Abstract
Receptors for human immunoglobulin (Ig)G and IgA initiate potent cytolysis of antibody (Ab)-coated targets by polymorphonuclear leukocytes (PMNs). Mac-1 (complement receptor type 3, CD11b/CD18) has previously been implicated in receptor cooperation with Fc receptors (FcRs). The role of Mac-1 in FcR-mediated lysis of tumor cells was characterized by studying normal human PMNs, Mac-1–deficient mouse PMNs, and mouse PMNs transgenic for human FcR. All PMNs efficiently phagocytosed Ab-coated particles. However, antibody-dependent cellular cytotoxicity (ADCC) was abrogated in Mac-1−/− PMNs and in human PMNs blocked with anti–Mac-1 monoclonal Ab (mAb). Mac-1−/− PMNs were unable to spread on Ab-opsonized target cells and other Ab-coated surfaces. Confocal laser scanning and electron microscopy revealed a striking difference in immunologic synapse formation between Mac-1−/− and wild-type PMNs. Also, respiratory burst activity could be measured outside membrane-enclosed compartments by using Mac-1−/− PMNs bound to Ab-coated tumor cells, in contrast to wild-type PMNs. In summary, these data document an absolute requirement of Mac-1 for FcR-mediated PMN cytotoxicity toward tumor targets. Mac-1−/− PMNs exhibit defective spreading on Ab-coated targets, impaired formation of immunologic synapses, and absent tumor cytolysis.
Introduction
Antibodies have been studied extensively for their use in immunotherapy of cancer.1-4 It is evident that receptors for the Fc portion of immunoglobulins (FcRs) on myeloid cells are critical in triggering antitumor cytotoxicity in vivo.5,6 Antibody-dependent cellular cytotoxicity (ADCC), considered crucial for antibody-mediated tumor cell degradation, can be mediated by polymorphonuclear leukocytes (PMNs), monocytes/macrophages, eosinophils, and natural killer (NK) cells.7,8 These effector cells use different cytotoxic mechanisms, depending on their activation state and the nature of the target.7,9-12 PMNs, representing the most populous type of white blood cell, exhibit fast recruitment activity in vivo. Potent and very rapid (within 30 minutes) PMN cytotoxicity toward various tumor targets has been documented.10,13-15 Two classes of immunoglobulin (Ig)G receptors (FcγRIIa, CD32, and FcγRIIIb, CD16) and one class of IgA receptor (FcαRI, CD89) have been identified on human PMNs, whereas FcγRI (CD64) expression is inducible on PMNs on stimulation with granulocyte colony-stimulating factor.16PMNs can trigger ADCC by engagement of FcγRI, FcγRIIa, and FcαRI. FcαRI, however, has been observed to be the most effective FcR for PMN-mediated tumor cell killing.14 17
Mac-1 (CR3, CD11b/CD18) is a member of the β2 integrin family, which includes Mac-1, LFA-1 (CD11a/CD18), and gp150/95 (CR4, CD11c/CD18).18,19 These receptors, sharing a common β-chain (CD18), can bind multiple ligands and can regulate various leukocyte functions. Mac-1 represents the predominant β2integrin on PMNs and is furthermore expressed on monocytes/macrophages and NK cells. Several PMN functions are regulated by Mac-1, including adhesion, migration, chemotaxis, phagocytosis, respiratory burst activity, and degranulation.18 On activation, Mac-1 is able to initiate signaling by its linkage to the actin cytoskeleton and associated signaling proteins.20,21 A number of studies described Mac-1 cooperation with different receptors on PMNs, indicating Mac-1 to be a signaling partner for other receptors.22 These include FMLP receptors, LPS/LBP receptors (CD14), urokinase plasminogen activator receptor (CD87), and Fc receptors.22-26
Mac-1 was found to trigger Ab-dependent phagocytosis by FcγRIIIb in fibroblasts transfected with both Mac-1 and FcγRIIIb, whereas cells expressing only Mac-1 or FcγRIIIb were unable to ingest Ab-opsonized particles.27 Mac-1 cooperation with FcγRIIIb in the generation of PMN respiratory burst has been described as well.28 Furthermore, Mac-1 restored IgG-dependent phagocytosis of transfectants with FcγRIIa tail-minus mutants.29 Importantly, PMNs from patients with leukocyte adhesion deficiency, who lack CD18, were shown to be severely impaired in mediating phagocytosis and ADCC.18,30-32 Although the relative contribution of individual β2 integrins remains to be determined, various studies point to an important role of Mac-1 in FcR-mediated cytotoxicity. Targets studied include tumor cells, virus-infected cells, Schistosoma mansoni, and sheep erythrocytes.33-38 The underlying mechanism of Mac-1 involvement in ADCC, however, remains unclear. This study focused on the role of Mac-1 in FcR-mediated cytotoxicity toward tumor targets by studying Mac-1–deficient mouse PMNs, mouse PMNs transgenic for human FcR, and normal human PMNs. Our data document Mac-1 to be crucial for spreading on Ab-coated targets, formation of intact immunologic synapses, and tumor cytolysis.
Materials and methods
Antibodies
Bispecific antibodies (BsAbs) [A77 × αCan] and [A77 × 520C9], recognizing both FcαRI and Candida albicans, or HER-2/neu, respectively, were produced as described.39 Briefly, F(ab′) fragments of mAb 520C9 (Medarex, Annandale, NJ) and F(ab′)2 fragments of anti–C albicans IgG (Biodesign, Kennebunk, ME) were chemically cross-linked to F(ab′) fragments of mAb A77 (mIgG1; Medarex). Anti–C albicans IgG and BsAb [A77 × αCan] were used to study C albicans phagocytosis and killing. MAb 520C9 (mIgG1 anti–HER-2/neu) and BsAb [A77 × 520C9] were used in ADCC experiments. Antihuman CD11b mAbs were purified from supernatants of hybridomas 44a and M1/70 (ATCC, Rockville, MD) and were used in human Mac-1–blocking experiments.
Isolation of human PMNs
PMNs were isolated from heparinized venous blood of healthy volunteers by Ficoll-Histopaque (Sigma, St. Louis, MO) density gradient centrifugation. PMN purity determined by cytospin preparations exceeded 95%, and cell viability was more than 98%, as determined by trypan blue exclusion.
Mouse PMNs
CD11b knockout mice (Mac-1−/−) were generated by homologous recombination in C57bl/6 × 129SV background.40 To study Mac-1 involvement in human FcαRI-mediated functions, mice were crossed back with human FcαRI (CD89) transgenic (Tg) FVB/N mice,37 yielding 4 different genotypes: nontransgenic (Ntg) Mac-1+/−, Ntg Mac-1−/−, Tg Mac-1+/−, and Tg Mac-1−/−. Mac-1 deficiency has no influence on PMN FcR expression.37 To increase blood PMN counts, mice were injected subcutaneously with 15 μg polyethylene glycol granulocyte colony-stimulating growth factor (kindly provided by Dr J. Andresen, Amgen, Thousand Oaks, CA), and blood was collected 3 days later. Erythrocytes were removed by hypotonic lysis, followed by washing the remaining leukocytes with RPMI 1640 medium (Gibco BRL, Grand Island, NY) with 10% fetal calf serum (FCS). Cell viability was always more than 95%. Flow cytometry analysis on a FACScan (Becton Dickinson, San Jose, CA) revealed leukocytes to consist of 55% to 60% PMNs, 35% to 40% lymphocytes, approximately 3% monocytes, and approximately 1% eosinophils.
C albicans phagocytosis and kill
C albicans (ATCC 448585) phagocytosis was analyzed by flow cytometry and light microscopy, and fungicidal activity was assayed by radiometric killing assays, as previously described.41 Percentages of specific FcR-mediated phagocytosis and fungal death were calculated by subtracting control values (no antibody present).
ADCC assays
The capacity of PMNs to lyse tumor cells was evaluated in51Chromium release assays.13 Briefly,51Cr-labeled SK-BR-3 (human breast carcinoma) cells (ATCC, HTB-30) were plated in round-bottom 96-well plates (5 × 103 cells/well) in RPMI 1640 medium (with 10% FCS). Isolated human or mouse PMNs were added in the absence or presence of 0.5 μg/mL BsAb [A77 × 520C9] or 2 μg/mL mAb 520C9, giving different effector (E)/target (T) ratios, and incubated for 4 hours at 37°C after which 51Cr-release was measured in supernatants. In blocking experiments, human PMNs were incubated with anti–Mac-1 mAb 44a (10 μg/mL), mAb M1/70 (10 μg/mL), or 0.1MN-acetyl-d-glucosamine (NADG; Sigma) during ADCC. All experiments were performed in the absence of serum complement to exclude complement-dependent cytotoxicity.
Cellular adhesion and spreading
Mouse PMNs were incubated with SK-BR-3 cells (ratio 50:1) in RPMI 1640 medium (with 10% FCS) with or without BsAb [A77 × 520C9] (0.5 μg/mL) on glass coverslips (Marienfeld, Lauda-Königshofen, Germany) at 37°C for 30, 60, or 120 minutes. Cells were fixed, stained (Diff-Quick staining, Dade Behring, Düdingen, Germany), and analyzed randomly by light microscopy to determine attachment indices (AIs; number of attached PMNs per 100 tumor cells).
To study PMN spreading, SK-BR-3 cells were cultured on glass coverslips overnight at 37°C and incubated with mouse PMNs (with or without BsAb) for 30 minutes at 37°C. After carefully washing away unbound cells, cells were fixed in 3.8% paraformaldehyde, and actin was stained with phalloidin-fluorescein isothiocyanate (FITC; 1:200; Sigma) for 15 minutes at 20°C. In an additional set of experiments, glass slides were coated with 100 μg/mL human serum IgA (ICN, Aurora, OH) or IgG (CLB, Amsterdam, The Netherlands) for 3 hours at 37°C and blocked with 0.5% (wt/vol) bovine serum albumin (BSA). Isolated PMNs were plated on the coated slides for 30 minutes at 37°C, fixed, and stained with phalloidin-FITC. Samples were mounted, and cell spreading was analyzed by confocal laser scanning microscopy using a Leitz DMIRB fluorescence microscope (Leica, Voorburg, The Netherlands). Cell morphology was imaged just above (0.2 μm) the coated surfaces inx/y and x/z directions, sectioning both PMN and underlying surfaces.
Neutrophil degranulation
Human PMNs were incubated with SK-BR-3 cells (with or without 0.5 μg/mL BsAb [A77 × 520C9]) in Hepes+ buffer (20 mM Hepes pH 7.4 with 132 mM NaCl, 6 mM KCl, 1 mM MgSO4, 1.2 mM NaH2PO4, 1 mM CaCl2, 5 mM glucose, and 0.5% [wt/vol] BSA) for 30 minutes at 37°C. Cytochalasin B (10 μg/mL) (Sigma) was added for 10 minutes at 4°C to detach PMNs from tumor cells. In addition, PMNs were incubated in IgA- and IgG-coated 96-well plates (see above) in Hepes+buffer for 30 minutes at 37°C. As positive controls, PMNs were stimulated with 100 ng/mL phorbol myristate acetate (PMA) (Sigma) or with 10−6 M FMLP (Sigma) and cytochalasin B for 10 minutes at 37°C. In Mac-1–blocking experiments, mAb 44a (10 μg/mL) was added during PMN stimulation. In all cases, supernatants were collected for β-glucuronidase and lactoferrin analysis. The β-glucuronidase activity was measured by incubating supernatants (in triplicate) with 1.1 mM phenolphthalein-β-glucosiduronic acid (Sigma) in 0.1 M acetate buffer (pH 4.5) overnight at 37° C. Reactions were stopped by 0.1 M glycine (pH 10.2) and measured by spectrophotometry at 560 nm. Lactoferrin release was quantified by sandwich enzyme–linked immunosorbent assay (ELISA) by using rabbit antilactoferrin IgG (1:2500; Sigma) to coat 96-maxisorp plates (Nunc, Roskilde, Denmark) and alkaline phosphatase–conjugated rabbit antilactoferrin IgG (1:5000; Sigma) to detect bound lactoferrin. Total lactoferrin and β-glucuronidase contents of PMNs were determined by lysing cells with 0.2% Triton X-100.
PMN fractionation assays
PMN fractionation assays42 were adjusted for mouse cells. Twenty-four-well plates were coated (see above) with IgA or BSA. Isolated PMNs (Ntg Mac-1+/−, Tg Mac-1+/−, and Tg Mac-1−/−) were stimulated with PMA (100 ng/mL), or incubated on BSA-coated plates, or IgA-coated plates (5 × 106 PMN/well) for 30 minutes at 37°C. Cells were detached with PBS containing 100 mM EDTA at 4°C, washed with PBS, and resuspended in 1 mL ice-cold oxidase buffer (75 mM NaCl, 10 mM Hepes, 170 mM sucrose, 1 mM MgCl2, 0.5 mM EGTA, 10 μM ATP and 2 mM azide, pH 7.0). The cells were then homogenized in a “cell cracker” (EMBL, Heidelberg, Germany). After centrifugation (10 minutes at 800g), 800 μL postnuclear supernatants were layered on discontinuous sucrose gradients in SW60 tubes (Beckman, Palo Alto, CA) containing layers of 10% sucrose, 35% sucrose, 50% sucrose, and 60% sucrose each of 800 μL in oxidase buffer. After ultracentrifugation (45 minutes at 100 000g), 600 μL supernatant (cytosol fraction), 600 μL of the 10%/35% interphase (plasma membrane), 600 μL of the 35%/50% interphase (specific granules), and 600 μL of the 50%/60% interphase (azurophilic granules) were harvested. Protein contents of fractions were determined with Bradford protein assays (Biorad, München, Germany). For immunodetection, fractions (2 μg of protein) were separated on 10% reducing SDS–polyacrylamide gel electrophoresis. P22-phoxwas detected by Western blotting, using rabbit polyclonal Ab (a kind gift from Dr M. C. Dinauer, Indiana University, Indianapolis, IN43), followed by horseradish peroxidase (HRP)-conjugated swine antirabbit IgG (Dako, Glostrup, Denmark).
Respiratory burst measurements
The (iso)luminol-enhanced chemiluminescence method was used for analysis of real time respiratory burst activity. Luminol (membrane permeable) was used for detection of total (intracellular and extracellular) oxygen radical production, isoluminol (membrane impermeable) served as the substrate for oxygen radical detection outside membrane-enclosed compartments.44 Mouse PMNs (5 × 105) were gently centrifuged (40g for 3 minutes at 4°C) on SK-BR-3 cells (1 × 104) opsonized with or without BsAb [A77 × 520C9] in RPMI 1640 medium (with 10% FCS) and were placed in a 953 LB Biolumat (Berthold, Wildbad, Germany). Luminol (150 μM) or isoluminol (56 μM) (Sigma) was injected in all tubes, and light emission was recorded continuously for 30 minutes at 37°C. Respiratory burst measurements using isoluminol were performed in the presence of HRP (4 U; Sigma) to overcome the limited availability of amplifying peroxidase outside membrane-enclosed compartments.44 As positive controls PMNs, were stimulated with PMA, as negative controls, PMNs were incubated with HRP and (iso)luminol only.
Analysis of immunologic synapse formation
Freshly grown SK-BR-3 cells (approximately 2 × 106) were biotinylated with 2 mg/mL sulfo-NHS-biotin (Sigma) in PBS for 10 minutes at 4°C. Tumor cells were subsequently incubated in PBS with 50 mM NH4Cl at 4°C to quench reactions. After washing in PBS, cell viability was checked by trypan blue exclusion, and cells were adhered to glass coverslips for 1 hour at 37°C. Subsequently, mouse PMNs were incubated on tumor cells (with or without 0.5 μg/mL BsAb [A77 × 520C9]) for 30 minutes at 37°C. After washing to remove unbound PMNs, cells were fixed in methanol. Samples were washed, incubated with streptavidin-FITC (1:100; Dako) for 10 minutes at 4°C for plasma membrane staining, and incubated with propidium iodide (1:1000; Sigma) for 1 minute at 4°C for nuclei staining. Slides were mounted, and tumor cell membrane FITC staining between PMNs and tumor cells was analyzed by confocal laser scanning microscopy (see above). Sections of approximately 0.75 μm were acquired, recording 8 images from top to bottom of cells. For quantification, at least 50 PMNs that adhered to tumor targets were analyzed randomly per sample for the presence or absence of membrane FITC staining at tumor cell–PMN interaction sites.
Electron microscopy
For transmission electron microscopy, SK-BR-3 cells were cultured to confluence in RPMI 1640 medium (10% FCS) on 6.5-mm 0.4-μm pore-size transwell filters (Costar, Cambridge, MA). Mouse PMNs (Tg Mac-1+/−, Tg Mac-1−/−, and Ntg Mac-1+/−) were incubated on the tumor cells with and without 0.5 μg/mL BsAb [A77 × 520C9] for 40 minutes at 37°C. After the filters were rinsed with RPMI 1640 medium, cells were fixed with 2.5% glutaraldehyde in cacodylate (Sigma) buffer (pH 7.2) overnight. Subsequently, filters were washed in cacodylate buffer, postfixed in cacodylate-buffered 1% OsO4 (Johnson Matthey Chem, Roystone, United Kingdom) for 30 minutes, and embedded in Epon 812 (Merck, Darmstadt, Germany). Ultrathin sections were cut on an ultratome (LKB Instruments, Bromma, Sweden) and contrasted with 3% aqueous uranyl acetate for 45 minutes and lead citrate for 2 minutes at 20°C. The sections were examined in a Jeol 1200EX electron microscope (Jeol, Tokyo, Japan).
Statistical analysis
Unpaired 2-tailed Student t tests were used to determine differences. Significance was accepted at theP < .05 level.
Results
To assess the role of Mac-1 in human FcR-mediated functions, we analyzed PMNs from Mac-1–deficient mice crossed with human FcR transgenic mice. This enabled us to selectively study Mac-1, in the presence of other β2 integrins, in human FcR functioning. In addition, we analyzed the effect of Mac-1 blocking on FcR functions mediated by human PMNs. Molecular cloning revealed the interspecies conservation of Mac-1 to be very high.31
Mac-1–deficient PMNs potently mediate phagocytosis and killing via FcRs
To examine the role of Mac-1 in FcαRI-mediated uptake of microorganisms, phagocytosis of BsAb [A77 × αCan]-opsonizedC albicans by PMNs (Tg Mac-1+/− and Tg Mac-1−/−) was monitored at different times. Light microscopic analyses revealed both Mac-1+/− and Mac-1−/− PMNs to be efficient in uptake of C albicans (Figure 1A). FcαRI-mediated phagocytosis, assessed by FACS analysis, was quantified on subtraction of uptake in the absence of BsAb. No differences were observed in FcαRI-mediated phagocytosis of C albicans between Mac-1+/− and Mac-1−/−PMNs (Figure 1B). To assess whether Mac-1 was important in PMN microbicidal activity following FcαRI-mediated uptake, C albicans killed by Mac-1+/− and Mac-1−/− PMNs was analyzed after 2 hours (Figure 1C). Both Mac-1+/− and Mac-1−/− PMNs killed C albicans with equal efficacy (28% ± 7% versus 25% ± 7%, n = 4). Similar results were obtained with IgG-coated C albicans. Ntg Mac-1+/− PMNs mediated neither phagocytosis nor killing of BsAb-coated C albicans (data not shown). These results indicate that Mac-1 is not required for FcR-mediated PMN phagocytosis and intracellular killing of C albicans.
Mac-1–deficient PMNs efficiently phagocytose and kill Ab-opsonized
C albicans. Tg mouse PMNs (Mac-1+/− and Mac-1−/−) were incubated with C albicansin the presence of FcαRI-directed BsAb. (A) Phagocytosis was analyzed by light microscopy after 30 minutes. (B) Flow cytometric analysis of FcαRI-mediated C albicans uptake by Tg Mac-1+/− (▪) and Tg Mac-1−/− PMNs (░). Results are expressed as percentage of PMNs that mediated phagocytosis (mean ± SEM [n = 4]). (C) FcαRI-mediated killing of C albicans by Tg PMNs (Mac-1+/− and Mac-1−/−) after 2 hours. Results are mean ± SEM based on 4 individual experiments. Bars indicate 10 μm.
Mac-1–deficient PMNs efficiently phagocytose and kill Ab-opsonized
C albicans. Tg mouse PMNs (Mac-1+/− and Mac-1−/−) were incubated with C albicansin the presence of FcαRI-directed BsAb. (A) Phagocytosis was analyzed by light microscopy after 30 minutes. (B) Flow cytometric analysis of FcαRI-mediated C albicans uptake by Tg Mac-1+/− (▪) and Tg Mac-1−/− PMNs (░). Results are expressed as percentage of PMNs that mediated phagocytosis (mean ± SEM [n = 4]). (C) FcαRI-mediated killing of C albicans by Tg PMNs (Mac-1+/− and Mac-1−/−) after 2 hours. Results are mean ± SEM based on 4 individual experiments. Bars indicate 10 μm.
Mac-1 is crucial for PMN ADCC
To study Mac-1 involvement in PMN FcR-mediated extracellular cytotoxicity, lysis of human breast carcinoma cells (SK-BR-3 cells) by both mouse and human PMNs was examined. Mac-1–expressing Tg mouse PMNs efficiently killed tumor targets in the presence of FcαRI-directed BsAb [A77 × 520C9] at different E/T ratios (Figure2). However, ADCC capacity of Mac-1–deficient Tg mouse PMNs was absent. Similarly, control ADCC experiments performed with mouse IgG1 (mAb 520C9) demonstrated tumor cytotoxicity of Mac-1+/− PMNs but not of Mac-1−/− PMNs (data not shown). Ntg (Mac-1+/−) mediated cytotoxicity toward tumor cells in the presence of 520C9 but not in the presence of FcαRI-directed BsAb (not shown). These results show that dependence of ADCC on Mac-1 is not restricted to cytotoxicity mediated via FcαRI. ADCC mediated via mouse FcγR or human FcγRI (tested in FcγRI-Tg mice15) was similarly dependent on Mac-1. Furthermore, the Mac-1 requirement for ADCC was observed for different tumor cell lines (not shown). To further assess the requirement for Mac-1, experiments with human PMNs in the presence of anti–Mac-1 antibodies were performed. Human PMNs mediated cytotoxicity of BsAb [A77 × 520C9]-opsonized SK-BR-3 cells with similar efficacy as Tg Mac-1+/− mouse PMNs (Figure 2B). Blocking Mac-1 by anti–Mac-1 antibodies (M1/70, 44a) or NADG resulted in significantly reduced FcR-mediated cytotoxicity (Figure 2B). PMN viability and FcR expression were not altered on Mac-1 blocking, and isotype controls (rat IgG2 and mouse IgG1) did not affect ADCC (data not shown). These data implicate Mac-1 to be important for both mouse and human PMN FcR-mediated cytotoxicity.
Mac-1 is crucial for FcR-mediated PMN cytotoxicity of tumor targets.
(A) Lysis of human breast carcinoma (SK-BR-3) cells mediated by Tg Mac-1+/− PMNs (▪) and Tg Mac-1−/− PMNs (░) in the absence (control) or presence of FcαRI-directed BsAb [A77 × 520C9]. Cytotoxicity was analyzed after 4 hours at different E/T ratios (20:1 and 80:1). Results are presented as mean ± SEM (n = 6), (*P < .0001). (B) FcαRI-mediated cytotoxicity toward SK-BR-3 cells by human PMNs (E/T ratio; 50:1) in the absence (control) or presence of BsAb [A77 × 520C9]. The effect of anti–Mac-1 mAb 44a or M1/70, and NADG on ADCC was analyzed as described in “Materials and methods.” Data are presented as mean ± SEM (n = 3). *Significant difference compared with BsAb alone (P < .002).
Mac-1 is crucial for FcR-mediated PMN cytotoxicity of tumor targets.
(A) Lysis of human breast carcinoma (SK-BR-3) cells mediated by Tg Mac-1+/− PMNs (▪) and Tg Mac-1−/− PMNs (░) in the absence (control) or presence of FcαRI-directed BsAb [A77 × 520C9]. Cytotoxicity was analyzed after 4 hours at different E/T ratios (20:1 and 80:1). Results are presented as mean ± SEM (n = 6), (*P < .0001). (B) FcαRI-mediated cytotoxicity toward SK-BR-3 cells by human PMNs (E/T ratio; 50:1) in the absence (control) or presence of BsAb [A77 × 520C9]. The effect of anti–Mac-1 mAb 44a or M1/70, and NADG on ADCC was analyzed as described in “Materials and methods.” Data are presented as mean ± SEM (n = 3). *Significant difference compared with BsAb alone (P < .002).
PMN binding and spreading on Ab-coated targets
On observing an essential role for Mac-1 in FcR-mediated killing, we investigated the underlying mechanism. First, we aimed to analyze whether PMNs devoid of Mac-1 were defective in binding Ab-opsonized tumor cells. AIs were determined for Mac-1–expressing and Mac-1–deficient PMNs. Both Tg Mac-1+/− and Tg Mac-1−/− PMNs effectively bound Ab-coated tumor targets within 30 minutes, albeit the AIs of Mac-1−/− PMNs were decreased compared with Mac-1–expressing PMNs (Figure3A). Adherence of Ntg Mac-1+/− PMNs to FcαRI-directed BsAb [A77 × 520C9]-coated tumor cells revealed hardly any binding (< 50 PMNs/100 SK-BR-3 cells, n = 4). In the absence of Ab, PMNs did not adhere to tumor targets (data not shown). Because the binding of Mac-1−/− PMNs to Ab-coated tumor cells was intact, we next studied PMN interactions with Ab-coated targets in more detail. Comparing Mac-1+/− PMNs with Mac-1−/− PMNs bound to Ab-coated surfaces (either IgA or IgG) revealed obvious morphologic differences. Figure 3B displays confocal microscopic images of the actin cytoskeleton of Mac-1+/− PMNs (left) and Mac-1−/− PMNs (right) adhered to IgA-coated glass slides. Mac-1–expressing PMNs exhibited spreading (clearly visible after analysis in x/z direction, inserts) after only 10 minutes, in contrast to Mac-1–deficient PMNs. PMNs bound to BSA-coated glass did not exhibit spreading (data not shown). Similarly, PMNs spreading on Ab-coated tumor cells was dependent on Mac-1 (not shown). These results show Mac-1−/− PMNs able to bind but unable to spread on Ab-coated targets.
PMN binding and spreading on Ab-coated targets.
(A) Numbers of Ntg Mac-1+/− PMNs (■), Tg Mac-1+/− PMNs (■), and Tg Mac-1−/− PMNs (░) bound to BsAb [A77 × 520C9]-opsonized SK-BR-3 cells were determined after 30 and 60 minutes. Results represent mean ± SEM from 3 individual experiments. (B) Tg Mac-1+/− PMNs (left) and Tg Mac-1−/− PMNs (right) were plated on IgA-coated glass slides for 30 minutes and stained for actin. Cell morphology was imaged in x/y and x/z directions (inserts), using confocal microscopy. Bars indicate 15 μm. Experiments were performed at least 4 times, yielding similar results.
PMN binding and spreading on Ab-coated targets.
(A) Numbers of Ntg Mac-1+/− PMNs (■), Tg Mac-1+/− PMNs (■), and Tg Mac-1−/− PMNs (░) bound to BsAb [A77 × 520C9]-opsonized SK-BR-3 cells were determined after 30 and 60 minutes. Results represent mean ± SEM from 3 individual experiments. (B) Tg Mac-1+/− PMNs (left) and Tg Mac-1−/− PMNs (right) were plated on IgA-coated glass slides for 30 minutes and stained for actin. Cell morphology was imaged in x/y and x/z directions (inserts), using confocal microscopy. Bars indicate 15 μm. Experiments were performed at least 4 times, yielding similar results.
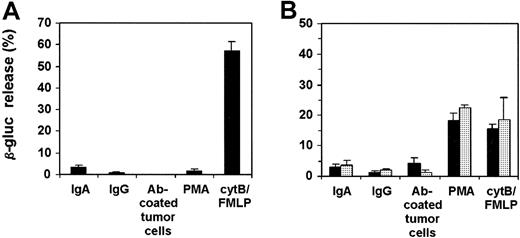
PMN degranulation and respiratory burst activity
We next investigated the role of Mac-1 in PMN degranulation and respiratory burst activity. First, exocytosis of azurophilic (primary) granules of mouse PMNs adhered to BsAb [A77 × 520C9]-coated tumor targets was analyzed. The β-glucuronidase activities were measured in supernatants after PMNs were detached from tumor cells. PMNs did not release β-glucuronidase after binding Ab-coated tumor cells, in contrast to the PMNs stimulated with cytB/FMLP or PMA (Figure4A). To rule out the possibility that β-glucuronidase interacts with tumor targets, precluding measurement of its activity in supernatants, total β-glucuronidase contents of PMNs incubated with tumor cells in the absence or presence of Ab were determined, again revealing no differences in β-glucuronidase activity. Extension of incubation periods to 4 hours did not result in detectable β-glucuronidase activity. Similar to these results, mouse and human PMNs adhering to IgA- or IgG-coated surfaces did not release detectable β-glucuronidase activity (Figure 4). To confirm the absence of PMN azurophilic granule mobilization after stimulation on Ig-coated surfaces, an alternative detection method was used. Separation of different PMN fractions (cytosol, plasma membrane, and granules) after PMN adherence to Ig-coated glass revealed β-glucuronidase to be retained within the granule fraction (data not shown). In contrast, β-glucuronidase activity of PMNs stimulated with cytB/FMLP was detectable in supernatants, consistent with the results above.
Mobilization of azurophilic granules by human (A) and mouse (B) PMNs.
Human PMNs, Tg Mac-1+/− PMNs (▪), and Tg Mac-1−/− PMNs (░) were incubated on IgA- or IgG-coated surfaces or on Ab-coated SK-BR-3 cells for 30 minutes. PMNs were detached from tumor cells by cytochalasin B, and supernatants were analyzed for β-glucuronidase (β-gluc) activity. In addition, PMNs were stimulated for 15 minutes with PMA or with FMLP in the presence of cytochalasin B. The β-glucuronidase release is presented as the percentage of total activity present in PMNs. Results are expressed as mean ± SEM from either 4 (A) or 3 (B) individual experiments.
Mobilization of azurophilic granules by human (A) and mouse (B) PMNs.
Human PMNs, Tg Mac-1+/− PMNs (▪), and Tg Mac-1−/− PMNs (░) were incubated on IgA- or IgG-coated surfaces or on Ab-coated SK-BR-3 cells for 30 minutes. PMNs were detached from tumor cells by cytochalasin B, and supernatants were analyzed for β-glucuronidase (β-gluc) activity. In addition, PMNs were stimulated for 15 minutes with PMA or with FMLP in the presence of cytochalasin B. The β-glucuronidase release is presented as the percentage of total activity present in PMNs. Results are expressed as mean ± SEM from either 4 (A) or 3 (B) individual experiments.
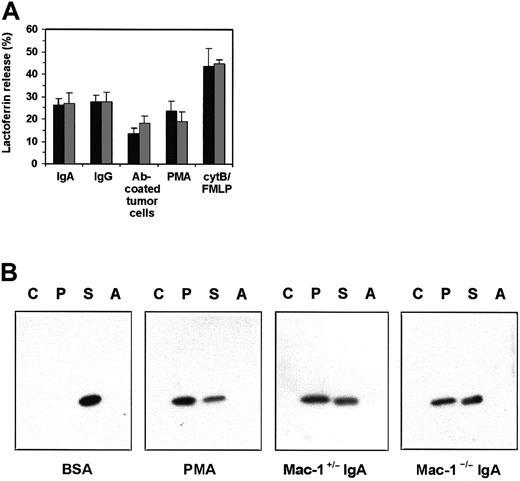
Specific (secondary) granule mobilization was analyzed by studying lactoferrin release and the translocation of p22-phox, a component of PMN NADPH oxidase complexes.45 Human PMNs released lactoferrin on adherence to IgA- or IgG-coated glass slides, in contrast to PMNs bound to BSA-coated plates (Figure5A). More importantly, PMNs bound to Ab-opsonized tumor cells released lactoferrin within 30 minutes, in contrast to PMNs incubated with uncoated tumor cells. This release was detectable only after detaching PMN from Ab-coated tumor targets. Human PMNs incubated with anti–Mac-1 mAb retained their ability to release specific granules after activation (Figure 5A). As our lactoferrin ELISA did not react with mouse lactoferrin, we developed another method to study exocytosis of specific granules. Mouse PMNs (Tg Mac-1+/−, Tg Mac-1−/−, and Ntg Mac-1+/−) were incubated on BSA- or IgA-coated surfaces. After detachment and homogenization, PMN fractions (cytosol, plasma membrane, specific and azurophilic granules) were analyzed for the presence of p22-phox in Western blots.43Translocation of p22-phox from the specific granules to the plasma membrane was observed with Tg PMNs stimulated by IgA or PMA but not BSA (Figure 5B). Importantly, Mac-1–deficient Tg PMNs mobilized p22-phox on IgA-stimulation with similar efficacy as Mac-1–expressing Tg PMN.
Mobilization of specific granules by human and mouse PMNs.
(A) Human PMNs were incubated on IgA- or IgG-coated surfaces or on Ab-coated SK-BR-3 cells for 30 minutes. PMNs were detached from tumor cells by cytochalasin B, and supernatants were tested for presence of lactoferrin. In addition, PMNs were stimulated for 15 minutes with PMA or with FMLP in the presence of cytochalasin B. The different stimuli were tested in the absence (▪) or presence (░) of anti–Mac-1 mAb 44a. Results are expressed as mean ± SEM (n = 3). (B) Mouse PMNs (Tg Mac-1+/− and Tg Mac-1−/−, n = 3) were incubated on BSA- (control) or IgA-coated surfaces. Cells were detached after 30 minutes and homogenized. Sucrose gradient ultracentrifugation separated cytoplasm (C), plasma membrane (P), specific granule (S), and azurophilic granule (A) fractions. Translocation of p22 from specific granules to the plasma membrane was detected by Western blotting using α-mouse p22-phox antiserum. PMA-stimulated mouse PMNs served as positive control.
Mobilization of specific granules by human and mouse PMNs.
(A) Human PMNs were incubated on IgA- or IgG-coated surfaces or on Ab-coated SK-BR-3 cells for 30 minutes. PMNs were detached from tumor cells by cytochalasin B, and supernatants were tested for presence of lactoferrin. In addition, PMNs were stimulated for 15 minutes with PMA or with FMLP in the presence of cytochalasin B. The different stimuli were tested in the absence (▪) or presence (░) of anti–Mac-1 mAb 44a. Results are expressed as mean ± SEM (n = 3). (B) Mouse PMNs (Tg Mac-1+/− and Tg Mac-1−/−, n = 3) were incubated on BSA- (control) or IgA-coated surfaces. Cells were detached after 30 minutes and homogenized. Sucrose gradient ultracentrifugation separated cytoplasm (C), plasma membrane (P), specific granule (S), and azurophilic granule (A) fractions. Translocation of p22 from specific granules to the plasma membrane was detected by Western blotting using α-mouse p22-phox antiserum. PMA-stimulated mouse PMNs served as positive control.
Next, respiratory burst activity of human and mouse PMNs bound to Ab-coated tumor targets was analyzed. Oxygen radical production was detected in time, using 2 types of substrates. Luminol, which is able to pass cell membranes, detects total (intracellular and extracellular) PMN oxygen radical production. On the contrary, isoluminol is membrane impermeable and detects radicals outside membrane-enclosed compartments only.44 PMA-stimulated Mac-1–expressing and Mac-1–deficient PMNs exhibited similar respiratory burst activity (data not shown). Mac-1+/− and Mac-1−/− Tg mouse PMNs adherent to BsAb [A77 × 520C9]-coated tumor cells initiated fast respiratory burst activities (within 10 minutes) detected with luminol, in contrast to Ntg Mac-1+/− PMNs (Figure 6A). PMNs incubated with uncoated tumor cells did not produce respiratory burst activity (data not shown). However, when using isoluminol, oxygen radical production by Mac-1−/− Tg PMNs was detectable outside membrane-enclosed compartments in response to Ab-coated tumor targets, in contrast to Mac-1–expressing Tg PMNs (Figure 6B). Respiratory burst activity of human PMNs adherent to Ab-coated tumor cells was detectable only by using luminol as substrate, similar to Mac-1–expressing mouse PMNs (data not shown).
Respiratory burst activity of Mac-1−/−PMNs is detectable outside membrane-enclosed compartments.
Mouse PMNs (Ntg Mac-1+/− [■], Tg Mac-1+/−[●], and Tg Mac-1−/− [○]) were incubated with BsAb-[A77 × 520C9]–coated SK-BR-3 cells, (♦) represents blanco (no cells). Oxygen radical production was measured in time by chemiluminescence using either membrane-permeable luminol (A) or impermeable isoluminol (B) as a substrate. Experiments were repeated 3 times, yielding similar results.
Respiratory burst activity of Mac-1−/−PMNs is detectable outside membrane-enclosed compartments.
Mouse PMNs (Ntg Mac-1+/− [■], Tg Mac-1+/−[●], and Tg Mac-1−/− [○]) were incubated with BsAb-[A77 × 520C9]–coated SK-BR-3 cells, (♦) represents blanco (no cells). Oxygen radical production was measured in time by chemiluminescence using either membrane-permeable luminol (A) or impermeable isoluminol (B) as a substrate. Experiments were repeated 3 times, yielding similar results.
Taken together, we observed effective mobilization of specific granules and respiratory burst activity of Mac-1–deficient PMNs stimulated by Ab-coated targets. Strikingly, however, oxygen radical production by Mac-1−/− PMNs was detectable outside membrane-enclosed compartments, in contrast to Mac-1–expressing PMNs.
Immunologic synapse formation between PMNs and tumor cells
Because of differences in spreading and respiratory burst activity by Mac-1–deficient PMNs, we hypothesized immunologic synapse formation between Mac-1−/− PMNs and tumor targets to be defective. Biotinylated tumor cells incubated with PMNs (Tg Mac-1+/−, Tg Mac-1−/−, and Ntg Mac-1+/−) in the absence or presence of FcαRI-directed BsAb [A77 × 520C9] were stained with streptavidin-FITC. Binding sites between PMNs and tumor cells were analyzed for the presence of tumor cell membrane FITC staining by “sectioning” at all levels through whole cells by confocal microscopy. Quantification of absence (closed synapses) and presence (open synapses) of membrane FITC staining showed Mac-1−/− PMNs to exhibit significantly higher numbers of open immunologic synapses than Mac-1+/− PMNs (Figure7A). Ntg Mac-1+/− PMNs did not adhere to Ab-coated tumor cells nor did Tg (Mac-1+/−and Mac-1−/−) PMNs bind tumor cells in the absence of Ab, which was in all cases revealed by continuous membrane FITC staining (data not shown).
Impaired immunologic synapse formation between Mac-1−/− PMNs and BsAb-coated tumor cells.
(A) SK-BR-3 cells were biotinylated and incubated with Tg mouse PMNs (Mac-1+/− and Mac-1−/−) in the presence of BsAb [A77 × 520C9]. On fixation after 30 minutes, cells were stained with streptavidin-FITC. Immunologic synapses were analyzed by confocal microscopy and were quantified by scanning cells through thex/y direction for the presence or absence of membrane-FITC staining at interaction sites. At least 50 PMNs adhered to SK-BR-3 cells were analyzed randomly per sample. Results are expressed as the percentage of open immunologic synapses (mean ± SEM [n = 4]), (*P < .005). (B) Electron micrographs of Tg Mac-1+/− PMNs (upper) and Tg Mac-1−/− PMNs (lower) adhered to BsAb-coated tumor cells. Original magnifications × 9000.
Impaired immunologic synapse formation between Mac-1−/− PMNs and BsAb-coated tumor cells.
(A) SK-BR-3 cells were biotinylated and incubated with Tg mouse PMNs (Mac-1+/− and Mac-1−/−) in the presence of BsAb [A77 × 520C9]. On fixation after 30 minutes, cells were stained with streptavidin-FITC. Immunologic synapses were analyzed by confocal microscopy and were quantified by scanning cells through thex/y direction for the presence or absence of membrane-FITC staining at interaction sites. At least 50 PMNs adhered to SK-BR-3 cells were analyzed randomly per sample. Results are expressed as the percentage of open immunologic synapses (mean ± SEM [n = 4]), (*P < .005). (B) Electron micrographs of Tg Mac-1+/− PMNs (upper) and Tg Mac-1−/− PMNs (lower) adhered to BsAb-coated tumor cells. Original magnifications × 9000.
These data were further supported by electron microscopic analysis of PMN–tumor cell interactions. Both Tg Mac-1+/− and Tg Mac-1−/− PMNs adhered to BsAb-coated tumor cells. However, Mac-1–expressing PMNs made very intimate contacts with the tumor targets, whereas Mac-1−/− PMNs formed pseudopod contacts only (Figure 7B). Ntg Mac-1+/− PMNs did not bind BsAb-coated tumor cells (data not shown). These results indicate Mac-1 to be required for the formation of intact immunologic synapses.
Discussion
Earlier studies documented Mac-1 to augment various FcR functions in myeloid cells. In the present work, we established a crucial role for Mac-1 in PMN-mediated ADCC. Mac-1 was found to be essential for PMN spreading and the formation of intact intercellular synapses. We propose these defective cytoskeleton-mediated processes to be responsible for defective FcR-mediated cytotoxicity by Mac-1–deficient PMNs.
Various studies documented involvement of Mac-1 in phagocytosis of Ab-coated targets.26,46-48 It is well known that antibody and complement can cooperate in the opsonization of pathogens for phagocytosis. Complement-independent Mac-1 cooperation with FcγR has also been documented in phagocytosis.47,49 Transfectant studies showed Mac-1 to mediate IgG-dependent uptake via the glycosyl-phosphatidylinositol–linked FcγRIIIb and to restore uptake by FcγRIIa tail-minus mutants.27,29 In particular, an immobile population of Mac-1, which is linked to the cytoskeleton, has been implicated in phagocytosis.47,49 In the present work, we observe that Mac-1 is not critical for FcR-mediated phagocytosis ofC albicans. This finding was not restricted to one particular target; we found effective uptake of Ab-opsonized bacteria (such as Bordetella pertussis and Escherichia coli; data not shown) by Mac-1–deficient PMNs as well. Furthermore, Mac-1−/− PMNs showed microbicidal activity on FcR-mediated phagocytosis. Although we found Mac-1 not to be crucial for FcR-mediated uptake, Mac-1 may still enhance Ab-mediated phagocytosis under suboptimal conditions (ie, low levels of opsonization). Alternatively, Mac-1 is suggested to be involved in the amplification of Ab-mediated phagocytosis in response to (cytokine) stimulation.32
A number of earlier studies documented Mac-1 to be involved in FcR-mediated extracellular cytotoxicity.33-38 Furthermore, Mac-1 was documented to be critical in FcγR-dependent glomerulonephritis.50 Our present data confirm an absolute requirement of Mac-1 for FcγR- and FcαR-mediated tumor cytotoxicity by both mouse and human PMNs. Anti–Mac-1 mAb 44a and M1/70 both recognize the C3bi-binding domain (I domain), whereas NADG interacts with the Mac-1 lectin-binding domain. Our blocking studies indicate both Mac-1 domains to be involved in PMN ADCC. Glycosyl-phosphatidylinositol–linked molecules, including FcγRIIIb, were found to interact with the lectin-binding domain of Mac-1 and were suggested to play a role in PMN ADCC.35,38 Moreover, Mac-1 was primed for cytotoxicity on lectin-binding domain engagement.51 FcγRIIIb has been described to “cocap” with Mac-1 and to cooperate in PMN respiratory burst activity.28,52,53 This cocapping was inhibitable by sugars like D-mannose and NADG.51 These studies point to possible physical interactions between Mac-1 and FcRs during ADCC. Mac-1 is linked to the actin cytoskeleton, which is proposed to act as a “platform” to bring signal transduction proteins in close proximity to various surface receptors (including integrins).20,21,54-56 We observed partial colocalization of Mac-1 with FcαRI on human PMNs on FcαRI cross-linking, which may represent the immobile (cytoskeleton-associated) population of Mac-1 (data not shown). All our experiments were performed in the absence of serum complement to exclude complement-mediated cytotoxicity. Mac-1 on human PMNs is capable of clustering in the absence of ligand.26 57 From our data, we can, however, not exclude the possibility that Mac-1 binds to some type of ligand on tumor targets.
To investigate the underlying mechanism of the requirement for Mac-1 in PMN ADCC, we first analyzed the interaction between PMN and Ab-coated targets. Mac-1 proved not to be crucial for mere FcR-mediated binding, although AIs of Mac-1−/− PMNs were lower than AIs of Mac-1+/− PMNs. The decreased adhesion of Mac-1−/− PMNs to Ab-coated targets was unlikely to be fully responsible for a total absence of cytotoxicity. We, therefore, analyzed the interaction of PMNs with Ab-coated tumor cells in more detail, revealing an essential role of Mac-1 in PMNs spreading on Ab-coated targets. The β2 integrin importance in adhesion and spreading onto integrin ligands (such as extracellular matrix proteins) is well established.31,58 However, β2 integrins may also be involved in spreading mediated via other receptors (such as FcRs and T-cell receptor/CD3 complexes). Our findings correspond with impaired spreading and F-actin redistribution of Mac-1−/− PMNs onto immune complex–coated glass.50 The β2integrin–induced spreading is paralleled by PMN respiratory burst activity,59-61 although some controversy in literature on the role of Mac-1 in respiratory burst activity exists.58,62-65 Because oxygen radical production and degranulation are both important for PMN cytotoxicity, we analyzed the role of Mac-1 in PMN activation. Unexpectedly, we observed Mac-1−/− PMNs to be capable of oxygen radical production and specific granule mobilization on binding Ab-coated targets, with similar activity as Mac-1+/− PMNs. Blocking studies performed with human PMNs supported the findings with mouse PMNs; blocking Mac-1 impaired ADCC but did not affect PMN degranulation. Apparently, engagement of FcRs in the absence of Mac-1 is sufficient to initiate downstream signaling, culminating in degranulation and respiratory burst. Signal transduction pathways that lead to granule exocytosis and respiratory burst activity are incompletely characterized, but G proteins and Ca++ control these processes.66 We observed similar rises in intracellular free Ca++ levels in Mac-1–deficient PMNs and Mac-1–expressing PMNs on FcR cross-linking (data not shown).
The apparent discrepancy between impaired ADCC and intact activation of Mac-1–deficient PMNs was resolved by investigating the initiation of respiratory burst activity in more detail. Although total oxygen radical production by Mac-1−/− and Mac-1+/−PMNs adherent to Ab-coated tumor cells was similar, respiratory burst activity of Mac-1−/− PMNs was detectableoutside PMN–tumor cell complexes. Normally, closed compartments are formed between phagocytes and Ab-coated targets, thereby creating a microenvironment.67 Our findings can be explained by incomplete intercellular interactions between Mac-1–deficient PMNs and Ab-coated tumor cells. Indeed, studying immunologic synapses between Ab-coated tumor cells and Mac-1−/− or Mac-1+/− PMNs by both confocal and electron microscopy, revealed Mac-1 to be required for the formation of intact immunologic synapses. Our findings are consistent with a previous study, which documents a role for Mac-1 in localization of ICAM-1–induced respiratory burst activity to membrane-enclosed compartments.65 Open intercellular clefts, in addition to resulting in the inappropriate delivery of toxic agents (“leakage”), may also prevent the pH in the cleft to decrease, which is essential for optimal activity of toxic enzymes. Although the signaling pathway(s) stimulated by Mac-1 leading to immunologic synapse formation remains to be elucidated, Mac-1 linkage to the actin cytoskeleton is likely to play an important role in this process. A critical role of the actin cytoskeleton was recently described for immunologic synapse formation followed by the recruitment of signaling complexes in T cells.68,69 We did not observe the percentage of open immunologic synapses between Ab-coated tumor cells and Mac-1−/− PMNs to be 100% (Figure 7A). LFA-1 (and to a lesser extent CR4) or other actin cytoskeleton–linked proteins might partly substitute for Mac-1 in cleft formation, although this was clearly insufficient for potent PMN cytotoxicity. We hypothesize the impaired spreading and cleft formation of Mac-1−/− PMNs to underlie defective ADCC. In addition, we are currently testing whether Mac-1−/− PMNs release their toxic contents in an uncontrolled manner (ie, to all plasma membrane sites instead of “local release” at contact sites). The critical role of Mac-1 seemed to be restricted to PMNs: Mac-1–deficient macrophages were fully capable of mediating ADCC (data not shown). This may well be attributable to a different mechanism used by macrophages and PMNs in the lysis of tumor targets. PMNs mediate cytotoxicity of large targets, mainly extracellularly.59,70 Macrophages, however, are able to phagocytose tumor targets.36,71 72 This corresponds with the intact phagocytosis of Ab-coated targets by Mac-1–deficient cells observed in our study.
Antibody therapies have been documented to be effective in the eradication of different human tumors.2,3 The interaction of therapeutic antibodies with FcRs has been reported crucial for therapeutic efficacy.6 PMNs, having potent cytolytic capacity, gained recent interest as effector cells in Ab-mediated antitumor immunity.14,39 73 Analyzing antibody treatment of tumor-bearing Mac-1+/− and Mac-1−/− mice can further unravel the role of Mac-1 in Ab treatment of malignancies in vivo. A better understanding of Ab-mediated cytotoxicity toward tumor cells will be of importance for novel immunotherapeutic approaches for cancer.
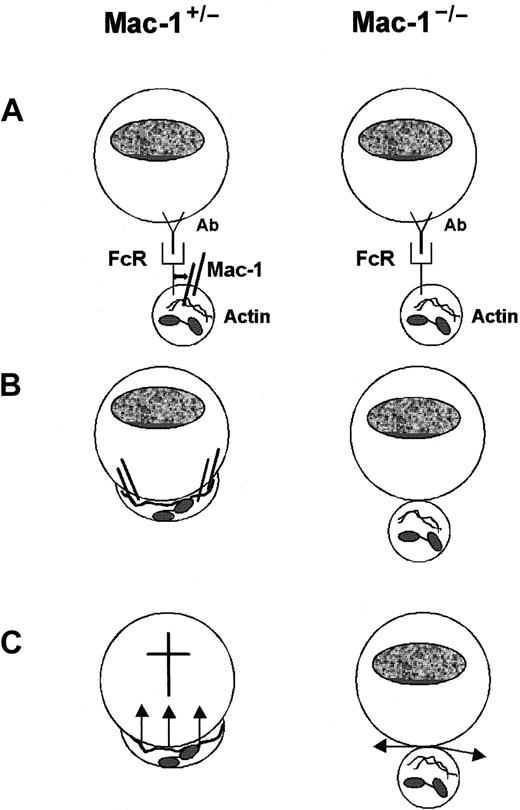
In summary, we defined a crucial role for Mac-1 in FcR-mediated extracellular PMN cytotoxicity and unraveled the underlying mechanism of Mac-1 involvement in ADCC (Figure 8). Mac-1 is not essential for the mere binding of PMNs to Ab-coated targets and subsequent PMN activation (ie, degranulation and respiratory burst activity). However, PMN spreading on Ab-coated targets is fully dependent on Mac-1. Moreover, Mac-1 is required for intact immunologic synapse formation between PMNs and Ab-coated tumor cells. We postulate these impaired cytoskeleton-mediated processes to be responsible for absent tumor cytolysis.
Schematic model for the role of Mac-1 in FcR-mediated PMN cytotoxicity.
(A) Recognition and binding of PMNs (Mac-1+/− and Mac-1−/−) to Ab-coated tumor targets initiated via FcRs. (B) PMN spreading onto tumor targets proceeded by immunologic synapse formation is dependent on Mac-1. This is accompanied by reorganization of the actin cytoskeleton. (C) Both Mac-1+/− and Mac-1−/− PMNs become activated, resulting in mobilization of specific granules and respiratory burst activity. Mac-1−/− PMNs are, however, unable to release toxic agents in close proximity of tumor targets, resulting in defective cytolysis.
Schematic model for the role of Mac-1 in FcR-mediated PMN cytotoxicity.
(A) Recognition and binding of PMNs (Mac-1+/− and Mac-1−/−) to Ab-coated tumor targets initiated via FcRs. (B) PMN spreading onto tumor targets proceeded by immunologic synapse formation is dependent on Mac-1. This is accompanied by reorganization of the actin cytoskeleton. (C) Both Mac-1+/− and Mac-1−/− PMNs become activated, resulting in mobilization of specific granules and respiratory burst activity. Mac-1−/− PMNs are, however, unable to release toxic agents in close proximity of tumor targets, resulting in defective cytolysis.
The authors thank Toon Hesp, Els Dorresteijn, Herma Boersma, and Anja van de Sar for excellent assistance with the animal experiments; Dr M. C. Dinauer for kindly providing α-mouse p22-phox antiserum; and Dr W-L. van der Pol for critically reading the manuscript.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Jan G. J. van de Winkel, Immunotherapy Laboratory, University Medical Center Utrecht, Rm KC02.085.2, Lundlaan 6, 3584 EA, Utrecht, The Netherlands; e-mail:j.vandewinkel@lab.azu.nl.

![Fig. 1. Mac-1–deficient PMNs efficiently phagocytose and kill Ab-opsonized. / C albicans. Tg mouse PMNs (Mac-1+/− and Mac-1−/−) were incubated with C albicansin the presence of FcαRI-directed BsAb. (A) Phagocytosis was analyzed by light microscopy after 30 minutes. (B) Flow cytometric analysis of FcαRI-mediated C albicans uptake by Tg Mac-1+/− (▪) and Tg Mac-1−/− PMNs (░). Results are expressed as percentage of PMNs that mediated phagocytosis (mean ± SEM [n = 4]). (C) FcαRI-mediated killing of C albicans by Tg PMNs (Mac-1+/− and Mac-1−/−) after 2 hours. Results are mean ± SEM based on 4 individual experiments. Bars indicate 10 μm.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/97/8/10.1182_blood.v97.8.2478/5/m_h80810929001.jpeg?Expires=1765889083&Signature=sMMOy1DpzvEJFTX71o1lsci0j4Q~gMEdtI-RPMS4IozlfBnzjdQRbVcAeAzuPJxa9m~GgigvsX3ZtN464M~jDUDm0Xjphri6fcjYQwiDsBqCBM1nMrQdIqnaCM8bZaBIByu3QfQngXznK65tLpmLFln0NEif5lmILd1CCYtn~skMTDK3MdBT3dyHsyrb5tmy3qJHz0SCh45EbXpGjz6zyTIOsyRrKon-qXv2r9mq9g-CK0Lzkh4ZouExth8ctJ3Qo8-K6xuZjE~LBPkbdQMnZBsBGscLhmhWTa~R-mf3zA0c11akguPjVrtOsQ6pTsuYu8Kj7gsAjaJ-BxuG1kKN1Q__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 2. Mac-1 is crucial for FcR-mediated PMN cytotoxicity of tumor targets. / (A) Lysis of human breast carcinoma (SK-BR-3) cells mediated by Tg Mac-1+/− PMNs (▪) and Tg Mac-1−/− PMNs (░) in the absence (control) or presence of FcαRI-directed BsAb [A77 × 520C9]. Cytotoxicity was analyzed after 4 hours at different E/T ratios (20:1 and 80:1). Results are presented as mean ± SEM (n = 6), (*P < .0001). (B) FcαRI-mediated cytotoxicity toward SK-BR-3 cells by human PMNs (E/T ratio; 50:1) in the absence (control) or presence of BsAb [A77 × 520C9]. The effect of anti–Mac-1 mAb 44a or M1/70, and NADG on ADCC was analyzed as described in “Materials and methods.” Data are presented as mean ± SEM (n = 3). *Significant difference compared with BsAb alone (P < .002).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/97/8/10.1182_blood.v97.8.2478/5/m_h80810929002.jpeg?Expires=1765889083&Signature=YYsGGx8krYOIQAxjjTDGxgAVsHZz6qCV8QYJk6V3-8unvgd7TPRnqSjZ0cv3j5Mkl4wPph1e3bQNR9jRm0N5RfNEVMcYkUBrVK3ebuA4kjx1IhVsC8pJdfqeVp6rp4GIFRpkmp8~guUm8QGLB90fIYrnlRa8G8XG27Cbv6idy6a~okqb-OE0jg3T3Llj2Tx49cP0YcB6CZSD5RR4qdUxkLnqBUkdyeOdabtFeUR5g0UcIVvP14dLawMPNEUjcFa0HlaRzX9xVJMJNbSJXXHQXwNtiGIZraL1LR2TXP8rBjZpzpo9LEN~Uf4x1Z2veDmwpHt4K5HNobUdeHEdvdLuOg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 3. PMN binding and spreading on Ab-coated targets. / (A) Numbers of Ntg Mac-1+/− PMNs (■), Tg Mac-1+/− PMNs (■), and Tg Mac-1−/− PMNs (░) bound to BsAb [A77 × 520C9]-opsonized SK-BR-3 cells were determined after 30 and 60 minutes. Results represent mean ± SEM from 3 individual experiments. (B) Tg Mac-1+/− PMNs (left) and Tg Mac-1−/− PMNs (right) were plated on IgA-coated glass slides for 30 minutes and stained for actin. Cell morphology was imaged in x/y and x/z directions (inserts), using confocal microscopy. Bars indicate 15 μm. Experiments were performed at least 4 times, yielding similar results.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/97/8/10.1182_blood.v97.8.2478/5/m_h80810929003.jpeg?Expires=1765889083&Signature=dMpgXZBsXG~ECqmi0t6ONlySpL4IoH7HhnLKq1IfIbVE2hGWiAE2Dw8f~j-e-lyoe33U2VZ-lngJBiSbuUvEl2ntI-X-ACSlyibR95Mvf76Hg0Br0zcBHwt0N5duJ~9oTB1SkztzQ6PL0FBUx9Kvt618gPdZ4U2einI5Y3B2ZKu21cw7qlux7Y86zNEM09bEjWLqappzYzxbD4695gXp5IusfYC-RlQKVqnKyAx73we29Ilrzo629i08jZLcptDDor-z~Z082ktecjlemaSgkO9PKBA75Y468DYQjlhHoBmNjTbA0VH8KLSKw6hOYzBpAKvJMk8ZJDFHlLNJ5RqdYQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 6. Respiratory burst activity of Mac-1−/−PMNs is detectable outside membrane-enclosed compartments. / Mouse PMNs (Ntg Mac-1+/− [■], Tg Mac-1+/−[●], and Tg Mac-1−/− [○]) were incubated with BsAb-[A77 × 520C9]–coated SK-BR-3 cells, (♦) represents blanco (no cells). Oxygen radical production was measured in time by chemiluminescence using either membrane-permeable luminol (A) or impermeable isoluminol (B) as a substrate. Experiments were repeated 3 times, yielding similar results.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/97/8/10.1182_blood.v97.8.2478/5/m_h80810929006.jpeg?Expires=1765889083&Signature=IZiz-~9nLmy1rVcFUoYeZr-f-k6ugIMPY7El8VdqSjX9fP3Bb5UpA4a7GsO9ciyvM4hwnuec8DBo00rpf0tu~y2luPsGmZGB7vFHh-2AEU-WNVJ~nPF93bxa84AXqNGvOtQHUJA3vKt8iAkpYH1BI5UUbkJtOGojkEJVOl3JUc5W5WqT9Yg-leKlIuoChqVZSfPgwGdrIakllxYalqHIpMPIXfYO~kikMUt6nUpCzlvrvl7-~-yJD8Q6jnYXGXH67ePmn8u~oloG9KnAYHkhfOtLBjA9kpEVI7iDCVyR5UqYVHKat8H-vNkJpKZk~wBL96oCY98WyOgfrQcNecLO4g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 7. Impaired immunologic synapse formation between Mac-1−/− PMNs and BsAb-coated tumor cells. / (A) SK-BR-3 cells were biotinylated and incubated with Tg mouse PMNs (Mac-1+/− and Mac-1−/−) in the presence of BsAb [A77 × 520C9]. On fixation after 30 minutes, cells were stained with streptavidin-FITC. Immunologic synapses were analyzed by confocal microscopy and were quantified by scanning cells through thex/y direction for the presence or absence of membrane-FITC staining at interaction sites. At least 50 PMNs adhered to SK-BR-3 cells were analyzed randomly per sample. Results are expressed as the percentage of open immunologic synapses (mean ± SEM [n = 4]), (*P < .005). (B) Electron micrographs of Tg Mac-1+/− PMNs (upper) and Tg Mac-1−/− PMNs (lower) adhered to BsAb-coated tumor cells. Original magnifications × 9000.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/97/8/10.1182_blood.v97.8.2478/5/m_h80810929007.jpeg?Expires=1765889083&Signature=IFMtIMLlEPX3m84nQ7oBkAc8Il-wk80HD-Wfxxn9WA-E19gepOWXnnJf-eca5yuEGvWZBSMWbOF-lhEfu24m1GlIvScMYq-gzQHfFksOccFXUzkBRgh87ZSW5UOCXi5Jmhw1XED2n472qNYIfUgWa-D5i7UuCgK1lhRfvgOqfOA3RmyrO3YI0nTyNBwk5CIp~AKEwktu6G6z53wc2wRK6wAZ~o0weIzNKz00fOBd9S2vaeWU6FowxbNHLa4sgnyYslzJJM6xBXwDK6Eor5UDZcvq0giSYRhoNRyFHUeZpdiFCi049nQSGFOS1salIFzhHAohXppe0AlxMXPyws6GHw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal