Abstract
The alpha chemokine receptor CXCR4 has been shown to be expressed on human hematopoietic progenitor cells and during the megakaryocytic differentiation pathway. Stromal cell–derived factor 1 (SDF-1) is the ligand for CXCR4. In this study, the role of SDF-1α in megakaryocytopoiesis was investigated. CD34+ progenitors purified from peripheral blood were grown in serum-free liquid suspension culture supplemented with thrombopoietin to obtain a virtually pure megakaryocytic progeny. In this condition, the addition of SDF-1α gives rise to megakaryocytes (MKs) showing an increased DNA content and a rise of lobated nuclei, as compared with untreated cells: at day 5, approximately 20% of the cells already showed the presence of more than one nuclear lobe versus fewer than 5% in the control cells; at day 12, approximately 85% of the cells were of large size and markedly polyploid, whereas approximately 60% of the control cells were polyploid, showed fewer lobes, and were a smaller size. This effect was dose-dependent and did not affect the megakaryocytic proliferation. Experiments with the mitogen-activated protein kinase (MAPK) inhibitor PD98059 suggested a role for MAPK pathway on SDF-1α–induced endomitosis. Furthermore, SDF-1α induced a significant increase in the number of proplatelet-bearing MKs and promoted the migration of megakaryocytic cells. Treatment with SDF-1α caused reduction in CXCR4 abundance on the plasma membrane, seemingly owing to receptor internalization. Furthermore, the presence of SDF-1α did not affect the expression of megakaryocytic markers, indicating that differentiation and polyploidization are independently regulated events.
Introduction
Chemokines are peptides of 70 to 100 amino acids secreted by different cell types in response to injury and infection. Chemokines activate and induce migration of leukocytes in a cell-specific fashion to sites of inflammation.1 Stromal cell–derived factor 1 (SDF-1) is a CXC chemokine defined on the basis of a typical sequence of cysteine residues.2 Characterized as a pre-B stimulatory factor at the beginning,3 SDF-1α is constitutively expressed by many tissues.4,5 Initially cloned from bone marrow stroma,6 it has been identified as a highly efficient chemotactic factor for T cells, monocytes, and CD34+ human progenitors4,7 and, more recently, as a potent chemotactic factor for mature megakaryocytes (MKs).8 Furthermore, it has been shown that it inhibits the colony formation of the myeloid progenitor cell line 32D, which suggests also a myelosuppressor role for this molecule.9
The signaling of SDF-1 is mediated by CXCR4, a G-protein–coupled receptor.10,11 The intracellular signals induced in myeloid precursor cells were monitored by increases in the level of intracellular Ca++.9 Ligand binding induced rapid internalization and down-modulation of the receptor on the cell surface.12 Data on CEM cell line and leukocytes indicated that, after removal of exogenous SDF-1α, CXCR4 was again localized on cell surface as a consequence of recycling from the internalized pool.13,14 Internalization by SDF-1 does not involve protein kinase C activation.12
Knockout of the SDF-1 gene15 in mice is lethal with severe abnormalities in B-cell lymphopoiesis and bone marrow myelopoiesis; similar defects were observed in knockout of the CXCR4 gene.16,17 Particularly, the defect in myelopoiesis also involved the megakaryocytic lineage, resulting in a markedly reduced number of these cells in the bone marrow of CXCR4−/− animals.16 The CXCR4 expression on MKs at different stages of differentiation and maturation was demonstrated by flow cytometry and reverse transcriptase–polymerase chain reaction (RT-PCR).18-20Wang et al18 found that the percentage of CXCR4 expression in CD34+ and CD61+ cells was different from one donor to another. The coexpression of CXCR4 along with CD34 or CD61 ranged from 13% to 93% and from 13% to 89%, respectively. Rivière et al19 demonstrated a close correlation between CD41a and CXCR4 expression on cell surface: the level of CXCR4 increased in parallel with the CD41a antigen during megakaryocytic differentiation. We recently analyzed protein and messenger RNA (mRNA) expression of CXCR4 on CD34+ and monocytic, megakaryocytic, erythroid, granulocytic-derived progenies: in particular, in megakaryocytic-lineage CXCR4 expression is sustained up to late stages of maturation, with an increase in the percentage of positive cells from 50% in undifferentiated hematopoietic progenitor cells (HPCs) to 90% in mature MKs.20 The expression of CXCR4 on platelets suggests that SDF-1 might also modulate platelet functions.8 18
The role of SDF-1 on human megakaryocytic cells at early or late stages of differentiation has been investigated with controversial results.8,18,19 Wang et al18 showed that megakaryocytic populations with large forward- and side-scatter properties, which correspond to MKs of larger size and higher ploidy levels, exhibited stronger staining for CXCR4 than MKs with relatively smaller ploidy levels. Rivière et al19 showed a preferential attraction of immature MKs by SDF-1, whereas Hamada et al8 suggested a potent chemotactic effect on mature MKs. In spite of these discrepancies, all these studies reported the CXCR4 expression on MK and suggested a role for SDF-1α on megakaryocytic function.
Recently Hodohara et al21 demonstrated that SDF-1α exerts a direct growth-promoting effect on MK progenitors (MK colony-forming units [CFU-MK]) purified from murine bone marrow cells, when combined with thrombopoietin (TPO).
CXCR4 receptor is known as one of the major CD4 coreceptors that allow the T-tropic human immunodeficiency virus (HIV) strains entry by cell-membrane fusion.22,10 We previously demonstrated T-tropic HIV infection of HPC-derived megakaryocytic precursors23 and the involvement of CXCR4, showing that SDF-1α–treated cells were essentially resistant to HIV infection.20
In the present report, we have analyzed the effect of SDF-1α on CD34+ cells obtained from peripheral blood (PB) and induced to megakaryocytic differentiation in serum-free liquid suspension culture in the presence of saturating amounts (100 ng/mL) of TPO.
Materials and methods
Hematopoietic growth factors and culture media
Recombinant human interkeukin 3 (rhIL-3) (2 × 106U/mg), granulomonocytic colony-stimulating factor (rhGM-CSF) (1.7 × 105 U/mg), and IL-6 (rhIL-6) (2 × 106 U/mg) were supplied by the Genetics Institute (Cambridge, MA); erythropoietin (rhEPO) (5 × 104U/L) by Amgen (Thousand Oaks, CA); flt3-ligand (rhFL) (1.9 × 106 U/mg) and kit-ligand (rhKL) (1 × 105 U/mg) by Immunex (Seattle, WA); granulocytic colony-stimulating factor (rhG-CSF) (1 × 108 U/mg) and monocytic colony-stimulating factor (rhM-CSF) (6 × 107U/mg) from R&D Systems (Minneapolis, MN); and rhTPO (1 × 106 U/mg) from Peprotech (Rocky Hill, NJ). A synthetic preparation of SDF-1α11 was kindly provided by Dr Ian Clark-Lewis, Biomedical Research Centre, University of British Columbia, Vancouver, BC, Canada; a recombinant preparation of human SDF-1α was purchased from R&D Systems; and Iscove modified Dulbecco medium (Gibco, Grand Island, NY) was prepared weekly before each purification experiment.
HPC purification
Adult PB was obtained from 20- to 40-year-old healthy male donors after informed consent. HPCs were purified as described in Gabbianelli et al24 and Labbaye et al.25 In particular, a negative selection of low-density cells was performed with a cocktail of anti-T, anti-B, and anti–natural killer cell lymphocytes and antimonocyte, antigranulocyte monoclonal antibodies (mAbs) supplemented with anti-CD45, anti-CD11a, and anti-CD71 mAbs (Becton Dickinson, Mountain View, CA).
HPC clonogenetic assay was performed as previously described.26 Briefly, HPCs were seeded (1 × 102 cells per milliliter dish, in triplicate) and cultured in 0.9% methylcellulose in the presence or absence of fetal calf serum (FCS). In FCS− culture, FCS was substituted by bovine serum albumin (BSA), pure human transferrin, human low-density lipoproteins, insulin, sodium pyruvate, L-glutamine (2 μM), rare inorganic elements supplemented with iron sulfate (4 × 10−8 mM), and nucleosides. Both FCS+and FCS− cultures were supplemented with FL (100 ng/mL), KL (100 ng), IL-3 (100 U), TPO (100 ng), GM-CSF (10 ng), EPO (3 U), M-CSF (250 U), and G-CSF (500 U). Granulocytic/erythroid/monocytic/megakaryocytic colony-forming unit, erythroid burst-forming unit, and granulocytic/macrophage colony-forming unit colonies were scored on days 14 to 15 and 16 to 17 in FCS+ and FCS− cultures, respectively.
MK cultures
Liquid suspension culture.
Purified HPCs were grown in FCS− liquid culture at 4 × 104 cells per milliliter,27 in the presence of a saturating dose of TPO (100 ng/mL) alone or with SDF-1α (1 μg/mL). Cells were incubated in a fully humidified atmosphere of 5% CO2, 5% O2, 90% N2. The cells were counted and the concentration was adjusted to 1 × 105 cells per milliliter twice a week.
In some experiments, HPCs were grown in the presence of PD98059 (Calbiochem, La Jolla, CA), a specific inhibitor of mitogen-activated protein kinase (MAPK) kinase (MEK)/extracellular signal–regulated protein kinase (ERK) pathway.28 PD98059 was dissolved in dimethylsulfoxide (DMSO), cell culture grade (Sigma, St Louis, MO), and added to cells 20 minutes before stimulation by TPO or TPO + SDF-1α at final concentration of either 25 or 50 μM/L.28 In the mock culture, an equivalent amount of DMSO was added.
Detection of proplatelet-forming MKs.
From day 9 onward, the cultures were examined daily for the emergence of proplatelets. An MK bearing one or more cytoplasmic processes (whose length was longer than the cell body diameter) was considered a proplatelet-displaying MK.29 The percentage of MKs displaying one or more cytoplasmic processes was determined by visual examination of video prints of the culture wells.
Platelet analysis.
Platelets were isolated from culture supernatants by centrifugation at room temperature for 10 minutes at 120g, washed with Tyrode-Hepes buffer (Sigma; 136.9 mM NaCl, 2.7 mM KCl, 11.9 mM NaHCO3, 2 mM CaCl2, 1 mM MgCl2, 5.5 mM glucose, 10 mM Hepes, pH 7.4), pelleted at room temperature for 20 minutes at 900g, and then counted in a Bürker camera with a contrast phase microscopy. In parallel, platelets were stained with an anti-CD61 mAb (Becton Dickinson) and analyzed by flow cytometry with a setting optimized for platelet analysis. The functionality of platelets released from cultured MKs was evaluated by the study of P-selectin (CD62) expression following 1 U/mL thrombin stimulation, according to Choi et al.29 CD62 expression was assayed by flow cytometry by means of a phycoerythrin (PE)–labeled anti–P-selectin mAb (Becton Dickinson).
MK characterization
Morphological analysis.
Cells collected at different days of culture were cytocentrifuged onto glass slides, stained with May-Grünwald Giemsa (Sigma), and then identified by morphology analysis.
Flow cytometric analysis.
The following mAbs directly conjugated with fluorescein isothiocyanate (FITC) or PE were used to characterize the membrane phenotype of cell samples: anti-CD34, anti-CD61, anti-CD62, anti-CD42b (Becton Dickinson), and anti-CD41a (Serotec, Oxford, UK). PE-labeled anti-CXCR4 mAb (clone 12G5, PharMingen, San Diego, CA) was used for the study of the chemokine receptor expression. Cells were washed twice in phosphate-buffered saline (PBS) and then incubated for 45 minutes at 4C° in the presence of appropriate amounts of specific mAbs. After 3 washes with cold PBS containing 1 g/L BSA, cells were resuspended in 0.2 mL PBS/2.5% formaldehyde (Sigma) and analyzed by FACScan (Becton Dickinson) by means of the Lysis II program for fluorescence intensity analysis.26
DNA staining.
MKs' ploidy was analyzed by flow cytometry after DNA staining with propidium iodide (PI) (Sigma) according to the procedure described in Dolzhanskiy et al.30 Cells were washed and resuspended in medium containing 0.5% Tween-20 for 30 minutes to permeabilize the cell membranes. Then, an equal volume of medium containing 0.5% Tween-20 and 2% paraformaldehyde was added. After 5 minutes at 4°C, the cells were pelleted, and freshly prepared PI was added. The suspension was stored overnight in the dark at 4°C. The following day, 50 μg/mL of RNAse A was added for 30 minutes at room temperature in the dark, and the cells were analyzed by flow cytometry.
Chemotaxis.
We used 5-μm pore filter (Transwell 24-well cell clusters; Costar, Cambridge, MA) for migration study.8 18 We loaded 1 × 105 MKs (200 μL in FCS− medium) into each Transwell filter pretreated with RPMI 1640 (Gibco) containing 0.3% human serum albumin (Sigma). Filters were then transferred to another well containing SDF-1α at a concentration of 200 ng/mL in serum-free medium. After 3 hours' incubation at 37°C in 5% CO2, cell migration was calculated by counting the recovered cells from the lower chamber. The viability of the cells was assessed by trypan blue dye exclusion. Cytospin preparations of the migrated cells were stained with May-Grünwald Giemsa.
Immunolabeling for confocal microscopy.
Cells derived from liquid culture were washed in PBS and immobilized on poly-d-lysine–coated glass coverslips, fixed in PBS/3.7% paraformaldehyde, quenched in 0.1 M glycine, and permeabilized by incubation with 0.05% saponin-PBS/0.2% BSA for 15 minutes.13 The cells were then incubated for 45 minutes at room temperature with anti-CXCR4 mAb and labeled with a secondary FITC-conjugated goat antimouse immunoglobulin G antibody (Dako, Glostrup, Denmark). After 3 washes in PBS, some samples were double-stained with a PE-conjugated anti-CD41a mAb. Cells were again washed and mounted in 50% glycerol and examined by means of a TCS 4D confocal microscope (Leica, Nussloch, Germany) interfaced with argon/krypton lasers. Single fluorescence was analyzed with a 488-nm laser line for FITC dye excitation; simultaneous double-fluorescence acquisition was performed by means of 488- to 568-nm laser lines to excite FITC-PE dyes.
RT-PCR analysis.
RT-PCR was performed on megakaryocytic cells as previously described.31 Briefly, 1 to 3 × 104 cells were lysed in 200 μL of 4 M guanidine isothiocyanate, and total RNA was extracted by the CsCl gradient technique in the presence of 12 μg rRNA of Escherichia coli as carrier. Samples were reverse transcribed according to the manufacturer instructions (Boehringer, Mannheim, Germany). RT-PCR products were normalized for Sp26; amplification within the linear range was achieved by 20 PCR cycles: denaturation at 95°C for 30 seconds, annealing at 56°C for 30 seconds, and extension at 72°C for 45 seconds. To evaluate the expression of CXCR4 gene, aliquots of RT-RNA were amplified within the linear range by 30 cycles: denaturation at 95°C for 30 seconds, annealing at 56°C for 30 seconds, and extension at 72°C for 45 seconds. PCR products were then electrophoresed on agarose gel, transferred onto nylon membranes, and hybridized with the specific probes. The following primers and probes were used:
Sp26: forward 5′-GCCTCCAAGATGACAAAG-3′; reverse 5′-CCAGAGAATAGCCTGTCT-3′; and probe 5′-GAGCGTCTTCGATGCCTATGTGCTTCCCAA-3′.
CXCR4: forward 5′-CCTCTATGCTTTCCTTGG-3′; reverse 5′-CCTGAAGACTCAGAC TCA-3′; and probe 5′-AGCAGAGGGTCCAGCCTCAAGATCCTCT-3′.
Statistical analysis.
The significance of differences in mean value was determined by means of the Student t test.
Results
HPC characterization and MK differentiation and maturation
HPCs purified from PB as described in “Materials and methods” were characterized by 93% ± 1% CD34+ cells (mean ± SEM from 8 separate experiments), as evaluated by flow cytometry and 91% ± 1% HPC frequency (mean ± SEM from 8 separate experiments), as evaluated by clonogenetic assay.
Purified HPCs grown in liquid suspension culture in the presence of TPO (100 ng/mL) undergo a gradual wave of differentiation and maturation along the MK lineage (Figure 1, left panel), giving rise to a virtually pure MK population (98% to 99% of the cells were CD61+).23 The differentiation stages were characterized during the whole culture by morphologic and phenotypic analysis: at day 0, cells were essentially composed of small undifferentiated blasts; at day 5, most cells were larger than at day 0 and had one nuclear lobe, representing MK precursors. At day 8, a significant proportion of cells were 2N and 4N and showed a more dense chromatin. At day 12, a high proportion of MKs (60%) showed lobulated polyploid nuclei with a highly granular cytoplasm. Platelets were produced at the end of the culture as evaluated with contrast phase microscopy. However, a significant proportion (approximately 40%) of MKs at this day still displayed only one nuclear lobe, suggesting that factors other than TPO are required for optimal polyploidization of MK precursors.
Morphology of megakaryocytic cells grown in presence of TPO alone or combined with SDF-1α.
Cell morphology of HPCs, derived from PB, cultured in serum-free liquid suspension medium in the presence of TPO (100 ng/mL) or TPO + SDF-1α (1 μg/mL) (representative results). Cells at different days of culture are presented (May-Grünwald staining; original magnification × 400).
Morphology of megakaryocytic cells grown in presence of TPO alone or combined with SDF-1α.
Cell morphology of HPCs, derived from PB, cultured in serum-free liquid suspension medium in the presence of TPO (100 ng/mL) or TPO + SDF-1α (1 μg/mL) (representative results). Cells at different days of culture are presented (May-Grünwald staining; original magnification × 400).
Effect of SDF-1α
At day 0 after purification, HPCs were treated with different amounts of synthetic SDF-1α: 0.1; 0.25; 0.5; 1 μg/mL in the presence of saturating amounts of TPO (100 ng/mL).
The morphological analysis and DNA content of the cells cultured in the presence of both TPO and SDF-1α revealed a significant increase in the number of nuclear lobes (Figure 1) and ploidy (Figure2) compared with the control cells grown in the presence of TPO alone (Figure 1, right panel). Cell polyploidization started earlier in culture supplemented with SDF-1α (1 μg/mL) than in the controls: at day 5, 20% of the resulting cells were already tetraploid (4N) or even more highly lobated, whereas in the control cultures almost all the cells were mononuclear; in SDF-1α–treated MKs, an increased polyploidization was maintained until the end of the culture, where, at day 12, more than 85% of the cells were larger than untreated control cells and showed a significant increase in the number of nuclear lobes per cell (Figure 1). This increase was dose dependent: at 0.1 μg, we did not observe any differences in respect to the untreated cells; at 0.25 and 0.5 μg, a clear increase in the number of polynucleated cells was evident, with a prevalence of 4N (Figure 3). Additional experiments were performed with a preparation of rhSDF-1α. Optimal stimulation of MK ploidization was observed at a concentration of 100 ng/mL (data not shown). In cytospin preparations, the presence of proplatelets was more consistent when SDF-1α was used (Table1), and the number of platelets counted at day 12 in the supernatant from SDF-1α–supplemented cultures was increased compared with the control (5 × 105 vs 18 × 105 platelets per 105 MKs). These platelets were functional according to standard criteria, ie, thrombin-induced increase of membrane CD62 expression (data not shown).
DNA content of MKs.
Polyploidy was evaluated by flow cytometry analysis, at day 9 of culture, on MKs grown either in the absence (A) or the presence (B) of SDF-1α (1 μg/mL). MKs were stained with PI as described in “Materials and methods.” A representative experiment from 3 separate experiments is shown.
DNA content of MKs.
Polyploidy was evaluated by flow cytometry analysis, at day 9 of culture, on MKs grown either in the absence (A) or the presence (B) of SDF-1α (1 μg/mL). MKs were stained with PI as described in “Materials and methods.” A representative experiment from 3 separate experiments is shown.
MK polyploidization increase after treatment with SDF-1α.
Effect of SDF-1α dose-response treatment on the polyploidization of the HPC-derived MK cells. Data relative to day 9 of culture are presented as mean ± SEM of 3 independent experiments. Asterisks denote a significant difference (P < .05) in comparison with control.
MK polyploidization increase after treatment with SDF-1α.
Effect of SDF-1α dose-response treatment on the polyploidization of the HPC-derived MK cells. Data relative to day 9 of culture are presented as mean ± SEM of 3 independent experiments. Asterisks denote a significant difference (P < .05) in comparison with control.
Effects of stromal cell–derived factor 1α on proplatelet formation
| Experiment no. . | Growth factor . | Proplatelet-bearing MKs, % . |
|---|---|---|
| 1 | TPO | 29 |
| TPO + SDF-1α | 48 | |
| 2 | TPO | 24 |
| TPO + SDF-1α | 55 | |
| 3 | TPO | 19 |
| TPO + SDF-1α | 41 | |
| Mean ± SEM | TPO | 24 ± 2.8 |
| TPO + SDF-1α | 48 ± 4* |
| Experiment no. . | Growth factor . | Proplatelet-bearing MKs, % . |
|---|---|---|
| 1 | TPO | 29 |
| TPO + SDF-1α | 48 | |
| 2 | TPO | 24 |
| TPO + SDF-1α | 55 | |
| 3 | TPO | 19 |
| TPO + SDF-1α | 41 | |
| Mean ± SEM | TPO | 24 ± 2.8 |
| TPO + SDF-1α | 48 ± 4* |
Data refer to HPC-derived MKs grown in serum-free liquid culture at day 12. TPO (100 ng/mL) was used with and without SDF-1α (1 μg/mL).
SDF-1α indicates stromal cell–derived factor 1α; TPO, thrombopoietin; MKs, megakaryocytes; HPC, hematopoietic progenitor cell.
P < .05 (Student t test) compared with cultures containing TPO alone.
The effect of SDF-1α on megakaryocytic cultures was relevant when SDF-1α was added in the HPC culture medium at day 0 and abolished if it was added at day 5 of culture, when the cells were already differentiated to MK precursors (data not shown).
When we used lower levels of TPO (1 to 10 ng/mL), we observed a decrease in the total number of cells although the relative percentage of MKs was not affected and remained around 99%: in these conditions, the addition of synthetic SDF-1α (1 μg/mL) still induced an increase in the ploidy of the cells (Table2). However, cell growth was not significantly affected by the presence of the chemokine, at any concentration used, and no MK growth was observed when SDF-1α was used alone. In Figure 4, the growth curves of the cells during the whole culture (0 to 12 days) in the presence or absence of 1 μg/mL SDF-1α are reported.
Effect of graded amounts of thrombopoietin in the presence or absence of stromal cell–derived factor 1α (1 μg/mL) on megakaryocyte percentage, proliferation, and polyploidization
| TPO . | MKs, % . | No. MKs × 104/mL . | Polylobated MKs, % . | |||
|---|---|---|---|---|---|---|
| −SDF-1α . | +SDF-1α . | −SDF-1α . | +SDF-1α . | −SDF-1α . | +SDF-1α . | |
| 1 ng/mL | 99 | 99 | 31.2 ± 10 | 30.3 ± 9 | 23 ± 7 | 50 ± 7* |
| 10 ng/mL | 99 | 99 | 43.7 ± 8 | 38.5 ± 8 | 28 ± 5 | 54 ± 6* |
| 100 ng/mL | 99 | 99 | 56.4 ± 11 | 55.7 ± 10 | 51 ± 2 | 73 ± 3* |
| TPO . | MKs, % . | No. MKs × 104/mL . | Polylobated MKs, % . | |||
|---|---|---|---|---|---|---|
| −SDF-1α . | +SDF-1α . | −SDF-1α . | +SDF-1α . | −SDF-1α . | +SDF-1α . | |
| 1 ng/mL | 99 | 99 | 31.2 ± 10 | 30.3 ± 9 | 23 ± 7 | 50 ± 7* |
| 10 ng/mL | 99 | 99 | 43.7 ± 8 | 38.5 ± 8 | 28 ± 5 | 54 ± 6* |
| 100 ng/mL | 99 | 99 | 56.4 ± 11 | 55.7 ± 10 | 51 ± 2 | 73 ± 3* |
Data refer to hematopoietic progenitor cells grown in serum-free liquid culture at day 9. Based on mean ± SEM from 5 separate experiments.
For abbreviations, see Table 1.
P < .05 when compared with −SDF-1α by Studentt test.
The MK proliferation is not affected by the presence of SDF-1α.
Proliferation of HPCs along the megakaryocytic lineage in the presence of TPO (100 ng/mL) alone (●) or in combination with SDF-1α (1 μg/mL, ○). Data are expressed as the mean ± SEM of 9 separate experiments. No significant differences were observed when statistical analysis was performed by means of the Student t test for paired data.
The MK proliferation is not affected by the presence of SDF-1α.
Proliferation of HPCs along the megakaryocytic lineage in the presence of TPO (100 ng/mL) alone (●) or in combination with SDF-1α (1 μg/mL, ○). Data are expressed as the mean ± SEM of 9 separate experiments. No significant differences were observed when statistical analysis was performed by means of the Student t test for paired data.
When we characterized the cells for their membrane phenotype during the differentiation and maturation process, we observed the same level of expression for the specific megakaryocytic markers, ie, CD61 and CD42b for cells grown in either the absence or the presence of SDF-1α. In both culture systems, direct immunofluorescence for the expression of CD34, CD61, and CD42b antigens confirmed gradual decrease of CD34 and an inverse increase of both CD61 and CD42b, which are expressed on more than 98% and 90% of cells respectively at day 12 of the culture (data not shown).
Effect of SDF-1α on the migration of differentiating MKs
We examined the capacity of differentiating MKs to migrate in response to a positive concentration of SDF-1α through a 5-μm Transwell cell filter.
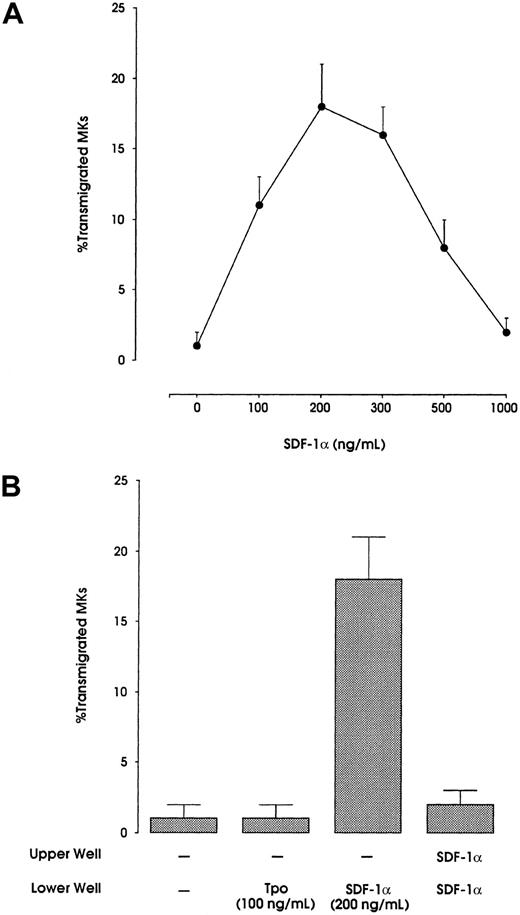
As shown in Figure 5A, these cells migrated in response to SDF-1α in a dose-dependent manner. The concentration of 200 ng/mL of SDF-1α in the lower chamber resulted in significant migration of day-10-culture–derived MKs: on average, 18% of MKs added to the upper chamber had the capacity to migrate. Megakaryoblastic cells at day 4 of culture also showed a migration but at a level of 10% (data not shown).
SDF-1α migration experiments of HPC-derived MKs at day 10 of cultures.
(A) Migration of MKs at different doses of SDF-1α. (Β) Migration of MKs under different stimuli: no addition; TPO (100 ng/mL) in the lower well; SDF-1α (200 ng/mL) in the lower well; and SDF-1α in both the upper and lower wells.
SDF-1α migration experiments of HPC-derived MKs at day 10 of cultures.
(A) Migration of MKs at different doses of SDF-1α. (Β) Migration of MKs under different stimuli: no addition; TPO (100 ng/mL) in the lower well; SDF-1α (200 ng/mL) in the lower well; and SDF-1α in both the upper and lower wells.
Neutralization of SDF-1α gradient by adding the chemokine to both upper and lower chambers completely abrogated the migration; replacement of SDF-1α with TPO also failed to induce cell migration (Figure 5B).
Analysis of CXCR4
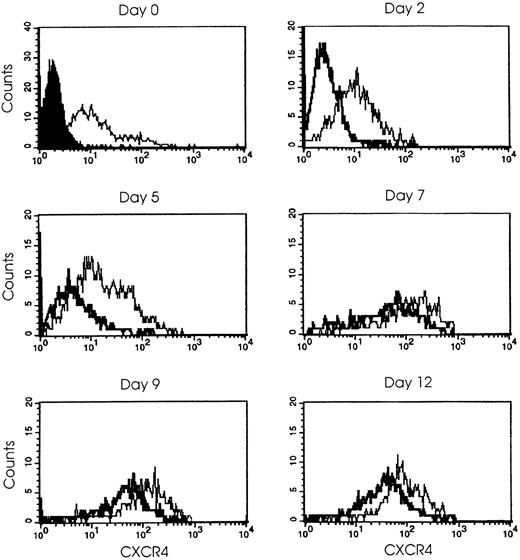
The effects of SDF-1α are mediated by the membrane receptor CXCR4. We evaluated the CXCR4 expression in quiescent HPCs and in MK-differentiating cells in the presence or absence of SDF-1α. Immunofluorescence studies with anti-CXCR4 mAb showed that about 50% of HPCs were CXCR4+, and this percentage increased to 90% at the end of the culture (day 12). In the presence of SDF-1α, CXCR4 was rapidly and markedly down-modulated (day 2) and remained scarcely expressed at membrane level up to day 5; at day 7 of culture, CXCR4 expression was partially recovered and then became only moderately lower than that observed in control cultures (Figure6). On the contrary, RT-PCR analysis performed on RNA obtained from the same cells showed similar CXCR4 mRNA levels in both control and SDF-1α–supplemented cultures (Figure7).
SDF-1α affects CXCR4 expression.
Cells were treated at day 0 after HPC purification with TPO (100 ng/mL) (thin line) or with TPO + SDF-1α (1 μg/mL) (broad line) and analyzed at intervals during the culture (days 2 through 12) by flow cytometry with the use of PE-conjugated CXCR4 mAb as described in “Materials and methods.” The staining with negative control antibody is shown, as solid profile, at day 0, and appears similar in the following days. Representative results from 1 out of 5 independent experiments are shown.
SDF-1α affects CXCR4 expression.
Cells were treated at day 0 after HPC purification with TPO (100 ng/mL) (thin line) or with TPO + SDF-1α (1 μg/mL) (broad line) and analyzed at intervals during the culture (days 2 through 12) by flow cytometry with the use of PE-conjugated CXCR4 mAb as described in “Materials and methods.” The staining with negative control antibody is shown, as solid profile, at day 0, and appears similar in the following days. Representative results from 1 out of 5 independent experiments are shown.
CXCR4 mRNA expression during MK differentiation.
Representative results of CXCR4 mRNA expression analyzed by RT-PCR in HPCs after purification (97% CD34+) and during MK differentiation in the presence or absence of SDF-1α. Total RNA from CEM cell line and from UT-7 cell line was used as a positive (C+) and negative (C−) control, respectively. RT-PCR samples were normalized for Sp26.
CXCR4 mRNA expression during MK differentiation.
Representative results of CXCR4 mRNA expression analyzed by RT-PCR in HPCs after purification (97% CD34+) and during MK differentiation in the presence or absence of SDF-1α. Total RNA from CEM cell line and from UT-7 cell line was used as a positive (C+) and negative (C−) control, respectively. RT-PCR samples were normalized for Sp26.
Confocal laser microscopic analysis was performed to investigate cellular distribution of CXCR4 in MKs grown in the absence or presence of SDF-1α (Figure 8). Cells maintained in the presence of TPO alone or of TPO combined with SDF-1α were compared for spatial distribution of CXCR4 receptor, ie, internalized after treatment with its specific ligand. CXCR4 receptor endocytosis was monitored on the control MKs and at different times after SDF-1α addition (ie, 30 minutes, 1 hour, 3 days, and 8 days). Untreated cells showed a homogeneous expression of CXCR4 on their surfaces (Figure 8A). Internalization of CXCR4 was evidenced as early as 30 minutes after SDF-1α addition, as suggested by a decrease of membrane reactivity, coupled with the appearance of internal endosomic spots (Figure 8B). This phenomenon became more evident after 1 hour of SDF-1α treatment (Figure 8C). At day 3, cells were double-stained with anti-CXCR4 and anti-CD41 mAbs: most of the SDF-1α–treated cells showed the presence of intracytoplasmic CXCR4 (Figure 8D), whereas the membrane megakaryocytic-specific marker CD41 was localized on cell surface (Figure 8E); the 2 markers do not colocalize (Figure 8F). Control double-stained cells displayed both the CXCR4 (Figure 8G) and the CD41a (Figure 8H) exclusively on the cell membrane, with colocalization pointed out by numerous yellow areas (Figure 8I).
Internalization of CXCR4 after exposure to SDF-1α.
Internalization of CXCR4 was analyzed by confocal laser scanning microscopy in HPCs. (A) Untreated cells. (B) Cells after exposure to 1 μg/mL of SDF-1α for 30 minutes. (C) Cells after exposure to 1 μg/mL of SDF-1α for 1 hour. (D-I) Double immunofluorescence for CXCR4 (green) and CD41 (red) on day 3 cells, cultured in the presence of SDF-1 (D-F), compared with control cells (G-I). The yellow area in panel I indicates the colocalization of both the receptors on the membrane (bar = 10 μm).
Internalization of CXCR4 after exposure to SDF-1α.
Internalization of CXCR4 was analyzed by confocal laser scanning microscopy in HPCs. (A) Untreated cells. (B) Cells after exposure to 1 μg/mL of SDF-1α for 30 minutes. (C) Cells after exposure to 1 μg/mL of SDF-1α for 1 hour. (D-I) Double immunofluorescence for CXCR4 (green) and CD41 (red) on day 3 cells, cultured in the presence of SDF-1 (D-F), compared with control cells (G-I). The yellow area in panel I indicates the colocalization of both the receptors on the membrane (bar = 10 μm).
MAPK pathway contributes to the stimulatory effect of SDF-1α on megakaryocytic endomitosis
To evaluate a possible functional role of MAPK activation in mediating the effects of SDF-1α on MK endomitosis, purified HPCs stimulated with TPO alone or in combination with SDF-1α were cultured in either the absence or the presence of the specific MEK inhibitor PD98059. These experiments showed that the inhibitor significantly reduced the MK endomitosis elicited both by TPO alone and by TPO in combination with SDF-1α (Figure 9), as evaluated through morphological analysis of the number of nuclear lobes. This result was also confirmed by flow cytometric analysis of DNA content (data not shown). In these experiments, PD98059 was used at a concentration of either 25 or 50 μM/L, both of which were reported to inhibit phosphorylation of ERKs in cultured MKs.28 The low amount of DMSO used in the culture had no adverse effects on MK ploidy as compared with untreated culture.
MK polyploidization decrease after treatment with PD98059.
The effect of MAPK inhibitor PD98059 was evaluated on differentiating MKs grown in the presence of TPO (100 ng/mL) alone or in combination with SDF-1α (1 μg/mL). Percentage of polylobated MKs derived from cultures grown in the presence of either 25 μM/L (░) or 50 μM/L (▪) PD98059 was evaluated by morphological analysis. An equal volume of diluent (DMSO) added to mock culture (■) had no adverse effects on polyploidization as compared with the untreated culture. Data from day 9 culture are presented as mean values ± SEM of 3 independent experiments.
MK polyploidization decrease after treatment with PD98059.
The effect of MAPK inhibitor PD98059 was evaluated on differentiating MKs grown in the presence of TPO (100 ng/mL) alone or in combination with SDF-1α (1 μg/mL). Percentage of polylobated MKs derived from cultures grown in the presence of either 25 μM/L (░) or 50 μM/L (▪) PD98059 was evaluated by morphological analysis. An equal volume of diluent (DMSO) added to mock culture (■) had no adverse effects on polyploidization as compared with the untreated culture. Data from day 9 culture are presented as mean values ± SEM of 3 independent experiments.
Discussion
In this study, we investigated the effect of the chemokine SDF-1α on megakaryocytopoiesis. The expression of CXCR4 receptor on HPCs, MKs, and circulating platelets18-20 indicates a potential role for its ligand on these cells. We observed that the polyploidization process was clearly enhanced when PB-purified CD34+ cells were induced to differentiate into MK lineage in serum-free liquid suspension culture in the presence of TPO and SDF-1α, as compared with TPO alone. The stimulatory effect of cell maturation induced by SDF-1α was apparently specific for MKs: this is shown by the absence of any effect of the cytokine on HPCs induced to differentiate along the granulocytic pathway, where the CXCR4 receptor was expressed at high level during the whole differentiative and maturative process (Chelucci et al20 and our unpublished results, December 2000).
SDF-1α accelerated the process of megakaryocytic polyploidization: at day 5, a significant percentage of these cells already showed the presence of more than one nuclear lobe, and at day 12, most of the cells were of large size and markedly polyploid, with a consistent percentage (20%) of cells more than 16N. At the end of the culture, the SDF-1α–supplemented MKs were huge, with large cytoplasm displaying a granular appearance and lobulated polyploid nuclei; we also observed a significant increase of proplatelet-bearing MKs. Finally, the stimulatory effect of SDF-1α was more pronounced in MK culture supplemented with very low doses of TPO.
Previous studies indicated that TPO supports full MK differentiation in vitro, including proplatelets and platelet formation,27,32but is unable to induce an optimal polyploidization.33,34Hypothetically, additional MK-active cytokines are essential for maximal differentiation and polyploidization of human MKs.34 Using a synthetic preparation of SDF-1α, we observed optimal effects on MK ploidization at a relatively high concentration (1 μg/mL). However, using recombinant SDF-1α, we observed a comparable stimulation of MK ploidization at a concentration (100 ng/mL) near to the levels released by stromal cells under physiological conditions.5
This is the first report showing that the chemokine SDF-1α enhances the polyploidization of MKs driven by TPO.
CXCR4 is known as the only receptor for SDF-111 and presumably could mediate different MK functions at different maturation stages of the megakaryocytic lineage from hematopoietic progenitors to platelets. Cytofluorimetric analysis indicated that treatment of CD34+ cells with SDF-1α reduced CXCR4 cell surface expression35: we observed that this reduction was maintained during the early stage of MK differentiation, but the initial expression level was almost completely restored starting from day 7 of culture. In line with these findings, confocal microscopy analysis showed that 1 hour after SDF-1α addition, a significant CXCR4 reactivity was found as intracellular spots, seemingly representing endocytic vesicles; however, at day 8 of culture, CXCR4 reactivity reappeared on the MK cell membrane. Although it is well established that MK cells express CXCR4 and bind SDF-1 during all differentiation and maturation steps,36 the specific role of the cytokine in the different stages of megakaryocytopoiesis is unclear. Recent studies on the effect of SDF-1 on migration and proliferation of MK progenitor cells8,18 indicate a role on the migration of MK progenitors and on cell adhesion of mature marrow MKs to endothelium, whereas only a moderate effect is reported on MK proliferation. More recently, Hodohara et al21demonstrated direct proliferative effect of SDF-1α on purified CD41bright/ c-kitbright CFU-MK derived from TPO-treated mice. In our liquid culture system, SDF-1α did not significantly affect MK proliferation. The discrepancy between our results and those reported by Hodohara et al21 on MK proliferation could be related to the different hemopoietic progenitors used in these 2 studies (total CD34+ human HPCs in our study and murine CD41bright/ c-kitbrightcells in the study of Hodohara et al). Furthermore, in the study by Hodohara et al,21 the hemopoietic progenitors were isolated from mice treated in vivo with TPO, and one cannot exclude the possibility that this treatment could sensitize MK progenitors/precursors to the stimulatory effect of SDF-1α on MK proliferation.
The mechanism responsible for the enhanced megakaryocytic maturation elicited by SDF-1α in cooperation with TPO is unclear. Recent studies showed that SDF-1α, in addition to its effect on stimulation of Ca++ influx, induced stimulation of the Janus kinase/signal transducers and activators of transcription (JAK/STAT) pathway, particularly of STAT3 and STAT5,37 and activated ERK1 and ERK2 and its upstream kinase MAPK kinase.38 These observations were also confirmed by recent studies performed directly on human MK precursors.39 In addition, it was shown that PD98059, a specific MEK inhibitor, reduced the TPO-induced MK polyploidy, thus suggesting an important role for MAPK in MK endomitosis.28,40 In line with these studies, we observed that the addition of PD98059 to MK cultures stimulated by TPO alone or by TPO in combination with SDF-1α inhibited the MK polyploidy, suggesting a role for MAPK in the stimulatory effect of SDF-1α on ploidy. This conclusion is also supported by a previous study showing that SDF-1α clearly potentiated the ERK-1/ERK-2 phosporylation induced by TPO.28
Our results demonstrated an effect on the polyploidization of the MK cells only when SDF-1α was added to purified CD34+cells; no effect was observed on the morphology of the cells when SDF-1α was added in the TPO-culture medium at day 5 on MK precursors. However, at this time, the CXCR4 coreceptor is expressed and SDF-1α was able to block the HIV entry in the same MK population,20 thus confirming the existence of a specific binding between CXCR4 and SDF-1 also at the MK- precursor stages.
Hamada et al8 demonstrated that SDF-1 induced rapid transmigration of intact polyploid MKs through bone marrow endothelial cells, followed by fragmentation of MKs into platelets within 12 to 24 hours after migration. On the basis of the effect of SDF-1α on MK ploidy, we suggest that the cytokine not only affects transmigration,8 but also induces an increase in the ploidy of MK cells. Kowalska et al36 reported that only the more immature cells responded to the chemotactic stimulus of SDF-1. In line with this observation, our transmigration results indicate that differentiating MKs respond to a positive gradient of SDF-1α: 10% of MKs were already able to transmigrate at day 4 of culture, when about 60% of the cells were positive for CXCR4; this percentage grew to 18% at day 10, when 90% of the cells expressed CXCR4 on the membrane. Morphological analysis on day-10–transmigrated MKs indicated that only monolobated or bilobated cells were able to pass through the membrane into the lower chamber.
The presence of the CXCR4 receptor on platelet surface has not been correlated with a specific function. However, a recent study suggests that SDF-1α acts as a weak agonist of platelet activation when added alone and as an enhancer of platelet activation in response to low doses of adenosine 5′-diphosphate.41
Our data suggest that the increased number of the nuclear lobes per cell induced by SDF-1α is coupled with an increase in platelet formation. Indeed, the number of proplatelet-bearing MKs is higher in SDF-1α–supplemented cultures than in controls. The number of generated platelets was also more elevated in the presence of SDF-1α. Mechanisms of platelet production and release by mammalian MKs are poorly understood. Mice lacking transcription factor NF-E2 have a late arrest in megakaryocytic maturation, resulting in profound thrombocytopenia.42 Our results on NF-E2 mRNA analyzed on megakaryocytic culture supplemented with SDF-1α and TPO or with TPO alone did not show any significant difference in the expression of this transcription factor (unpublished data, 1999).
Since the expression of the phenotypical markers analyzed (ie, CD61, CD62, CD41, CD42b) was similar in both TPO- supplemented and TPO + SDF-1α–supplemented cultures, we suggest that acquisition of membrane differentiation markers and polyploidization are independently regulated events, as was already proposed when the megakaryocytic differentiation process was investigated with the use of the UT-7 cell line.43
In conclusion, our results indicate that the chemokine SDF-1α, combined with TPO, is involved not only in migration but also in polyploidization of MKs and in proplatelet and platelet production, providing a novel insight into the physiological regulation of megakaryocytopoiesis.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Hamisa Jane Hassan, Dept of Clinical Biochemistry, Istituto Superiore di Sanità, Viale Regina Elena 299, 00161 Rome, Italy; e-mail: j.hassan@iss.it.








This feature is available to Subscribers Only
Sign In or Create an Account Close Modal