Abstract
During differentiation, megakaryocytes increase ploidy through a process called endomitosis, whose mechanisms remain unknown. As it corresponds to abortive mitosis at anaphase and is associated with a multipolar spindle, investigation of chromosome segregation may help to better understand this cell-cycle abnormality. To examine this variation, a new method was developed to combine primed in situ labeling to label centromeres of one chromosome category and immunostaining of tubulin. Human megakaryocytes were obtained from normal bone marrow culture. By confocal microscopy, this study demonstrates an asymmetrical distribution of chromosomes (1 or 7) either between the spindle poles at anaphase stage of endomitosis and between the different lobes of interphase megakaryocyte nuclei. The metaphase/anaphase checkpoint appears normal on the evidence that under nocodazole treatment megakaryocytes progressively accumulate in pseudo-metaphase, without spontaneous escape from this blockage. Immunostaining of p55CDC/hCDC20 with similar kinetochore localization and dynamics as during normal mitosis confirms this result. HCdh1 was also expressed in megakaryocytes, and its main target, cyclin B1, was normally degraded at anaphase, suggesting that the hCdh1-anaphase–promoting complex checkpoint was also functional. This study found the explanation for these unexpected results of an asymmetrical segregation coupled to normal checkpoints by careful analysis of multipolar endomitotic spindles: whereas each aster is connected to more than one other aster, one chromosome may segregate symmetrically between 2 spindle poles and still show asymmetrical segregation when the entire complex spindle is considered.
Introduction
Megakaryocytes are polyploid cells that increase their DNA content through an original process called endomitosis.1 Megakaryocyte progenitors proliferate through normal 2N to 4N cycles under hematopoietic growth factor control. They begin terminal differentiation with synthesis of specific platelet proteins (promegakaryoblast stage), followed by a switch from a mitotic to an endomitotic process, which is characterized by a complete DNA replication without karyokinesis and cytokinesis. This leads to cells that contain a single polylobulated typical nucleus with a 2xN ploidy. Thereafter, terminal cytoplasmic maturation occurs, leading to proplatelet formation and platelet production.
The main consequence of megakaryocyte polyploidization is to augment cell size and, thus, to increase platelet production that originates from its cytoplasmic fragmentation.2-4 Some researchers have also hypothesized that the level of megakaryocyte ploidy may modify platelet size and functions by altering gene regulation (reviewed by Zimmet and Ravid5).
Two teams have shown that endomitosis in mouse and human megakaryocytes correspond to an abortive mitotic process.6,7Megakaryocytes undergo a normal cycle progression with G1, S, and G2 phases. It could be demonstrated by immunofluorescence that the first stages of mitosis also normally occur with chromosome condensation and a single multipolar spindle. More surprisingly, a nuclear membrane breakdown was observed that is in contradiction with the theoretical definition of an endomitosis. The first stage of anaphase (anaphase A) characterized by sister chromatid separation and chromosome to pole movement was also present. In both studies, however, absence of spindle pole to pole elongation and chromosome segregation via microtubule retraction was noticed and was interpreted by Nagata et al6as a defect of anaphase B. Finally, telophase or cytokinesis was never observed. Thus, megakaryocyte endomitosis is incomplete mitosis characterized by a skip of late mitosis stages. However, the molecular mechanisms responsible for this cell-cycle abnormality remain to be determined.
Late mitosis, from metaphase to cytokinesis, is characterized by timed and coordinated events, which imply proper mechanisms of regulation (reviewed in Morgan et al8). Most of these mechanisms have been largely studied in genetic yeast models, leading to the identification of key protein networks. Homologues of the main components have been found in other species, including human, revealing that this system is highly conserved through the evolution. The fundamental principle of coordination of late mitotic events is based on the sequential ubiquitin-dependent proteolysis of regulatory proteins, catalyzed by the ubiquitin-ligase anaphase-promoting complex (APC).8 The sequential association of the APC with 2 activators, namely CDC20 at the metaphase/anaphase transition, then Cdh1 (p55CDC/hCDC209 and hCdh1 are the human homologues10) at anaphase seems partially responsible for the specific substrate targeting and timing.
The first critical step in late mitosis is an accurate chromosome segregation, which ensures an equivalent DNA repartition between the 2 daughter cells and confers genomic stability. It is initiated as early as prometaphase, which allows the bipolar attachment of chromosomes, followed by their alignment on the equatorial plate at metaphase. Although all the chromosomes are attached, the p55CDC-APC complex triggers the anaphase inhibitor Pds1 for degradation, leading to the sister chromatid separation with cohesin release (reviewed in Nasmyth et al11). This process is monitored by a conserved spindle checkpoint that is able to delay the anaphase onset and to inhibit the mitotic exit, in case of spindle assembly abnormality or a failure in chromosome attachment (reviewed by Rudner and Murray12 and Hardwick13). Several proteins including Mad1, 214,15 and Bub1, 316,17 are implicated that form complexes at the kinetochores of unattached chromosomes.18 If a single chromosome is misaligned, the main component Mad2 blocks the activation of the p55CDC-APC and maintains the metaphase.19 20
The second crucial event during the mitosis is the cyclin B1 degradation, leading to Cdk1 inactivation. In somatic vertebrate cells, cyclin B1 destruction has been shown to be initiated at metaphase and complete at the beginning of anaphase,21 consistent with the notion that p55CDC-APC might target cyclin B1 (as well as anaphase inhibitors) for proteolysis.8 Late mitotic abnormalities such as anaphase delay, aberrant anaphase, and a failure of mitotic exit have also been described after microinjection of an anti-p55CDC antibody in a human cell line, arguing for a similar function of P55CDC in humans.22 In yeast, cyclin destruction by the CDC20-APC initiates a drop in Cdk1 activity, leading to the subsequent activation of the Cdh1-APC.23,24 Therefore, high Cdh1-APC activity at the end of mitosis induces a complete cyclin B1 degradation that allows cytokinesis, and the maintenance of a mitotic cyclin low level allows G1 phase transition followed by DNA replication.25Furthermore, a separate pathway of the spindle checkpoint has been defined, implying Bub2 protein located at the spindle poles,26 which monitors the completion of anaphase and prevents activation of Cdh1-APC, degradation of the B type cyclin, and the final mitosis exit (reviewed by Burke27). The precise role of Cdh1 during mitosis has not been clearly defined in eukaryotes,28 although homologues of this regulation pathway, such as hCdc14 phosphatase and GAPCenA, have been identified in humans.29 Different timing of p55CDC and hCdh1 has been explained through distinct mechanisms of regulation. p55CDC expression has been demonstrated to fluctuate during cell cycle, in parallel to change in cellular localization. Its level peaks at the onset of the mitosis, when the protein is localized in the nucleus at prophase and concentrates at the kinetochores at prometaphase. Finally, p55CDC is abruptly degraded at the end of mitosis. HCdh1 also displays fluctuations through the cell cycle, whereas its main activation is regulated by the nonphosphorylated status of the protein.28
In this report, we investigated how chromosome segregation occurred on a multipolar spindle in endomitotic megakaryocytes. Indeed, previous immunofluorescence studies with kinetochores staining were limited to properly analyze separation and segregation of chromosomes. Because of the high number of chromosomes in polyploid cells, they only gave a rough appreciation of this phase. Whether this process was complete for all the chromosomes remained an important point to define, because an abnormal anaphase A might prevent cells from undergoing the following anaphase B and cytokinesis. To perform this study, we elaborated a new approach, associating both primed in situ labeling (PRINS) to stain chromosome centromeres and immunofluorescence to visualize megakaryocyte spindle. This procedure allowed us to demonstrate that the mitotic checkpoint is functional in megakaryocytes. However, an asymmetrical repartition of chromosomes in polyploid megakaryocytes occurs that is the consequence of the spindle organization but not to a defect in sister chromatid separation. Moreover, in contrast with previous published results,30 mechanisms of endomitosis seem independent of cyclin B1 regulation.
Materials and methods
In vitro cultures of megakaryocytes from CD34+cells
CD34+ cells were obtained from human bone marrow of healthy patients undergoing hip surgery, with their informed consent. Cells were separated over a Ficoll-metrizoate gradient (Ficoll separating solution, Biochrom KG, Berlin, Germany), then CD34+ cells were isolated, using the immunomagnetic beads technique (Miltenyi Biotec, Paris, France).31 The purity was usually in the range of 70% to 90%.
CD34+ cells were grown for 5 days in Iscoves modified Dulbecco medium (Gibco BRL, Cergy Pontoise, France) that contained penicillin (100 U/mL), streptomycin (100 μg/mL), glutamine (2 mM) (Sigma Chemical, St Louis, MO), α-monothioglycerol (76 nM, Sigma), 1.5% deionized bovine serum albumin (BSA) (Cohn fraction V, Sigma), 1% insulin–transferrin–selenium X (Gibco BRL), and sonicated lipids (20 μg/mL).32 The culture medium was supplemented with polyethylene glycol (PEG)–rhu megakaryocyte growth and differentiation factor (MGDF) (10 ng/mL) and recombinant human stem cell factor (SCF; 50 ng/mL) (generous gifts from Kirin Brewery, Tokyo, Japan; and Amgen, Thousand Oaks, CA, respectively).
PRINS and immunofluorescence
Megakaryocytes were harvested, suspended in 1 × phosphate-buffered saline (PBS)/0.1% EDTA/2% BSA, and cytocentrifuged onto slides at 500 rpm for 4 minutes (cytospin, Shandon, Pittsburgh, PA) They were fixed in 90% methanol/10% acetic acid for 5 minutes, rinsed in 1 × PBS, then permeabilized with 0.1% Triton-X100 (Sigma) for 8 minutes and dehydrated in 3%, 70%, 85% and 100% ethanol washes. DNA was denatured in 70% formamide (Merck, Darmstadt, Germany) and 2 × SSC, pH 7 to 7.2 at 71°C for 2 minutes and stopped in a series of ethanol washes of 70%, 80%, 90%, and 100% at 4°C for 2 minutes each. PRINS reaction was performed as previously described.33 For each slide, a mix was prepared, containing specific primer of chromosomes 1 or 7 centromeres, nucleotides mixture that included Fluorescein-12-dUTP (Boeringher Mannheim, Meylan, France), Taq polymerase buffer, and 2 units of enzyme (Taq DNA polymerase; Appligene Oncor, Illkirsh, France). The denatured slides were put on the plate block of a programmable thermal cycler, whereas the reaction mix was deposited on the slides and spread with coverslips. The PRINS reaction consists of 2 steps: 15 minutes at 55°C corresponding to the specific annealing temperature of the centromeric primers of chromosomes 1 and 7 and 15 minutes at 72°C to allow the in situ chain elongation. Then slides are washed 3 times in 4 × SSC-Tween for 4 minutes each and dehydrated in 3 ethanol washes of 70%, 85%, and 100% for 2 minutes each. The indirect immunofluorescence procedure is performed in the dark. Primary mouse anti–β-tubulin antibody was applied (1/50 dilution in 1 × PBS; Sigma) for 30 minutes, followed by 3 washes in 1 × PBS and incubation with donkey tetrarhodamine isothiocyanate (TRITC)-labeled antimouse F(ab′)2 fragments (Jackson Immunoresearch, West Grove, PA) for 30 minutes. Nuclei were finally counterstained with 1/3000 TOTO3-iodide (in dimethyl sulfoxide 1 mM; Molecular Probes, Eugene, OR) for 30 minutes, slides were mounted with Vectashield antifading solution (Vector Laboratories, Burlingame, CA).
Fluorescence in situ hybridization and immunofluorescence
Similar megakaryocyte preparations as described above were used. Immunodetection of the von Willebrand factor (vWF) was first performed by using a rabbit polyclonal antibody (1/200; Dako, Trappes, France). Then slides were fixed 5 minutes again in methanol 75/acetic acid 25 and dehydrated in ethanol 70%, 85%, and 100% before the following fluorescence in situ hybridization (FISH) procedure on DNA. A labeled 11q probe was used according to the distributor recommendations (LSI 11q, Vysis, Voinsins-le Bretonneux, France). Codenaturation of probe and specimens was made in a single step (2 minutes at 85°C) followed by 8 hours of hybridization at 37°C by placing the slides on a hot plate. Slides were immediately immersed in 0.4 × SSC/0.3% NP-40 at 72°C for 2 minutes, also in 2 × SSC/0.1% NP-40 for 30 seconds, and were finally mounted, using 4′,6-diamidino-2phenilindole (DAPI) and Vectashield (Vector Laboratories).
Indirect immunofluorescence
Megakaryocytes were cytocentrifuged onto slides, fixed in 2% paraformaldehyde (Serva, Heidelberg, Germany) for 10 minutes, and rinsed in 1 × PBS. Cells were permeabilized with 0.1% Triton X-100 before incubation with primary antibodies for 30 minutes. Three washes in 1 × PBS preceded incubation with secondary antibodies for 30 minutes. DNA was stained with TOTO3-iodide diluted at 1/1500 for 1 hour, or DAPI finally mounted with antifading. The primary antibodies included mouse anti–α, anti–β-tubulin (1/50, Sigma) and anti–cyclin B1 (1/100, GNS-1 Pharmingen) antibodies, rabbit anti-p55CDC (1/200, Amgen), anti-hCdh1 (1/200, kindly provided by J. M. Peters, Research Institute of Molecular Pathology, Vienna, Austria) antibodies, a goat anti-MKLP1 (Santa Cruz Biotechnology, Santa Cruz, CA) antibody, and a human antikinetochore antibody (1/200, obtained from patients with calcinosis cutis, Raynaud phenomenon, esophageal motility disorder, sclerodactyly, and telangiectasis [CREST] syndrome and kindly provided by J. C. Brouet, Hopital St Louis, Paris, France). Secondary antibodies were donkey fluorescein isothiocyanate (FITC)- or TRITC-labeled antimouse, FITC- or TRITC-conjugated antirabbit, TRITC-labeled antigoat, and Cy5-labeled antihuman F(ab′)2 fragments (1/50, Jackson Immunoresearch Laboratories).
Image acquisition and analysis
Confocal images were captured using a laser scanning confocal microscope LMS 510 (Zeiss, Oberkochen, Germany) either with Plan-apochromat × 63/NA1.4 or × 100/NA1.4 objectives. Three laser excitations were used (ie, 488 nm, 543 nm, 633 nm for FITC, TRITC, and Cy5 or TOTO 3-iodide, respectively). Serial optical sections of 0.7 μm (images collected at 0.5-μm intervals) in the z-axis of the cell were collected sequentially for each marker and overlaid to obtain a 3-dimensional reconstruction.
Other images were captured with the use of an epifluorescence microscope, using either × 60 or × 40 objectives (Nikon, Tokyo, Japan) and processed using Adobe Photoshop 5.0.
Results
To observe chromosome segregation during megakaryocyte polyploidization, we elaborated a new method, which couples PRINS for labeling centromeres of one chromosome and tubulin immunostaining to visualize the endomitotic spindle. In that case, PRINS provided an advantageous approach to label centromeres of chromosomes in comparison to classical FISH for 2 reasons. First, it is a quicker method, giving best quality cell samples for the subsequent immunostaining procedure; second, in situ primer elongation gave a stronger and more specific centromeric signal, compared to what can be obtained by probe hybridization. Chromosomes 1 or 7 centromeres were arbitrarily chosen for labeling because specific probes were available for these 2 chromosomes.33 Simultaneous painting of more than one chromosome would have been confusing for the image analysis because of the high number of generated signals in polyploid cells.
Interphase nuclei contained green (FITC) dots, corresponding to centromeres of chromosomes 1 or 7, depending on the specific primers used for the experiments. Their number could be correlated to cell ploidy, because most interphase nuclei contained 2 copies when they were diploid, and 2xN copies when their DNA content was polyploid. During mitosis, these dots were localized on the spindle labeled in red (TRITC) by immunofluorescence (Figure1).
Correlation between the number of centromeres of chromosome 1 and the cell ploidy.
Human CD34+ cells were grown in the presence of PEG-rhuMGDF and SCF. Day 5 megakaryocytes were treated with the PRINS and immunofluorescence procedure described in “Materials and methods.” They were cytospun, fixed with methanol/acetic acid, and permeabilized with Triton. Centromeres of chromosome 1s were probed in green by PRINS, using specific centromeric primers and FITC-labeled nucleotides. Microtubules were stained in red by indirect immunofluorescence with an anti–β-tubulin antibody, followed by incubation with TRITC-labeled antimouse F(ab′)2 fragments. Slides were examined by confocal microscopy with a 3-dimensional reconstruction and superimposition of the 2 signals. The upper-left corner of the figure shows many diploid interphase cells containing 2 dots representing 2 chromosome 1s; the lower-right corner shows a mitotic polyploid cell that contains a tetrapolar spindle and 4 chromosome 1s (white arrows). Bar, 10 μm.
Correlation between the number of centromeres of chromosome 1 and the cell ploidy.
Human CD34+ cells were grown in the presence of PEG-rhuMGDF and SCF. Day 5 megakaryocytes were treated with the PRINS and immunofluorescence procedure described in “Materials and methods.” They were cytospun, fixed with methanol/acetic acid, and permeabilized with Triton. Centromeres of chromosome 1s were probed in green by PRINS, using specific centromeric primers and FITC-labeled nucleotides. Microtubules were stained in red by indirect immunofluorescence with an anti–β-tubulin antibody, followed by incubation with TRITC-labeled antimouse F(ab′)2 fragments. Slides were examined by confocal microscopy with a 3-dimensional reconstruction and superimposition of the 2 signals. The upper-left corner of the figure shows many diploid interphase cells containing 2 dots representing 2 chromosome 1s; the lower-right corner shows a mitotic polyploid cell that contains a tetrapolar spindle and 4 chromosome 1s (white arrows). Bar, 10 μm.
To precisely analyze chromosome segregation, the whole nucleus was analyzed by confocal microscopy. In diploid cells from interphase to metaphase, 2 green dots (2 copies of the same chromosome) were observed; 4 dots were present when sister chromatids separated from the beginning of anaphase to the onset of cytokinesis, 2 on each side of the equator, as would be expected from a regulated chromosome segregation (data not shown). In case of polyploid cells, which had complex spherical multipolar spindles, a simultaneous nuclear DNA painting was necessary to properly determine the precise stage of endomitosis.
Asymmetrical chromosomes repartition in polyploid megakaryocytes
CD34+ cell–derived cultures did not contain only megakaryocytes (about 50% purity). A double labeling with anti-vWF and antitubulin antibodies was first performed, which confirmed that polyploid cells were exclusively megakaryocytes, and allowed us to use the protocol described above without need of a megakaryocytic marker. In the subsequent set of experiments, we analyzed endomitotic megakaryocytes and observed that the centromeres of chromosome 1 were not symmetrically distributed along the multipolar spindle of all endomitotic cells examined. Indeed, some spindle poles did not bear any chromosome 1s as illustrated in Figure 2in either 4N (Figure 2A), 8N (Figure 2B), or 16N (Figure 2C) megakaryocytes. Identical experiments were performed by labeling the centromere of chromosome 7s, leading to the same results (data not shown). These observations demonstrate that the chromosome repartition is performed asymmetrically on the multipolar spindle, regardless of the chromosome category (either chromosome 1 or 7) or the degree of cell ploidy.
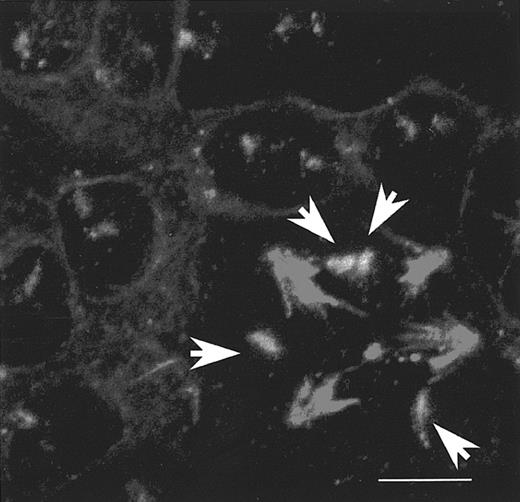
Asymmetrical repartition of chromosome 1s on multipolar spindle of endomitotic megakaryocytes.
Double staining obtained by PRINS and immunofluorescence procedure, as reported in Figure 1, in endomitosis 4N to 8N (A), 8N to 16N (B), and 16N to 32N (C). Centromeres of chromosome 1s (green dots) are asymmetrically distributed among the different spindle poles, as no green dot is associated with some asters. Bar, 5 μm. (D) Asynchronous separation of sister chromatids in a 4N to 8N endomitosis. Additional DNA staining (in blue) was performed: chromosomes have congressed to a complex metaphase plate typical of the metaphase/anaphase transition. The white arrow shows the single chromosome 1 whose chromatids have already separated (2 dots). Panel E corresponds to an interphase megakaryocyte with positive vWF staining (in green): 8 chromosomes (red) are unevenly labeled by FISH (distributed between the 4 lobes of the nucleus [in blue]).
Asymmetrical repartition of chromosome 1s on multipolar spindle of endomitotic megakaryocytes.
Double staining obtained by PRINS and immunofluorescence procedure, as reported in Figure 1, in endomitosis 4N to 8N (A), 8N to 16N (B), and 16N to 32N (C). Centromeres of chromosome 1s (green dots) are asymmetrically distributed among the different spindle poles, as no green dot is associated with some asters. Bar, 5 μm. (D) Asynchronous separation of sister chromatids in a 4N to 8N endomitosis. Additional DNA staining (in blue) was performed: chromosomes have congressed to a complex metaphase plate typical of the metaphase/anaphase transition. The white arrow shows the single chromosome 1 whose chromatids have already separated (2 dots). Panel E corresponds to an interphase megakaryocyte with positive vWF staining (in green): 8 chromosomes (red) are unevenly labeled by FISH (distributed between the 4 lobes of the nucleus [in blue]).
Megakaryocytes have a normal metaphase/anaphase checkpoint but an asymmetrical segregation of chromosomes at anaphase
The adequate bipolar attachment of chromosome to microtubules from different centrosomes occurs at prometaphase and metaphase and is essential for the ensuing accurate segregation of chromosomes at anaphase onset. This stage is normally monitored by the mitotic checkpoint. The observation of an asymmetrical repartition of chromosomes along the megakaryocytic spindle suggested an abnormality of chromosome attachment to microtubules, which had been missed by the mitotic checkpoint. Therefore, we investigated if the control of this checkpoint was normal in megakaryocytes as had been previously suggested.6 To explore the metaphase/anaphase checkpoint in megakaryocytes, nocodazole was added to the cultures, and cells were studied at various times up to 24 hours (0, 3, 5, 7, and 24 hours). Mitotic index was determined using nuclear staining, and cells were considered to be in pseudo-metaphase arrest when chromosomes were condensed. A total of 400 cells were examined at each time period, and the average of a triplicate determination was calculated. Typical pseudo-metaphase arrest of megakaryocytes was observed as early as 3 hours following exposure to nocodazole, whereas mitotic index increased from 1% up to 25% after 24 hours. The pseudo-metaphase arrest was maintained as long as the drug was present. In contrast, after nocodazole withdrawal, megakaryocytes exit from mitosis (Figure3A) as judged by decondensed chromosomes. As megakaryocyte culture contains a portion of diploid cells with normal mitotic cell cycle, an additional score on 500 cells was similarly determined, taking into account only polyploid cells. Similar results were observed (Figure 3B)
Nocodazole treatment of megakaryocytes.
Nocodazole (▪, 2 μg/mL) was added to the medium of culture of megakaryocytes obtained from CD34+ cells with PEG-rhuMGDF and SCF. Megakaryocytes were regularly sampled (at 0, 3, 5, 7, and 24 hours) centrifuged onto slides and fixed with paraformaldehyde, then nuclei were counterstained with DAPI. Mitotic index was calculated for 400 cells at each time and determined on the average of triplicate experiments. In parallel, nocodazole was withdrawn (▨) at 7 hours and mitotic indexes were similarly determined at 24 and 48 hours (A). Panel B shows a similar experiment in which only polyploid megakaryocytes were taken into account to determine the mitotic index (based on a 500-cell count for each sample).
Nocodazole treatment of megakaryocytes.
Nocodazole (▪, 2 μg/mL) was added to the medium of culture of megakaryocytes obtained from CD34+ cells with PEG-rhuMGDF and SCF. Megakaryocytes were regularly sampled (at 0, 3, 5, 7, and 24 hours) centrifuged onto slides and fixed with paraformaldehyde, then nuclei were counterstained with DAPI. Mitotic index was calculated for 400 cells at each time and determined on the average of triplicate experiments. In parallel, nocodazole was withdrawn (▨) at 7 hours and mitotic indexes were similarly determined at 24 and 48 hours (A). Panel B shows a similar experiment in which only polyploid megakaryocytes were taken into account to determine the mitotic index (based on a 500-cell count for each sample).
As the mitotic checkpoint is normal in megakaryocytes, it suggests that chromatid separation should occur normally. By careful analysis of endomitotic megakaryocytes we had previously analyzed, we observed that the number of hybridization dots was similar to that of spindle poles: it meant that the sister chromatids had not yet separated (Figure2A-C). The endomitosis of these previous experiments corresponded to prometaphase or metaphase, but not to anaphase, which is consistent with the respective duration of both phases. Thereafter, improved culture conditions and microscopic examination of up to 500 endomitotic megakaryocytes allowed us to visualize anaphases and chromatid separation. At this stage, regardless of ploidy, the nucleus was characterized by a unique shape with chromosomes forming rings around each aster (Figure 4). This organization was the consequence of chromosome segregation and migration to each spindle pole. In that case, the dot number, which is equivalent to the labeled sister chromatid number (chromosome 1), was twice the aster number, as expected. It was clearly visualized during endomitosis of 4N megakaryocytes, which have 8 labeled chromosome 1s on their tetrapolar-spindle at anaphase (Figure 4A). These data support the contention that sister chromatid separation was complete and normal in megakaryocytes as previously suggested.6 However, the numbers of chromosome 1s at each aster were different (from 0 to 4 during 4N to 8N endomitosis), suggesting that chromosome migration toward the spindle poles is asymmetrical. Then, at the end of anaphase, the number of each category of chromosome is not identical for each aster in polyploid megakaryocytes (Figure 4A-C). Identical results were obtained with centromere labeling of chromosome 7 (not shown). However, 2 potential biases could limit the proper interpretation of these results: first, PRINS as well as other in situ fluorescence labeling techniques can give false signals. These false signals were clearly distinguished from the real signals by confocal microscopy, considering the colocalization of the last one with the labeled DNA. Second, 2 signals could superimpose on a cell view and appear as one dot: it was resolved by analyzing different angles of the same cell using the 3-dimensional cell reconstruction (not shown). Thus, we were able to clearly demonstrate that chromosome segregation was asymmetrical; this asymmetry was independent of the chromosome category (either chromosome 1 or 7) and the degree of cell ploidy.
Asymmetrical segregation of chromosome 1 at anaphase of megakaryocyte endomitosis.
PRINS and immunofluorescence procedures were used on day 5 megakaryocytes as previously described in Figure 1. Centromeres of chromosome 1 are probed by PRINS in green (FITC). Spindle microtubules are immunostained in red (TRITC), and nuclei are counterstained with TOTO3-iodide that appear in blue. Merging of the 3 markers is visible on the first column. (A) 4N to 8N endomitosis at anaphase: chromosomes cluster into a ring around each aster. The number of green spots on DNA area is twice the spindle poles number. Note that there are normally 2 green spots at 2 poles (white arrows), but 3 and 1 dots are observed at the 2 resting poles (yellow arrows). The other green points correspond to false signals. (B) 8N to 16N endomitosis: the number of green dots shown by white arrows is indicated when greater than one. (C) Endomitosis of high ploidy: surimposition of both PRINS-probed centromeres of chromosome 1 in green and counterstained nuclei in blue. Bar, 5μm.
Asymmetrical segregation of chromosome 1 at anaphase of megakaryocyte endomitosis.
PRINS and immunofluorescence procedures were used on day 5 megakaryocytes as previously described in Figure 1. Centromeres of chromosome 1 are probed by PRINS in green (FITC). Spindle microtubules are immunostained in red (TRITC), and nuclei are counterstained with TOTO3-iodide that appear in blue. Merging of the 3 markers is visible on the first column. (A) 4N to 8N endomitosis at anaphase: chromosomes cluster into a ring around each aster. The number of green spots on DNA area is twice the spindle poles number. Note that there are normally 2 green spots at 2 poles (white arrows), but 3 and 1 dots are observed at the 2 resting poles (yellow arrows). The other green points correspond to false signals. (B) 8N to 16N endomitosis: the number of green dots shown by white arrows is indicated when greater than one. (C) Endomitosis of high ploidy: surimposition of both PRINS-probed centromeres of chromosome 1 in green and counterstained nuclei in blue. Bar, 5μm.
P55CDC/hCDC20 protein is expressed and normally regulated in polyploid megakaryocytes
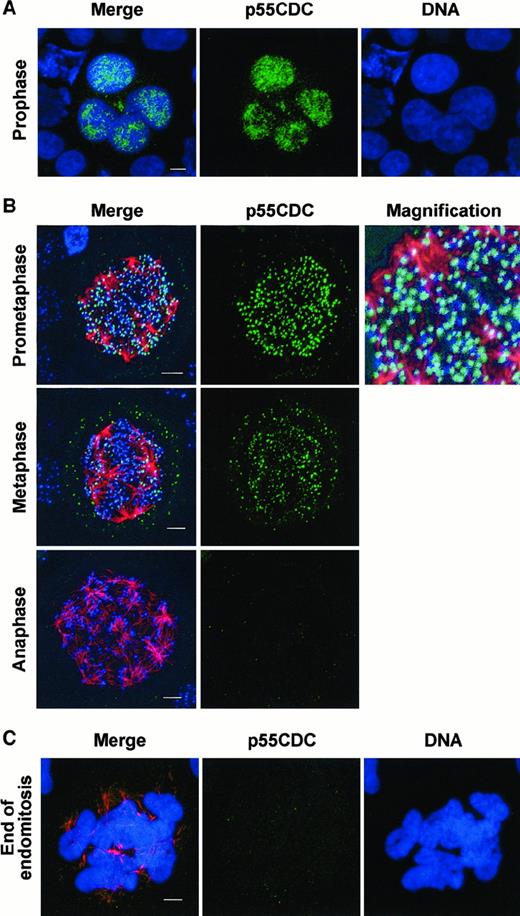
P55CDC/hCDC20 is involved in metaphase/anaphase checkpoint, which appears to be normal in megakaryocytes but also may regulate late phases of the mitosis.22 We, therefore, studied p55CDC/hCDC20 expression in megakaryocytes and examined its cell-cycle changes during endomitosis. By immunostaining experiments, p55CDC was detected in megakaryocyte nuclei (Figure5A). Double labeling with a human CREST antibody colocalized p55CDC to each kinetochore at prometaphase and metaphase. Finally, when using a third staining with an antitubulin antibody, the p55CDC localization and the kinetics of its disappearance could be studied during an endomitotic cell cycle (Figure 5B,C). The results were consistent with those previously obtained by Kallio et al22 during a normal mitosis. P55CDC accumulated in the nucleus at prophase; it concentrated at kinetochores at prometaphase with a heterogeneous staining intensity between chromosomes: Those closest to the spindle poles, which have not yet been correctly attached to the microtubules, had a brighter staining than those close to the metaphase plate. At metaphase, all chromosomes accumulated in the center of the spindle, forming a complex metaphase plate, had a quite uniform and less intense p55CDC signal. At anaphase p55CDC could not be detected. Thus, p55CDC has a normal expression in megakaryocytes.
P55CDC dynamics and localization in endomitotic megakaryocytes.
Immunofluorescence experiments were performed on human megakaryocytes at day 5, following CD34+ cells cultured with PEG-rhuMGDF and SCF. Cells were cytocentrifuged, fixed with paraformaldehyde, and permeabilized with triton. (A) p55CDC immunostaining was performed by using a rabbit antibody followed by incubation with donkey FITC-labeled antirabbit (in green), and DNA was counterstained with TOTO3-iodide (in blue). (B) Triple immunostaining was performed by using rabbit anti-p55CDC, mouse antitubulin, and human antikinetochore antibodies, followed by incubation with donkey FITC-labeled antirabbit (in green), TRITC-conjugated antimouse (in red), and Cy-5 labeled antihuman (in blue), F(ab′)2 fragments, respectively. (C) Double immunostaining anti-p55CDC (in green) and antitubulin (in red) and nuclei counterstained with TOTO3-iodide (in blue). In A, B, and C, cells were examined by confocal microscopy with a 3-dimensional reconstruction and superimposition of the markers. Triple staining merge is shown on the first column. (A) Endomitotic megakaryocyte at prophase. (B) 8N to 16N endomitosis at prometaphase, metaphase, anaphase. P55CDC and kinetochore signals colocalization provides a fusion pale-blue signal (corresponding magnification). (C) Megakaryocyte of high ploidy at the end of an endomitosis. Bar, 5μm.
P55CDC dynamics and localization in endomitotic megakaryocytes.
Immunofluorescence experiments were performed on human megakaryocytes at day 5, following CD34+ cells cultured with PEG-rhuMGDF and SCF. Cells were cytocentrifuged, fixed with paraformaldehyde, and permeabilized with triton. (A) p55CDC immunostaining was performed by using a rabbit antibody followed by incubation with donkey FITC-labeled antirabbit (in green), and DNA was counterstained with TOTO3-iodide (in blue). (B) Triple immunostaining was performed by using rabbit anti-p55CDC, mouse antitubulin, and human antikinetochore antibodies, followed by incubation with donkey FITC-labeled antirabbit (in green), TRITC-conjugated antimouse (in red), and Cy-5 labeled antihuman (in blue), F(ab′)2 fragments, respectively. (C) Double immunostaining anti-p55CDC (in green) and antitubulin (in red) and nuclei counterstained with TOTO3-iodide (in blue). In A, B, and C, cells were examined by confocal microscopy with a 3-dimensional reconstruction and superimposition of the markers. Triple staining merge is shown on the first column. (A) Endomitotic megakaryocyte at prophase. (B) 8N to 16N endomitosis at prometaphase, metaphase, anaphase. P55CDC and kinetochore signals colocalization provides a fusion pale-blue signal (corresponding magnification). (C) Megakaryocyte of high ploidy at the end of an endomitosis. Bar, 5μm.
Spindle organization explains the paradox between an asymmetrical segregation of chromosomes and a normal mitotic checkpoint
Endomitotic megakaryocytes are characterized by a multipolar spindle. According to immunofluorescence section analysis of these spindles, several asters are bound together and form a complex array of interconnected microtubules (Figure 6). At metaphase, chromosomes migrated to the center of the spindle and formed a multibranched structure. At anaphase, sister chromatid separation occurred asynchronously, depending on the spindle localization (Figure 2D). The separated chromatids migrated toward each spindle pole, resulting in an asymmetrical distribution between the different poles. This asymmetry was related to the complex structure of the spindle, provided one aster is connected to more than one other aster. Thus, at the level of a “bipolar spindle” (ie, connection between 2 asters), chromosome segregation was symmetrical, but it became asymmetrical when the entire spindle was considered. In a manner similar to mitotic diploid cells, chromosomes clustered into a ring around each aster. In the absence of spindle elongation and disassociation movements of the chromosomes surrounding the aster, the different rings remained connected to each other. Consequently, the nuclear membrane may reform around a single polyploid nucleus. At this stage the spindle was degraded, and the chromosomes decondensed. Each chromosome ring appears to correspond to one lobe of the megakaryocytic nucleus (Figure 5C).
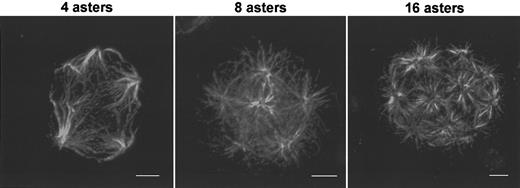
Structure of multipolar spindle in megakaryocytes.
Human megakaryocytes were obtained as previously described in Figure 1. Spindle immunostaining was performed with mouse antitubulin antibodies followed by incubation with TRITC-conjugated antimouse F(ab′)2 fragments. Endomitotic megakaryocytes with tetrapolar spindle, 8-polar spindle, and 16-polar spindle. Bar, 5 μm.
Structure of multipolar spindle in megakaryocytes.
Human megakaryocytes were obtained as previously described in Figure 1. Spindle immunostaining was performed with mouse antitubulin antibodies followed by incubation with TRITC-conjugated antimouse F(ab′)2 fragments. Endomitotic megakaryocytes with tetrapolar spindle, 8-polar spindle, and 16-polar spindle. Bar, 5 μm.
This hypothesis was verified by further analysis of interphase megakaryocytes. We observed a heterogeneous distribution of chromosome 11 labeled by FISH in the different lobes of the polyploid nuclei (Figure 2E). The significance of the megakaryocyte nucleus polylobulation was not well understood, but these results suggest that each lobe corresponds to the incompletely segregated chromosomes surrounding one aster as a consequence of the anaphase B defect.
Timing of cyclin B1 degradation is conserved in polyploid megakaryocytes
As we have first demonstrated that p55CDC/hCDC20 and the “first branch” of the spindle checkpoint were functional in polyploid megakaryocytes, we investigated the second main regulation pathway leading to mitotic exit.27
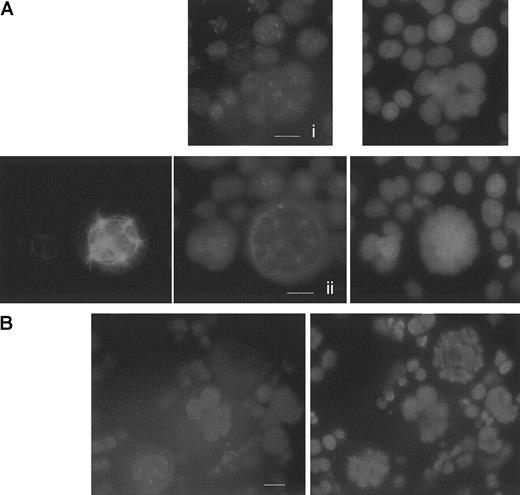
We first investigated expression of hCdh1, the second APC coactivator, by immunofluorescence experiments. We could observe that this protein was normally expressed in polyploid megakaryocytes (Figure7A). The nuclear punctuate staining was homogeneous in interphase (Figure 7Ai), consistent with previous experiments in human neuronal cells.34 During endomitosis, hCdh1 colocalized with the spindle poles (a common localization with cyclin B1) (Figure 7Aii,B), and these results were confirmed in the myeloid KG1a cell line. Nevertheless, as hCdh1 is activated through dephosphorylation more than through its expression level, these results do not demonstrate that this molecule is functional during endomitosis. Thus, we decided to study indirectly Cdh1 function through the analysis of the main hCdh1-APC complex substrate: cyclin B1. With the use of cyclin B1 immunostaining, we confirmed expression of cyclin B1 in polyploid megakaryocytes. Moreover, by analyzing many endomitosis situations, we showed that timing of the mitotic cyclin expression was analogous to that previously described in normal mitosis21(Figure 7B). During endomitosis, cyclin B1 displayed a strong nuclear labeling in prophase, then colocalized with spindle poles in prometaphase, whereas staining completely disappears at anaphase. Altogether, these results demonstrate that cyclin B1 is expressed in endomitotic megakaryocytes with a normal timing. Normal degradation of cyclin B1 concomitant with expression of hCdh1 provides a molecular environment25 in megakaryocytes favorable for G1-phase transition accomplishment and DNA re-replication despite an abortive mitosis.
Studies of hCdh1 and cyclin B1 expression in megakaryocytes.
Immunofluorescence was performed on day 6 megakaryocyte culture as described in Figure 5. (A) HCdh1 immunodetection in human megakaryocytes. (i) Interphase polyploid megakaryocyte shows a nuclear hCdh1 staining, using a polyclonal rabbit antibody followed by donkey TRITC-labeled F(ab′)2 fragments. DNA was counterstained with DAPI. (ii) Double immunostaining of hCdh1 and tubulin with DNA painting. HCdh1 was localized at the spindle poles during endomitosis. Bar 10μm. (B) Timing of cyclin B1 degradation in endomitosis. Cyclin B1 was immunodetected by using a mouse antibody followed by incubation with TRITC-conjugated antimouse F(ab′)2 fragments. DNA was labeled with DAPI. Three sequential phases of endomitosis were observed on a single view: G2/M megakaryocyte in the middle with a strong nuclear staining, prometaphase megakaryocyte in the lower-left corner with cyclin B1 localized at the spindle poles, and anaphase in the upper-right corner in which cyclin B1 is undetectable. Bar, 10 μm.
Studies of hCdh1 and cyclin B1 expression in megakaryocytes.
Immunofluorescence was performed on day 6 megakaryocyte culture as described in Figure 5. (A) HCdh1 immunodetection in human megakaryocytes. (i) Interphase polyploid megakaryocyte shows a nuclear hCdh1 staining, using a polyclonal rabbit antibody followed by donkey TRITC-labeled F(ab′)2 fragments. DNA was counterstained with DAPI. (ii) Double immunostaining of hCdh1 and tubulin with DNA painting. HCdh1 was localized at the spindle poles during endomitosis. Bar 10μm. (B) Timing of cyclin B1 degradation in endomitosis. Cyclin B1 was immunodetected by using a mouse antibody followed by incubation with TRITC-conjugated antimouse F(ab′)2 fragments. DNA was labeled with DAPI. Three sequential phases of endomitosis were observed on a single view: G2/M megakaryocyte in the middle with a strong nuclear staining, prometaphase megakaryocyte in the lower-left corner with cyclin B1 localized at the spindle poles, and anaphase in the upper-right corner in which cyclin B1 is undetectable. Bar, 10 μm.
Expression of MKLP1 in megakaryocytes
Finally, we investigated whether one of the main kinesin MKLP1 implicated in anaphase and cytokinesis35 36 was expressed in megakaryocytes. Immunofluorescence experiments showed that MKLP1 was normally present in the midzone of the multipolar spindle of megakaryocytes (Figure 8) like in other diploid mitotic cells, revealing that the first stages of the cleavage furrow organization occurs. Subsequent bundles of the microtubules were, however, not observed, as expected.
Immunolocalization of MKLP1 at anaphase.
Double immunostaining was performed on day 6 megakaryocyte culture by using polyclonal goat anti-MKLP1 and mouse antitubulin antibodies followed by incubation with donkey TRITC-conjugated antigoat (C) and FITC-labeled antimouse (D) F(ab′)2 fragments, respectively. DNA was painted with DAPI (B). Panel A shows the merging of the 3 markers. Bar, 10 μm.
Immunolocalization of MKLP1 at anaphase.
Double immunostaining was performed on day 6 megakaryocyte culture by using polyclonal goat anti-MKLP1 and mouse antitubulin antibodies followed by incubation with donkey TRITC-conjugated antigoat (C) and FITC-labeled antimouse (D) F(ab′)2 fragments, respectively. DNA was painted with DAPI (B). Panel A shows the merging of the 3 markers. Bar, 10 μm.
Discussion
Previous studies have provided some evidence that endomitosis could be a consequence of both a multipolar spindle that limits chromatid separation and the absence of cytokinesis.6 7Our purpose was to investigate whether chromosomes segregate normally during endomitosis. We addressed this question by localizing centromeres of either chromosome 1 or 7 in endomitotic human megakaryocytes.
Our main difficulty was to elaborate a reliable strategy to visualize one category of chromosomes and to follow their localization along the spindle during the different stages of mitosis. PRINS labeling is an alternative to FISH that has already proved its ability to label repeated sequences of DNA-like centromeres.37 For our purpose, it turned out to be an advantageous approach because of its simplicity, quickness, specificity, and the possibility to couple it with an immunostaining procedure. With this technique we observed an asymmetrical repartition of labeled chromosome 1s along the spindle at prometaphase and metaphase of each endomitosis from 4N to 32N cells. Indeed, megakaryocytes usually had the expected number of labeled chromosome 1s, but they were not symmetrically coupled to a spindle pole. A defined subnuclear localization of chromosomes, depending on their category, has been described.38 To demonstrate that the asymmetrical repartition was not due to a particular localization of chromosome 1s, we performed the same experiments with labeling of chromosome 7 centromeres and observed a similar asymmetrical repartition of this chromosome along the spindle of polyploid megakaryocytes.
The metaphase/anaphase checkpoint explored by spindle disruption with nocodazole treatment was normal because the endomitotic index increased with time in the presence of the drug, suggesting that the entry in anaphase is normally controlled. Thus, our goal was to demonstrate how chromosome segregation per se occurs during the endomitotic anaphase. A major obstacle was the scarceness of anaphase on slides, inasmuch as endomitotic megakaryocytes corresponded to less than 1% of the cell samples. Therefore, there was an obvious need to analyze a very large number of asynchronous endomitosis to observe a few anaphases. By this approach we could prove that chromosome disjunction was always a complete process for chromosome 1, 7, or 11. This result is in agreement with previous experiments, which have suggested by immunostaining that chromatid separation occurs in megakaryocytes.6 7 However, despite this normal centromere dissociation, the final repartition of sister chromatids was always asymmetrical between all the spindle poles leading to different numbers of chromosomes at each pole. It was possible to extrapolate these conclusions whatever the ploidy of human megakaryocytes (from 4N to 64N).
Furthermore, by studying late stages of endomitosis, we found that each lobe of the megakaryocyte nucleus corresponded to chromosomes that have migrated to one aster and thus are the equivalent of one nucleus. However, this asymmetrical repartition of chromosomes explains why previously it has been shown that each individual lobe does not have a 2N ploidy.39
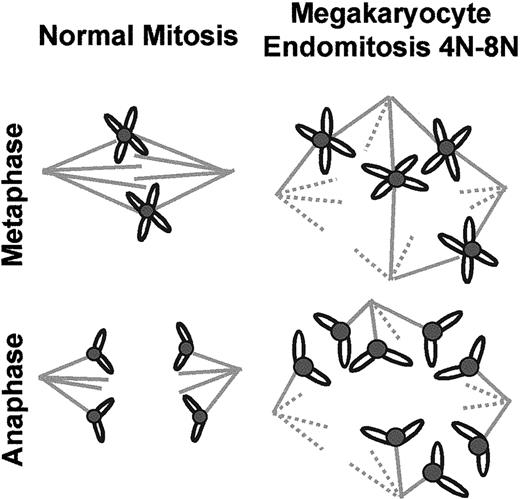
To our knowledge, there is no precedent that an “atypical” segregation of chromosomes is allowed by a normal mitotic checkpoint, 2 statements which are apparently contradictory. One explanation could be found in the structure of megakaryocytic spindles. A 2-pole spindle has only 2 microtubule arrays, and there is theoretically no problem of “choice” for chromosome attachment. In contrast, immunofluorescence studies have shown that the megakaryocytic spindle contained several asters that turned out to be interconnected. In that case, the chromosomes can be effectively bound in a stable manner to microtubules emanating from 2 different asters, which seem to be randomly chosen. Then sister chromatids separate normally and migrate symmetrically between these 2 poles. However, chromosome distribution is ultimately asymmetrical when considering the entire multipolar spindle. Thus, the definitive asymmetrical localization of chromosomes appears to be due to the existence of multiple interconnections between the different asters. These results are summarized in a model in Figure9.
Schematic model of chromosome segregation during megakaryocytic endomitosis.
The left drawing shows the symmetrical chromosome segregation along the bipolar spindle of a normal diploid cell. The right drawing shows an example of asymmetrical chromosome segregation due to a multipolar complex spindle in a 4N to 8N megakaryocyte.
Schematic model of chromosome segregation during megakaryocytic endomitosis.
The left drawing shows the symmetrical chromosome segregation along the bipolar spindle of a normal diploid cell. The right drawing shows an example of asymmetrical chromosome segregation due to a multipolar complex spindle in a 4N to 8N megakaryocyte.
Previously, the phosphoepitope 3F3/2 was shown to disappear during endomitosis,7 a result also in favor of a normal regulation of the metaphase/anaphase transition.40 To further demonstrate this hypothesis, we investigated the expression and localization of P55CDC/hCDC20 in megakaryocytes. P55CDC plays a major role in mitosis by conferring the specificity of the proteolysis that occurs at the metaphase/anaphase transition and may also be involved in the regulation of mitotic exit. The level of P55CDC is regulated during the cell cycle with a peak at mitotic entry and a fall at mitotic exit. Immunofluorescence demonstrates that p55CDC is maximally expressed at prometaphase and is localized on kinetochores. The staining is lost in anaphase. The same dynamics and localization of P55CDC were observed in megakaryocytes at all levels of ploidy.
Previous studies have involved cyclin B1 in the endomitotic process with controversial results. Two found a high level of cyclin B1 in polyploid endomitotic megakaryocytes.6,7 In contrast, other studies in megakaryocytic cell line and primary megakaryocytes showed a reduced level of cyclin B1 correlated with a modified dynamics during endomitosis, related to an enhanced ability to degrade cyclin B1.30 Our immunofluorescence experiments definitively show that cyclin B1 has strictly the same timing in endomitotic megakaryocytes as in other human cells with a strong nuclear staining in G2 prophase, a localization at the spindle poles in prometaphase, followed by an abrupt disappearance at anaphase.21 By the same method, hCdh1 was detected in megakaryocytes, but its functionality and role in endomitosis remain to be confirmed. Nonetheless, as described in other vertebrate somatic cells, the Cdh1-APC is supposed to be functional in G1 phase, allowing the re-replication of the genome since megakaryocytes have degraded cyclin B1 and exit of endomitosis.25
Taken together, our observations lead to the conclusion that neither chromosome segregation nor cyclin B1 regulation abnormalities are implicated in the endomitotic mechanism. Thus, endomitosis might be the consequence of microtubule motor proteins, as previously suggested by Nagata et al,6 so that the nuclear membrane finally reforms around a unique polyploid nucleus without telophase and cytokinesis. In diploid cells, the metaphase spindle steady-state structure is the result of antagonistic forces generated on one hand by the C terminal kinesins and on another hand by bipolar kinesins and the MKLP1 kinesins located at the midzone (reviewed in Sharp et al41). Anaphase B spindle elongation is usually correlated with the inactivation of “inward force.” Then, during endomitosis, the absence of anaphase B might correspond to 3 mechanisms. The first mechanism is the lack of microtubule motor proteins that participate in the separation of the 2 polar microtubule arrays. This seems unlikely as the first states of spindle assembly and metaphase plate normally occur and microtubule motors likely operate in a group with extensive overlapping function.42,43 Immunofluorescence studies also confirmed that the MKLP1 protein was present in megakaryocytes with an expected localization at the midzone of the spindle at anaphase. More than a structural defect, the endomitotic mechanism could effectively reside in an abnormal regulation of such “outward proteins” that may depend on the aurora kinase and polo kinase pathways.36,44 In favor of this hypothesis, the aurora kinase AIM1 has been shown to be down-regulated in the megakaryocyte.45 Finally, a defect of C kinesin degradation might also compromise the anaphase B process in megakaryocytes.
All these hypotheses have to be confirmed in the future with the help of other eukaryote cell models.
The authors thank J. M. Peters (Research Institute of Molecular Pathology, A-1030 Vienna, Austria) and J. C. Brouet (Hôpital St Louis, Paris, France) for the human antibodies, anti-hCdh1 and antikinetochores, respectively.
Supported by Fondation de la Recherche Médicale (FRM) (L.R.) and Association de la Recherche contre le Cancer (ARC 9728) (N.D.) from La Ligue Nationale contre le Cancer.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
William Vainchenker, INSERM U362, PR1, Institut Gustave Roussy, 39 rue Camille Desmoulins, Villejuif, 94805, France; e-mail: verpre@igr.fr.

![Fig. 2. Asymmetrical repartition of chromosome 1s on multipolar spindle of endomitotic megakaryocytes. / Double staining obtained by PRINS and immunofluorescence procedure, as reported in Figure 1, in endomitosis 4N to 8N (A), 8N to 16N (B), and 16N to 32N (C). Centromeres of chromosome 1s (green dots) are asymmetrically distributed among the different spindle poles, as no green dot is associated with some asters. Bar, 5 μm. (D) Asynchronous separation of sister chromatids in a 4N to 8N endomitosis. Additional DNA staining (in blue) was performed: chromosomes have congressed to a complex metaphase plate typical of the metaphase/anaphase transition. The white arrow shows the single chromosome 1 whose chromatids have already separated (2 dots). Panel E corresponds to an interphase megakaryocyte with positive vWF staining (in green): 8 chromosomes (red) are unevenly labeled by FISH (distributed between the 4 lobes of the nucleus [in blue]).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/97/8/10.1182_blood.v97.8.2238/5/m_h80810897002.jpeg?Expires=1765951118&Signature=Jzupa9F1ygMC6UZP0aUWDzTKzltXIiYJW7jhkbYcm6AtgZevvsYt6EPr2r4poCAvouC69~Pv6SDQWyES4utYF7gLkdDnibi2eVfds-RQpqQCSijUosMbOaVIQANFGi-~58mo4bNDRwX5bksJU74606qKV30T86-MV39AjDKb0oYRwG61LVYxERmtSdEoZ-stYEgcfVbAvEQlsY1-gJ-cP6kvL1oPkan3zmkIvoSUte3oR1ztTflFeM7sH3QBlhKc2vLu~VfTUNrNtFQqBQXaoSnawhjYcfNJLuauRa7OK8Iumtp34b6taqeHb6FB0jlmhK1gCaCZ6C8bzc6z0s8GGA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal