screening). The superoxide generation was measured as change in light absorption at 550 nm over 5 minutes at 1-minute intervals. From at least 4 independent experiments, peptides with active amino acids at each position were chosen. Superoxide generation by the identified peptides was measured by the same method. Spurious reduction of cytochrome c was ruled out by checking that the peptide-induced ones are superoxide dismutase–inhibitable in all experiments (data not shown).
Measurement of [Ca++]i
The level of [Ca++]i was determined by Grynkiewicz's method using fura-2/am.21Briefly, prepared cells were incubated with 3 μM fura-2/am at 37°C for 50 minutes in fresh serum-free RPMI 1640 medium under continuous stirring. A total of 2 × 106 cells were aliquoted for each assay in Ca++-free Locke's solution (154 mM NaCl, 5.6 mM KCl, 1.2 mM MgCl2, 5 mM HEPES [pH 7.3], 10 mM glucose, and 0.2 mM EGTA). The fluorescence changes at the dual excitation wavelengths of 340 nm and 380 nm and the emission wavelength of 500 nm were measured, and the calibrated fluorescence ratio was translated into [Ca++]i.
Chemotaxis assay
Chemotaxis assays were performed using multiwell chambers (Neuroprobe, Gaithersburg, MD).20 Briefly, prepared human monocytes were suspended in RPMI at a concentration of 1 × 106/mL, and 25 μL suspension was placed onto the upper well of a chamber that was separated by a 5 μm polyhydrocarbon filter (3 μm diameter pores not coated with polyvinylpyrrolidone for neutrophils) from peptides or fMLF containing a lower well. After incubation for 2 hours (90 minutes for neutrophils) at 37°C, nonmigrated cells were removed by scarping them out, and cells that migrated across the filter were dehydrated, fixed, and stained with hematoxylin (Sigma). The stained cells in 5 randomly chosen high-power fields (HPF; 400 ×) in that well were then counted.21 For checkboard analysis, cells were resuspended in RPMI containing various concentrations of the peptide just before transferring them to the upper wells as described before.22
Statistical analysis
The results are expressed as mean ± SE of data obtained from the indicated number of experiments performed. Statistical significance was determined using the Student ttest.
Results
Identification of peptides that stimulate superoxide generation in human monocytes
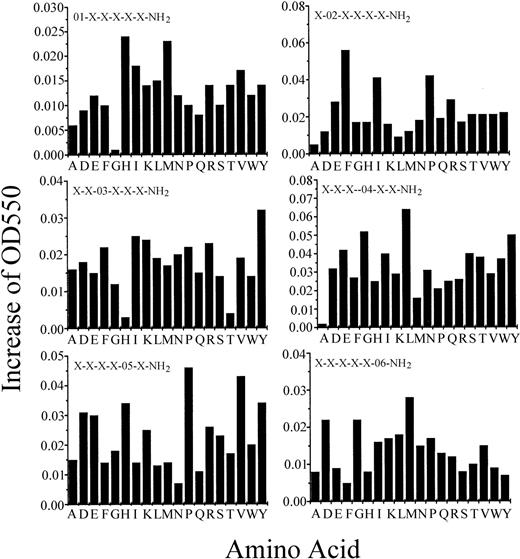
We screened 114 peptide pools (around 47 million peptides) from hexapeptide PS-SPCLs to identify peptides that stimulate superoxide generation in human monocytes. Figure 1shows the results of the initial screening. Each amino acid that was fixed at each position induced different levels of superoxide generation. The most active peptides for each position were as follows: His (H) or Met (M) in the first position, Phe (F) in second, Tyr (Y) in third, Leu (L) in fourth, Pro (P) or Val (V) in fifth, and Met (M), Asp (D), or Gly (G) in sixth.
The initial screening of the PS-SPCLs for peptides stimulating superoxide generation of human monocytes.
Each panel shows the results obtained with the peptide pools with known amino acids at each of the 6 positions of the hexapeptide. The 6 positions were individually defined (eg, O1, O2) by one of the 19 L-amino acids. The remaining 5 positions consist of mixtures (X) of the 19 L-amino acids (except for cysteine). Human monocytes (9 × 105/100 μL) were used for each assay. Cytochrome c reduction was monitored as described in “Materials and methods.” The results represent 1 of 4 independent experiments.
The initial screening of the PS-SPCLs for peptides stimulating superoxide generation of human monocytes.
Each panel shows the results obtained with the peptide pools with known amino acids at each of the 6 positions of the hexapeptide. The 6 positions were individually defined (eg, O1, O2) by one of the 19 L-amino acids. The remaining 5 positions consist of mixtures (X) of the 19 L-amino acids (except for cysteine). Human monocytes (9 × 105/100 μL) were used for each assay. Cytochrome c reduction was monitored as described in “Materials and methods.” The results represent 1 of 4 independent experiments.
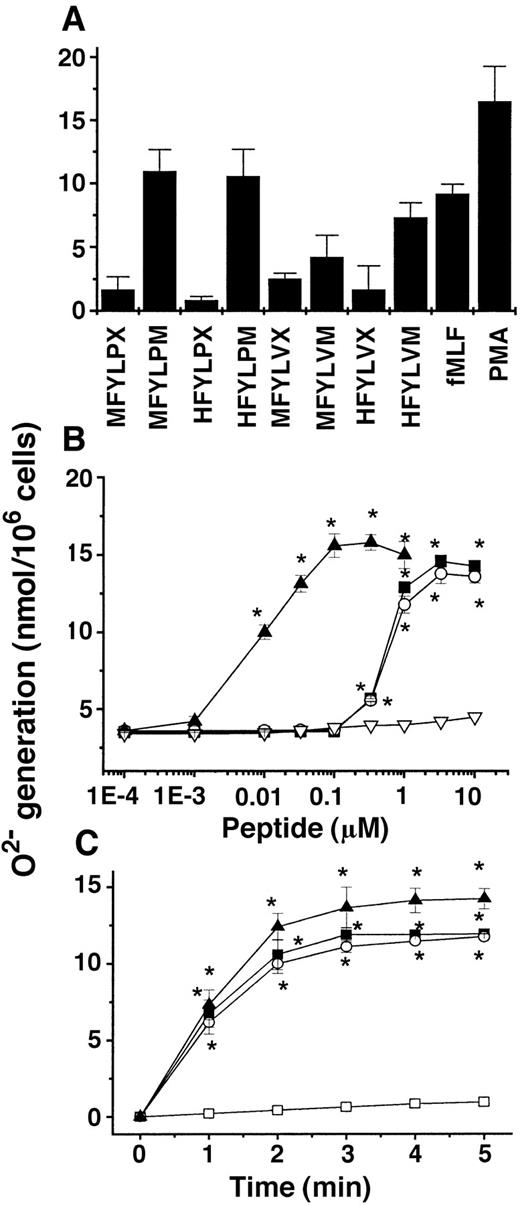
Based on the results of the first screening of the peptide libraries, we generated by reiterative synthesis a peptide pool containing 2 × 1 × 1 × 1 × 2 × 3 = 12 individual hexapeptides. The peptide mixture was purified into 8 fractions that contained MFYLPX, MFYLPM, HFYLPX, HFYLPM, MFYLVX, MFYLVM, HFYLVX, and HFYLVM (where X is D or G) by reverse-phase high-performance liquid chromatography with a C18 column (Vydac, 218TP1022, 22 × 250 mm). We then tested the effectiveness of the peptides for the superoxide generation in human monocytes by the same method as used in the initial screening (Figure 2A). Finally, we identified HFYLPM and MFYLPM as the most active peptides in terms of superoxide generation by human monocytes (Figure 2B). The stimulation of monocytes with various concentrations of the 2 peptides induced superoxide generation in a concentration-dependent manner with maximal activity at 3.3 μM (Figure 2B). In our previous report, we showed that substitution of the sixth M from an L type to a D type highly enhanced the affinity of a synthetic peptide, WKYMVM, to its corresponding receptor.23 In this study we also developed a modified peptide from HFYLPM carrying the sequence HFYLPm. The HFYLPm also enhanced superoxide generation, showing the maximal activity with around 100 nM (Figure 2B). The peptide-stimulated superoxide generation was rapid, showing the maximal effect within 3 minutes of the stimulation (Figure 2C).
Effect of several candidate peptides synthesized based on the screening results of the PS-SPCLs with regard to superoxide generation in human monocytes.
(A) Human monocytes were stimulated with 1 μM concentrations of several peptides, 1 μM fMLF, or 100 nM phorbol myristate acetate, and the generated superoxide was measured using cytochrome c reduction assay. (B) The concentration dependency of the superoxide generation induced by peptides in monocytes. HFYLPM, ▪; MFYLPM, ○; HFYLPm, ▴; LFMYHP, ▿. (C) Time course of the peptide-induced superoxide generation in human monocytes; 3.3 μM of HFYLPM and 330 nM of HFYLPm were used. The results are presented as means ± SE of 3 independent experiments. *P < .01 versus vehicle treatment. Medium, ■; HFYLPM, ▪; MFYLPM, ○; HFYLPm, ▴.
Effect of several candidate peptides synthesized based on the screening results of the PS-SPCLs with regard to superoxide generation in human monocytes.
(A) Human monocytes were stimulated with 1 μM concentrations of several peptides, 1 μM fMLF, or 100 nM phorbol myristate acetate, and the generated superoxide was measured using cytochrome c reduction assay. (B) The concentration dependency of the superoxide generation induced by peptides in monocytes. HFYLPM, ▪; MFYLPM, ○; HFYLPm, ▴; LFMYHP, ▿. (C) Time course of the peptide-induced superoxide generation in human monocytes; 3.3 μM of HFYLPM and 330 nM of HFYLPm were used. The results are presented as means ± SE of 3 independent experiments. *P < .01 versus vehicle treatment. Medium, ■; HFYLPM, ▪; MFYLPM, ○; HFYLPm, ▴.
Effect of novel peptides on [Ca++]i rise in human monocytes
Many extracellular agonists that stimulate superoxide generation in human phagocytic cells are involved in intracellular calcium rise.24 We tested the effect of the peptides on the [Ca++]i of monocytes. As shown in Figure3A, HFYLPM, MFYLPM, and HFYLPm caused intracellular Ca++ mobilization in monocytes. A scrambled sequence of the HFYLPM, LFMYHP, did not affect [Ca++]i in the cells (Figure 3A). Concentration dependency of the peptide-induced intracellular Ca++ mobilization was checked. HFYLPM and MFYLPM showed a maximal [Ca++]i increase at 1 μM concentrations (Figure 3B). HFYLPm could stimulate the monocytes at a lower concentration, showing a maximal effect at 100 nM (Figure 3B). A scrambled sequence of the HFYLPM, LFMYHP, had no effect on the [Ca++]i increase up to 10 μM stimulation (Figure 3B).
Effect of peptides on [Ca++]irise in human monocytes.
Fura-2–loaded monocytes were stimulated with effective concentrations of HFYLPM (1 μM, ); MFYLPM (1 μM,▪); HFYLPm (100 nM,
); MFYLPM (1 μM,▪); HFYLPm (100 nM, ); and LFMYHP (10 μM,▾). The change in 340 nm/380 nm was monitored. (A) The results are representative of 6 independent experiments. The cells were stimulated with various concentrations of each peptide. The peak level of the increase in [Ca++]i was monitored. (B) Data are presented as means ± SE of 4 independent experiments. *P < .01 versus vehicle treatment.
); and LFMYHP (10 μM,▾). The change in 340 nm/380 nm was monitored. (A) The results are representative of 6 independent experiments. The cells were stimulated with various concentrations of each peptide. The peak level of the increase in [Ca++]i was monitored. (B) Data are presented as means ± SE of 4 independent experiments. *P < .01 versus vehicle treatment.
Effect of peptides on [Ca++]irise in human monocytes.
Fura-2–loaded monocytes were stimulated with effective concentrations of HFYLPM (1 μM, ); MFYLPM (1 μM,▪); HFYLPm (100 nM,
); MFYLPM (1 μM,▪); HFYLPm (100 nM, ); and LFMYHP (10 μM,▾). The change in 340 nm/380 nm was monitored. (A) The results are representative of 6 independent experiments. The cells were stimulated with various concentrations of each peptide. The peak level of the increase in [Ca++]i was monitored. (B) Data are presented as means ± SE of 4 independent experiments. *P < .01 versus vehicle treatment.
); and LFMYHP (10 μM,▾). The change in 340 nm/380 nm was monitored. (A) The results are representative of 6 independent experiments. The cells were stimulated with various concentrations of each peptide. The peak level of the increase in [Ca++]i was monitored. (B) Data are presented as means ± SE of 4 independent experiments. *P < .01 versus vehicle treatment.
Cell type specificity of the novel peptides
The fact that HFYLPM, MFYLPM, and HFYLPm stimulate human monocytes led us to look for effects of the peptides on other leukocytes such as neutrophils. Stimulation of neutrophils with the 3 peptides resulted in an internal calcium increase (Figure 4A). All the peptides also enhanced superoxide generation by neutrophils with a similar concentration dependency as observed for the [Ca++]i increase in these cells (Figure 4B). U937 human promonocytic cells were also activated by 3 of the peptides (Figure 4A). Next, we examined the effects of HFYLPM, MFYLPM, and HFYLPm on intracellular calcium release in several nonleukocytic cell lines. NIH3T3, 3Y1, 3T3L1, and PC12 cells showed no response to the 3 peptides in terms of [Ca++]i rise (Figure4A). The results suggest that the effect of the peptides is specific for human leukocytes.
Cell specificity of peptides.
Prepared human primary monocytes, neutrophils, and cultured HL60, Jurkat, U937, NIH3T3, 3Y1, 3T3L1, and PC12 cells were loaded with fura-2 and stimulated with effective concentrations of the 3 peptides (1 μM for monocytes and neutrophils; 10 μM for HL60 and Jurkat; 20 μM for U937, NIH3T3, 3Y1, 3T3L1, and PC12). (A) The peak level of the [Ca++]i increase was recorded. (B) Monocytes and neutrophils were stimulated with 10 μM of each peptide. Superoxide generation was measured using cytochrome c reduction assay. Data are presented are means ± SE from at least 3 independent experiments.
Cell specificity of peptides.
Prepared human primary monocytes, neutrophils, and cultured HL60, Jurkat, U937, NIH3T3, 3Y1, 3T3L1, and PC12 cells were loaded with fura-2 and stimulated with effective concentrations of the 3 peptides (1 μM for monocytes and neutrophils; 10 μM for HL60 and Jurkat; 20 μM for U937, NIH3T3, 3Y1, 3T3L1, and PC12). (A) The peak level of the [Ca++]i increase was recorded. (B) Monocytes and neutrophils were stimulated with 10 μM of each peptide. Superoxide generation was measured using cytochrome c reduction assay. Data are presented are means ± SE from at least 3 independent experiments.
Jurkat human T-cell leukemia cells were stimulated by HFYLPM and HFYLPm, showing maximal activity at 10 μM and 30 μM peptide concentration, respectively (Figure 4A). Meanwhile, MFYLPM did not cause a similar intracellular calcium mobilization in Jurkat cells even upon 30 μM stimulation (Figure 4A). This suggests that only HFYLPM and HFYLPm, but not MFYLPM, can bind to receptor(s) on Jurkat T cells. HL60 human promyelocytic cells were also tested for stimulation by the 3 peptides. As depicted in Figure 4A, MFYLPM and HFYLPm caused a [Ca++]i increase in HL60 cells. In this case, HFYLPM did not evoke an intracellular calcium rise in HL60 cells even after a 20 μM stimulation (Figure 4A). Thus, HL60 cells seem to specifically respond to MFYLPM and HFYLPm but not to HFYLPM.
Effect of the peptides on differentiated HL60 cells
Although we observed that HFYLPM, MFYLPM, and HFYLPm stimulated human neutrophils to increase their [Ca++]iand to generate superoxides (Figure 4A,B), we observed that only MFYLPM and HFYLPm, but not HFYLPM, induced an intracellular calcium rise in HL60 human promyelocytic cells (Figure 4A). We then tested the 3 peptides on HL60 cells that had differentiated into granulocytes after having been cultured in the presence of 1.25% DMSO for 4 days. Differentiation of the cells into granulocytes was confirmed by monitoring the morphologic change and the generation of superoxide upon fMLF treatment as described before.25 The differentiated HL60 cells were stimulated with various concentrations of HFYLPM, MFYLPM, and HFYLPm. We found that HFYLPM affected [Ca++]i in differentiated but not undifferentiated HL60 cells in a concentration-dependent manner with maximal effect at 5 μM (Figure 5A). Figure 5B,C shows that MFYLPM and HFYLPm stimulated both undifferentiated and differentiated HL60 cells, resulting in [Ca++]i rise. Stimulation of the differentiated HL60 cells with HFYLPM, MFYLPM, and HFYLPm also resulted in superoxide generation within similar concentrations as required for the [Ca++]i increase (data not shown).
Differentiation specificity of the peptides.
HL60 cells were cultured in RPMI medium containing 20% fetal calf serum. To induce differentiation of the cells into granulocytes, the cells were cultured in the presence 1.25% DMSO for 4 days. Healthy HL60 (■) and differentiated HL60 cells (dHL60, ▪) were loaded with fura-2 and treated with various concentrations of HFYLPM (A), MFYLPM (B), and HFYLPm (C). The peak level of the [Ca++]i increase was recorded. The data are means ± SE from 4 independent experiments. *P < .01 versus vehicle treatment.
Differentiation specificity of the peptides.
HL60 cells were cultured in RPMI medium containing 20% fetal calf serum. To induce differentiation of the cells into granulocytes, the cells were cultured in the presence 1.25% DMSO for 4 days. Healthy HL60 (■) and differentiated HL60 cells (dHL60, ▪) were loaded with fura-2 and treated with various concentrations of HFYLPM (A), MFYLPM (B), and HFYLPm (C). The peak level of the [Ca++]i increase was recorded. The data are means ± SE from 4 independent experiments. *P < .01 versus vehicle treatment.
Overall, our results indicate that HFYLPM, MFYLPM, and HFYLPm are potent stimulators of human phagocytic cells and that HFYLPM affects only differentiated HL60 cells and does not affect undifferentiated myelocytes.
Effect of fMLF on the novel peptide–induced [Ca++]i increase
Among various agonists, fMLF is known to cause superoxide production and a [Ca++]i increase in phagocytic cells by binding to its specific receptor.7 To check whether the novel peptides can cross-desensitize the signaling by fMLF, we examined the effect of fMLF on the novel peptide–induced [Ca++]i increase and vice versa. Fura-2–loaded differentiated HL60 cells were stimulated with a peptide, and complete desensitization of its corresponding receptor was confirmed by the fact that any additional [Ca++]i increase was not observed upon the subsequent stimulation with the same peptide. However, the considerable responses were shown by the stimulation with saturating concentrations of 3 novel peptides following the treatment with a saturating concentration of fMLF (Figure 6). Conversely, a saturating concentration of 1 of 3 novel peptides could completely block the calcium response to a following stimulation with a saturating concentration of fMLF (Figure 6). These results suggest that the novel peptides can cross-desensitize fMLF signaling and novel peptides can also bind additional receptor other than the fMLF receptor in the cells.
Effect of fMLF on the peptide-induced [Ca++]i increase.
Ratios in fluorescence were monitored on fura-2–loaded differentiated HL60 cells before and during sequential addition of agonists at the times indicated by the arrows. The concentrations used were 1 μM Hm (HFYLPm), 1 μM fMLF, 10 μM HM (HFYLPM), or 10 μM MM (MFYLPM). The tracings shown are from 1 experiment representative of at least 3 independent experiments.
Effect of fMLF on the peptide-induced [Ca++]i increase.
Ratios in fluorescence were monitored on fura-2–loaded differentiated HL60 cells before and during sequential addition of agonists at the times indicated by the arrows. The concentrations used were 1 μM Hm (HFYLPm), 1 μM fMLF, 10 μM HM (HFYLPM), or 10 μM MM (MFYLPM). The tracings shown are from 1 experiment representative of at least 3 independent experiments.
Chemotactic effect of the novel peptides for human phagocytes
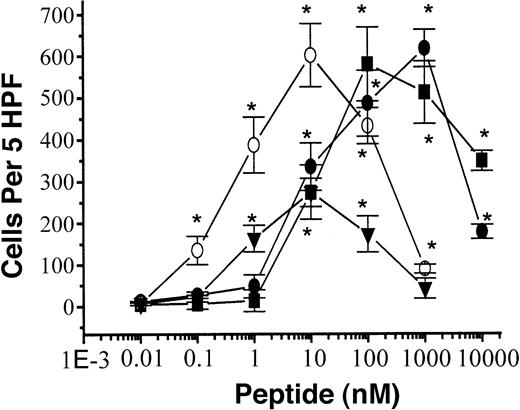
We demonstrated that the novel peptides stimulate superoxide generation and a [Ca++]i increase in human phagocytic cells. These peptide-induced phagocyte activation phenomena are similar to chemoattractant-induced ones. Therefore, we checked whether the peptides exhibited chemotactic activity on the cells. HFYLPM, MFYLPM, and HFYLPm induced migration of human monocytes within a 0.01 to 10 μM (0.001-1 μM for HFYLPm) concentration, showing a bell-shaped concentration response curve in human monocytes (Figure7) similar to the fMLF-induced one as reported previously.26 The maximal cellular migration-inducing activity mediated by the peptides was more than 200% of that of a 100 nM fMLF-induced one (Figure 7). The 3 peptides also induced cellular migration in human neutrophils within similar concentrations as for monocyte migration (data not shown). In 3 experiments with independently prepared leukocytes, the peptides showed cellular migration-inducing activity with similar patterns. To distinguish whether the peptide-induced monocyte migration is chemotaxis or chemokinesis, we performed a checkboard analysis as described previously.22 27 As shown in Table1, a gradually increasing concentration gradient of each peptide between the lower chamber and upper chamber induced significant migration of monocytes to the peptides. This implies that the 3 peptides induce chemotaxis in human monocytes.
Chemotactic effect of peptides.
Assays were performed using a modified Boyden chamber assay as described in “Materials and methods.” Isolated human monocytes (1 × 106 cells per milliliter of serum-free RPMI) were added to the upper wells of a 96-well chemotaxis chamber, and migration across the polycarbonate membrane with 5 μm pore size was assessed after 2 hours of incubation at 37°C. The number of migrated cells was determined by counting them in high-power field (400 ×). The data are presented as mean ± SE of 3 independent experiments each performed in duplicate. *P < .01 versus vehicle treatment. HFYLPM, ; MFYLPM, ▪; HFYLPM,
; MFYLPM, ▪; HFYLPM,  ; fMLF, ▾.
; fMLF, ▾.
Chemotactic effect of peptides.
Assays were performed using a modified Boyden chamber assay as described in “Materials and methods.” Isolated human monocytes (1 × 106 cells per milliliter of serum-free RPMI) were added to the upper wells of a 96-well chemotaxis chamber, and migration across the polycarbonate membrane with 5 μm pore size was assessed after 2 hours of incubation at 37°C. The number of migrated cells was determined by counting them in high-power field (400 ×). The data are presented as mean ± SE of 3 independent experiments each performed in duplicate. *P < .01 versus vehicle treatment. HFYLPM, ; MFYLPM, ▪; HFYLPM,
; MFYLPM, ▪; HFYLPM,  ; fMLF, ▾.
; fMLF, ▾.
Checkboard analysis of monocyte migration across polycarbonate membrane after treatment with HFYLPM, MFYLPM, and HFYLPm
| Below (nM) . | Above (nM) . | |||
|---|---|---|---|---|
| 0 . | 10 . | 100 . | 1000 . | |
| HFYLPM | ||||
| 0 | 10.5 ± 3.42 | 11.0 ± 2.54 | 13.3 ± 2.40 | 10.0 ± 2.57 |
| 10 | 312 ± 23.0 | 47.7 ± 7.43 | 14.0 ± 3.97 | 10.7 ± 2.21 |
| 100 | 494 ± 34.0 | 366 ± 26.1 | 54.0 ± 14.5 | 19.3 ± 11.4 |
| 1000 | 612 ± 66.7 | 505 ± 53.7 | 210 ± 45.6 | 62.3 ± 21.0 |
| MFYLPM | ||||
| 0 | 16.3 ± 4.51 | 11.3 ± 2.42 | 15.0 ± 3.50 | 11.0 ± 2.67 |
| 10 | 198 ± 32.3 | 33.7 ± 8.93 | 16.0 ± 3.16 | 12.3 ± 3.43 |
| 100 | 557 ± 29.5 | 425 ± 48.3 | 66.3 ± 14.9 | 18.0 ± 4.05 |
| 1000 | 484 ± 43.6 | 395 ± 38.5 | 198 ± 26.7 | 76.7 ± 18.9 |
| HFYLPm | ||||
| 0 | 16.3 ± 3.22 | 11.3 ± 3.20 | 15.0 ± 4.82 | 11.0 ± 2.33 |
| 10 | 313 ± 32.4 | 53.7 ± 12.4 | 20.0 ± 5.56 | 12.3 ± 7.91 |
| 100 | 527 ± 29.5 | 325 ± 28.4 | 56.3 ± 9.37 | 28.0 ± 8.06 |
| 1000 | 425 ± 74.0 | 295 ± 56.6 | 98.3 ± 26.9 | 76.7 ± 17.8 |
| Below (nM) . | Above (nM) . | |||
|---|---|---|---|---|
| 0 . | 10 . | 100 . | 1000 . | |
| HFYLPM | ||||
| 0 | 10.5 ± 3.42 | 11.0 ± 2.54 | 13.3 ± 2.40 | 10.0 ± 2.57 |
| 10 | 312 ± 23.0 | 47.7 ± 7.43 | 14.0 ± 3.97 | 10.7 ± 2.21 |
| 100 | 494 ± 34.0 | 366 ± 26.1 | 54.0 ± 14.5 | 19.3 ± 11.4 |
| 1000 | 612 ± 66.7 | 505 ± 53.7 | 210 ± 45.6 | 62.3 ± 21.0 |
| MFYLPM | ||||
| 0 | 16.3 ± 4.51 | 11.3 ± 2.42 | 15.0 ± 3.50 | 11.0 ± 2.67 |
| 10 | 198 ± 32.3 | 33.7 ± 8.93 | 16.0 ± 3.16 | 12.3 ± 3.43 |
| 100 | 557 ± 29.5 | 425 ± 48.3 | 66.3 ± 14.9 | 18.0 ± 4.05 |
| 1000 | 484 ± 43.6 | 395 ± 38.5 | 198 ± 26.7 | 76.7 ± 18.9 |
| HFYLPm | ||||
| 0 | 16.3 ± 3.22 | 11.3 ± 3.20 | 15.0 ± 4.82 | 11.0 ± 2.33 |
| 10 | 313 ± 32.4 | 53.7 ± 12.4 | 20.0 ± 5.56 | 12.3 ± 7.91 |
| 100 | 527 ± 29.5 | 325 ± 28.4 | 56.3 ± 9.37 | 28.0 ± 8.06 |
| 1000 | 425 ± 74.0 | 295 ± 56.6 | 98.3 ± 26.9 | 76.7 ± 17.8 |
Monocyte migration was analyzed for 2 hours across a polycarbonate membrane with 5 μm pore size. Various concentrations of HFYLPM, MFYLPM, or HFYLPm were placed in the upper and lower compartments of the chambers. Data are presented as mean ± SE for migrated monocytes in 5 high-power fields counted in triplicate of 2 independent experiments.
Effect of PTX on the peptide-induced chemotatic migration in monocytes
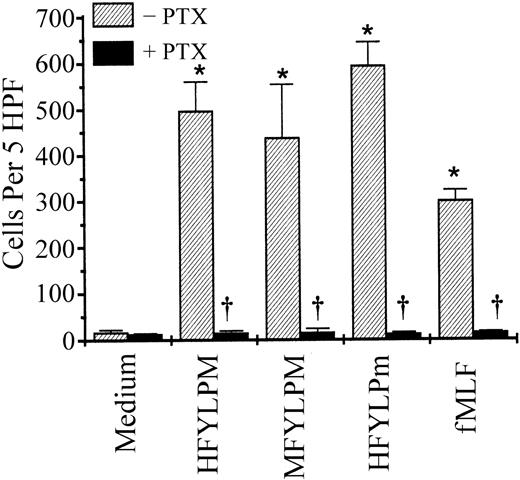
Several extracellular signals, including many chemoattractants, activate phagocytic cells via PTX-sensitive G protein(s).28-30 We therefore examined the involvement of PTX-sensitive G protein(s) on the peptide-induced monocyte activation. Preincubation of monocytes with PTX (1 μg/mL) for 90 minutes led to complete inhibition of the HFYLPM-, MFYLPM-, and HFYLPm-induced monocyte chemotaxis (Figure 8). Under the same condition, monocyte chemotaxis induced by fMLF, a well-known chemoattractant, was also completely blocked (Figure 8). The results, therefore, imply that HFYLPM, MFYLPM, and HFYLPm stimulate human monocytes via PTX-sensitive G protein(s).
Effect of PTX on the monocyte chemotactic migration induced by the peptides.
Monocytes were preincubated with PTX (1 μg/mL) or vehicle only for 90 minutes at 37°C. The cells were used for chemotaxis assay with HFYLPM (1 μM), MFYLPM (1 μM), HFYLPm (100 nM), and fMLF (100 nM). The number of migrated cells was determined by counting them in high-power field (400 ×). The data are presented as mean ± SE of 2 independent experiments each performed in duplicate. *P < .01 versus medium treatment, †P < .01 versus peptide treatment, respectively.
Effect of PTX on the monocyte chemotactic migration induced by the peptides.
Monocytes were preincubated with PTX (1 μg/mL) or vehicle only for 90 minutes at 37°C. The cells were used for chemotaxis assay with HFYLPM (1 μM), MFYLPM (1 μM), HFYLPm (100 nM), and fMLF (100 nM). The number of migrated cells was determined by counting them in high-power field (400 ×). The data are presented as mean ± SE of 2 independent experiments each performed in duplicate. *P < .01 versus medium treatment, †P < .01 versus peptide treatment, respectively.
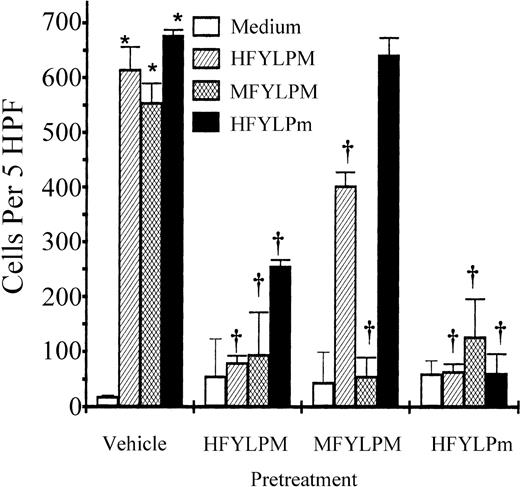
Receptor specificity of the 3 peptides
The 3 peptides, which have similar sequences, exerted similar effects on human leukocytes in that they stimulated superoxide generation and chemotactic migration involving PTX-sensitive G protein(s) (data not shown and Figure 8). We therefore attempted to reveal whether the 3 peptides acted through the same or different receptor(s). For this purpose, monocytes were preincubated with 10 μM of each peptide for 10 minutes or remained untreated. After 2 washings, the cells were used for chemotaxis assay. As shown in Figure9, preincubation of the cells with one peptide caused complete inhibition of the same peptide-induced cell migration, indicating homologous receptor down-regulation. When the cells were preincubated with HFYLPM, MFYLPM- or HFYLPm-induced cell migration was completely or partially inhibited. Pretreatment of MFYLPM caused partial inhibition of HFYLPM-induced chemotaxis and no inhibition of an HFYLPm-induced one (Figure 9). Pretreatment of HFYLPm caused almost complete inhibition of all the peptide-induced chemotactic migration of monocytes (Figure 9).
Specificity of each peptide-induced monocyte chemotaxis.
Monocytes were pretreated with HFYLPM (10 μM), MFYLPM (10 μM), or HFYLPm (10 μM) for 10 minutes at 37°C, washed twice, and thereafter allowed to migrate toward the maximal effective concentrations of each peptide (1 μM HFYLPM, 1 μM MFYLPM, or 10 nM HFYLPm) or medium. After fixing and staining of the membrane, migrated cells were quantified microscopically. The data are presented as mean ± SE of 3 independent experiments each performed in duplicate. *P < .01 versus medium treatment, †P < .01 versus peptide treatment, respectively.
Specificity of each peptide-induced monocyte chemotaxis.
Monocytes were pretreated with HFYLPM (10 μM), MFYLPM (10 μM), or HFYLPm (10 μM) for 10 minutes at 37°C, washed twice, and thereafter allowed to migrate toward the maximal effective concentrations of each peptide (1 μM HFYLPM, 1 μM MFYLPM, or 10 nM HFYLPm) or medium. After fixing and staining of the membrane, migrated cells were quantified microscopically. The data are presented as mean ± SE of 3 independent experiments each performed in duplicate. *P < .01 versus medium treatment, †P < .01 versus peptide treatment, respectively.
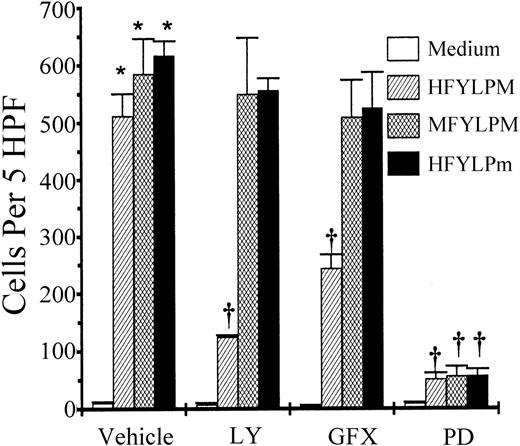
Comparison of the signalings for chemotaxis by the peptides
For this purpose, monocytes were pretreated either with LY294002 (50 μM), GF109203X (5 μM), or PD98059 (50 μM) or remained untreated for the control. After an incubation of the indicated periods (15 minutes for LY294002 and GF109203X, 60 minutes for PD98059), the cells were washed twice and used for chemotaxis assay. As shown in Figure 10, phosphatidylinositol-3 kinase (PI-3K) inhibitor (LY294002) and protein kinase C (PKC) inhibitor (GF109203X) inhibited HFYLPM-induced chemotaxis about 75% and 50%, respectively, but the inhibitors had no effect on MFYLPM- and HFYLPm-induced chemotaxis. These results indicate that HFYLPM, but not MFYLPM or HFYLPm, induces monocyte chemotaxis via PI-3K and PKC activation. Pretreatment of the cells with mitogen-activated protein kinase kinase (MEK) inhibitor (PD98059) caused almost complete inhibition of each peptide-induced chemotaxis (Figure 10). This implies that MEK activation is a critical event for chemotaxis induced by the 3 peptides.
Effect of enzyme inhibitors on monocyte chemotaxis induced by the peptides.
Monocytes remained untreated or were treated for 15 minutes (60 minutes for PD98059) with LY (LY294002, 50 μM), GFX (GF109203X, 5 μM), or PD (PD98059, 50 μM). After washing twice, 25 μL of monocytes at 1 × 106/mL was added to the upper wells and allowed to migrate for 2 hours at 37°C (humidified atmosphere; 5% CO2) toward each peptide (1 μM HFYLPM, 1 μM MFYLPM, or 10 nM HFYLPm) or medium. After fixing and staining of the membrane, the number of migrated cells was determined by counting them in high-power field (400 ×). The data are presented as mean ± SE of 3 independent experiments each performed in duplicate. *P < .01 versus medium treatment, †P < .01 versus peptide treatment, respectively.
Effect of enzyme inhibitors on monocyte chemotaxis induced by the peptides.
Monocytes remained untreated or were treated for 15 minutes (60 minutes for PD98059) with LY (LY294002, 50 μM), GFX (GF109203X, 5 μM), or PD (PD98059, 50 μM). After washing twice, 25 μL of monocytes at 1 × 106/mL was added to the upper wells and allowed to migrate for 2 hours at 37°C (humidified atmosphere; 5% CO2) toward each peptide (1 μM HFYLPM, 1 μM MFYLPM, or 10 nM HFYLPm) or medium. After fixing and staining of the membrane, the number of migrated cells was determined by counting them in high-power field (400 ×). The data are presented as mean ± SE of 3 independent experiments each performed in duplicate. *P < .01 versus medium treatment, †P < .01 versus peptide treatment, respectively.
Discussion
An organism has to defend itself against invading pathogens to survive. Therefore, hosts have developed efficient defense mechanisms. Phagocytic cells including monocytes, macrophages, and neutrophils have important roles in the earliest defenses against pathogens and other harmful agents.1-3 Because ROS including superoxide are part of the armory of the host defense system against invading microorganism, the identification of novel agents that can modulate phagocyte function and can help to elucidate its action is of paramount importance. However, approaches to identify novel agents have been very limited until now. In this study we adopted the screening of hexapeptide combinatorial libraries that contained more than 47 million different peptide sequences, and we identified 2 novel hexapeptides (HFYLPM and MFYLPM) that could induce superoxide generation in human monocytes. An analogue of HFYLPM, HFYLPm, was also developed as a potent stimulator for human leukocytes.
In our experiments investigating cell specificity of the peptides, we observed that the 3 peptides affected only human leukocytic cells, including phagocytes and Jurkat T cells. Nonleukocytic cells such as fibroblasts and neuronal cells were not affected by the novel peptides (Figure 4A). Among human leukocytes, Jurkat T cells were stimulated only by HFYLPM and HFYLPm but not by MFYLPM (Figure 4A). However, undifferentiated HL60 cells were activated only by MFYLPM and HFYLPm but not by HFYLPM (Figure 4A). Surprisingly, that just one amino acid change would create cell specificity, differentiating between different leukocytic cells, such as Jurkat T cells and HL60 promyelocytic cells. The specificity for Jurkat T cells versus undifferentiated HL60 cells resides in the first residue (H vs M) of the hexapeptide containing the consensus sequence (XFYLPM). The fact that HL60 cells were stimulated by HFYLPm but not by HFYLPM suggests that the 2 peptides have different receptor specificity and that at least a certain receptor specific for HFYLPm exists on HL60 cells. Although MFYLPM and HFYLPm acted on undifferentiated HL60 and HL60 cells differentiated into granulocytes, HFYLPM acted exclusively on differentiated HL60 cells. These results suggest that an unidentified receptor(s) for HFYLPM might exist on the differentiated HL60 cells but not be present on the undifferentiated cells. The differentiation of HL60 cells into granulocytes is accompanied by several expression pattern changes for various proteins.31-33 For instance, the fMLF receptor is not expressed on undifferentiated HL60 cells.34 However, HL60 cells differentiated into granulocytes do express a functional receptor for fMLF that plays an important role in the defense against invading pathogens.26 Although both the fMLF-receptor(s) and the HFYLPM-receptor(s) are restricted to differentiated HL60 cells, HFYLPM-receptor(s) but not fMLF-receptor(s) are expressed on Jurkat T cells.35 This indicates that HFYLPM can bind to receptors that are distinct from fMLF-receptor(s).
The novel peptides identified in this study, HFYLPM, MFYLPM, and HFYLPm, induced intracellular calcium mobilization, superoxide generation, and chemotactic activity for human phagocytic cells (Figures 2, 3, 7). HFYLPM and MFYLPM stimulate monocytes in a concentration range of 0.001 to 10 μM (0.33-10 μM for superoxide production; 0.01-1 μM for [Ca++]i release; 0.001-1 μM for chemotaxis). HFYLPm is more potent than 2 of the peptides, stimulating the cells within 0.01 to 100 nM (1-100 nM for superoxide generation; 0.1-100 nM for [Ca++]irelease; 0.001-10 nM for chemotaxis). Through checkboard analysis we confirmed that the peptide-induced activities are chemotaxis rather than chemokinesis (Table 1). Previous reports demonstrated that many leukocyte chemoattractants such as fMLF and various chemokines activate leukocytes via PTX-sensitive G protein–coupled receptors and phospholipase C β, resulting in cellular responses such as [Ca++]i rise, superoxide generation, and chemotaxis of the cells.25 36 Based on these circumstances, we can suggest that 3 novel peptides, HFYLPM, MFYLPM, and HFYLPm, can act as chemoattractants for human phagocytic cells.
Pretreatment of the cells with effective concentrations of HFYLPM, MFYLPM, or HFYLPm for 10 minutes caused complete inhibition of the same peptide-induced cell migration, indicating homologous receptor down-regulation (Figure 9). When the cells were preincubated with HFYLPM or HFYLPm for 10 minutes, it led to almost complete inhibition of MFYLPM-induced migration of monocytes (Figure 9). However, pretreatment of the cells with MFYLPM only partially or never affected HFYLPM- or HFYLPm-induced monocyte migration, respectively (Figure 9). These results suggest that HFYLPM and HFYLPm may have at least one unique and an independent receptor for MFYLPM on monocytes.
Through the study of the intracellular signaling pathways by the 3 peptides, we found that the HFYLPM-induced migration, but not the MFYLPM- and HFYLPm-induced migration, is sensitive to LY294002 and GF109203X, indicating PI-3K– and PKC-dependent (Figure 10) participation. However, all 3 peptide-induced migrations are sensitive to PD98059, implying that MEK activity is critical for the migration. These results suggest that the peptide HFYLPM may bind distinct receptors for MFYLPM and HFYLPm, and the receptor that HFYLPM binds would be coupled to PI-3K and PKC in monocytes. Surprisingly, peptides sharing essentially the same sequence and differing in stereospecificity by only one amino acid can bind some distinct receptor and induce distinct intracellular signaling.
Specific ligands act via specific receptors. This specificity results from a combination of subtle conformational and amino acid sequence differences. We previously described a synthetic hexapeptide, Trp-Lys-Tyr-Met-Val-D-Met-CONH2 (WKYMVm), that stimulated several human leukocytes except T cells.15,23,37,38Recently Le et al reported that the WKYMVm peptide acts at 2 fMLF receptor subtypes.39 Although HFYLPM and HFYLPm have an effect similar to WKYMVm on human phagocytic cells, they additionally exhibit specificity for Jurkat T cells. In our previous report we demonstrated that the Y in the third position of the hexapeptide and the M in the sixth position were important for the activity of WKYMVm on phosphoinositide hydrolysis in human leukocytes.23 The new peptides (HFYLPM, MFYLPM, and HFYLPm) also have a Y and an M in the third and the sixth position of the hexapeptide (Figure 2). Remembering that WKYMVm was modified from the lead sequence (M/W)KYM(P/V)M,15 23 we suggest that while Y at the third, P or V at the fifth, and M at the sixth position of the hexapeptide are important for the binding to a certain type of receptor, the first residue of the hexapeptide can determine the specificity of the peptide for receptor.
It seems reasonable to suggest that there might be a pool of receptors for a certain group of chemoattractants including our novel peptides on the leukocyte surface. Each leukocytic cell may have a distinct expression profile. The results that Jurkat cells were stimulated by HFYLPM and HFYLPm but not by MFYLPM, while HL60 cells were stimulated by HFYLPm and MFYLPM but not by HFYLPM, support our notion. Each peptide among 3 novel chemotactic peptides may bind to some of the receptors. Some receptors can be occupied by more than 2 peptides, and some can be occupied only one. Based on our observations that (1) the 3 peptides have different cell type specificities among leukocytes, (2) MFYLPM-induced monocyte chemotaxis could be inhibited by the other 2 peptides but not vice versa, and (3) the HFYLPM-induced cell migration was mediated by a different signaling than MFYLPM and HFYLPm, we can suggest that the 3 novel peptides act on some shared and some distinct receptors on a certain group in leukocytes.
Because HFYLPM, MFYLPM, and HFYLPm were identified by screening artificially synthesized peptides and one peptide was modified, we guess that these peptides can be mimetic of some natural ligands. We looked for sequence similarities between the novel peptides and known proteins by searching the SWISS-PROT and TrEMBL databases. We did not find a protein carrying the exact same sequence as the peptides; however, we found that several viral proteins such as the major capsid protein of the pseudorabies virus contain the X(F/K)Y(L/M)(V/P)M sequence. At this time, the significance of this sequence homology between our novel peptides and the viral protein is not clear.
Although various chemokines and chemoattractants have been identified, few short peptides acting on human leukocytes have been known until now. WKYMVm15,23,37,38 and fMLF26,33,34 40have been useful tools for studying phagocyte activation. Because the novel peptides HFYLPM, MFYLPM, and HFYLPm stimulate human phagocytes including monocytes and neutrophils (Figure 4A), these 3 peptides can also be used as tools to study phagocyte signaling. In the area of T-cell activation and signaling, there has been no report yet on small peptides acting on T cells. Because 2 of the novel peptides, HFYLPM and HFYLPm, stimulate Jurkat T cells, inducing [Ca++]i rise, they can serve as a tool to characterize T-cell activation.
We thank D. S. Cho and his colleagues for kind preparation of peripheral blood leukocytes.
Supported in part by programs of the Highly Advanced National Project and the National Research Laboratory from the Ministry of Science and Technology and by the Center for Cellular Signaling Research in Korea (S.H.R.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Sung Ho Ryu, Division of Molecular and Life Sciences, Pohang University of Science and Technology, Pohang, 790-784, Korea; e-mail:sungho@postech.ac.kr.

![Fig. 3. Effect of peptides on [Ca++]irise in human monocytes. / Fura-2–loaded monocytes were stimulated with effective concentrations of HFYLPM (1 μM,); MFYLPM (1 μM,▪); HFYLPm (100 nM,); and LFMYHP (10 μM,▾). The change in 340 nm/380 nm was monitored. (A) The results are representative of 6 independent experiments. The cells were stimulated with various concentrations of each peptide. The peak level of the increase in [Ca++]i was monitored. (B) Data are presented as means ± SE of 4 independent experiments. *P < .01 versus vehicle treatment.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/97/9/10.1182_blood.v97.9.2854/6/m_h80910966003.jpeg?Expires=1765900715&Signature=aBJL4RNvv6x3DaElRKXeRxBnhUBsofkn-gkK6ObX6UxoOjS2e5vvx1OuqP7qABaP9dvMbIYxf5XSSjRAOzhMoGEBcFjq2ajAECeV6qVJM8TgmHhAWbG63hgnVLTDODHJa3IW8oIQ0ydI5ynUhq2sS69cZ51C1KTVmvHAAximdu3lkX4EVUDw3CwmjCsuplPgSNgq2ftZsKNP4TXbY2GY1os85Qp7qBKXFzeHCQ1WzvGWlFfwloYgHNe9p86nxeSr-WlqAZolXUMrv4PqdvXBFjJGspCwLnkE~YEs290WctLvJEFaMCMPlH8ZkDExu2QXvchiFkBg3Ge16vRHQP4nUQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 4. Cell specificity of peptides. / Prepared human primary monocytes, neutrophils, and cultured HL60, Jurkat, U937, NIH3T3, 3Y1, 3T3L1, and PC12 cells were loaded with fura-2 and stimulated with effective concentrations of the 3 peptides (1 μM for monocytes and neutrophils; 10 μM for HL60 and Jurkat; 20 μM for U937, NIH3T3, 3Y1, 3T3L1, and PC12). (A) The peak level of the [Ca++]i increase was recorded. (B) Monocytes and neutrophils were stimulated with 10 μM of each peptide. Superoxide generation was measured using cytochrome c reduction assay. Data are presented are means ± SE from at least 3 independent experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/97/9/10.1182_blood.v97.9.2854/6/m_h80910966004.jpeg?Expires=1765900715&Signature=JlyapnPGPdjll8hsEu0kCrYoZQ6h~NlyTlUopazipQoX3~TgHLILLWdVNkr~714AKIytcV9BK542ojiePVvn13kDAVh4fQzKXLeGzsVgEHPLD2wNj0lJNGBfCmvXCzaefFNYiBtkrtIn2~H87p0-I~vFGg0fT3b54esLO~UWLOSN80q1rPT-hrSsXUOZJyLDPVhcL-Oby7BB10GwINeHPvlYS4Nn8mPkYTm85tvVQNp4mziB1pEY1BO2Dkqzv871gJkFPEsrofRGSY70~2qXnoNGvoSab1ZdpXJsIJbyeyTRXQbFfiqJz-EozOvKrhkSMtr13UkCjzYsxLUarF5rNw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 5. Differentiation specificity of the peptides. / HL60 cells were cultured in RPMI medium containing 20% fetal calf serum. To induce differentiation of the cells into granulocytes, the cells were cultured in the presence 1.25% DMSO for 4 days. Healthy HL60 (■) and differentiated HL60 cells (dHL60, ▪) were loaded with fura-2 and treated with various concentrations of HFYLPM (A), MFYLPM (B), and HFYLPm (C). The peak level of the [Ca++]i increase was recorded. The data are means ± SE from 4 independent experiments. *P < .01 versus vehicle treatment.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/97/9/10.1182_blood.v97.9.2854/6/m_h80910966005.jpeg?Expires=1765900715&Signature=KVjKb0nyEUidCZGbYMLXbkodbo20m31BLzJAyHtVMa6vrUvkXfifHeE1rLSCOkLcyWJeXP9Whyd8M2G3aBq33Obb3kMRpk0dDnER9g05b4vxDfpzSRRZx-p0OuQKZceA55yBik-l2s1DpOjVUzmHXAV5pQnlsY6IPIRtUAJBr3-9mYbujFYHGa7YnKCgkZCOWp-2dT-IpgJQq5q97vzBo3BvN0Le39bWUqYDl7gtdxpjIuW4u5Hun5bDRugGXv4K4MjjMajg3YxN5GsgODoy4QJHk6nydS6iQb2CjQQ1WFZjKejF21cbPPLH3K0PVMT5J5THiRu2RQ3UTEkLyvI4~w__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 6. Effect of fMLF on the peptide-induced [Ca++]i increase. / Ratios in fluorescence were monitored on fura-2–loaded differentiated HL60 cells before and during sequential addition of agonists at the times indicated by the arrows. The concentrations used were 1 μM Hm (HFYLPm), 1 μM fMLF, 10 μM HM (HFYLPM), or 10 μM MM (MFYLPM). The tracings shown are from 1 experiment representative of at least 3 independent experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/97/9/10.1182_blood.v97.9.2854/6/m_h80910966006.jpeg?Expires=1765900715&Signature=vIbSIY9Va40wr5nG0ki8qBXCEhVNvGALcRl3OcVtcJtuLPgkR0IufuOxIriNqA3HrjUcvI9LIVuiony6HRDF05hRLr7l-Iw0MUisHsHPjP8HHADew0EJasP54BO3xFnnBnBkMfYL4paccq6sxhnRjH7V4VEudwUiYBPweQb2X6wlzsneJ9eGw3gEeyXvDYZYxTHX0Ledp-NYAr9wOXHJnqoldt3Y8DwoxNLWIcq1TkbA2uyiQ-0SJ-kZb3ns-YRVzs2MLqTudtYAVdVBrJQMXn1~yf6J6kETC0INQfuokwmaRxUSqJbbwYfPM2~uT70p~~KEZDJjrIawbYfwfGOD3w__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal