Abstract
A factor VIII–deficient knockout mouse was used as a model for severe hemophilia A to characterize the immune response to recombinant human factor VIII (fVIII) and to study new approaches for induction of immune tolerance to fVIII. Mice initially received periodic injections of fVIII in doses similar to those used for the treatment of human hemophilia A. To induce immune tolerance, a hamster monoclonal antibody specific for murine CD40 ligand (CD40L or CD154) was injected with fVIII. Control mice received fVIII alone or fVIII and hamster immunoglobulin G. After treatment, humoral and cellular immune responses were evaluated. Ninety-five percent of anti-CD40L–treated mice had lower titers of anti-fVIII antibody (less than 1 μg/mL) compared with fVIII-injected control mice (mean, 18 μg/mL). To determine whether anti-CD40L treatment induces long-term immune tolerance, mice were rechallenged 3 times with fVIII alone. At 150 days after treatment, 12 of 22 anti-CD40L–treated mice remained tolerant to fVIII (anti-fVIII antibody titers less than 1 μg/mL). However, tolerant mice immunized with tetanus toxoid (TT) developed high anti-TT antibody, demonstrating that tolerance is fVIII specific. T cells from tolerant mice showed impaired proliferative responses after stimulation with fVIII in vitro and lack of production of the cytokines interleukin-2 (IL-2), IL-4, interferon γ, and IL-10. These results demonstrate that long-term immune tolerance to fVIII was effectively induced after early blockade of CD40-CD40L interaction. In addition, the lack of tolerance in this model was associated with the expression of a Th2 phenotype.
Introduction
Hemophilia A is an X-linked, recessive bleeding disorder that results from a deficiency of human blood clotting factor VIII (fVIII), and it affects 1 in 10 000 males.1 Severe hemophilia A (less than 1% normal fVIII levels) is associated with spontaneous or trauma-induced bleeding episodes.1 Such patients are commonly treated by replacement therapy consisting of frequent intravenous (IV) infusions of a purified human fVIII concentrate. However, a serious treatment complication is the development of a humoral immune response to fVIII in approximately 30% of patients with severe hemophilia A.2 These antibodies bind to fVIII and reduce its function by increasing fVIII plasma clearance and/or directly inhibiting its function as an enzymatic cofactor.3,4 Patients with such antibodies require additional therapies to eliminate the undesired anti-fVIII immune response. The current strategy to induce immune tolerance is frequent infusions of high doses of fVIII,5,6and this is usually successful but very expensive.7
In this study, we have used fVIII knockout mice generated by targeted disruption of exon 16 of the fVIII gene.8,These mice have undetectable fVIII activity in their plasma, and they represent a suitable model for severe hemophilia A. Knockout mice either periodically injected with fVIII or used for gene-therapy approaches develop an anti-fVIII immune response.9-14 It has previously been shown in this mouse model that the administration of cytotoxic T-lymphocyte–associated antigen 4–immunoglobulin (CTLA4-Ig) to block CD28-B7 interaction resulted in an effective prevention of anti-fVIII antibody (Ab) development. However, immune tolerance to fVIII was not achieved because mice developed Ab responses after subsequent challenges with fVIII.14
The activation of naive T cells requires at least 2 signals. Signal 1 is antigen/major histocompatibility complex (MHC) stimulation of the T-cell receptor/CD3 complex, and signal 2 is delivered through interaction of costimulatory molecules.15 The interaction of B7.1 and B7.2 proteins (CD80 and CD86, respectively) on antigen-presenting cells (APCs) with their receptor, CD28, on T cells is a well-described form of costimulation.16
Likewise, the interaction of CD40 ligand (CD40L), a member of the tumor necrosis factor (TNF) family, with CD40 on APCs has been identified as a significant pathway for the generation of many T-cell–mediated immune responses.17 CD40L is expressed mainly on activated CD4+ T cells.18 Expression of CD40L has also been reported on CD8+ T cells, mast cells, basophils, and on activated platelets.19,20 CD40, the receptor for CD40L, is a member of the TNF receptor family. It is expressed on APCs such as B cells, macrophages, and dendritic cells.21 It has been shown that CD40 ligation induces the expression of accessory molecules such as CD80, CD86, and MHC II on the APC population.16This APC activation step allows these cells to successfully activate naive T cells and to drive their differentiation to effector cells. Therefore, activation of APCs through CD40-CD40L interaction appears to be a mechanism by which cell-mediated immune responses are generated.22 Studies with immunodeficient CD40L knockout mice strengthen the role of this pathway in the development of effective immunity.23,24 In addition, humans with hyper-IgM syndrome who fail to express functional CD40L have increased susceptibility to opportunistic pathogens.25
In this study, we tested the hypothesis that costimulatory signaling via CD40-CD40L interaction is required for the development of the immune response to fVIII in mice with hemophilia A. To evaluate whether anti-fVIII Ab development could be prevented and whether immune tolerance could be induced in fVIII-deficient mice periodically infused with fVIII, we administered anti-CD40L monoclonal Ab to block CD40-CD40L interaction in vivo.
Materials and methods
Mice
Eight- to 14-week-old, age-matched, exon-16 fVIII knockout male and female mice were used in this study.8 The colony was derived by crossing 129SV female knockout founder mice with C57BL/6 male mice. The heterozygous female offspring were further back-crossed with normal C57BL/6 males for 3 additional generations, and subsequently affected males and females were interbred. The hemophilic mice used in the present study were thereby only partially inbred into a C57BL/6 genetic background. The genotypes of 4-week-old pups were determined by polymerase chain reaction (PCR) analysis of genomic DNA extracted from tail segments (Puregene; Gentra Systems, Minneapolis, MN), as described previously.26 The PCR reactions were slightly modified. The DNA was denatured for 6 minutes at 94°C and then subjected to 30 cycles of amplification. Each cycle consisted of 60 seconds at 94°C, 75 seconds at 60°C, and 60 seconds at 72°C. DNA from normal C57BL/6 mice was used as a control. The genotypes of hemophilic males and females were confirmed at the beginning of each experiment.
Antibody
The B-cell hybridoma secreting hamster antimouse CD40L IgG (MR1) was obtained from the American Type Culture Collection (Rockville, MD). The cells were grown in Hybridoma serum-free medium (Gibco BRL, Rockville, MD), and the monoclonal Ab was purified from the culture supernatant using a protein-G Sepharose column (Pharmacia, Uppsala, Sweden). The Ab concentration was determined by the Bio-Rad protein assay (Hercules, CA).
Immunization and tolerance induction of mice
Recombinant human fVIII (kindly provided by Baxter Healthcare, Glendale, CA) was used in this study. Control hemophilic mice were immunized by IV injections every 2 weeks with 0.2 μg fVIII, as described previously.10 To induce immune tolerance, mice were injected with 230 μg anti-CD40L IV 4 days before the first injection of fVIII. At days 0, 14, and 28, these mice received anti-CD40L plus fVIII, and control mice received 230 μg hamster IgG (Pierce, Rockford, IL) plus fVIII or fVIII alone per IV injection by the same schedule. After this treatment, all mice were injected 3 times 7 weeks apart with 0.2 μg fVIII. Blood samples were taken 10 days after immunization or treatment by retro-orbital bleeding, and plasma samples were stored at −80°C until processed. Mice were injected intraperitoneally with 2 flocculation units of tetanus toxoid (TT) vaccine (Aventis Pasteur, Swiftwater, PA). Plasma samples were obtained after 3 weeks, and anti-TT Ab titers were determined by luminometric enzyme-linked immunosorbent assay (ELISA).
Luminometric ELISA for Ab determination
Microlite 2 microtiter plates (Dynatech, Chantilly, VA) were coated overnight at 4°C with 2 μg/mL fVIII in sodium carbonate buffer (0.1 M, pH 9). Plates were washed 3 times with 100 μL per well of Tris-buffered saline (TBS), 0.05% Tween 20, and blocked for 1 hour with 100 μL per well of TBS, 1% bovine serum albumin (BSA). After washing, the plates were incubated for 1 hour at 37°C with 100 μL of mouse plasma samples diluted in TBS and 1% BSA in duplicate wells. Plates were washed as above and incubated for 1 hour at 37°C with 100 μL horseradish peroxidase (HRP)–labeled goat antimouse IgG (Pierce). After 3 final washes, 100 μL Super Signal (Pierce) was added to each well for 6 minutes. Chemiluminescence was measured in an ML2250 Microtiter Plate Luminometer (Dynatech). The concentration of anti-fVIII Ab was calculated as the average of at least 2 dilutions that were within the linear portion of the standard curve by using the coefficients of linear regression analysis. The standard curve consisted of a mixture of 2 monoclonal antihuman fVIII Abs (413 and C5) specific for fVIII heavy chain3 27 and Ab ESH8 (American Diagnostica, Greenwich, CT) directed to the fVIII C2 domain. The limit of detection of the ELISA was 1 ng/mL. For determination of IgG isotypes, we incubated fVIII-coated plates with plasma samples diluted as above. After washing, the plates were incubated with HRP antimouse IgG1 or IgG2a (Southern Biotech, Birmingham, AL). For anti-TT Ab determination, plates were coated overnight with 5 μg/mL purified TT protein (List Biological Laboratories, Campbell, CA). After washing as above and blocking with TBS, 3% BSA, diluted plasma samples were added to the plates and incubated for 2 hours at 37°C. After washing, 100 μL antimouse HRP-labeled Ab was added, and chemiluminescence was measured as above. Ab titers were calculated as end-point titers, the dilution factor that gave a signal more than 2 SDs above the background.
Splenic T-cell proliferation and cytokine assays
Spleens were removed aseptically, and single-cell suspensions were prepared in RPMI 1640 medium (Gibco BRL) with 15 mM HEPES buffer, 2 mM L-glutamine, 1 mM sodium pyruvate, 100 U/mL penicillin, 100 μg/mL streptomycin, and 10% heat-inactivated fetal calf serum. Red blood cells were lysed with sterile 17 mM Tris and 140 mM NH4Cl buffer, pH 7.4. Mononuclear spleen cells were washed extensively with X-vivo medium (Biowhittaker, Walkersville, MD) and used as a source of responder T cells. For T-cell enrichment, we performed B-cell depletion with BioMag goat antirat IgG magnetic beads (Polysciences, Warrington, PA) coated with monoclonal Ab B220 (Pharmingen, San Diego, CA). The remaining enriched T cells contained more than 80% CD3+ cells as determined by flow cytometry with a BP FACScan machine (Becton Dickinson, San Jose, CA), and they were 95% viable as shown by trypan blue exclusion. Peritoneal macrophages obtained as described previously28 were washed twice with RPMI 1640 medium (Gibco BRL), counted, diluted appropriately, and added to 96- and 24-well plates to allow adherence for 2 hours at 37°C in 5% CO2. After incubation, nonadherent cells were removed by extensive washing with X-vivo medium. The resulting APCs were used in T-cell proliferation and cytokine-production assays,29 and they consisted of more than 95% CD11b+ cells and 95% CD3− cells as determined by FACS analysis. Enriched T cells (105cells/well) were cultured with APCs (5 × 104 cells/well) and serial dilutions of fVIII in X-vivo medium for 4 days in 96-well flat-bottom plates.
To measure T-cell proliferation, we added 1 μCi [3H]-thymidine (Sigma, St Louis, MO) for the final 18 hours of incubation. The cells were harvested, and the amount of [3H]-thymidine incorporation was determined by scintillation counting. For cytokine assays, T cells (2 × 106/well) were cultured with APCs (6 × 104/well) and several concentrations of human fVIII in X-vivo medium. After 2 days of incubation for interleukin (IL)-2 and 5 days for interferon γ (IFN-γ), IL-4, and IL-10, culture supernatants were harvested and stored at −80°C. They were analyzed for each cytokine by ELISA using Ab pairs and standards purchased from Pharmingen.
Statistics
When required, the unpaired Student t test was performed, and P < .05 was considered statistically significant.
Results
Characterization of humoral and cellular immune responses in mice with severe hemophilia A
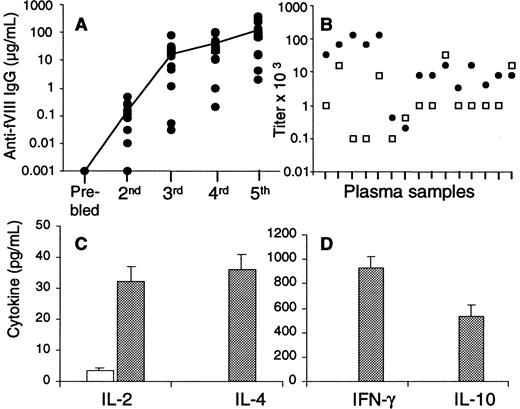
To characterize the immune response in hemophilia A knockout mice, we injected the animals with 0.2 μg human fVIII every 2 weeks, as described in “Materials and methods.” Figure1A shows the kinetics of the anti-fVIII Ab response of 16 hemophilic mice after 5 consecutive injections each with fVIII. The mice responded with increasing concentrations of anti-fVIII Ab after each of the injections with fVIII, as measured by ELISA. Although the Ab titers were low after the second injection (range, 10-480 ng/mL), the anti-fVIII Ab response increased rapidly after subsequent fVIII injections. After the third injection, 14 of 16 mice developed Ab titers greater than 1 μg/mL (range, 1.2-78 μg/mL), and 2 others had titers of 30 and 50 ng/mL.
Characterization of the immune response in mice with hemophilia A.
(A) Sixteen mice injected with 0.2 μg fVIII were bled after each of 5 consecutive injections, and Ab titers were determined by ELISA. The closed circles represent Ab concentrations for individual mice, and the line is the mean of Ab titers after each bleed. (B) Anti-fVIII IgG1 (gray) and IgG2a (white) were determined after 5 injections of fVIII in plasma samples of 15 mice. Results are expressed as end-point titers, the dilution factor that gave a signal greater than 2 SDs above the background, a 1:10 dilution of naive hemophilic plasma. (C,D) T cells and peritoneal macrophages were pooled from 2 mice injected 5 times with fVIII (gray) or 2 naive mice (white) and cultured with 100 nM fVIII in X-vivo medium. After 2 days (IL-2) and 5 days (IL-4, IL-10, and IFN-γ), cytokine ELISAs were performed from culture supernatants. The cytokine results are expressed as the mean and SD of duplicates.
Characterization of the immune response in mice with hemophilia A.
(A) Sixteen mice injected with 0.2 μg fVIII were bled after each of 5 consecutive injections, and Ab titers were determined by ELISA. The closed circles represent Ab concentrations for individual mice, and the line is the mean of Ab titers after each bleed. (B) Anti-fVIII IgG1 (gray) and IgG2a (white) were determined after 5 injections of fVIII in plasma samples of 15 mice. Results are expressed as end-point titers, the dilution factor that gave a signal greater than 2 SDs above the background, a 1:10 dilution of naive hemophilic plasma. (C,D) T cells and peritoneal macrophages were pooled from 2 mice injected 5 times with fVIII (gray) or 2 naive mice (white) and cultured with 100 nM fVIII in X-vivo medium. After 2 days (IL-2) and 5 days (IL-4, IL-10, and IFN-γ), cytokine ELISAs were performed from culture supernatants. The cytokine results are expressed as the mean and SD of duplicates.
In additional experiments not shown, we evaluated another 55 mice injected 3 times each with fVIII. Fifty-one of these mice responded with Ab titers greater than 1 μg/mL, and only 4 mice responded with low Ab titers of 300 to 800 ng/mL. Consistent with a previous publication,10 these data demonstrate that after 3 consecutive injections with fVIII, anti-fVIII Ab titers are detected in all mice. After subsequent injections with fVIII, the Ab titers increased in a dose-dependent fashion. After the fifth injection, 14 of 16 mice responded with more than 10 μg/mL anti-fVIII Ab, whereas plasma samples of the other 2 mice had 2 and 4 μg/mL, respectively. As shown by our results and reported previously by others,10 human fVIII is highly immunogenic in all mice (Figure 1A).
To determine whether periodic administration of fVIII induces a Th1 and/or a Th2 type of immune response, we determined fVIII-specific IgG1 and IgG2a subtypes (Figure 1B) and cytokine production by T cells after the fifth injection with fVIII (Figure 1C,D). IgG1 was detected in 15 plasma samples from individual mice with a range of end-point dilution factors of 200 to 128 000, and IgG2a was also detected with a dilution range of 100 to 32 000.
T cells isolated from pooled spleens of 2 naive hemophilic mice neither proliferated (not shown) nor produced measurable amounts of cytokines upon in vitro stimulation with fVIII (Figure 1C,D). However, T cells pooled from 2 mice injected only with fVIII and cultured in vitro with fVIII produced IL-2, IFN-γ, IL-4, and IL-10. Similar results were observed in 2 additional experiments. These cytokines were not detected in culture supernatants of T cells from fVIII-injected mice or naive mice stimulated in vitro with the unrelated protein hen egg lysozyme (not shown). These data demonstrate that fVIII-specific cytokine-producing T cells are induced in vivo after consecutive injections of fVIII in mice. In addition, these T cells produced Th1 cytokines (IL-2 and IFN-γ) and Th2 cytokines (IL-4 and IL-10) upon in vitro stimulation with the antigen. The cytokine profiles were consistent with the presence of both IgG1 and IgG2a fVIII-specific isotypes.
The anti-fVIII humoral immune response is significantly suppressed after blockade of CD40-CD40L interaction
To determine whether CD40-CD40L costimulatory signaling plays a role in the induction of the primary immune response to fVIII, we used a hamster monoclonal Ab specific for mouse CD40L to block CD40-CD40L interaction in vivo. Mice were treated with anti-CD40L or hamster IgG 4 days before the first injection with fVIII. At days 0, 14, and 28, mice received fVIII plus anti-CD40L. Controls were injected with fVIII alone or hamster IgG plus fVIII. At the end of the treatments, anti-fVIII Ab titers were determined by ELISA (Table1). A total of 56 mice were initially included in the experiments using anti-CD40L for immunosuppression, but 17 (30%) of these mice died because hemophilic mice have an increased risk of death due to bleeding during handling and/or spontaneous hemorrhage.26 In addition, 10 mice were killed to perform the T-cell proliferation and cytokine experiments shown below. For that reason, with the exception of the hamster IgG controls, sequential data from 27 mice surviving at the end of the study are shown in Table1. The rate of death in mice treated with anti-CD40L did not differ from that of untreated mice.
Humoral immune response after CD40-CD40L blockade
| Treatment . | Anti-fVIII antibody titer, μg/mL . | |||
|---|---|---|---|---|
| End of treatment . | Challenge with fVIII (days after treatment) . | |||
| 50 . | 100 . | 150 . | ||
| Hamster IgG plus fVIII | 1.3 | 66 | Died | — |
| 14 | 110 | Died | — | |
| 15 | Died | — | — | |
| 50 | 232 | 661 | 474 | |
| 145 | 103 | 180 | 185 | |
| fVIII alone | 3.6 | 77 | 104 | 165 |
| 4.3 | 83 | 168 | 65 | |
| 12 | 134 | 123 | 300 | |
| 19 | 77 | 51 | 120 | |
| 53 | 166 | 175 | 250 | |
| Anti-CD40L + fVIII (nontolerant) | 0.02 | 0.96 | 14.8 | 182 |
| 0.03 | 0.2 | 4.2 | 180 | |
| 0.05 | 0.9 | 36 | 185 | |
| 0.1 | 0.36 | 1.2 | 20 | |
| 0.15 | 60 | 258 | 300 | |
| 0.15 | 53 | 617 | 550 | |
| 0.39 | 4.3 | 186 | 276 | |
| 0.65 | 19 | 165 | 116 | |
| 0.9 | 0.08 | 117 | 338 | |
| 1.5 | 5.8 | 25 | 150 | |
| Anti-CD40L + fVIII (tolerant) | 0.02 | 0.01 | 0.01 | 0.05 |
| 0.05 | 0.06 | 0.08 | 0.05 | |
| 0.05 | 0.01 | 0.05 | 0.02 | |
| 0.05 | 0.09 | 0.08 | 0.04 | |
| 0.06 | 0.04 | 0.05 | 0.05 | |
| 0.08 | 0.04 | 0.07 | 0.05 | |
| 0.09 | 0.02 | 0.06 | 0.04 | |
| 0.1 | 0.15 | 0.11 | 0.5 | |
| 0.1 | 0.04 | 0.48 | 0.85 | |
| 0.12 | 0.1 | 0.05 | 0.08 | |
| 0.15 | 0.05 | 0.18 | 0.22 | |
| 0.35 | 0.06 | 0.04 | 0.16 | |
| Treatment . | Anti-fVIII antibody titer, μg/mL . | |||
|---|---|---|---|---|
| End of treatment . | Challenge with fVIII (days after treatment) . | |||
| 50 . | 100 . | 150 . | ||
| Hamster IgG plus fVIII | 1.3 | 66 | Died | — |
| 14 | 110 | Died | — | |
| 15 | Died | — | — | |
| 50 | 232 | 661 | 474 | |
| 145 | 103 | 180 | 185 | |
| fVIII alone | 3.6 | 77 | 104 | 165 |
| 4.3 | 83 | 168 | 65 | |
| 12 | 134 | 123 | 300 | |
| 19 | 77 | 51 | 120 | |
| 53 | 166 | 175 | 250 | |
| Anti-CD40L + fVIII (nontolerant) | 0.02 | 0.96 | 14.8 | 182 |
| 0.03 | 0.2 | 4.2 | 180 | |
| 0.05 | 0.9 | 36 | 185 | |
| 0.1 | 0.36 | 1.2 | 20 | |
| 0.15 | 60 | 258 | 300 | |
| 0.15 | 53 | 617 | 550 | |
| 0.39 | 4.3 | 186 | 276 | |
| 0.65 | 19 | 165 | 116 | |
| 0.9 | 0.08 | 117 | 338 | |
| 1.5 | 5.8 | 25 | 150 | |
| Anti-CD40L + fVIII (tolerant) | 0.02 | 0.01 | 0.01 | 0.05 |
| 0.05 | 0.06 | 0.08 | 0.05 | |
| 0.05 | 0.01 | 0.05 | 0.02 | |
| 0.05 | 0.09 | 0.08 | 0.04 | |
| 0.06 | 0.04 | 0.05 | 0.05 | |
| 0.08 | 0.04 | 0.07 | 0.05 | |
| 0.09 | 0.02 | 0.06 | 0.04 | |
| 0.1 | 0.15 | 0.11 | 0.5 | |
| 0.1 | 0.04 | 0.48 | 0.85 | |
| 0.12 | 0.1 | 0.05 | 0.08 | |
| 0.15 | 0.05 | 0.18 | 0.22 | |
| 0.35 | 0.06 | 0.04 | 0.16 | |
Mice were injected with hamster IgG plus fVIII, fVIII alone, or with anti-CD40L Ab plus fVIII (treatment); they were further challenged with 0.2 μg fVIII 3 times after the treatment. Anti-fVIII Ab titers were determined by ELISA.
Control mice injected with hamster IgG plus fVIII or only with fVIII had mean Ab titers of 45 and 18 μg/mL, respectively (P > .05), at the end of the treatment. This indicates that hamster IgG does not affect the ability of fVIII to elicit a humoral immune response. In contrast, all 22 anti-CD40L–treated mice produced significantly lower titers of anti-fVIII Ab at the end of the treatment (less than 1.5 μg/mL; mean, 0.23 μg/mL) compared with fVIII-injected control mice (mean, 18 μg/mL;P < .0002). These results demonstrate significant suppression of the humoral immune response in mice treated with anti-CD40L, indicating that CD40-CD40L interaction plays an important role in the development of anti-fVIII Ab in these mice.
Defective fVIII-specific T-cell activity after blocking CD40-CD40L interaction
To determine whether coadministration of fVIII and anti-CD40L exerts a direct suppressive effect on fVIII-specific T cells, we measured T-cell proliferation in response to fVIII in fVIII-injected control and anti-CD40L–treated mice at the end of the treatment (Figure 2). Pooled T cells from 2 control mice showed a dose-dependent proliferation upon restimulation in vitro with fVIII. The dramatic reduction in Ab formation after anti-CD40L treatment was accompanied by defective T-cell activity, as pooled T cells from 2 treated mice were unable to proliferate after restimulation in vitro with fVIII. Similar results were observed in one additional experiment using 3 mice in each group.
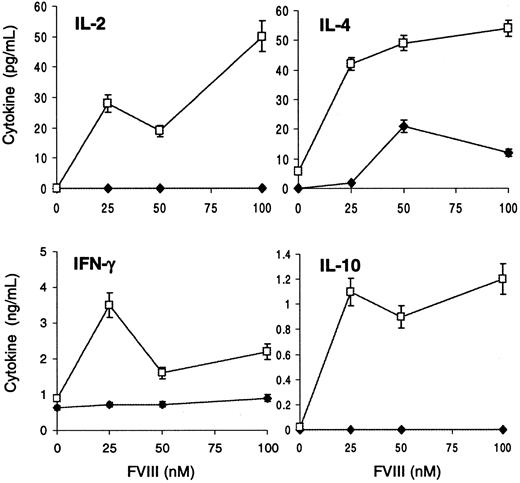
fVIII-Specific T-cell proliferative response is suppressed after blocking CD40-CD40L interaction in vivo.
T cells and peritoneal macrophages from 2 mice injected with fVIII (■) or 2 mice treated with anti-CD40L plus fVIII (●) were harvested at the end of the treatment and cocultured with increasing concentrations of fVIII. After 4 days, cells were pulsed with 1 μCi [3H]-thymidine, and incorporation of radioactivity was measured. Results are expressed as the mean of counts per minute (CPM) ± 1 SD of triplicate wells.
fVIII-Specific T-cell proliferative response is suppressed after blocking CD40-CD40L interaction in vivo.
T cells and peritoneal macrophages from 2 mice injected with fVIII (■) or 2 mice treated with anti-CD40L plus fVIII (●) were harvested at the end of the treatment and cocultured with increasing concentrations of fVIII. After 4 days, cells were pulsed with 1 μCi [3H]-thymidine, and incorporation of radioactivity was measured. Results are expressed as the mean of counts per minute (CPM) ± 1 SD of triplicate wells.
A variety of cytokine-dependent regulatory pathways have been suggested to be important in suppressor mechanisms as well as in tolerance induction.30 31 To determine whether regulatory cytokines were involved in the unresponsiveness observed, we measured cytokines released into the culture supernatants of T cells from 3 anti-CD40L–treated mice and 3 fVIII-injected control mice. High levels of IL-10, IL-2, IFN-γ, and IL-4 were detected in culture supernatants of T cells from mice injected only with fVIII (Figure3). However, T cells from anti-CD40L–treated mice did not produce detectable amounts of IL-10, IL-2, and IFN-γ. Some IL-4 was detected in culture supernatants of T cells from these mice, but the proliferative response of these cells was markedly suppressed. The experiment was repeated with 2 mice from each group, and similar results were observed. These results indicate that impaired T-cell activity after CD40-CD40L blockade is not likely to be regulated by an active suppressor mechanism mediated by cytokines. In contrast, the evident reduction of Th1-type (IL-2 and IFN-γ) and Th2-type (IL-4 and IL-10) cytokines suggests that early blockade of CD40-CD40L interaction prevents the expansion of both types of T-cell subsets.
FVIII-specific cytokine production is inhibited after blocking CD40-CD40L interaction in vivo.
Peritoneal macrophages and T cells from 3 fVIII-injected (■) and 3 anti-CD40L plus fVIII-treated mice (♦) were pooled and cultured with or without fVIII. Culture supernatants were harvested at 2 days (IL-2) and 4 days (IL-4, IFN-γ, and IL-10), and cytokine ELISAs were performed. Results show the mean ± 1 SD of duplicates.
FVIII-specific cytokine production is inhibited after blocking CD40-CD40L interaction in vivo.
Peritoneal macrophages and T cells from 3 fVIII-injected (■) and 3 anti-CD40L plus fVIII-treated mice (♦) were pooled and cultured with or without fVIII. Culture supernatants were harvested at 2 days (IL-2) and 4 days (IL-4, IFN-γ, and IL-10), and cytokine ELISAs were performed. Results show the mean ± 1 SD of duplicates.
Long-term induction of immune tolerance to fVIII after blocking CD40-CD40L interaction
To determine whether the immune suppression observed by anti-CD40L treatment confers immune tolerance to fVIII as defined by unresponsiveness after subsequent antigenic stimulation, we rechallenged the mice with fVIII alone (0.2 μg IV) at 50 days after the last anti-CD40L administration. Because the half-life of anti-CD40L in the mouse circulation is 2 to 3 weeks,17 we can assume that by the time of the first rechallenge with fVIII, the circulating anti-CD40L has been reduced to approximately 20% of the original plasma level. Because there are no published data that could indicate whether this theoretical amount of anti-CD40L would be immunosuppressive, 3 injections of fVIII alone 50 days apart were administered. If some anti-CD40L is present at the time of the first challenge with fVIII, we can presume that by the time of the second and third injections with fVIII alone, the anti-CD40L level would be reduced enough so that a suppressive effect is unlikely to be observed. After each fVIII challenge, anti-fVIII Ab titers were determined as described earlier (Table 1).
Mice treated with hamster IgG plus fVIII responded with anti-fVIII Ab titers similar to those of control mice injected only with fVIII (Table1). However, 17 of 22 mice initially immunosuppressed by anti-CD40L treatment remained unresponsive and produced anti-fVIII Ab titers of less than 1 μg/mL after the first challenge with fVIII (Table 1; means 0.2 (controls) and 108 μg/mL (high-titer antibody group); P < .005). When mice received the second challenge of fVIII, 13 of 22 anti-CD40L–treated mice still remained significantly unresponsive to fVIII compared with fVIII-injected control mice. The final challenge with fVIII was done at 150 days after treatment. The results in Table 1 demonstrate that 12 of 22 anti-CD40L–treated mice remained tolerant to fVIII, as the Ab titers observed in these mice were significantly reduced (less than or equal to 0.85 μg/mL; mean, 0.18 μg/mL) compared with those of fVIII-injected control mice, which produced high titers of anti-fVIII Ab (mean, 180 μg/mL; P < .02). This finding indicates that long-term induction of immune tolerance to fVIII was successfully achieved after early CD40-CD40L blockade in a significant number of treated mice.
The development of anti-fVIII Ab is significantly delayed by early blockade of the CD40-CD40L interaction
Some mice initially immunosuppressed after anti-CD40L treatment were able to produce anti-fVIII Ab after subsequent challenge with the antigen, demonstrating a lack of tolerance (Table 1). When the mice were challenged with fVIII 50 days after the last anti-CD40L administration, 5 treated mice responded with significantly higher titers of anti-fVIII Ab compared with the response of tolerant mice (means 28 and 0.06 μg/mL, respectively; P < .002). Nevertheless, Ab production by these treated/nontolerant mice still remained 4-fold suppressed compared with the Ab response of fVIII-injected controls (means 28 and 107 μg/mL, respectively;P < .009).
After the second challenge with fVIII 100 days after treatment, 5 anti-CD40L–treated mice were still suppressed by 8-fold or less compared with control mice injected only with fVIII (means 16 and 124 μg/mL, respectively; P < .008). This result indicates that even though these 5 treated/nontolerant mice have anti-fVIII Ab at 50 days after treatment, they still remain significantly suppressed up to 100 days beyond the last anti-CD40L administration. These data demonstrate that some mice initially immunosuppressed by CD40-CD40L blockade overcame the unresponsiveness after subsequent challenge with fVIII. Although the 5 treated/nontolerant mice developed anti-fVIII Ab, the early blockade of CD40-CD40L interaction delayed by 100 days the development of anti-fVIII Ab.
Tolerance induction after CD40-CD40L blockade is fVIII specific
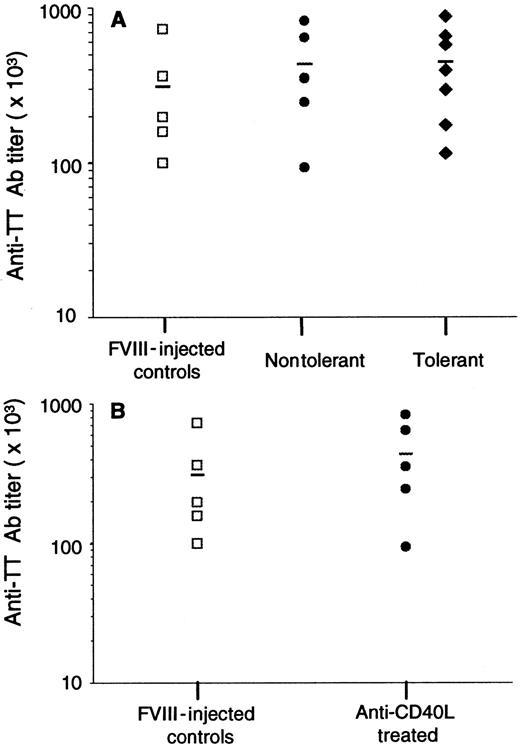
To determine whether the suppression of Ab production is specific for fVIII, 12 mice shown in Table 1 were immunized intraperitoneally with TT vaccine 5 weeks after the last challenge with fVIII. As controls, 5 mice were injected 4 times with fVIII. Anti-TT Ab titers were measured by ELISA in plasma samples at day 21 after immunization (Figure 4A). The results demonstrated that anti-CD40L–treated tolerant mice were able to mount a T-cell–dependent immune response to a different antigen, which is similar to the Ab response observed in controls and nontolerant mice. This result indicates that the suppression observed in Table 1 is fVIII specific. In addition, we determined the immune response to TT in fVIII-injected control and anti-CD40L–treated mice at the time they received the first challenge with fVIII at 7 weeks after the last anti-CD40L administration (Figure 4B). These results also demonstrated that the suppression of Ab formation was fVIII specific.
Humoral immune response to tetanus toxoid.
(A) Five fVIII-injected controls (■), 5 anti-CD40L–treated/nontolerant mice (●), and 7 anti-CD40L–treated mice tolerant to fVIII (♦) were injected intraperitoneally with 2 flocculation units of TT vaccine 5 weeks after the last challenge with fVIII. (B) Five control mice (■) injected 3 times with fVIII alone and 5 mice treated with fVIII plus anti-CD40L Ab (●) were immunized with TT 7 weeks after treatment. Results show the end-point titer of each sample, and the line is the mean for each group.
Humoral immune response to tetanus toxoid.
(A) Five fVIII-injected controls (■), 5 anti-CD40L–treated/nontolerant mice (●), and 7 anti-CD40L–treated mice tolerant to fVIII (♦) were injected intraperitoneally with 2 flocculation units of TT vaccine 5 weeks after the last challenge with fVIII. (B) Five control mice (■) injected 3 times with fVIII alone and 5 mice treated with fVIII plus anti-CD40L Ab (●) were immunized with TT 7 weeks after treatment. Results show the end-point titer of each sample, and the line is the mean for each group.
Anti-CD40L–treated/nontolerant mice express a predominant Th2 phenotype
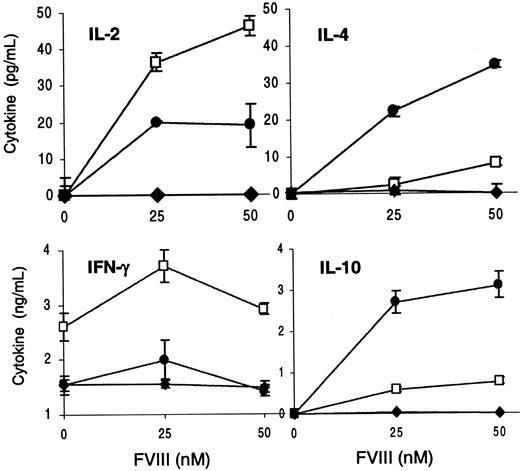
To assess whether the production of anti-fVIII Ab in anti-CD40L–treated/nontolerant mice could be the result of a lack of tolerance in either Th1 or Th2 cells, we measured cytokine production in culture supernatants. T cells and APCs were isolated from 3 fVIII-injected, 3 anti-CD40L–treated/nontolerant, and 3 treated/tolerant mice after the third challenge with fVIII. The cells from each group were pooled and cultured to measure the in vitro proliferative response upon fVIII stimulation (not shown) and cytokine levels in the culture supernatants (Figure5).
Cytokine production by T cells from control, anti-CD40L–treated/nontolerant, and tolerant mice.
T cells and peritoneal macrophages from 3 fVIII-injected mice (■), 3 anti-CD40L–treated/nontolerant mice (●), and 3 anti-CD40L–treated/tolerant mice (♦) were cultured and tested as in Figure 3. Results show the mean ± 1 SD of duplicates.
Cytokine production by T cells from control, anti-CD40L–treated/nontolerant, and tolerant mice.
T cells and peritoneal macrophages from 3 fVIII-injected mice (■), 3 anti-CD40L–treated/nontolerant mice (●), and 3 anti-CD40L–treated/tolerant mice (♦) were cultured and tested as in Figure 3. Results show the mean ± 1 SD of duplicates.
T cells from treated mice that were tolerant to fVIII (Ab less than 1 μg/mL) did not proliferate, remaining unresponsive upon in vitro restimulation with fVIII (not shown). These T cells were also unable to produce measurable amounts of IL-2, IL-4, IL-10, and IFN-γ (Figure5). On the contrary, T cells from treated/nontolerant mice (Ab greater than or equal to 20 μg/mL) showed a significant proliferative response after in vitro restimulation with fVIII (not shown). Although these T cells showed proliferation similar to the responses of T cells from control mice, the pattern of cytokine production differed markedly. T cells from treated/nontolerant mice produced greater amounts of IL-10 and IL-4 than T cells from control mice. This increase in Th2 cytokines produced by T cells from the nontolerant mice was accompanied by a reduction of the Th1-type cytokines IL-2 and IFN-γ (Figure 5). The experiment was repeated using 2 mice from each group, and similar results were observed.
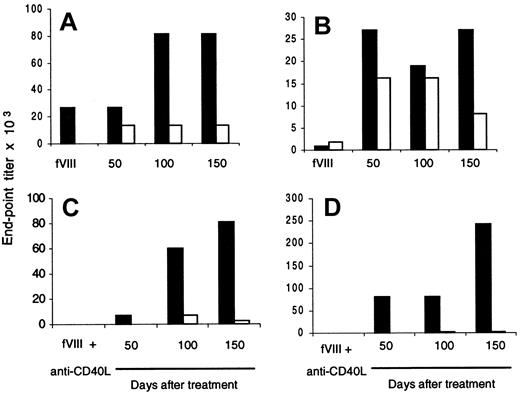
To further confirm that the Th2 phenotype is predominantly expressed in treated/nontolerant mice, we determined fVIII-specific IgG1 and IgG2a titers in plasma samples from fVIII-injected controls and anti-CD40L–treated/nontolerant mice. Results from 2 control mice (Figure 6A,B) and 2 treated/nontolerant mice (Figure 6C,D) are shown. FVIII-specific IgG1 and IgG2a were produced in significant titers in control mice (Figure 6A,B). A different pattern was observed in plasma samples from treated/nontolerant mice (Figure 6C,D). Both nontolerant mice showed higher titers of fVIII-specific IgG1 isotype, and IgG2a was barely detectable. These data are consistent with the cytokine profile of T cells from treated/nontolerant mice shown above, for which Th2 cytokines (IL-4 and IL-10) were increased compared with Th1 cytokines (IL-2 and IFN-γ) (Figure 5).
fVIII-Specific IgG1 is increased after blocking CD40-CD40L interaction in nontolerant mice.
Two mice were injected 3 times each with fVIII alone (A,B) or treated with anti-CD40L plus fVIII (C,D). Ten days after each treatment, blood was drawn, and anti-fVIII IgG1 (black bar) and anti-fVIII IgG2a (white bar) titers were determined by ELISA. Mice were challenged with fVIII alone (0.2 μg) 3 times after treatment, and plasma samples were tested for anti-fVIII IgG1 and IgG2a. Results are expressed by end-point titration.
fVIII-Specific IgG1 is increased after blocking CD40-CD40L interaction in nontolerant mice.
Two mice were injected 3 times each with fVIII alone (A,B) or treated with anti-CD40L plus fVIII (C,D). Ten days after each treatment, blood was drawn, and anti-fVIII IgG1 (black bar) and anti-fVIII IgG2a (white bar) titers were determined by ELISA. Mice were challenged with fVIII alone (0.2 μg) 3 times after treatment, and plasma samples were tested for anti-fVIII IgG1 and IgG2a. Results are expressed by end-point titration.
Discussion
Current approaches to induce tolerance in animal models include costimulatory blockade. Blockade of the CD40-CD40L pathway has proved effective in inducing tolerance in transplantation models.32-34 The administration of anti-CD40L in combination with the B7-specific fusion protein CTLA4-Ig prolonged renal allograft survival in Rhesus monkeys.33 However, this combination failed to lead to indefinite graft survival.34 In addition, the combined therapy of anti-CD40L plus CTLA4-Ig reduced skin-graft survival in mice.32 Because the results of using anti-CD40L were more encouraging in the induction of long-term engraftment as well as the prevention of autoimmune disease, we focused our attention on CD40-CD40L blockade to determine whether immune tolerance could be achieved in mice with hemophilia A by using monotherapy with anti-CD40L. The results demonstrate for the first time that early blockade of CD40-CD40L interaction successfully induces long-term immune tolerance to fVIII in mice with hemophilia A.
Different approaches to prevent the development of anti-fVIII Ab in mice with hemophilia A included the administration of CTLA-4Ig to block CD28-B7 interaction14 and also a brief treatment with anti-CD40L Ab.35 These strategies effectively inhibited the primary immune response to fVIII; however, tolerance induction was not achieved by these treatments because initially immune-suppressed mice rechallenged with fVIII responded with anti-fVIII Ab.14 35
The results of the present study demonstrate that the therapy with anti-CD40L Ab results in effective suppression of the primary immune response as well as successful induction of long-term immune tolerance to fVIII in some treated mice. Twenty-one of 22 mice treated with anti-CD40L showed marked suppression of anti-fVIII Ab development. In addition, 12 of 22 mice that received anti-CD40L treatment and were subsequently rechallenged with fVIII alone remained unresponsive for more than 150 days without receiving additional maintenance doses of anti-CD40L. These results establish that CD40-CD40L interaction plays an important role in anti-fVIII Ab development in the hemophilic mouse model, and, more important, that tolerance to fVIII can be induced by early CD40-CD40L blockade.
We also demonstrated that anti-CD40L–treated tolerant mice retain the ability to mount a normal immune response to the unrelated antigen TT. This result indicates that blockade of CD40-CD40L signaling at the time of antigen presentation generates fVIII-specific unresponsiveness while leaving intact the ability to mount a humoral immune response to other T-cell–dependent antigens.
The mechanism by which anti-CD40L therapy inhibits rejection in transplantation and modifies the course of autoimmune diseases remains to be fully elucidated. In addition, there is a lack of information about the mechanism by which prevention of Ab formation occurs in mice with hemophilia A after treatment with CTLA-4Ig.14 Among the mechanisms that have been proposed to be involved in the induction and maintenance of tolerance by costimulatory blockade is the active suppression mediated by individual subsets of T cells or cytokines.36-39 To explore the mechanism of immune tolerance in this mouse model, we first tested the hypothesis that regulatory cytokines may be involved in tolerance induction. We demonstrated that the suppression of the humoral immune response is accompanied by severe disability of T cells from anti-CD40L–treated mice to respond to fVIII after in vitro restimulation. T cells from these mice were also unable to proliferate and to secrete IL-2, IL-10, and IFN-γ. We also observed reduced IL-4 production by these T cells. We further demonstrated that T cells from tolerant mice remained functionally disabled upon in vitro restimulation with fVIII for more than 150 days. The lack of proliferation and the inability to produce both Th1-type (IL-2, IFN-γ) and Th2-type (IL-4, IL-10) cytokines indicate that blocking the signaling via CD40-CD40L interaction in vivo affects both T-cell subsets. Furthermore, the absence from tolerant mice of T cells able to respond in vitro to fVIII stimulation indicates that the mechanism(s) by which CD40-CD40L blockade prevents Ab formation and maintains tolerance is apparently not mediated by cytokines.
Other possible mechanisms proposed for tolerance induction after costimulatory blockade are anergy and/or deletion by apoptosis of antigen-specific T lymphocytes. Using transgenic mice with aBcl-x gene mutation, it was demonstrated that tolerance induction by costimulatory blockade was inhibited.40 The blockade of both CD28-B7 and CD40-CD40L interactions did not allow stable skin allografts. However, treatment with rapamycin plus blockade of both of these pathways resulted in massive apoptosis of alloreactive T cells and produced stable skin allograft tolerance.41Tolerance induction was associated with increased apoptotic CD4+ T cells after costimulatory blockade in pancreatic islet xenografts.42 It has also been demonstrated that blockade of CD86 and CD40 induced anergy.43 Based upon these observations, it appears that in our model, the mechanism(s) by which CD40-CD40L blockade induced tolerance might be anergy and/or apoptosis of fVIII-specific T cells. This is suggested by the lack of proliferation and the absence of cytokine release by T cells from tolerant mice.
As reported by others,11,9 12 we observed heterogeneity in the humoral immune response to fVIII. Although all mice develop anti-fVIII Ab when injected periodically with fVIII, the Ab titers vary considerably among individual mice. In addition, some mice that were initially immunosuppressed by anti-CD40L administration gradually responded by producing anti-fVIII Ab. It is not yet clear whether genetic variability plays a role in the complexity of the immune response to fVIII. Because the mice we used were back-crossed only 4 times with C57BL/6 mice, we cannot yet determine whether a more homogeneous genetic background would lead to less heterogeneity of the immune response.
We demonstrated that T cells expressing type-1 (IL-2, IFN-γ) and type-2 (IL-10, IL-4) cytokines are induced after periodic immunization with fVIII alone. Consistent with the production of IFN-γ by T cells from control mice, we detected significant titers of fVIII-specific IgG2a. Similarly, splenic T cells from fVIII-injected mice produced IL-4, which is correlated with the presence of anti-fVIII IgG1 in their plasma. These data demonstrate for the first time that periodic IV injections with fVIII in mice with hemophilia A lead to the development of both Th1 and Th2 responses. However, 10 of 22 mice that were initially immunosuppressed by anti-CD40L treatment and gradually recovered the ability to respond to fVIII after subsequent challenges expressed a predominant Th2 phenotype. T cells isolated from anti-CD40L–treated/nontolerant mice produced greater amounts of IL-4 and IL-10 compared with T cells from control mice. This increase in the production of Th2-type cytokines was accompanied by an increase of anti-fVIII–specific IgG1 titers in plasma samples of treated/nontolerant mice. In addition, T cells from such mice produced lower amounts of IL-2, and they did not produce IFN-γ. FVIII-specific IgG2a was barely detectable in the mouse plasma samples.
It is not yet clear why the Th2 phenotype is related to the lack of tolerance in anti-CD40L–treated mice. This response has been associated with the induction of tolerance after costimulatory blockade in allograft models, but it can also be observed in the context of transplant rejection.30,31,34 Considering that CD40-CD40L blockade significantly suppressed the immune response to fVIII, it may be that fVIII-specific Th2 cells are either escaping or surviving the immunosuppressive therapy. The presence of some IL-4, determined immediately after treatment in culture supernatants of T cells from immune-suppressed mice, may favor this hypothesis. Although we have not addressed the role of IL-4, it has been suggested that IL-4 is not sufficient for tolerance induction to donor-specific transfusions and CD40-CD40L blockade.32 Costimulation via CD28 has been shown to promote Th2 differentiation,44 and new members of the B7 family stimulate the production of IL-10.45 Taken together, these observations suggest that anti-fVIII Ab development in nontolerant mice may be mediated by signals delivered via other costimulatory molecules, such as CD28-B7, thereby promoting the expansion of Th2 clones. Recent data indicate that additional strategies are likely to be necessary for induction of tolerance in the group of nontolerant animals.
We thank Baxter Healthcare Corporation for providing us with purified recombinant human fVIII.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Dorothea Scandella, American Red Cross, 15601 Crabbs Branch Way, Rockville, MD 20855; e-mail:scandell@usa.redcross.org.

![Fig. 2. fVIII-Specific T-cell proliferative response is suppressed after blocking CD40-CD40L interaction in vivo. / T cells and peritoneal macrophages from 2 mice injected with fVIII (■) or 2 mice treated with anti-CD40L plus fVIII (●) were harvested at the end of the treatment and cocultured with increasing concentrations of fVIII. After 4 days, cells were pulsed with 1 μCi [3H]-thymidine, and incorporation of radioactivity was measured. Results are expressed as the mean of counts per minute (CPM) ± 1 SD of triplicate wells.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/97/9/10.1182_blood.v97.9.2750/6/m_h80911007002.jpeg?Expires=1765121833&Signature=qbjMBHpRI0Vxct~psFBrlU94hJIwYahRuJDUzSD9JpMZQpXHjzJ7z7durxZLnudQuSGmmr7daNiNtZI4tPT7GOlIVhkHl2QpyN8I50Nyr0XN8LZGzxJ8IE2T9AaGo5dMdu9GpWYZBHMILlZl55WP0Xc0yTFXksV8Z~f2DhM34Rgzvqohs3FYq8KsKEzBZZPGo51XO3PAPSYA2z6R4uf19XX4rbITJdeLcsPnnLTaOs0IUu4ffRGKZp0N1xvuXzkPQ3hbXrRE5v5ZeeqqCoHRVGQQy6QakOI5cp~-0xVfhwHZ-TuWw1fQtmUXCoGPNEDU-rP~fOFrHgR-J2-9MYn0Lg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal