Abstract
The gene for hemochromatosis (HFE) is expressed in a variety of cells, including those not thought to be affected by this disease. The impact of HFE on iron transport was examined in B-lymphoid cell lines developed from a patient with hemochromatosis with the HFE C282Y mutation (C282Y cells) and an individual with the wild-type HFE gene (WT cells). Whereas both cell lines expressed HFE protein, C282Y cells displayed less HFE protein at the cell surface. Transferrin receptor (TfR) number was 2- to 3-fold greater in WT cells than in C282Y cells, while TfR affinity for transferrin (Tf) was slightly lower in C282Y cells. TfR distribution between intracellular and cell-surface compartments was similar in both cell lines. Iron uptake per cell was greater in WT cells but was not increased proportional to TfR number. When considered relative to cell-surface TfR number, however, iron uptake and Tf internalization were actually greater in C282Y cells. Surprisingly, Tf-independent iron uptake was also significantly greater in C282Y cells than in WT cells. The ferritin content of C282Y cells was approximately 40% that of WT cells. Exposure of cells to pro-oxidant conditions in culture led to a greater inhibition of proliferation in C282Y cells than in WT cells. Our results indicate that in this B-lymphoid cell line, the HFE C282Y mutation affects both Tf-dependent and -independent iron uptake and enhances cell sensitivity to oxidative stress. The role of HFE in iron uptake by B cells may extend beyond its known interaction with the TfR.
Introduction
Hereditary hemochromatosis is one of the most common autosomal recessive diseases in the Caucasian population.1 The cloning of the hemochromatosis(HFE) gene has led to the recognition of 2 predominantHFE gene mutations: one in which C is replaced by Y (single-letter amino acid code) at amino acid 282 (C282Y) and another in which H is replaced by D at amino acid 63 (H63D).2These HFE mutations are responsible for hemochromatosis in the majority of patients of northern European ancestry.2-5
The hallmark of hemochromatosis is the increased gastrointestinal absorption of iron and its accumulation in parenchymal cells, resulting in tissue injury.6 In the circulation, iron is transported bound to its transport protein transferrin (Tf), and its uptake by cells is facilitated by cell-surface Tf receptors (TfRs).7,8 Iron may also be incorporated into some cells by a Tf-independent transport system.7 This latter mechanism of iron uptake may be relevant in hemochromatosis because the amount of iron in the circulation may exceed the binding capacity of Tf, resulting in the binding of iron to low-molecular-weight (non-Tf) ligands.9 10
The relation of HFE to iron metabolism has been the subject of intense investigation. In the cell, newly synthesized HFE protein binds to β2-microglobulin (β2M) and traffics to the cell surface in association with the TfR (as a β2M–HFE–TfR complex).11,12 TheHFE C282Y mutation disrupts the interaction between HFE and β2M, resulting in a block in HFE trafficking and a failure of HFE to be presented with TfR at the cell surface.11,13-15 Several studies have examined the effect of HFE overexpression in HeLa cells transfected with the wild-typeHFE gene and have shown that it results in a down-regulation of Tf-dependent iron uptake.16-18 Hence, HFE, through its binding to the TfR, appears to function as a negative regulator of TfR-mediated uptake of iron. The role of HFE mutations in the pathogenesis of hemochromatosis has been confirmed by the demonstration that mice with the HFE gene knockout or theHFE C282Y gene mutation develop iron overload that mimics hemochromatosis in humans.19 20
Whereas the major organ systems affected in hemochromatosis are the liver, heart, pancreas, joints, and skin,21,22HFE also appears to be expressed in a variety of different cell types, including those not thought to be directly involved in this disease.2 This raises important questions regarding the role of HFE in cells of different lineages. To examine the significance of HFE in lymphoid cells, we have developed B-lymphoblastoid cell lines from a normal individual and from a patient with hemochromatosis homozygous for the C282Y mutation. The latter cell line (C282Y cells) has provided us with the opportunity to investigate cellular iron transport in cells that express the mutant HFE C282Y protein constitutively rather than by gene transfection. Our studies show that B cells with the HFE C282Y mutation and B cells with wild-type HFE differ significantly with respect to TfR expression, iron uptake, and sensitivity to the cytotoxicity of oxidative stress.
Materials and methods
Materials
Human Tf, nitrilotriacetic acid (NTA), glucose oxidase, phenylmethylsulfonyl fluoride (PMSF), bovine serum albumin (BSA), and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) were purchased from Sigma Chemical Company (St Louis, MO). Pansorbin was obtained from Calbiochem (La Jolla, CA). 125I-Na and59FeCl3 were purchased from Amersham (Arlington Heights, IL). EZ-Link Sulfo-NHS-LC Biotin and Streptavidin conjugated to horseradish peroxidase (HRP) were purchased from Pierce (Rockford, IL). 59Fe-NTA and 59Fe-Tf were prepared as described by Bates and Schlabach23; 125I-Tf was prepared by the chloramine-T method.24 Rabbit antiserum to human HFE protein was a generous gift from Dr William Sly (St Louis University School of Medicine).
Cells
Peripheral blood was obtained from a normal individual withoutHFE mutations and from a patient with hemochromatosis homozygous for the HFE C282Y mutation. The study was approved by the Institutional Review Board of the Medical College of Wisconsin, and informed consent was obtained before obtaining blood to generate cell lines. Epstein-Barr virus–transformed B-lymphoblastoid cell lines were established by culturing peripheral blood mononuclear cells in the presence of culture supernatant from the B95.8 cell line. Human leukemic HL60 (myeloid) and CCRF-CEM (lymphoid) cell lines were obtained from American Type Culture Collection (Manassas, VA). Cells were grown in RPMI 1640 medium supplemented with L-glutamine and 10% fetal calf serum (FCS) and were maintained in an atmosphere of 6% CO2.
Analysis for HFE mutations in cells
Cells were analyzed for HFE gene mutations at the Molecular Diagnostics Laboratory of the Blood Center of Southeastern Wisconsin, Milwaukee. Genomic DNA isolated from cells was amplified by polymerase chain reaction (PCR) using HFE allele-specific primers to confirm the presence of the HFE C282Y mutation or the wild-type HFE gene, as described previously by Dinauer and Bellissimo,25 with modifications to avoid a polymorphism found in normal alleles.26 PCR amplifications for HFE H63D were performed using primers as described by Feder et al.2 Cells with the HFE C282Y/C282Y mutation and cells without HFE mutations are subsequently referred to as C282Y and wild-type (WT) cells, respectively.
Immunoprecipitation and Western blotting
HFE protein expression in cells was detected by immunoprecipitation of cell lysates followed by Western blotting with an ECL Western blotting detection system (Amersham), as described previously.12 Cells (107) were harvested after 24 hours of growth in fresh medium; washed twice by centrifugation with ice-cold 10 mM Tris/150 mM NaCl, pH 7.4 buffer (Tris-buffered saline); and lysed in 1 mL of 150 mM NaCl/10 mM Tris, pH 7.4/5 mM EDTA/1% Triton X-100 (NET-Triton) buffer containing 100 μg/mL PMSF and 75 μg/mL aprotinin. Cellular debris was removed by brief centrifugation, and the supernatant was saved for measurement of protein content by a BCA protein assay kit (Pierce). An aliquot of the supernatant containing 500 μg protein was preadsorbed by mixing with Pansorbin at 4°C for 1 hour. Pansorbin was removed by centrifugation, and the supernatant was incubated with 1.5 μL anti-HFE antiserum and 25 μL fresh Pansorbin to immunoprecipitate HFE. The HFE–antibody–Pansorbin pellet was washed extensively with RIPA buffer (150 mM NaCl/50 mM Tris, pH 7.4/1% Triton X-100/1% sodium deoxycholate/0.1% sodium dodecyl sulfate [SDS]) containing 15% sucrose and finally resuspended in Laemmli sample buffer.27 SDS-polyacrylamide gel electrophoresis (SDS-PAGE) of the samples was performed as described.27Proteins were transferred from the gel onto a nitrocellulose membrane, as described,28 using a Transblot system (Bio-Rad, Richmond, CA). For detection of HFE, membranes were first incubated for 1 hour at room temperature in blocking buffer (phosphate-buffered saline [PBS] with 0.1% Tween-20 containing 10% nonfat dry milk), followed by sequential washes in PBS-Tween. Membranes were then incubated for 1 hour at room temperature in PBS-Tween containing rabbit antiserum to human HFE (1:5000 dilution). Following this, the membranes were washed with PBS-Tween and incubated for 1 hour at room temperature in the same buffer containing donkey anti-rabbit Ig (1:10 000 dilution) conjugated to HRP. Membranes were then immersed in ECL detection solution and exposed to XAR-5 film for autoradiography.
Cell-surface protein labeling
HFE protein expressed at the cell surface was identified using a modification of a previously described method.15 WT and C282Y cells were harvested and washed 3 times with ice-cold PBS. Five hundred micrograms of EZ-link Sulfo-NHS-LC biotin was added to 2 × 107 cells in 1 mL of ice-cold PBS. After a 30-minute incubation on ice, the cells were washed extensively with PBS and lysed in 1 mL of NET-Triton buffer. Cellular debris was removed by centrifugation, and the protein content of the supernatant was measured. Five hundred micrograms of the sample was preadsorbed at 4°C for 1 hour with 50 μL Pansorbin. Biotinylated HFE protein in the preadsorbed sample was immunoprecipitated by incubating it for 2 hours at 4°C with 1.5 μL rabbit antiserum to human HFE and 25 μL fresh Pansorbin. The HFE–antibody–Pansorbin pellet was washed extensively with RIPA-sucrose and resuspended in Laemmli sample buffer, as described in the previous section. The sample was then resolved by SDS-PAGE and transferred to a nitrocellulose membrane. Biotinylated HFE on the membrane was identified by incubating the membrane with 2 μg/mL streptavidin-HRP for 1 hour, immersing it in ECL detection solution, and exposing it to XAR-5 film for autoradiography.
59Fe uptake studies
Cells were harvested, washed with medium, and replated (106 cells/mL) in 24-well plates in fresh medium containing 1% FCS. 59Fe-Tf (0-24 ng Fe/mL) was added to each well at the start of the incubation. After 45 to 270 minutes of incubation, cells were removed from the wells and washed by centrifugation with ice-cold PBS. The radioactivity in the cell pellet was counted to determine the amount of 59Fe taken up by cells. Tf-independent iron uptake was examined as described previously.29 Cells were washed with PBS, incubated at 37°C for 30 minutes in PBS with 1% BSA to deplete them of Tf, and then incubated in serum-free medium with 0 to 12 ng/mL Fe as59Fe-NTA. After 270 minutes of incubation, the amount of59Fe taken up by cells was determined as described above.
125I-Tf binding studies
Cell-surface TfR density was determined by 125I-Tf binding to intact cells. Proliferating cells were harvested, washed with ice-cold PBS containing 0.1% BSA, and assayed for125I-Tf binding at 4°C, as described previously.30 Maximal Tf binding and TfR affinity for Tf were determined according to the method of Scatchard.31 To determine the relative distribution of TfRs between cell surface and intracellular compartments, 125I-Tf binding studies were performed at 4°C (cell-surface TfR) and at 37°C (total TfR). The difference between 125I-Tf binding to cells at 37°C (total) and 4°C (cell surface) was taken to represent intracellular TfR. The fraction of intracellular TfRs was also determined by washing cells from the 37°C binding assay with an ice-cold acidic buffer to remove cell-surface–bound 125I-Tf and then counting the amount of radioactivity remaining in the cells.
125I-Tf internalization studies
The internalization by cells of Tf bound to cell-surface TfRs was examined as described previously.32 Briefly, exponentially growing cells were harvested, washed twice with ice-cold PBS-BSA, and then incubated with 125I-Tf at 4°C for 60 minutes to allow for Tf binding to cell-surface TfRs. At the end of this incubation, cells were washed extensively by centrifugation with ice-cold PBS-BSA to remove unbound 125I-Tf and then resuspended in 1 mL of serum-free medium at 37°C. At 2.5-minute intervals, aliquots of cell suspension were removed and centrifuged through ice-cold acid wash buffer. The supernatant was carefully removed, and the radioactivity in the cell pellet and supernatant was counted to determine the fraction of 125I-Tf internalized by cells (ie, acid-resistant cpm).
Measurement of cellular ferritin
Cells were harvested after a 24-hour incubation in fresh medium, washed with PBS, and disrupted by sonication. Cellular debris was removed by centrifugation (30 000g for 30 minutes), and the cytoplasmic fraction (supernatant) was assayed for protein content and for total ferritin with the Quantimune ferritin assay (Bio-Rad). The ferritin content was expressed as ng/mg cell protein.
Oxidative stress conditions
The sensitivity of cells to oxidative stress was determined by examining their growth under pro-oxidant conditions in culture medium generated by the presence of glucose and glucose oxidase.33 Cells (2 × 105 cells/mL) were plated in 96-well microwell plates in complete medium containing 20 mM D-glucose. Glucose oxidase (0-2 mU) was added to the wells at the start of incubation, and cell growth was determined by MTT assay after 48 hours, as described previously.34 Briefly, at the end of the 48-hour incubation, 10 μL of MTT (5 mg/mL stock solution) was added to each well, and the cells were incubated at 37°C for an additional 4 hours. Cells were solubilized by the addition of 100 μL of 0.04 N HCl in isopropanol to each well, and the absorbance of each well was determined spectrophotometrically at a dual wavelength of 570/630 nm using an EL 310 microplate auto-reader (Biotech Instruments, Winooski, VT). The effect of glucose oxidase on cell proliferation was determined by comparing the absorbance of the wells containing glucose oxidase with absorbance in those in which it was omitted.
Results
Cells with wild-type and mutant C282Y HFE gene
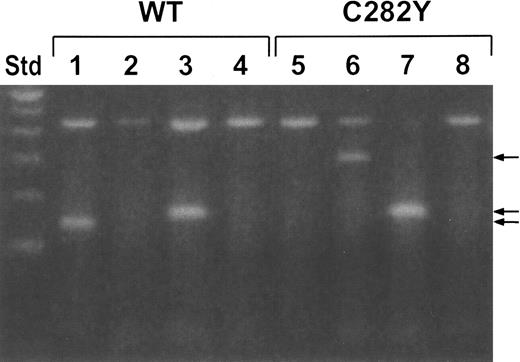
The C282Y cell line was derived from a patient with hemochromatosis homozygous for the HFE C282Y mutation, whereas the WT cell line was derived from a healthy control subject. The established cell lines were analyzed by PCR for the HFEC282Y and H63D mutations using allele-specific primers for wild-typeHFE (C282 and H63 primers) or for mutant HFEC282Y and H63D (Y282 and D63 primers, respectively). Figure1 shows the results of the PCR amplifications with WT and C282Y cells using the 4 specific primers with each cell line. Lanes 1 and 5, which represent the PCR amplification using the wild-type HFE C282-specific primers, show a positive PCR product with WT cells (lane 1) but not with C282Y cells (lane 5). Lanes 2 and 6, which represent the PCR amplification using the mutant Y282 primers, show a positive PCR product with C282Y cells (lane 6) but not with WT cells (lane 2). Both cell lines gave positive PCR products with the wild-type H63 primers (lanes 3 and 7), but not with mutant D63 primers (lanes 4 and 8), confirming the absence of the HFE H63D mutation in either cell line. These results confirm that the C282Y cells are homozygous for the HFEC282Y mutation, whereas the WT cells do not haveHFE mutations.
Identification of the HFE gene by PCR.
WT and C282Y cells were analyzed for HFE and HFEgene mutations by allele-specific PCR. Primers specific for wild-typeHFE (C282 and H63) and mutant HFE (Y282 and D63) were used in PCR reactions on DNA isolated from the 2 cell lines. Lanes 1 and 5, C282 primers; lanes 2 and 6, Y282 primers; lanes 3 and 7, H63 primers; lanes 4 and 8, D63 primers. The 430-bp band (larger mol. wt. band) in all lanes represents a control PCR product. The other bands (arrows) are specific for HFE. Std (far left lane) is a 100-bp ladder standard.
Identification of the HFE gene by PCR.
WT and C282Y cells were analyzed for HFE and HFEgene mutations by allele-specific PCR. Primers specific for wild-typeHFE (C282 and H63) and mutant HFE (Y282 and D63) were used in PCR reactions on DNA isolated from the 2 cell lines. Lanes 1 and 5, C282 primers; lanes 2 and 6, Y282 primers; lanes 3 and 7, H63 primers; lanes 4 and 8, D63 primers. The 430-bp band (larger mol. wt. band) in all lanes represents a control PCR product. The other bands (arrows) are specific for HFE. Std (far left lane) is a 100-bp ladder standard.
HFE expression
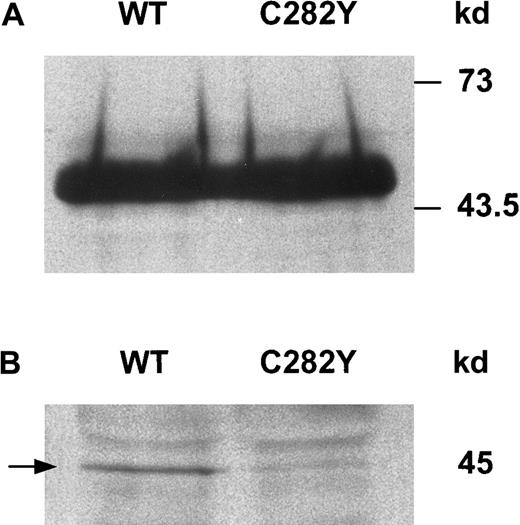
To determine whether HFE protein was expressed in WT and C282Y cells, we immunoprecipitated cell lysates with anti-HFE antiserum and analyzed the immunoprecipitates by Western blotting. As shown in Figure 2A, the HFE protein was detected as a band of approximately 45 kd in cell lysates from both WT and C282Y cells. Although these experiments confirmed the presence of HFE in both cell lines, it remained to be determined whether WT and C282Y cells differed with respect to the presence of HFE protein on the cell surface. Therefore, cell-surface proteins were labeled by biotinylation and immunoprecipitated with anti-HFE antiserum, and the immunoprecipitates were resolved by SDS-PAGE and transferred to nitrocellulose membranes. Biotinylated HFE on the membrane was identified by its binding to Streptavidin-HRP. As shown in Figure 2B, a 45-kd band consistent with biotin-labeled cell-surface HFE was detected with WT cells. A similar band of much lesser intensity was seen with C282Y cells. In several additional surface-labeling experiments (not shown), the differences in cell-surface HFE expression between WT and C282Y cells varied, but remained lower with C282Y cells.
HFE protein is expressed in WT and C282Y cells.
(A) Cell lysates from WT and C282Y cells were immunoprecipitated with anti-HFE antiserum and identified by Western blotting. HFE bands on membranes were identified by immunoblotting with anti-HFE antiserum, as described in the text. (B) HFE expression at the cell surface differs between WT and C282Y cells. Cell-surface proteins were labeled with biotin and immunoprecipitated with anti-HFE antiserum. Immunoprecipitates were resolved by SDS-PAGE and transferred to nitrocellulose membranes. Biotinylated HFE was identified with Streptavidin-HRP.
HFE protein is expressed in WT and C282Y cells.
(A) Cell lysates from WT and C282Y cells were immunoprecipitated with anti-HFE antiserum and identified by Western blotting. HFE bands on membranes were identified by immunoblotting with anti-HFE antiserum, as described in the text. (B) HFE expression at the cell surface differs between WT and C282Y cells. Cell-surface proteins were labeled with biotin and immunoprecipitated with anti-HFE antiserum. Immunoprecipitates were resolved by SDS-PAGE and transferred to nitrocellulose membranes. Biotinylated HFE was identified with Streptavidin-HRP.
59Fe-Tf uptake and TfR expression
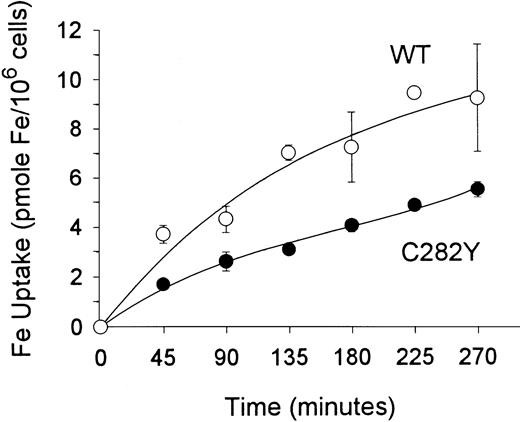
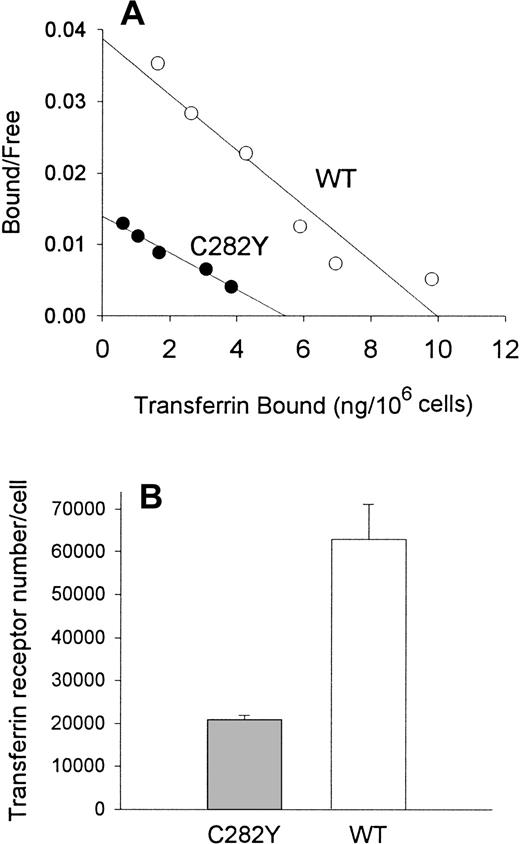
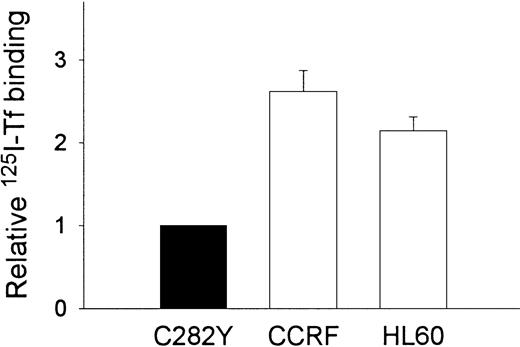
To examine the impact of the HFE C282Y mutation on iron transport into cells, we incubated cells with 59Fe-Tf and determined the amount of iron taken up by the cells. As shown in Figure3, these experiments suggested that the uptake of 59Fe (as Fe-Tf) per 106 cells was greater in WT than in C282Y cells. Additional experiments were conducted to determine whether differences in cell-surface TfR expression might explain the difference in iron uptake between C282Y and WT cells. Cells were therefore analyzed for TfR expression by125I-Tf binding assay and for receptor affinity for Tf by Scatchard analysis. In the experiment shown in Figure4A, maximal Tf binding to C282Y cells was found to be approximately 50% that of WT cells, while the KD was 2.4 nM and 1.6 nM for C282Y cells and WT cells, respectively. In 3 additional 125I-Tf binding studies, performed in conjunction with the 59Fe-Tf uptake studies, maximal Tf binding was found to be 5.5 ± 0.28 and 16.7 ± 2.2 ng Tf bound/106 cells for C282Y and WT cells, respectively (mean ± SE). These values were used to calculate the number of TfRs expressed on C282Y and WT cells, and they revealed that the number of TfRs on C282Y cells was approximately 33% that of WT cells (Figure4B). The 125I-Tf binding studies were extended to compare C282Y cells with lymphoid CCRF-CEM and myeloid HL60 cell lines. As shown in Figure 5, TfR expression on C282Y cells was significantly lower than that on these latter 2 cell types.
Total iron uptake per cell is greater in WT than in C282Y cells.
Cells (106/mL) were incubated with 59Fe-Tf (143 pmol Fe/mL) in fresh medium containing 1% FCS. 59Fe uptake by cells was determined at the times shown. Values shown represent means ± SE (n = 3).
Total iron uptake per cell is greater in WT than in C282Y cells.
Cells (106/mL) were incubated with 59Fe-Tf (143 pmol Fe/mL) in fresh medium containing 1% FCS. 59Fe uptake by cells was determined at the times shown. Values shown represent means ± SE (n = 3).
Tf binding sites and TfR number in C282Y and WT cells.
(A) Scatchard analysis shows that C282Y cells display fewer Tf binding sites than WT cells. Binding studies were performed at 4°C on intact cells. (B) TfR number is decreased on C282Y cells. TfR density on cells was determined by 125I-Tf binding. Bars represent the mean ± SE from 3 separate experiments.
Tf binding sites and TfR number in C282Y and WT cells.
(A) Scatchard analysis shows that C282Y cells display fewer Tf binding sites than WT cells. Binding studies were performed at 4°C on intact cells. (B) TfR number is decreased on C282Y cells. TfR density on cells was determined by 125I-Tf binding. Bars represent the mean ± SE from 3 separate experiments.
C282Y cells display fewer Tf binding sites than CCRF-CEM and HL60 cells.
125I-Tf binding studies were performed at 4°C on intact cells. Tf binding to CCRF-CEM and HL60 cells is expressed relative to C282Y cells. Values shown represent the means ± SE (n = 3-4 separate binding studies, each performed in duplicate).
C282Y cells display fewer Tf binding sites than CCRF-CEM and HL60 cells.
125I-Tf binding studies were performed at 4°C on intact cells. Tf binding to CCRF-CEM and HL60 cells is expressed relative to C282Y cells. Values shown represent the means ± SE (n = 3-4 separate binding studies, each performed in duplicate).
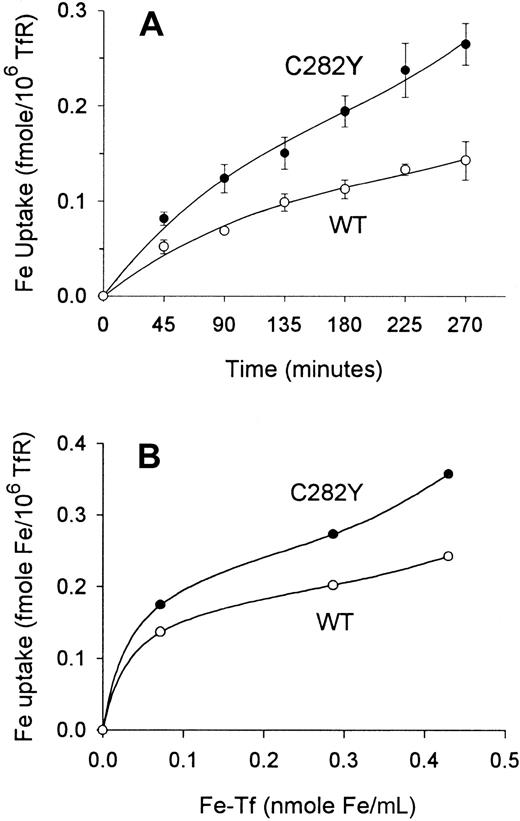
Having determined that the expression of TfRs on C282Y and WT cells differed, we analyzed cells for their uptake of 59Fe relative to the number TfRs expressed on the cell surface. In all of these experiments, TfR expression on cells was determined by125I-Tf binding immediately before measuring59Fe uptake. This analysis revealed that iron uptake per unit TfR was actually greater in C282Y cells (Figure6A). Furthermore, when the amount of exogenously added 59Fe-Tf in the medium was varied over a 6-fold range, the difference in iron uptake per unit TfR between C282Y and WT cells increased with increasing concentrations of iron in the medium (Figure 6B).
Iron uptake per TfR is greater in C282Y cells than in WT cells.
(A) Iron uptake over time is increased in C282Y cells. Cells (106/mL) were incubated with 59Fe-Tf (143 pmol Fe/mL) in fresh medium containing 1% FCS. 59Fe uptake by cells was determined at the times shown. Values shown represent means ± SE (n = 3). (B) Iron uptake over a range of exogenous Fe-Tf concentrations is increased in C282Y cells. 59Fe uptake was measured over a 270-minute incubation. A representative experiment is shown. In all experiments, cell-surface TfR expression was determined by 125I-Tf binding assay at the start of59Fe uptake.
Iron uptake per TfR is greater in C282Y cells than in WT cells.
(A) Iron uptake over time is increased in C282Y cells. Cells (106/mL) were incubated with 59Fe-Tf (143 pmol Fe/mL) in fresh medium containing 1% FCS. 59Fe uptake by cells was determined at the times shown. Values shown represent means ± SE (n = 3). (B) Iron uptake over a range of exogenous Fe-Tf concentrations is increased in C282Y cells. 59Fe uptake was measured over a 270-minute incubation. A representative experiment is shown. In all experiments, cell-surface TfR expression was determined by 125I-Tf binding assay at the start of59Fe uptake.
Tf-TfR internalization
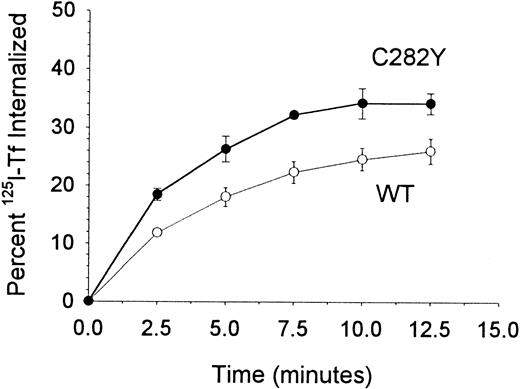
To examine whether the increase in 59Fe uptake by C282Y cells was accompanied by a corresponding change in Tf uptake kinetics, we compared the internalization of cell-surface TfR-bound125I-Tf by C282Y and WT cells. The fraction of cell-surface 125I-Tf internalized by the respective cell lines was normalized for their differences in TfR expression. As shown in Figure 7, C282Y cells internalized a significantly greater amount of cell-surface TfR-bound Tf than WT cells, consistent with their increased uptake of59Fe.
TfR-mediated internalization of Tf is greater in C282Y cells.
The percentage of cell-surface TfR-bound 125I-Tf internalized by cells was determined by centrifuging cells through an acidic buffer at the times shown. The data shown have been normalized for differences in TfR expression between the 2 cell lines. Values shown represent the means ± SE (n = 4-6).
TfR-mediated internalization of Tf is greater in C282Y cells.
The percentage of cell-surface TfR-bound 125I-Tf internalized by cells was determined by centrifuging cells through an acidic buffer at the times shown. The data shown have been normalized for differences in TfR expression between the 2 cell lines. Values shown represent the means ± SE (n = 4-6).
TfR distribution
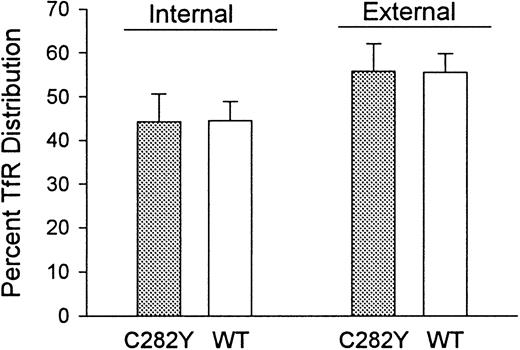
Because the 125I-Tf binding studies indicated that C282Y cells displayed a lower number of cell-surface TfRs than WT cells, we questioned whether this was due to a redistribution of TfRs from the cell surface to the cell interior or to a decrease in total cellular TfRs. To examine this, we determined the fraction of TfRs present on the cell surface versus in the intracellular compartments. As shown in Figure 8, the percentages of total cellular TfRs present intracellularly and on the cell surface were similar in C282Y and WT cells. These results suggest that the decreased number of TfRs on C282Y cells is not due to a redistribution of TfRs from the cell surface to the cytoplasm.
The distribution of TfRs between cell-surface and intracellular compartments is similar in C282Y and WT cells.
Cell-surface (external) and total cellular Tf binding sites were measured by 125I-Tf binding to C282Y and WT cells at 4°C and 37°C, respectively. Internal (intracellular) Tf binding sites represent the difference between 125I-Tf binding at 37°C and 4°C. The fraction of intracellular 125I-Tf binding sites was also determined by washing cells from the 37°C binding assay with an acidic buffer to remove cell-surface–bound125I-Tf, as discussed in the text. Bars represent means ± SE (n = 4).
The distribution of TfRs between cell-surface and intracellular compartments is similar in C282Y and WT cells.
Cell-surface (external) and total cellular Tf binding sites were measured by 125I-Tf binding to C282Y and WT cells at 4°C and 37°C, respectively. Internal (intracellular) Tf binding sites represent the difference between 125I-Tf binding at 37°C and 4°C. The fraction of intracellular 125I-Tf binding sites was also determined by washing cells from the 37°C binding assay with an acidic buffer to remove cell-surface–bound125I-Tf, as discussed in the text. Bars represent means ± SE (n = 4).
Tf-independent iron uptake
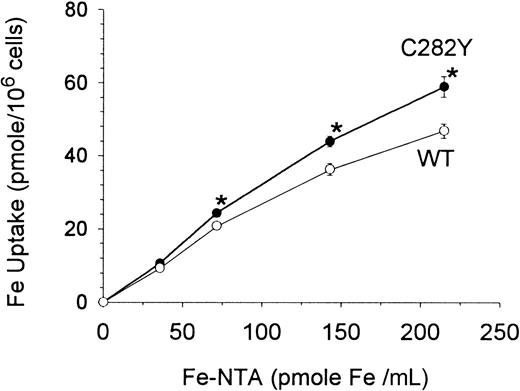
Others have shown that B-lymphoid cells can acquire iron by Tf-independent mechanisms35; however, the relation of this iron-transport system to HFE is unknown. In additional experiments, therefore, the Tf-independent cellular uptake of different concentrations of 59Fe-NTA was examined under serum-free, Tf-free incubation conditions. As shown in Figure9, Tf-independent Fe uptake by C282Y cells was significantly greater than by WT cells.
Tf-independent iron uptake is increased in C282Y cells.
Cells were washed and incubated in serum-free, Tf-free medium with increasing concentrations of 59Fe-NTA for 270 minutes. Values shown represent means ± SE of 3 separate experiments, each performed in quadruplicate. *P < .001 for differences in Fe uptake.
Tf-independent iron uptake is increased in C282Y cells.
Cells were washed and incubated in serum-free, Tf-free medium with increasing concentrations of 59Fe-NTA for 270 minutes. Values shown represent means ± SE of 3 separate experiments, each performed in quadruplicate. *P < .001 for differences in Fe uptake.
Effect of pro-oxidant conditions on cell growth
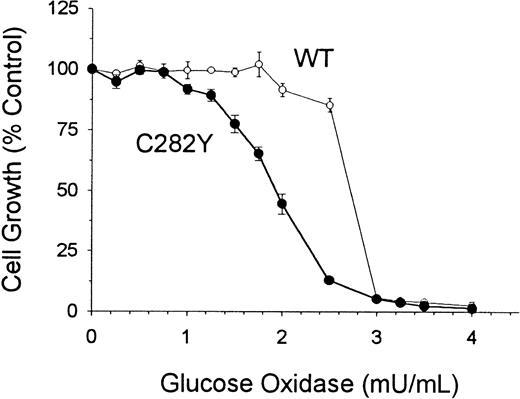
To determine whether C282Y and WT cells differed with respect to their sensitivity to oxidative stress, we examined cell proliferation in culture medium containing glucose and glucose oxidase. These additives have been shown to subject cells to continuous pro-oxidant conditions in culture.33 As shown in Figure10, C282Y cells were found to be more sensitive to the cytotoxicity of oxidative stress than WT cells. After a 48-hour incubation, the IC50 (concentration required to inhibit cell growth by 50%) of glucose oxidase was 2.7 mU/mL for WT cells and 1.9 mU/mL for C282Y cells. At a concentration of 2.5 mU/mL glucose oxidase, the growth of WT cells was inhibited by approximately 14%, whereas the growth of C282Y cells was inhibited by 88% (Figure 10).
C282Y cells are more sensitive than WT cells to the cytotoxicity of oxidative stress.
Cells were incubated for 48 hours in the presence of increasing concentrations of glucose oxidase. Control growth represents cell growth in the absence of glucose oxidase. Values represent means ± SE (n = 4).
C282Y cells are more sensitive than WT cells to the cytotoxicity of oxidative stress.
Cells were incubated for 48 hours in the presence of increasing concentrations of glucose oxidase. Control growth represents cell growth in the absence of glucose oxidase. Values represent means ± SE (n = 4).
Cellular ferritin content
Cellular ferritin was measured after a 24-hour incubation of cells in fresh medium. WT cells contained 70.1 ng ferritin/mg protein, whereas C282Y cells contained 33.4 ng ferritin/mg protein (average of 2 separate experiments).
Discussion
Iron uptake by cells is a highly regulated process that involves both TfR-dependent and TfR-independent iron-transport systems. The recent discovery that HFE protein can, by virtue of binding to the TfR, affect the receptor-mediated uptake of iron by cells raises important questions as to the significance of HFE in the biology of lymphocytes and other cells not thought to be directly involved in iron absorption and the pathogenesis of hemochromatosis. To investigate this, we developed a lymphoblastoid cell line from a patient homozygous for the HFE C282Y mutation and compared it with a lymphoblastoid cell line from an individual with the wild-type HFE gene. PCR analysis confirmed that C282Y cells were homozygous for the HFE C282Y mutation, whereas WT cells lacked HFE mutations. Although both cell lines were found to express HFE protein, C282Y cells expressed less (but detectable) HFE protein at the cell surface. Using gene-transfection experiments, other investigators have shown that the HFE C282Y mutation results in a block in the normal intracellular trafficking of HFE protein and a failure of the HFE protein to be presented at the cell surface.11,13-15 Our finding that HFE protein was present (but reduced) on the surface of C282Y cells suggests that in B lymphocytes, the HFE C282Y mutation does not result in a complete block in the transit of this protein to the cell surface. This conclusion is consistent with a recent report that demonstrated that peripheral blood monocytes from patients with hemochromatosis homozygous for HFE C282Y displayed immunoreactive HFE protein on their surfaces.36 Additional support for this conclusion is provided by the finding that mice homozygous for theHFE C282Y/C282Y mutation develop less severe iron overload than mice with the HFE null (−/−) mutation, suggesting that the HFE C282Y mutation does not completely block the trafficking of HFE to the cell surface.20
Our study shows that in proliferating B-lymphoid cells, theHFE C282Y mutation results in an increase in TfR-mediated iron uptake relative to the number of TfRs expressed on these cells. This finding was not readily obvious on initial analysis because when iron uptake by cells was measured without consideration of cell-surface TfR number, we found that total iron uptake per cell by WT cells was greater than by C282Y cells. Further analysis, however, revealed that whereas WT cells displayed almost 3 times the number of cell-surface TfRs than C282Y cells, their uptake of iron was not increased by the same magnitude. Measurement of iron uptake in terms of cell-surface TfR density clearly showed that the uptake of iron per unit receptor was significantly lower in WT cells than in C282Y cells.
An unexpected finding in our study was that Tf-independent iron uptake was significantly increased in C282Y cells relative to WT cells. Others have reported contradictory results regarding the effect of wild-type HFE on Tf-independent iron uptake. Roy et al16 found no change in Tf-independent iron uptake in HeLa cells transfected to overexpress the wild-type HFE gene. In contrast, Corsi et al17 found that overexpression of wild-type HFEin HeLa cells blocked Tf-iron uptake but led to an increase in Tf-independent iron uptake. Neither group examined the effect of theHFE C282Y mutation on Tf-independent iron uptake. Our investigation contrasts with both of these previous studies because we compared Tf-independent iron uptake in C282Y and WT cells. It is not clear how the HFE C282Y mutation affects non-Tf iron uptake; further studies will be needed to elucidate this mechanism and to determine whether it varies in cells of different lineages. Our results do suggest, however, that the function of HFE may extend beyond its known interaction with the TfR and that HFE mutations may also affect other steps in iron transport and metabolism.
The finding that C282Y cells display significantly fewer TfRs than WT cells is of interest. Although it could be argued that this may merely reflect inherent differences in TfR expression between 2 cell lines rather than a relation to HFE, the former explanation appears to be less likely. Comparison of 125I-Tf binding in C282Y, HL60, and CCRF-CEM cells also showed TfR expression to be lower in C282Y cells. This supports our contention that the decreased number of TfRs in C282Y cells is related to the HFE C282Y mutation. A possible explanation for the lower number of TfRs on C282Y cells is that they acquire more iron per TfR than other cells and therefore require fewer receptors to incorporate the critical amount of iron necessary for their viability and proliferation. In general, cells in culture appear to have a significant requirement for iron, as evidenced by high expression of TfRs and iron uptake. At the same time, cells in culture contain a substantial amount of ferritin, suggesting that not all the iron taken up is used and thus needs to be sequestered. In this context, the HFE C282Y mutation may allow cells to incorporate Tf-iron more efficiently and use it for cell function rather than store it in ferritin. The decreased level of ferritin in C282Y cells is consistent with this notion.
The role of iron as a catalyst in the generation of reactive oxygen species (ROS) is well established.37 Iron excess can enhance the cytotoxicity of oxidative stress, whereas iron deprivation may have the opposite effect.38 We questioned, therefore, whether the increase in TfR-dependent and -independent iron uptake by C282Y cells might lead to a larger amount of iron “in transit” available for ROS production and thus render them more susceptible to pro-oxidant challenge. Using glucose/glucose oxidase to generate a continuous pro-oxidant condition in culture, we found that C282Y cells were indeed more sensitive to the cytotoxicity of oxidative stress. These results, however, need to be interpreted with caution because other variables may greatly influence the response of cells to oxidative stress. One important modulator of the cytotoxicity of oxidative stress is ferritin. Increased levels of intracellular ferritin have been shown to reduce cell sensitivity to oxidative stress by sequestering iron and limiting its availability for metal-catalyzed formation of ROS.33 39 In our studies, the ferritin content of WT cells was approximately 2.4-fold greater than that of C282Y cells. Hence, it is possible that the lower content of ferritin, along with increased TfR-dependent and -independent iron uptake, may have contributed to the increased sensitivity of C282Y cells to oxidative stress.
The significance of HFE gene expression in cells of lymphoid lineage needs to be further elucidated. Conflicting data exist regarding the effect of iron overload on the immune system, with some studies suggesting an impairment of the immune function in hemochromatosis and others showing no such abnormality.40However, those studies were conducted before the discovery ofHFE gene mutations. More recently, Bahram et al41 performed a phenotypic analysis of various T-cell subpopulations in the lymphoid organs of an HFE-deficient mouse model of hemochromatosis and were unable to detect differences between control and HFE-deficient animals. Unfortunately, B cells were not examined in their study.41 In considering the significance of HFE in lymphoid cells, it should be noted that under normal conditions, lymphocytes are nonproliferating cells that express very few TfRs and that significant TfR expression occurs only when B or T cells are activated.42-44 Hence, HFEexpression and its interaction with the TfR in lymphoid cells may be relevant only in conditions associ-ated with lymphocyte activation and proliferation rather than in hemochromatosis per se. Further investigations are planned to develop immortalized lymphoblastoid cell lines from additional patients with hemochromatosis with the C282Y mutation. Studies with these cell lines will, one hopes, advance our understanding of the role of HFE in iron metabolism in B lymphocytes.
We are grateful to Rimas Orentas, PhD, for assistance in developing the lymphoblastoid cell lines; and to Daniel Bellissimo, PhD, for performing the PCR analysis.
Supported by a grant from the Medical College of Wisconsin Research Affairs Committee.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Christopher R. Chitambar, Division of Hematology/Oncology, Medical College of Wisconsin, 9200 West Wisconsin Avenue, Milwaukee, WI 53226; e-mail: chitambr@mcw.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal