Abstract
Echicetin, a heterodimeric snake C-type lectin from Echis carinatus, is known to bind specifically to platelet glycoprotein (GP)Ib. We now show that, in addition, it agglutinates platelets in plasma and induces platelet signal transduction. The agglutination is caused by binding to a specific protein in plasma. The protein was isolated from plasma and shown to cause platelet agglutination when added to washed platelets in the presence of echicetin. It was identified as immunoglogulin Mκ (IgMκ) by peptide sequencing and dot blotting with specific heavy and light chain anti-immunoglobulin reagents. Platelet agglutination by clustering echicetin with IgMκ induced P-selectin expression and activation of GPIIb/IIIa as well as tyrosine phosphorylation of several signal transduction molecules, including p53/56LYN, p64, p72SYK, p70 to p90, and p120. However, neither ethylenediaminetetraacetic acid nor specific inhibition of GPIIb/IIIa affected platelet agglutination or activation by echicetin. Platelet agglutination and induction of signal transduction could also be produced by cross-linking biotinylated echicetin with avidin. These data indicate that clustering of GPIb alone is sufficient to activate platelets. In vivo, echicetin probably activates platelets rather than inhibits platelet activation, as previously proposed, accounting for the observed induction of thrombocytopenia.
Introduction
Snakes produce venoms containing a wide variety of components that kill or weaken their prey. Whereas venoms from some snake families contain mostly neurotoxic proteins, others such as theViperidae and Crotalidae genera are mainly hemorrhagic. Among the protein families that have been shown to have hemorrhagic effects are the snake C-type (calcium-dependent) lectins. This family is named after the type of folding that occurs in classic C-type lectins such as mannose-binding protein1,2and the selectins.3 Many snake C-type lectins have now been characterized with effects on either coagulation factors or platelets. Those affecting platelets either inhibit or activate them by binding to specific receptors like glycoprotein (GP)Ib, α2β1, and GPVI. Those that act via GPIb to agglutinate platelets include alboaggregins,4-6flavocetin-A and -B,7 and mamushigin.8 Most of the inhibitory C-type lectins described so far bind to GPIb. These include echicetin,9 jararaca GPIb-binding protein,10,11 tokaracetin,12 CHH-A and -B,4 and agkicetin.13 Echicetin, a heterodimeric snake C-type lectin from Echis carinatus, has been shown by several authors to bind specifically to platelet GPIb and to block platelet interactions with von Willebrand factor (vWf)9 and with thrombin.14 There has been considerable interest in using C-type lectins, such as echicetin as antithrombotics, in blocking the interaction between vWf and platelets. However, when echicetin or similar snake C-type lectins have been injected into small animals to study their effects in vivo, induction of thrombocytopenia has often been reported.9 15Generally, the platelet count dropped to 20% to 30% of the control value and then gradually recovered over several hours. This phenomenon has remained unexplained. In addition, it is far from clear why a snake venom component blocking GPIb as single mode of action would have evolved. GPIb is one of the most common platelet receptors, with at least 25 000 copies per platelet (and more likely 50 000 based on monomeric snake C-type lectin binding), and needs to be inhibited to at least 80% to effect platelet function. The number of snake venom component molecules required to inhibit 80% of GPIb on all platelets in the circulation of even a small animal is quite considerable and would be an inefficient strategy for producing bleeding in the prey. Therefore, it seemed much more likely that this category of C-type lectins causes platelet activation by additional effects. In this paper we report that echicetin induces platelet agglutination in platelet-rich plasma (PRP) via a multimeric, plasma protein present in microgram amounts per milliliter. This protein was isolated and characterized, and its effects together with echicetin on platelets were investigated in detail.
Materials and methods
Materials
Lyophilized Echis carinatus sochureki andTrimeresurus albolabris venoms were from Latoxan (Rosans, France), protein A–Sepharose, bovine serum albumin, ristocetin, peroxidase-conjugated rabbit antimouse antibodies, biotinamidocaproate N-hydroxysuccinimide ester, bovine thrombin, and fluorescein isothiocyanate (FITC) were from Sigma (Buchs, Switzerland). N-ethylmaleimide and N-acetylglucosamine were from Fluka (Buchs, Switzerland). Octanyl N-methylglucamide was from Oxyl Chemie (Bobingen, Germany). Human fibrinogen (vWf and plasminogen-free) was from Enzyme Research Labs (South Bend, IN). Fibrinogen was conjugated to FITC as described earlier.16 Avidin was from Imtec (Moscow, Russia). FITC-coupled chicken anti–P-selectin (CD62P) and FITC-coupled chicken immunoglobulin Y (IgY) as control were from WAK-Chemie Medical (Bad Soden, Germany). The peptide GPRP (Gly-Pro-Arg-Pro) was from Calbiochem-Novabiochem (Bad Soden, Germany). Sephadex G-10 and Sepharose 4B were from Pharmacia Fine Chemicals (Uppsala, Sweden). Autoradiography films were from Fujifilm (Dielsdorf, Switzerland). Antiphosphotyrosine monoclonal antibody (4G10) and anti–phosphatidylinositol-3 kinase (PI-3K; 85-kd subunit) monoclonal antibody were from Upstate Biotechnology (Lake Placid, NY). Anti-p72SYK (4D10) monoclonal antibody, anti-pp125FAK (A-17) polyclonal antibodies, and anti-p53/56LYN rabbit polyclonal antibodies were from Santa Cruz Biotechnology (Santa Cruz, CA). The anti-GPIbα monoclonal antibody (SZ2) was from Coulter-Immunotech Diagnostics (Hamburg, Germany), and the monoclonal antibody to the thrombin-binding site on GPIbα, VM16d, was a kind gift from Dr A.V. Mazurov. FITC-labeled anti-CD36 monoclonal antibody (clone FA6.152) was from Immunotech (Marseille, France). The GPIIb-IIIa inhibitor Ro44-9883 and the anti-GPIbα monoclonal antibody, Ib-4, were kind gifts from Dr Beat Steiner, Hoffmann-La Roche (Basel, Switzerland). The adenosine 5′-diphosphate (ADP) receptor inhibitor AR-C66096 was a kind gift from Dr Bob Humphries, AstraZeneca (Loughborough, England). Polyvinylidene fluoride (PVDF) membranes were PolyScreen from DuPont NEN (Zaventem, Belgium). Alboaggregin A was purified from Trimeresurus albolabris venom by a method similar to that of Peng et al.17
Purification of echicetin
Lyophilized Echis carinatus sochureki venom was dissolved at 50 mg/5 mL in 50 mM sodium acetate, pH 5.0 (buffer A). Insoluble components were removed by centrifugation, and supernatant was loaded on a Fractogel EMD SO3-650(S) column (10 × 150 mm, Merck, Darmstadt, Germany) equilibrated with buffer A. Elution of echicetin was performed by a 0 to 1 M gradient of NaCl in buffer A. Fractions (5 mL) were collected at 1 mL/min flow rate. Activity of echicetin was determined by its ability to block alboaggregin A–induced agglutination of fixed platelets. The fractions containing echicetin were pooled and concentrated by SpeedVac. Further purification of the fractions containing echicetin was performed using reverse-phase chromatography (wide pore C-4).
Biotinylation of echicetin
Purified echicetin was dialyzed against 10 mM Na phosphate buffer, pH 8.0. Biotinamidocaproate N-hydroxysuccinimide ester in dimethyl sulfoxide (2 mg/mL) was added to echicetin at a molar ratio 2:1. The mixture was incubated at room temperature for 2 hours. Biotin-echicetin conjugate was separated from free biotin by gel filtration on a Sephadex G-10 column.
Protein determination
Protein determination was performed by the bovine serum albumin protein assay (Pierce, Sochochim, Lausanne, Switzerland) with bovine serum albumin as standard.
Sodium dodecyl sulfate–polyacrylamide gel electrophoresis and silver staining
Preparation of washed platelets and platelet aggregation
Human platelets were isolated from buffy coats less than 20 hours after blood collection obtained from the Central Laboratory of the Swiss Red Cross Blood Transfusion Service. To one buffy coat was added 30 mL of 100 mM citrate, pH 6.5. PRP and the platelet pellet were isolated by successive centrifugation steps. Platelets were resuspended in 113 mM NaCl, 4.3 mM K2HPO4, 4.3 mM Na2HPO4, 24.4 mM NaH2PO4, and 5.5 mM glucose (pH 6.5) (buffer B) and centrifuged at 250g for 5 minutes. The platelet-rich supernatant was centrifuged at 1000g for 10 minutes, and platelets were washed with buffer B once more. Washed platelets were resuspended in 20 mM HEPES, 140 mM NaCl, 4 mM KCl, and 5.5 mM glucose (pH 7.4) (buffer C), and the platelet count was adjusted to 5 × 108/mL by dilution with buffer C. Samples were kept at room temperature until used for aggregation studies. Platelet aggregation was monitored by light transmission in an aggregometer (Lumitec, France) with continuous stirring at 1100 rpm at 37°C. Platelets were preincubated in buffer C containing 2 mM CaCl2 and 2 mM MgCl2 at 37°C for 2 minutes before starting the measurement by adding the samples for analysis. All experiments were repeated at least 3 times with platelets from different donors.
Platelet biotinylation, Triton X-100 platelet lysate, wheat germ agglutinin affinity chromatography, and echicetin affinity chromatography
Human platelets were isolated from buffy coats as described above but in the presence of 10 μM Iloprost. Washed platelets were diluted with phosphate-buffered saline to 5 × 109/mL and incubated with 10 μg biotinamidocaproate N-hydroxysuccinimide ester for 1 hour at room temperature. Free biotinamidocaproate N-hydroxysuccinimide ester was removed by washing the platelets 3 times with phosphate-buffered saline, pH 6.8. Biotinylated platelets were solubilized in phosphate-buffered saline containing 1.2% Triton X-100, 1 mM phenylmethylsulfonyl fluoride, 100 μM leupeptin, 2 mM N-ethylmaleimide, and 2 mM sodium orthovanadate. After centrifugation (40 000g, 1 hour, 4°C), the supernatant was applied to a column of wheat germ agglutinin–Sepharose 4B equlibrated with 130 mM NaCl, 10 mM Tris-HCl (pH 7.4) (buffer D). The column was washed thoroughly with buffer D containing 0.2% octanoyl-N-methylglucamide (ONMG). The bound material was eluted with 2.5% N-acetylglucosamine in 10 mM Tris, 30 mM NaCl (pH 7.4) (buffer E) containing 0.2% ONMG. Fractions containing eluted membrane glycoproteins were pooled and loaded on the echicetin affinity chromatography column equilibrated with buffer D. The column was washed thoroughly with buffer D containing 0.2% ONMG. The echicetin-Sepharose with bound platelet proteins was boiled for 1 minute with buffer E containing 1% SDS. Eluted proteins were separated by electrophoresis and transferred to PVDF membrane.
Protein sequencing
Proteins were separated by SDS-PAGE and blotted to PVDF membrane. Protein bands were identified by staining parallel lanes, and the corresponding membrane piece was cut out and the protein sequenced on an Applied Biosystems model 477A pulsed liquid-phase protein sequencer with a model 120A online phenylthiohydantoin amino acid analyzer.
Flow cytometry
Samples were analyzed using a Becton Dickinson FACScan flow cytometer (Becton Dickinson, Heidelberg, Germany). Excitation was with an argon laser at 488 nm. The FACScan was used in a standard configuration with a 530 nm bandpass filter. Standard beads containing specific amounts of “mean equivalent soluble fluorescein molecules” were used for calibration. Standard beads or platelets were gated, and data were obtained from fluorescence channels in a logarithmic mode. A total of 5000 events were analyzed. Specific binding of antibodies was calculated by substracting unspecific binding as determined with a FITC-labeled mouse isotype-specific IgG or FITC-labeled chicken IgY. Specific binding of FITC-labeled fibrinogen was calculated by substracting unspecific binding as determined with a 10-fold excess of unlabeled fibrinogen.
P-selectin expression and fibrinogen binding to platelets
Washed platelets were diluted to 5 × 107/mL with HEPES buffer (buffer C). Platelets (100 μL) were activated with echicetin-IgMκ (5 μg/mL echicetin, 1 μg/mL IgMκ) for 5 minutes or thrombin (1 U/mL) in the presence of GPRP (1.25 mM) for 3 minutes and fixed with formaldehyde. After platelets were washed and resuspended in 10 mM Tris-HCl, pH 7.4, buffer, they were incubated with anti-CD62–FITC chicken antibodies (10 μg/mL). After 1 hour, platelets were again washed and analyzed by flow cytometry.
In the presence of GPRP (1.25 mM), washed platelets (100 μL, 5 × 107/mL in buffer C) were incubated with fibrinogen-FITC (100 μg/mL) for 10 minutes. Platelets were activated with echicetin-IgMκ (5 μg/mL echicetin, 1 μg/mL IgMκ) for 5 minutes or 1 U/mL thrombin for 3 minutes and fixed with formaldehyde. After platelets were washed and resuspended in Tris buffer, they were analyzed by flow cytometry.
Immunoprecipitation
For immunoprecipitation, aliquots (700 μL, 5 × 108/mL) of control, resting platelets as well as activated platelets were solubilized in phosphate-buffered saline containing 1.2% Triton X-100 with 1 mM phenylmethylsulfonyl fluoride, 5 mM ethylenediaminetetraacetic acid (EDTA), 2 mM N-ethylmaleimide, 2 mM benzamidine, and 2 mM sodium orthovanadate. After centrifugation, platelet lysates precleared with protein A–Sepharose were stirred for 2 hours with specific antibodies before the addition of 20 μL protein A–Sepharose followed by 6 to 8 hours of incubation.
Purification of echicetin-binding protein from blood plasma
Human blood plasma was depleted in fibrinogen, dialyzed against 50 mM Tris-HCl, pH 7.5, and loaded on a Fractogel EMD TMAE-650(S) column (10 × 150 mm, Merck) equilibrated with the same buffer. Echicetin-binding protein was eluted by a gradient of NaCl (0-1 mM in Tris buffer). Fractions (5 mL) were collected at 1 mL/min flow rate. Fractions containing echicetin-binding protein activity were pooled and purified further by affinity chromatography on an echicetin–Sepharose 4B column. Echicetin-binding protein was eluted from the echicetin-Sepharose with 100 mM citrate buffer, pH 2.5.
Results
Echicetin binds specifically to GPIb on the platelet surface
To establish which platelet receptor binds to echicetin, platelet surface proteins were labeled with biotin. A fraction enriched in platelet glycoproteins was prepared by affinity chromatography on a wheat germ agglutinin–Sepharose 4B column. This fraction was used for affinity chromatography on echicetin–Sepharose 4B or on Sepharose 4B as a control. The proteins bound to echicetin or Sepharose 4B were eluted and separated by gel electrophoresis. Proteins were transferred to a PVDF membrane, and the membrane was treated with anti-GPIb mAb (Ib-4), peroxidase-coupled goat antimouse second antibodies, and bound antibodies were detected by chemiluminescence. The membrane was restained with avidin-phosphatase conjugate to identify biotinylated platelet membrane proteins, which were bound to echicetin or Sepharose 4B. The results of this experiment are shown in Figure1. Echicetin–Sepharose 4B bound only GPIb and some of its proteolytic degradation products among the platelet membrane proteins. Sepharose 4B alone did not bind any membrane proteins from platelet lysate.
Binding of platelet proteins to echicetin-Sepharose.
Platelet surface proteins were labeled with biotin, platelets were lysed by Triton X-100, and the lysate was added to echicetin-Sepharose. The proteins eluted from the echicetin-Sepharose were separated by SDS-PAGE, transferred to PVDF membrane, and detected with avidin-phosphatase conjugate (lanes 1-3) or with anti-GPIb monoclonal antibody (lanes 4-6). Lanes 1 and 4: platelet lysates. Lanes 2 and 5: eluate from Sepharose (negative control). Lanes 3 and 6: eluate from echicetin-Sepharose. Specific bands detected by anti-GPIb monoclonal antibody (Ib-4) are indicated by (nonreduced [NR]) GPIb and glycocalicin (GC; the extracellular proteolytic fragment of GPIbα) and (reduced [R]) GPIbα and macroglycopeptide (MG; the mucinlike proteolytic fragment of GPIbα). Under reducing conditions GC and GPIbα comigrated.
Binding of platelet proteins to echicetin-Sepharose.
Platelet surface proteins were labeled with biotin, platelets were lysed by Triton X-100, and the lysate was added to echicetin-Sepharose. The proteins eluted from the echicetin-Sepharose were separated by SDS-PAGE, transferred to PVDF membrane, and detected with avidin-phosphatase conjugate (lanes 1-3) or with anti-GPIb monoclonal antibody (lanes 4-6). Lanes 1 and 4: platelet lysates. Lanes 2 and 5: eluate from Sepharose (negative control). Lanes 3 and 6: eluate from echicetin-Sepharose. Specific bands detected by anti-GPIb monoclonal antibody (Ib-4) are indicated by (nonreduced [NR]) GPIb and glycocalicin (GC; the extracellular proteolytic fragment of GPIbα) and (reduced [R]) GPIbα and macroglycopeptide (MG; the mucinlike proteolytic fragment of GPIbα). Under reducing conditions GC and GPIbα comigrated.
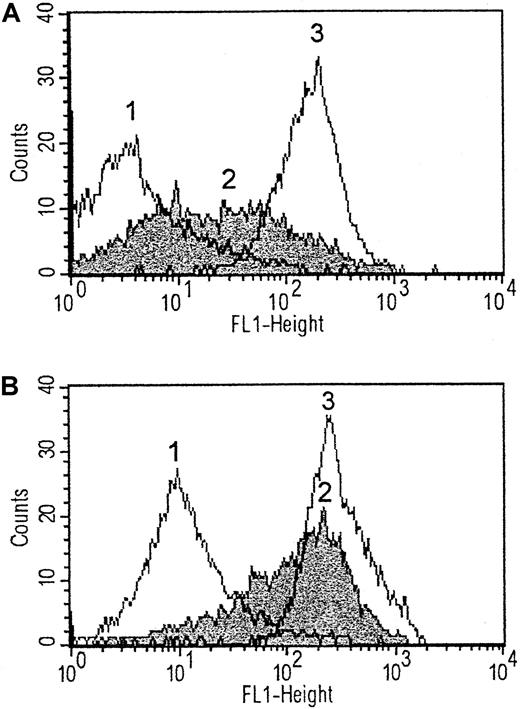
Echicetin-induced agglutination of platelets in plasma
High-purity echicetin isolated from Echis carinatusvenom was tested for its ability to inhibit platelet aggregation induced by vWf and alboaggregin A as well as by low doses of thrombin. This echicetin preparation had the same properties as those previously described.9 Echicetin (20 μg/mL) completely inhibited aggregation of washed platelets induced by vWf (5 μg/mL) plus ristocetin (0.5 mg/mL) or by alboaggregin A (0.1 μg/mL) (Figure2 A,B).
Inhibition of aggregation of washed platelets induced by vWF or alboaggregin A.
Upper curves: washed human platelets (500 μL, 5 × 108/mL) were stirred at 1100 rpm at 37°C and aggregation was induced by 5 μg/mL human vWF plus 0.5 mg/mL ristocetin (A) or by 0.1 μg/mL alboaggregin A (B). Lower curves: washed human platelets (500 μL, 5 × 108/mL) were stirred in the presence of 20 μg/mL echicetin, and the same agonists (A and B) were added after 1 minute.
Inhibition of aggregation of washed platelets induced by vWF or alboaggregin A.
Upper curves: washed human platelets (500 μL, 5 × 108/mL) were stirred at 1100 rpm at 37°C and aggregation was induced by 5 μg/mL human vWF plus 0.5 mg/mL ristocetin (A) or by 0.1 μg/mL alboaggregin A (B). Lower curves: washed human platelets (500 μL, 5 × 108/mL) were stirred in the presence of 20 μg/mL echicetin, and the same agonists (A and B) were added after 1 minute.
It was previously reported9 that intravenous injection of echicetin in small animals to test for antihemostatic or antithrombotic effects can provoke thrombocytopenia. Therefore, we investigated the action of echicetin on PRP. In contrast to experiments with washed platelets, where no agglutination was seen, in blood plasma echicetin induced platelet agglutination (Figure3). Whether echicetin and an echicetin-binding protein from plasma simply agglutinate platelets or whether, in addition, aggregation occurs by activation of IIb/IIIa receptors and formation of fibrinogen bridges between platelets was not clear. Therefore, a IIb/IIIa inhibitor was used to prevent fibrinogen binding to platelets, but it did not affect platelet agglutination induced by echicetin in plasma (Figure 3).
Echicetin-induced platelet agglutination in blood plasma independent of GPIIb/IIIa.
PRP (500 μL, curve 1) or washed human platelets (500 μL, 5 × 108/mL, curve 3) were stirred at 1100 rpm at 37°C, and echicetin (5 μg) was added to each sample. Curve 2: platelet agglutination in PRP (500 μL) induced by echicetin (5 μg) in the presence of GPIIb/IIIa inhibitor (Ro44-9883, 1 μM/mL).
Echicetin-induced platelet agglutination in blood plasma independent of GPIIb/IIIa.
PRP (500 μL, curve 1) or washed human platelets (500 μL, 5 × 108/mL, curve 3) were stirred at 1100 rpm at 37°C, and echicetin (5 μg) was added to each sample. Curve 2: platelet agglutination in PRP (500 μL) induced by echicetin (5 μg) in the presence of GPIIb/IIIa inhibitor (Ro44-9883, 1 μM/mL).
Echicetin binds specifically to plasma IgM with κ light chain
To identify the plasma component that binds to echicetin, plasma was fractionated by ion-exchange chromatography on a TMAE-Fractogel column followed by affinity chromatography of the active fractions on an echicetin–Sepharose 4B column. Eluates from this column contained a protein that showed a high molecular mass single band on SDS-PAGE under nonreduced conditions and 2 bands with masses of 70 kd and 25 kd under reduced conditions. The N-terminal amino acid sequence of the 70 kd chain was EVQLVESGGXL, which is typical for the variable III domain of the heavy chain of immunoglobulins. This protein was analyzed further by dot blot using specific antiheavy and antilight chain immunoglobulin antibodies. Antibodies to μ heavy chain and κ light chain bound specifically to this protein. Thus, the protein isolated from plasma that specifically binds echicetin is IgM with a κ light chain.
P-selectin expression and fibrinogen binding to platelets activated by echicetin-IgMκ complex
The expression of P-selectin on platelets after 5 minutes of activation by echicetin-IgMκ (5 μg/mL echicetin, 1 μg/mL IgMκ) or thrombin (1 U/mL) (as positive control) was determined by flow cytometry. After activation, platelets were fixed with formaldehyde, washed with Tris buffer, and stained by FITC-labeled anti–P-selectin antibodies (10 μg/mL). The amount of antibodies bound was measured by flow cytometry. Binding of anti–P-selectin antibodies increased strongly on both thrombin and echicetin-IgMκ–activated platelets. The thrombin-activated platelets expressed higher levels of P-selectin than those activated with echicetin-IgMκ (Figure4A).
P-selectin expression and fibrinogen binding to platelets activated by echicetin-IgMκ.
Platelets were activated by echicetin-IgMκ or thrombin as positive control, and binding of anti–P-selectin antibodies (A) or FITC-fibrinogen (B) was measured. In comparison to resting platelets (1), both echicetin-IgMκ–activated (2) and thrombin-activated (3) platelets bind higher amounts of anti P-selectin antibodies and FITC-fibrinogen. All measurements were repeated with platelets from 3 different donors.
P-selectin expression and fibrinogen binding to platelets activated by echicetin-IgMκ.
Platelets were activated by echicetin-IgMκ or thrombin as positive control, and binding of anti–P-selectin antibodies (A) or FITC-fibrinogen (B) was measured. In comparison to resting platelets (1), both echicetin-IgMκ–activated (2) and thrombin-activated (3) platelets bind higher amounts of anti P-selectin antibodies and FITC-fibrinogen. All measurements were repeated with platelets from 3 different donors.
To investigate GPIIb/IIIa activation, FITC-labeled fibrinogen (100 μg/mL) was added to a suspension of 100 μL of washed platelets in the presence of GPRP (1.25 mM), and platelets were activated as described above. After activation, platelets were fixed with formaldehyde and washed with Tris buffer, and the amount of bound FITC-fibrinogen was measured by flow cytometry.
Binding of fibrinogen-FITC increased on the surface of echicetin-IgMκ– or thrombin-activated platelets compared with resting platelets. Again, fibrinogen binding increased more strongly on platelets activated with thrombin than with echicetin-IgMκ (Figure 4B).
The increased fluorescence found with fibrinogen and anti–P-selectin antibodies after activation could possibly have been an artifact due to platelet agglutination by echicetin-IgMκ rather than a real increase in P-selectin expression and GPIIb/IIIa activation. Therefore, as a control, binding of anti-CD36 antibodies (10 μg/mL) to platelets activated under the same conditions was examined. It was shown previously that levels of CD36 do not change appreciably on the surface of activated platelets compared with resting platelets.20We also did not find any marked differences in expression level of CD36 on activated platelets in our experiments (data not shown).
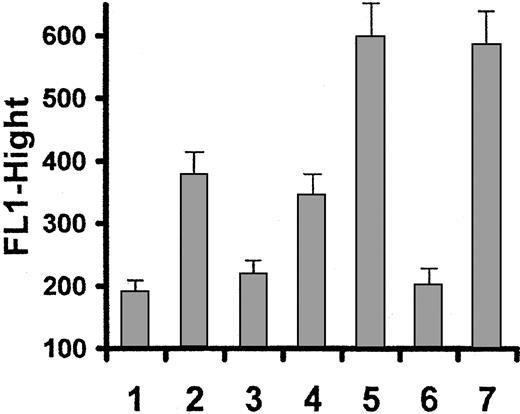
The specific GPIIb/IIIa inhibitor Ro44-9883 21 at 1 μmol/mL and ADP receptor inhibitor AR-C66096 22 at 1 μmol/mL were used to investigate the role of fibrinogen binding to platelets as well as involvement of ADP in GPIIb/IIIa activation in platelets stimulated by echicetin-IgMκ complex. Thrombin-activated platelets were used as a positive control. Ro44-9883 was able to completely inhibit fibrinogen binding to both echicetin-IgMκ– and thrombin-activated platelets (Figure 5). ADP receptor inhibitor slightly decreased binding of fibrinogen to the surface of echicetin-IgMκ–activated platelets but had no effect on binding of fibrinogen to thrombin-activated platelets (Figure 5).
Effect of GPIIb/IIIa inhibitor and ADP receptor inhibitor on fibrinogen binding to platelets.
Platelets pretreated with GPIIb/IIIa inhibitor (Ro44-9883, 1 μM/mL) or ADP receptor inhibitor (AR-C66096, 1 μM/mL) were activated with echicetin-IgMκ or thrombin as positive control, and binding of FITC-labeled fibrinogen was examined compared with resting platelets. All measurements were repeated 3 times with platelets from different donors. Graph shows fibrinogen binding to resting platelets (1), echicetin-IgMκ–activated platelets without inhibitors (2), echicetin-IgMκ–activated platelets with IIb/IIIa inhibitor (3), echicetin-IgMκ–activated platelets with ADP receptor inhibitor (4), thrombin-activated platelets without inhibitors (5), thrombin-activated platelets with IIb/IIIa inhibitor (6), and thrombin-activated platelets with ADP receptor inhibitor (7).
Effect of GPIIb/IIIa inhibitor and ADP receptor inhibitor on fibrinogen binding to platelets.
Platelets pretreated with GPIIb/IIIa inhibitor (Ro44-9883, 1 μM/mL) or ADP receptor inhibitor (AR-C66096, 1 μM/mL) were activated with echicetin-IgMκ or thrombin as positive control, and binding of FITC-labeled fibrinogen was examined compared with resting platelets. All measurements were repeated 3 times with platelets from different donors. Graph shows fibrinogen binding to resting platelets (1), echicetin-IgMκ–activated platelets without inhibitors (2), echicetin-IgMκ–activated platelets with IIb/IIIa inhibitor (3), echicetin-IgMκ–activated platelets with ADP receptor inhibitor (4), thrombin-activated platelets without inhibitors (5), thrombin-activated platelets with IIb/IIIa inhibitor (6), and thrombin-activated platelets with ADP receptor inhibitor (7).
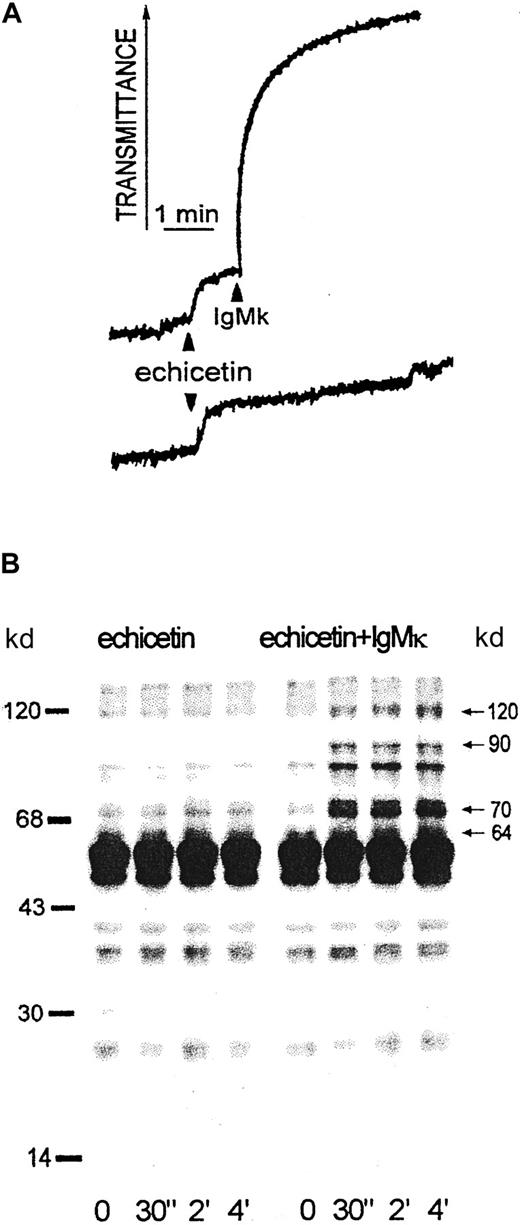
Protein tyrosine phosphorylation in platelets activated by echicetin-IgMκ complex
Echicetin alone at concentrations up to 20 μg/mL did not induce the agglutination of washed platelets. However, addition of echicetin-binding IgMκ to platelet suspensions containing echicetin induced agglutination (Figure 6A). Aliquots of platelets at various times after addition of IgMκ were lysed by SDS and examined for protein tyrosine phosphorylation. Echicetin-IgMκ complex induced marked changes in tyrosine phosphorylation of several platelet proteins with masses of 64, 70 to 90, and 120 kd (Figure 6B). The tyrosine phosphorylation of these proteins increased rapidly after addition of IgMκ but was not affected by echicetin alone. Fcγ and p44, which show strongly increased tyrosine phosphorylation in platelets in response to alboaggregin A,23 were not tyrosine phosphorylated in response to echicetin-IgMκ (Figure 6B).
Agglutination and protein tyrosine phosphorylation induced by echicetin-IgMκ complex in washed human platelets.
(A) Washed human platelets (500 μL, 5 × 108/mL) were stirred at 1100 rpm at 37°C in the presence of 5 μg echicetin. One minute after adding echicetin, 1 μg IgMκ (upper curve) or buffer for control (bottom curve) were added. (B) Proteins from SDS-lysed platelets were separated by SDS-PAGE, transferred to PVDF membrane, and stained with antiphosphotyrosine antibody (4G10). The left panel shows proteins from echicetin-treated platelets; the right panel shows tyrosine phosphorylation of proteins from platelets activated by echicetin-IgMκ complex.
Agglutination and protein tyrosine phosphorylation induced by echicetin-IgMκ complex in washed human platelets.
(A) Washed human platelets (500 μL, 5 × 108/mL) were stirred at 1100 rpm at 37°C in the presence of 5 μg echicetin. One minute after adding echicetin, 1 μg IgMκ (upper curve) or buffer for control (bottom curve) were added. (B) Proteins from SDS-lysed platelets were separated by SDS-PAGE, transferred to PVDF membrane, and stained with antiphosphotyrosine antibody (4G10). The left panel shows proteins from echicetin-treated platelets; the right panel shows tyrosine phosphorylation of proteins from platelets activated by echicetin-IgMκ complex.
Influence of EDTA, IIb/IIIa inhibitor, and acetylsalicylic acid on activation of platelets by echicetin-IgMκ complex
To study the involvement of fibrinogen binding in the aggregation of washed platelets by echicetin-IgMκ complex, a specific inhibitor of IIb/IIIa receptor (Ro44-9883, 1 μM/mL) was added to the platelet suspension 1 minute before adding echicetin-IgMκ. There was no difference in agglutination response between inhibited platelets and untreated platelets. The IIb/IIIa inhibitor also had no effect on protein tyrosine phosphorylation in platelets activated by echicetin-IgMκ (data not shown).
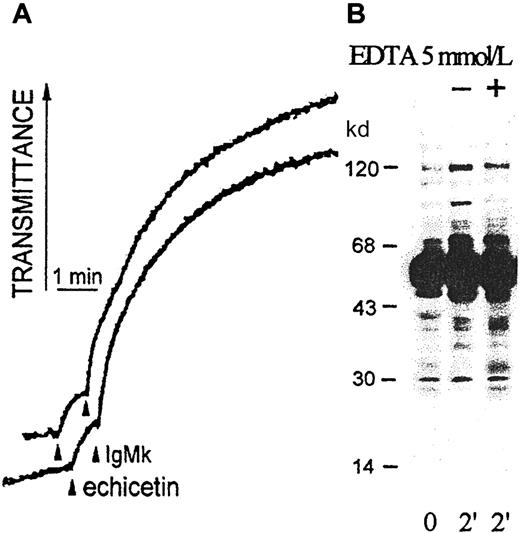
EDTA (5 mmol/mL) did not affect platelet agglutination induced by echicetin-IgMκ; however, EDTA slightly suppressed tyrosine phosphorylation of the 70- to 90-kd proteins (Figure7). Platelets incubated with acetylsalicylic acid (100 mM/mL) for 5 minutes before adding echicetin-IgMκ did not show differences in platelet agglutination or protein tyrosine phosphorylation compared with control platelets (data not shown).
Influence of EDTA on platelet agglutination and activation by echicetin-IgMκ complex.
(A) Washed human platelets (500 μL, 5 × 108/mL) were stirred at 1100 rpm at 37°C in the presence (upper line) or absence (bottom line) of 5 mM EDTA and agglutinated by echicetin (5 μg) plus IgMκ (1 μg). (B) After 2 minutes of agglutination with echicetin-IgMκ complex, platelets were lysed by SDS. Proteins were separated by SDS-PAGE, transferred to a PVDF membrane, and stained with antiphosphotyrosine antibody (4G10).
Influence of EDTA on platelet agglutination and activation by echicetin-IgMκ complex.
(A) Washed human platelets (500 μL, 5 × 108/mL) were stirred at 1100 rpm at 37°C in the presence (upper line) or absence (bottom line) of 5 mM EDTA and agglutinated by echicetin (5 μg) plus IgMκ (1 μg). (B) After 2 minutes of agglutination with echicetin-IgMκ complex, platelets were lysed by SDS. Proteins were separated by SDS-PAGE, transferred to a PVDF membrane, and stained with antiphosphotyrosine antibody (4G10).
Tyrosine kinases p72SYK and p53/56LYN but not p125FAK are involved in platelet activation by echicetin-IgMκ
Because agglutination of platelets by echicetin-IgMκ complex induced clear changes in tyrosine phosphorylation of several proteins, the involvement of candidate tyrosine kinases p72SYK, p53/56LYN, and PI-3K were investigated. Washed platelets were activated by echicetin-IgMκ (5 μg/mL echicetin, 1 μg/mL IgMκ), lysed in Triton X-100 (1.2%), and centrifuged to remove the cytoskeleton. Specific antibodies against p72SYK, p53/56LYN, and PI-3K with protein A–Sepharose were used for immunoprecipitation from the supernatant of platelet lysates.
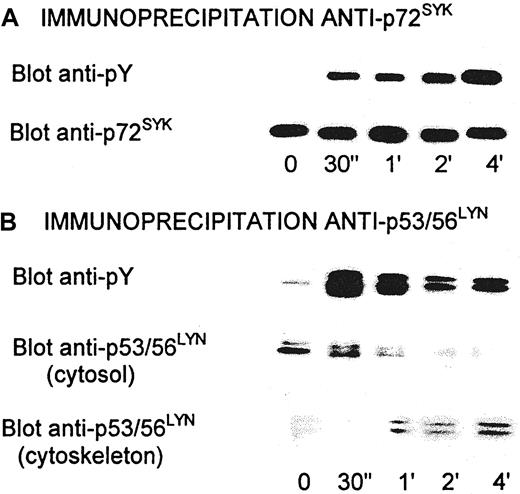
Activation of all of these kinases has been shown to be associated with tyrosine phosphorylation. Tyrosine phosphorylation of p72SYK and p53/56LYN increased rapidly after activation of platelets by echicetin-IgMκ (Figure8A). Tyrosine phosphorylation of p72SYK markedly increased for 30 seconds after adding IgMκ and then continued to increase slowly.
Tyrosine phosphorylation of p72SYK and p53/56LYN in platelets activated by echicetin-IgMκ complex.
After activation, platelets were lysed by 1.2% Triton X-100 and cytoskleton was removed by centrifugation at 100 000g. Supernatant was used for immunoprecipitation by specific anti-p72SYK (A) or anti-p53/56LYN (B) antibodies coupled to protein A–Sepharose 4B. Immunoprecipitated proteins were eluted by 1% SDS, separated by SDS-PAGE, transferred to PVDF membrane, and stained with antiphosphotyrosine antibody (4G10) or with specific anti-p72SYK and anti-p53/56LYNantibodies. Proteins from cytoskeleton were solubilized in 1% SDS, separated by SDS-PAGE, transferred to PVDF membrane, and stained with anti-p53/56LYN antibodies.
Tyrosine phosphorylation of p72SYK and p53/56LYN in platelets activated by echicetin-IgMκ complex.
After activation, platelets were lysed by 1.2% Triton X-100 and cytoskleton was removed by centrifugation at 100 000g. Supernatant was used for immunoprecipitation by specific anti-p72SYK (A) or anti-p53/56LYN (B) antibodies coupled to protein A–Sepharose 4B. Immunoprecipitated proteins were eluted by 1% SDS, separated by SDS-PAGE, transferred to PVDF membrane, and stained with antiphosphotyrosine antibody (4G10) or with specific anti-p72SYK and anti-p53/56LYNantibodies. Proteins from cytoskeleton were solubilized in 1% SDS, separated by SDS-PAGE, transferred to PVDF membrane, and stained with anti-p53/56LYN antibodies.
In contrast to p72SYK, phosphorylation of p53/56LYN increased rapidly for the first 30 seconds, a maximum, and then rapidly decreased. At the same time, the amount of p53/56LYN in the supernatant of platelet lysates also decreased. This decrease was due to p53/56LYN binding to cytoskeletal proteins (Figure 8B) and therefore probably not due to dephosphorylation.
There were no changes in tyrosine phosphorylation of PI-3K in response to echicetin-IgMκ. Platelets treated with wortmannin (1 μM), a specific inhibitor of PI-3K, for 5 minutes also did not show any differences in response to echicetin-IgMκ. These data support a role for p72SYK and p53/56LYN but not PI-3K in activation of platelets by the echicetin-IgMκ complex.
Activation and tyrosine phosphorylation of p125FAK as a result of signaling through activated and clustered GPIIb/IIIa was shown earlier.24 We examined tyrosine phosphorylation of p125FAK in platelets activated by echicetin-IgMκ to study any involvement of GPIIb/IIIa signaling. Activated, washed platelets were lysed with Triton X-100, p125FAK was immunoprecipitated, and tyrosine phosphorylation determined using 4G10 antibody. No changes in tyrosine phosphorylation of p125FAKin echicetin-IgMκ–activated platelets were detected compared to resting platelets (data not shown).
Echicetin-IgMκ complex agglutinates and activates platelets through GPIb only
Activation of washed platelets by echicetin-IgMκ complex is probably the result of GPIb clustering. However, immunoglobulins complexed with echicetin could possibly activate other platelet receptors. To confirm the essential role of GPIb in this process, monoclonal antibodies SZ2 and VM16d, which bind to different sites on GPIb molecule, were used to inhibit platelet agglutination induced by echicetin-IgMκ. SZ2 inhibited platelet agglutination only slightly even at high concentrations (Figure 9, curve 2). However, VM16d completely inhibited the agglutination. The inhibition was dependent on the VM16d mAb concentration in the sample (Figure 9, curves 3 and 4).
Inhibition of platelet agglutination induced by echicetin-IgMκ.
Washed human platelets (500 μL, 5 × 108/mL) were stirred at 1100 rpm at 37°C. A total of 5 μg echicetin was added to the platelet suspension and incubated for 1 minute. Agglutination was started by adding 1 μg IgMκ. Monoclonal antibody against GPIb was added to platelets 2 minutes before echicetin. Curve 1: platelet agglutination induced by echicetin-IgMκ without any inhibitors. Curve 2: in the presence of 16 μg/mL SZ2. Curve 3: in the presence of 3.8 μg/mL VM16d. Curve 4: in the presence of 11.4 μg/mL VM16d.
Inhibition of platelet agglutination induced by echicetin-IgMκ.
Washed human platelets (500 μL, 5 × 108/mL) were stirred at 1100 rpm at 37°C. A total of 5 μg echicetin was added to the platelet suspension and incubated for 1 minute. Agglutination was started by adding 1 μg IgMκ. Monoclonal antibody against GPIb was added to platelets 2 minutes before echicetin. Curve 1: platelet agglutination induced by echicetin-IgMκ without any inhibitors. Curve 2: in the presence of 16 μg/mL SZ2. Curve 3: in the presence of 3.8 μg/mL VM16d. Curve 4: in the presence of 11.4 μg/mL VM16d.
An alternative approach to clustering GPIb using biotinylated echicetin cross-linked by avidin was investigated. Biotin was coupled to echicetin to give a biotin:echicetin molar ratio of 1.5:1. The ability of biotinylated echicetin to bind to the surface of fixed, washed platelets was examined by flow cytometry. Biotinylated echicetin binds to the surface of fixed platelets in a saturable manner. Binding of biotinylated echicetin to fixed platelets was inhibited by an excess of unlabeled echicetin (data not shown). It was shown previously that alboaggregin A can agglutinate fixed platelets by binding to GPIb.4 We found that 0.2 μg/mL alboaggregin A agglutinated fixed platelets to give visible aggregates. Echicetin added to a suspension of fixed platelets 5 minutes before alboaggregin A inhibits this agglutination in a dose-dependent manner. Echicetin at a concentration of 20 μg/mL completely blocks the agglutination of fixed platelets by alboaggregin A (0.2 μg/mL). There were no differences in ability to inhibit alboaggregin A–dependent agglutination of fixed platelets between biotinylated echicetin and unlabeled echicetin (data not shown).
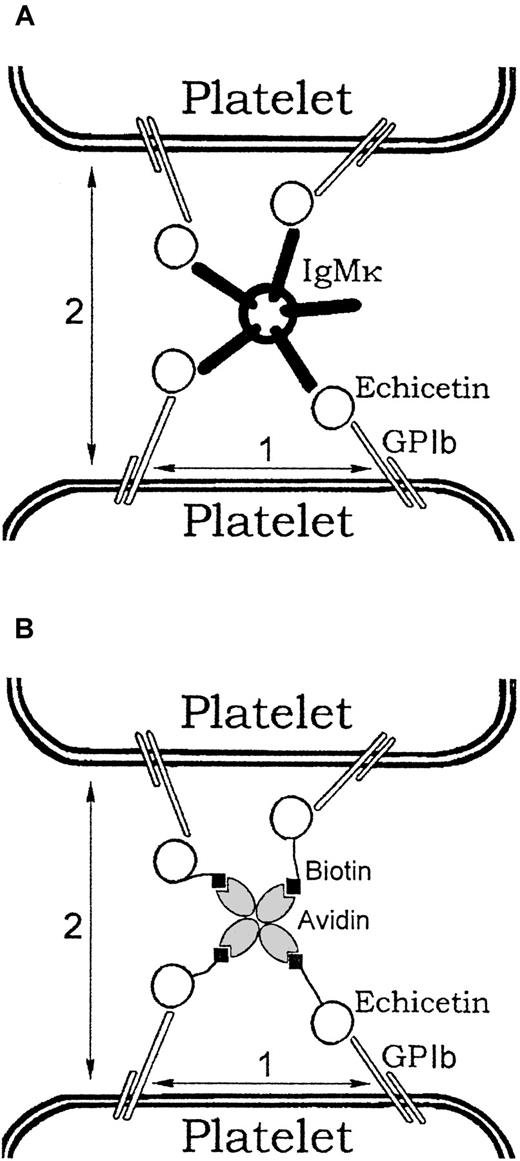
Biotinylated echicetin was used in a similar way to echicetin-IgMκ to activate platelets by cross-linking with avidin. The biotinylated echicetin/avidin complex induced agglutination of washed human platelets (Figure 10, curve 1) as effectively as echicetin-IgMκ or echicetin in PRP (compare with Figures 3 and 6A). Neither GPIIb/IIIa inhibitor nor EDTA blocked platelet agglutination induced by biotinylated echicetin and avidin (Figure 10, curves 2 and 3).
Agglutination of platelets induced by biotinylated echicetin/avidin complex.
Washed human platelets (500 μL, 5 × 108/mL) were stirred at 1100 rpm at 37°C. Biotinylated echicetin (5 μg) was added to the platelet suspension and incubated for 1 minute. Agglutination was started by adding 2 μg avidin. Curve 1: platelet agglutination induced by biotinylated echicetin/avidin. Curve 2: GPIIb/IIIa inhibitor (Ro44-9883, 1 μM/mL) was added to platelet suspension 1 minute before adding of biotinylated echicetin/avidin. Curve 3: EDTA (5 mM/mL) was added to platelet suspension 5 minutes before adding biotinylated echicetin/avidin. Curve 4: washed platelets plus biotinylated echicetin without avidin.
Agglutination of platelets induced by biotinylated echicetin/avidin complex.
Washed human platelets (500 μL, 5 × 108/mL) were stirred at 1100 rpm at 37°C. Biotinylated echicetin (5 μg) was added to the platelet suspension and incubated for 1 minute. Agglutination was started by adding 2 μg avidin. Curve 1: platelet agglutination induced by biotinylated echicetin/avidin. Curve 2: GPIIb/IIIa inhibitor (Ro44-9883, 1 μM/mL) was added to platelet suspension 1 minute before adding of biotinylated echicetin/avidin. Curve 3: EDTA (5 mM/mL) was added to platelet suspension 5 minutes before adding biotinylated echicetin/avidin. Curve 4: washed platelets plus biotinylated echicetin without avidin.
Protein tyrosine phosphorylation in platelets activated by biotinylated echicetin/avidin complex was also similar to that obtained with platelet activation by echicetin-IgMκ (data not shown).
Discussion
A number of proteins from different snake venoms bind to platelet GPlb. Some of these, such as flavocetin A and mamushigin, have been shown to activate platelets. Echicetin itself does not activate washed platelets but inhibited platelet activation by vWF, thrombin, or alboaggregin A9,14 (Figure 2). It is also known that echicetin can induce thrombocytopenia after injection into mice.9 15 This observation was previously unexplained. We found that platelets in PRP, unlike washed platelets, agglutinate in the presence of echicetin (Figure 3). Plasma was therefore fractionated by ion exchange chromatography and the fractions identified that induce agglutination of washed platelets in the presence of echicetin. A final purification to a single band (nonreduced) on SDS-PAGE was obtained by affinity chromatography on an echicetin–Sepharose 4B column. The purified product had a very high molecular mass nonreduced (> 500 kd) and reduced gave 2 bands at 70 and 25 kd. The N-terminal amino acid sequence for the 70-kd chain was found to be EVQLVESGGXL, which is typical for the variable III domain of the heavy chain of IgG and IgM. These results suggested that the protein was an immunoglobulin, and it was thus tested against a panel of heavy and light chain immunoglobulin specific antibodies. Antibodies to μ heavy chains and κ light chains gave a clear positive response whereas others were negative, indicating that the purified plasma protein binding to echicetin was IgM with κ light chains. This also explains the clustering of echicetin in plasma. Because IgM is pentameric, theoretically up to 5 molecules of echicetin can bind to one molecule of IgM. This mechanism can cluster several molecules of echicetin attached to the surface of one platelet and, consequently, cluster GPIb receptors. On the other hand, it can bind molecules of echicetin on the surface of different platelets and provide a mechanism for the agglutination of platelets (Figure 11A). This mechanism can explain the thrombocytopenia observed in mice after echicetin injection. In this case the increase in the bleeding time can be influenced by the decrease in platelet count as well as by inhibition of vWf/thrombin platelet activation by echicetin.
Mechanism of platelet agglutination and activation induced by echicetin-IgM complex or biotinylated echicetin/avidin.
Echicetin binds to GPIb. (A) One molecule of IgMκ can bind up to 5 molecules of echicetin. Binding of several molecules to the surface of one platelet results in clustering of GPIb molecules (1). Binding of echicetin molecules attached to the surface of different platelets results in agglutination (2). (B) One molecule of avidin can bind up to 4 molecules of biotin. Binding of several molecules of biotinylated echicetin to the surface of one platelet results in clustering of GPIb molecules (1). Binding of biotinylated echicetin molecules attached to the surface of different platelets results in agglutination (2).
Mechanism of platelet agglutination and activation induced by echicetin-IgM complex or biotinylated echicetin/avidin.
Echicetin binds to GPIb. (A) One molecule of IgMκ can bind up to 5 molecules of echicetin. Binding of several molecules to the surface of one platelet results in clustering of GPIb molecules (1). Binding of echicetin molecules attached to the surface of different platelets results in agglutination (2). (B) One molecule of avidin can bind up to 4 molecules of biotin. Binding of several molecules of biotinylated echicetin to the surface of one platelet results in clustering of GPIb molecules (1). Binding of biotinylated echicetin molecules attached to the surface of different platelets results in agglutination (2).
Binding of echicetin-IgMκ complex to platelets induced agglutination and partial activation of washed platelets. However, neither EDTA nor GPIIb/IIIa inhibitor prevent platelet agglutination in response to echicetin-IgMκ, raising the question whether GPIIb/IIIa is activated. It was shown before that GPIIb/IIIa can be activated and can bind fibrinogen without aggregation necessarily occurring.16 25FITC-fibrinogen binding to platelets activated by echicetin-IgMκ was examined by flow cytometric analysis and was significantly increased on activated platelets (Figure 4).
GPIIb/IIIa inhibitor completely abolished binding of FITC-fibrinogen to the surface of platelets activated by echicetin-IgMκ (Figure 5). These data show that GPIIb/IIIa is activated on the surface of these stimulated platelets. However, neither GPIIb/IIIa inhibitor nor EDTA affect agglutination/aggregation of platelets by either echicetin-IgMκ (Figure 3) or biotinylated echicetin/avidin (Figure10), demonstrating that aggregation via GPIIb/IIIa-fibrinogen does not occur. The mechanism of GPIIb/IIIa activation via GPIb is still far from clear. One possibility is direct activation of GPIIb/IIIa after GPIb clustering.26 Alternatively, GPIIb/IIIa activation may largely result from ADP receptor activation by ADP released from granules. Activation of platelets by echicetin-IgMκ also induces granule release as assessed by P-selectin expression measured by flow cytometry. Thus, ADP is released as well. We have examined the possibility that ADP is involved in GPIIb/IIIa activation by inhibition of the P2T ADP receptor. Some decrease (about 20%) in FITC-fibrinogen binding to the surface of platelets was observed. However, ADP receptor inhibition did not completely inhibit FITC-fibrinogen binding to platelets activated by echicetin-IgMκ. ADP receptor inhibitor did not prevent FITC-fibrinogen binding to platelets activated by thrombin. These data suggest that probably both mechanisms, direct activation of GPIIb/IIIa via clustering of GPIb as well as activation of GPIIb/IIIa by feedback through ADP, operate in platelets activated by echicetin-IgMκ. Clustering of GPIb by vWf has been previously proposed as a mechanism for initial activation of platelets under high shear stress conditions. Several articles support this hypothesis, including that of Falati et al23 where alboaggregin A and mutant forms of vWf were used to cluster GPIb. The authors showed that alboaggregin A induced tyrosine phosphorylation of Syk, Fyn, Lyn, phospholipase Cγ2, Fcγ, and proteins with mass 44, 56, and 59 kd. Platelet activation by echicetin-IgMκ (or biotinylated echicetin/avidin) also caused tyrosine phosphorylation of Syk and Lyn. However, in the experiments with platelets activated via echicetin clustering we did not find tyrosine phosphorylation of Fcγ and p44. These differences in experimental results may be due to binding of alboaggregin A to more than one class of receptor on the platelet surface. Strong activation of Fcγ in platelet activation by alboaggregin A23 might be induced via binding to GPVI, for example. Platelet activation by clustering GPIb receptors was also examined by Yanabu et al.27 In this case receptors were clustered using a GPIb-specific antibody NNKY5-5. The authors reported that activation of platelets in blood plasma by NNKY5-5 caused formation of small aggregates and tyrosine phosphorylation of p72SYK and a protein with mass of 64 kd. However, washed platelets only showed a minimal response to NNKY5-5.
In our experiments, cross-linking–washed platelets by echicetin-IgMκ or PRP by echicetin or by biotinylated echicetin/avidin induced very similar changes in light transmission, implying that the size of the aggregates in PRP and with washed platelets was similar as well. Visual inspection of the aggregates supported this interpretation. This implies a similar mechanism in each case, without participation of other plasma components, limited by some common factor such as GPIb density on platelets.
It was also shown that inhibition of GPIIb/IIIa by GRGDS peptide or by a specific monoclonal antibody completely suppressed platelet aggregation induced by NNKY5-5. This shows that GPIIb/IIIa was involved in aggregation, indicating that platelet activation had occurred. It was shown earlier that fibrinogen binding to activated GPIIb/IIIa induced the activation of p72SYK.28,29 In contrast to the data of Yanabu et al,27 we did not find any involvement of GPIIb/IIIa clustering by fibrinogen in the process of platelet agglutination/activation induced by echicetin-IgMκ (or biotinylated echicetin/avidin complex). However, we also found tyrosine phosphorylation of p72SYK and p64. It has also been shown that vWf can induce tyrosine phosphorylation of p72SYK and p64 in platelets independently of GPIIb/IIIa.30 Thus, the mechanism of tyrosine phosphorylation of p72SYK and p64 induced by NNKY5-5 is unclear, because the phosphorylation could be a result of signaling from either GPIb or GPIIb/IIIa or both.
Recently, Zaffran et al26 and Yap et al31 have shown that GPIb complexes transfected into Chinese hamster ovary cells, which already have GPIIb/IIIa transfected, are able to transmit signals to activate GPIIb/IIIa. The mechanisms involved are not clear and seem to depend upon the shear stress involved, lower shear being compensated by release and feedback of ADP and thromboxanes. In general, our results support these conclusions and suggest that signal transduction by engagement of GPIb alone in platelets is capable of activating GPIIb/IIIa to bind fibrinogen. Aggregation and further signaling via GPIIb/IIIa may require the cross-linking of GPIb with GPIIb/IIIa that normally occurs with vWf.
In conclusion, we have shown that echicetin can bind IgMκ from blood plasma. The complex of echicetin-IgMκ effectively induces platelet agglutination, which is not dependent on fibrinogen binding to GPIIb/IIIa. Cross-linking of GPIb by the echicetin-IgMκ complex also induces tyrosine phosphorylation of p72SYK, p53/56LYN, p64, p70 to p90, and p120. The echicetin-IgMκ complex should be a good reagent for exploring signal transduction mechanisms induced via GPIb complex independently of other platelet receptors. These results also suggest that the mechanisms of action of other inhibitory snake C-type lectins that bind to GPIb may require reinvestigation.
We thank Dr Edith Magnenat, Serono Pharmaceutical Research Institute, Geneva, Switzerland, for the peptide sequencing; Prof Beda Stadler, Department of Clinical Research, University of Berne, Switzerland, for the immunoglobulin analyses; and the Central Laboratory of the Swiss Red Cross Blood Transfusion Service for the supply of buffy coats, erythrocyte concentrates, and IgM fractions.
Supported by Swiss National Science Foundation grant 31-52396.97.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
K. J. Clemetson, Theodor Kocher Institute, University of Berne, Freiestrasse 1, CH-3012 Berne, Switzerland; e-mail: clemetson@tki.unibe.ch.

![Fig. 1. Binding of platelet proteins to echicetin-Sepharose. / Platelet surface proteins were labeled with biotin, platelets were lysed by Triton X-100, and the lysate was added to echicetin-Sepharose. The proteins eluted from the echicetin-Sepharose were separated by SDS-PAGE, transferred to PVDF membrane, and detected with avidin-phosphatase conjugate (lanes 1-3) or with anti-GPIb monoclonal antibody (lanes 4-6). Lanes 1 and 4: platelet lysates. Lanes 2 and 5: eluate from Sepharose (negative control). Lanes 3 and 6: eluate from echicetin-Sepharose. Specific bands detected by anti-GPIb monoclonal antibody (Ib-4) are indicated by (nonreduced [NR]) GPIb and glycocalicin (GC; the extracellular proteolytic fragment of GPIbα) and (reduced [R]) GPIbα and macroglycopeptide (MG; the mucinlike proteolytic fragment of GPIbα). Under reducing conditions GC and GPIbα comigrated.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/97/8/10.1182_blood.v97.8.2333/5/m_h80810914001.jpeg?Expires=1767705451&Signature=aQF~13wkQQn94IrErA-hn9D26Ba84vUasQNGb6z3r4f7yH4UnhZtbA~xZJjhch8ChEd7GezOkAb~LGWBDgm6GjU~QgfUz7KUARmaAUeTbHpRu5Ax1VtRQTHReNgb9cACMIOGS8XMw-tSDBRusvU213ZkB2yBavGLByxq5U5d0gRzeVTdEJV9BPpTEdxdbsESQLbxUe-SN6ci9t7sOZLhUVldNL54vv37Y9QXkSvfwynf5RfcGRWkNj5KcvWEOLWKavVRSrHg9nGyMhwJIX9x-ATs~XPEQseX2H73tlykgqJZPoOih80VcfYowcnB1eWaX3Ms88VKqCHpYDhITHcZlA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal