Abstract
Human T-cell leukemia virus type I (HTLV-I) Tax is a potent transcriptional regulator that can activate or repress specific cellular genes and that has been proposed to contribute to leukemogenesis in adult T-cell leukemia. Previously, HTLV-I– infected T-cell clones were found to be resistant to growth inhibition by transforming growth factor (TGF)-β. Here it is shown that Tax can perturb Smad-dependent TGF-β signaling even though no direct interaction of Tax and Smad proteins could be detected. Importantly, a mutant Tax of CREB-binding protein (CBP)/p300 binding site, could not repress the Smad transactivation function, suggesting that the CBP/p300 binding domain of Tax is essential for the suppression of Smad function. Because both Tax and Smad are known to interact with CBP/p300 for the potentiation of their transcriptional activities, the effect of CBP/p300 on suppression of Smad-mediated transactivation by Tax was examined. Overexpression of CBP/p300 reversed Tax-mediated inhibition of Smad transactivation. Furthermore, Smad could repress Tax transcriptional activation, indicating reciprocal repression between Tax and Smad. These results suggest that Tax interferes with the recruitment of CBP/p300 into transcription initiation complexes on TGF-β–responsive elements through its binding to CBP/p300. The novel function of Tax as a repressor of TGF-β signaling may contribute to HTLV-I leukemogenesis.
Introduction
Human T-cell leukemia virus type I (HTLV-I) is an etiologic agent of an acute malignancy of CD4+ T lymphocytes called adult T-cell leukemia (ATL).1,2 The virus-encoded regulatory protein, Tax, is critical for HTLV-I replication and is thought to contribute to ATL development. Several experimental observations indicate that Tax mediates the oncogenic activity of HTLV-I. For example, Tax immortalizes primary human T lymphocytes and transforms rodent fibroblasts in vitro.3-5In addition, transgenic mice expressing Tax develop mesenchymal tumors or large granular lymphocytic leukemia in vivo.6 7
The exact mechanism through which Tax exerts its oncogenic potential is still unknown. Tax was originally identified as a transcriptional activator for viral gene expression and then was shown to activate the expression of a number of cellular genes, many of which either encode proteins involved in the regulation of cellular proliferation (ie, interleukin [IL]-2,8 IL-2 receptor α chain,8,9 and proliferating cell nuclear antigen),10 or are proto-oncogenes (c-fos,11,12 c-jun,12and c-myc).13 In contrast to its transcriptional activation, Tax can also repress the expression of β polymerase, an enzyme important for DNA repair, and Bax, an accelerator of apoptosis.14,15 Furthermore, Tax alters the activity of a number of cell cycle regulators, including cyclin D,16,17 the mitotic checkpoint regulator MAD1,18 the cyclin-dependent kinases (Cdk) Cdk4 and Cdk6,19 the Cdk inhibitors p15INK4b, p16INK4a, and p18INK4b,20-22 the tumor suppressor p53, and the p53-related proteins p73 and p51.23-26 Thus, it is likely that Tax dysregulates the cell cycle through many different mechanisms, leading to the eventual immortalization and transformation of the infected cells.
Proliferation and differentiation of cells are tightly regulated by a delicate balance of growth factors and growth-inhibitory factors. Transforming growth factor (TGF)-β is one of the best-characterized members of growth-inhibitory factors. TGF-β can inhibit the growth of cells of epithelial, endothelial, and lymphoid origin.27Binding of TGF-β to the cell-surface type II TGF-β receptor (TβRII) results in the formation of a multimeric complex with type I TGF-β receptor (TβRI), followed by the phosphorylation and activation of TβRI by the TβRII kinase.28,29 The activated TβRI then interacts with an adaptor molecule, Smad anchor for receptor activation (SARA),30 which recruits downstream Smad2 and Smad3 proteins to be phosphorylated by TβRI.28 29
The Smad family proteins are critical components of the TGF-β signaling pathways.28,29 On stimulation by TGF-β, the pathway-restricted Smads, Smad2 and Smad3, interact with the TGF-β receptor complex and become phosphorylated on serine residues located at the carboxyl termini of the molecules.28,29Phosphorylated Smad2 and Smad3 then form multimeric complexes with the common mediator, Smad4, and translocate to the nucleus, where they can bind to the TGF-β–responsive promoter DNA either directly through the Smad-binding elements31-37 or in conjunction with other sequence-specific DNA-binding proteins such as FAST-1 and FAST-2.38-41 Smad proteins may form complexes with general transcriptional activators, such as cyclic adenosine monophosphate-responsive element-binding protein (CREB) binding protein (CBP)/p300 to regulate TGF-β signaling.42-46 All 3 Smad proteins are important tumor suppressors, and loss-of-function mutations in Smad2 and Smad4 have been found to associate with many types of human cancer.29
Previously, it was reported that HTLV-I–infected T-cell clones were resistant to growth inhibition by TGF-β, and this resistance correlated with the lack of prevention of retinoblastoma protein phosphorylation.47 Therefore, we investigated whether Tax might block TGF-β signaling. In this study, we show that the expression of Tax inhibits TGF-β–induced transactivation of the responsive promoters. Furthermore, we provide evidence to show that Tax inhibits the ability of the Smads to mediate TGF-β–induced transcriptional activation by interfering with the recruitment of CBP/p300. These results suggest that Tax may contribute to leukemogenesis by negatively regulating TGF-β signaling.
Materials and methods
Plasmid constructions
The SV40-driven expression vector for HTLV-I Tax, pH2R40M,4 the β-actin–driven expression vector for Tax, pβMT-2Tax, and for the Tax-derived mutants, pβTax703 (M47) and pβTaxM22,48 and the CMV-Tax expression vector and the CMV-driven expression vector for the Tax mutant, K88A,49have all previously been described. Expression vectors for Flag-Smad2, Flag-Smad3, Smad4-hemagglutinin (HA), TβRI-WT-HA, and TβRI-T204D-HA were generous gifts from Dr J. L. Wrana (Mount Sinai Hospital, Toronto, Canada). An expression plasmid for FAST-1 was provided by Dr M. Whitman (Harvard Medical School, Boston, MA). Expression plasmids for carboxyl terminal Flag-tagged CBP and p300 were obtained from Dr K. Miyazono (The Cancer Institute of Japanese Foundation for Cancer Research, Tokyo, Japan). The HTLV-I long terminal repeat (LTR) luciferase reporter plasmid, which contains the HindIII fragment from the HTLV-I LTR and κB-LUC,50 containing 5 tandem repeats of an NF-κB binding site from the IL-2 receptor α chain gene, were kindly provided by Dr I. Futsuki (Nagasaki University School of Medicine, Nagasaki, Japan) and Dr J. Fujisawa (Kansai Medical University, Osaka, Japan), respectively. p3TP-Lux was provided by Dr J. Massague (Memorial Sloan-Kettering Cancer Center, New York, NY).51 p800neoLuc and p15P113-Luc were generated as described previously.52 53 The ARE-Lux construct contains the activin response element (ARE) from the Xenopus Mix.2 gene.
Cell lines, transfections, and luciferase assays
HepG2, Mv1Lu, and COS7 cells were cultured in Dulbecco modified Eagle medium (DMEM) supplemented with 10% fetal bovine serum (FBS). For TGF-β–inducible luciferase reporter assays, HepG2 or Mv1Lu cells were seeded at a density of 3 × 105 per 6-cm plate. Cells were transfected 18 hours after seeding with various amounts of effector plasmids, along with the reporter plasmids and pRL-TK, an expression vector of renilla luciferase, using Lipofectamine (GIBCO-BRL, Gaithersburg, MD). Total amounts of DNA for each transfection were equalized by the addition of an empty vector. Cells were fed 7 hours later with equal volumes of DMEM containing 4% FBS and incubated for an additional 17 hours. Thereafter, cells were washed with phosphate-buffered saline twice and incubated for 24 hours in the absence or presence of 10 ng/mL TGF-β in DMEM containing 0.2% FBS. Luciferase assays were performed by using the Dual-Luciferase Reporter System (Promega, Madison, WI) in which relative luciferase activities were calculated by normalizing transfection efficiency according to the renilla luciferase activities. All transfection experiments were performed at least 3 times, and similar results were obtained.
Assay for cell proliferation
To generate stable Mv1Lu cell lines overexpressing Tax, cells were transfected with pH2R40M using lipofectamine. Cells were selected with G418 (800 μg/mL) for 2 to 3 weeks and then cloned using a cloning cylinder. The expression of Tax mRNA in cell clones was examined by reverse transcriptase-polymerase chain reaction as previously described.54 For cell proliferation studies, Tax-expressing clones and control clone were plated at a density of 5 × 103 cells/well in 96-well microtiter plates. After 24-hour plating, cells were treated with increasing concentrations of TGF-β for 48 hours and then assayed for cell growth with the use of a Cell Counting kit (Wako Chemical, Osaka, Japan) based on an MTT assay. Each experiment was performed at least 3 times, and typical results are shown.
Immunoprecipitation and Western blot analysis
COS7 cells transiently transfected with the indicated constructs were washed and lysed in TNE buffer. Whole-cell extracts or immunoprecipitates produced with anti-Flag, anti-HA, or anti-Tax (Lt-4)55 were visualized by immunoblotting with anti-Tax, anti-Flag, or anti-HA antibodies.
Results
Tax inhibits TGF-β–mediated transcriptional activation of the target promoter
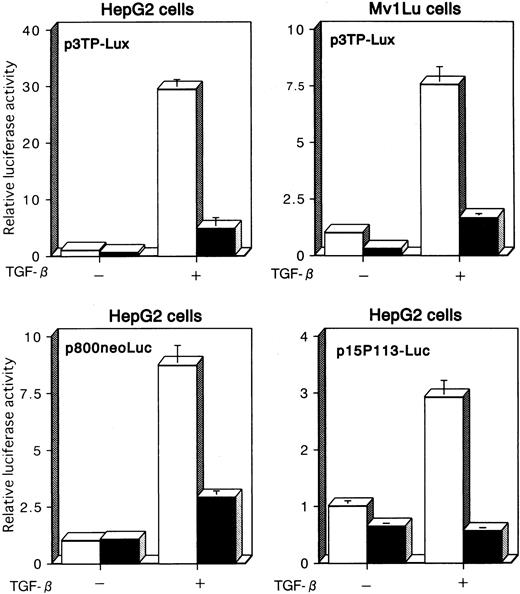
We examined the effects of Tax on TGF-β–mediated transcriptional responses using transient cotransfection assays. We first used p3TP-Lux, a TGF-β–responsive reporter plasmid that contains 3 repeats of a 12-O-tetradecanoylphorbol 13-acetate response element and a fragment from positions −636 to −740 of the human plasminogen activator inhibitor-1 (PAI-1) promoter.51,56-58 This construct has been shown to be efficiently stimulated by TGF-β through its receptors in a variety of cell lines. p3TP-Lux was transiently transfected together with either empty (pH2Rneo) or Tax expression vector (pH2R40M), and luciferase activity was measured in the extracts from untreated cells or cells treated with 10 ng/mL TGF-β for 24 hours. As a model for these experiments, we used HepG2 and Mv1Lu cells, which are frequently used for studies of TGF-β–induced transactivation because they express TGF-β receptors and are highly responsive to TGF-β and contain endogenous Smad2, Smad3, and Smad4. When p3TP-Lux alone was transfected into HepG2 or Mv1Lu cells, a significant increase in luciferase activity was observed in the presence of TGF-β (Figure1). These transactivations were repressed almost to control levels when Tax was transfected with p3TP-Lux. Similar repression occurred when we used another TGF-β–responsive reporter, p800neoLuc,52 which contained the PAI-1 promoter alone, and p15P113-Luc,53 which contained the p15 promoter (Figure 1). These results indicate that Tax may repress TGF-β signaling by interrupting intracellular signaling pathways.
TGF-β–mediated transcriptional responses are suppressed by Tax.
p3TP-Lux, p800neoLuc, or p15P113-Luc was cotransfected into HepG2 or Mv1Lu cells together with either pH2Rneo (■, −Tax) or pH2R40M (▪, +Tax). Cells were incubated for 24 hours in the presence or absence of 10 ng/mL TGF-β. Relative luciferase activities were measured in cell extracts, normalized to the renilla luciferase activity. Luciferase activity is presented as fold induction relative to the basal level measured in cells transfected with the reporter plasmid alone without further treatment. Data represent the mean ± SD from 3 separate experiments.
TGF-β–mediated transcriptional responses are suppressed by Tax.
p3TP-Lux, p800neoLuc, or p15P113-Luc was cotransfected into HepG2 or Mv1Lu cells together with either pH2Rneo (■, −Tax) or pH2R40M (▪, +Tax). Cells were incubated for 24 hours in the presence or absence of 10 ng/mL TGF-β. Relative luciferase activities were measured in cell extracts, normalized to the renilla luciferase activity. Luciferase activity is presented as fold induction relative to the basal level measured in cells transfected with the reporter plasmid alone without further treatment. Data represent the mean ± SD from 3 separate experiments.
To determine whether Tax could affect the antiproliferative effects of TGF-β in vivo, we established several Mv1Lu cell lines that stably expressed Tax mRNA at similar levels (Figure2A). In the presence of TGF-β, the growth of the control cell lines was effectively inhibited. In contrast, overexpression of Tax prevented the cells from undergoing growth arrest even after exposure to TGF-β (Figure 2B).
Resistance of Tax-expressing cells to TGF-β treatment.
(A) Expression of Tax mRNA in stable Mv1Lu transfectants. A clone Neo-10 (lane 1) is a control line obtained from Mv1Lu cells transfected with pH2Rneo, followed by G418 selection. Clones Tax-3 (lane 2), Tax-10 (lane 3), and Tax-15 (lane 4) were established from cells transfected with pH2R40M. As a positive control, total cellular RNA from HTLV-I–infected HUT-102 cells (lane 5) was used. (B) Tax-expressing (clones Tax-3 [●], Tax-10 [○], and Tax-15 [▵]) and unmodified (clone Neo-10 [■]) Mv1Lu cells were treated with the indicated concentrations of TGF-β for 48 hours. Proliferation of cells was examined by MTT assay. Results are expressed as percentages of the values obtained from control cultures that did not receive TGF-β. Each experiment was performed at least 3 times, and representative results are shown.
Resistance of Tax-expressing cells to TGF-β treatment.
(A) Expression of Tax mRNA in stable Mv1Lu transfectants. A clone Neo-10 (lane 1) is a control line obtained from Mv1Lu cells transfected with pH2Rneo, followed by G418 selection. Clones Tax-3 (lane 2), Tax-10 (lane 3), and Tax-15 (lane 4) were established from cells transfected with pH2R40M. As a positive control, total cellular RNA from HTLV-I–infected HUT-102 cells (lane 5) was used. (B) Tax-expressing (clones Tax-3 [●], Tax-10 [○], and Tax-15 [▵]) and unmodified (clone Neo-10 [■]) Mv1Lu cells were treated with the indicated concentrations of TGF-β for 48 hours. Proliferation of cells was examined by MTT assay. Results are expressed as percentages of the values obtained from control cultures that did not receive TGF-β. Each experiment was performed at least 3 times, and representative results are shown.
Tax inhibits Smad-induced responses to TGF-β
Smad proteins play an important role in mediating TGF-β–induced transcriptional activation of downstream genes. To investigate the effects of Tax on Smad signaling, we assayed transcriptional responses in the presence of various Smad proteins. As shown in Figure3, Tax inhibits Smad2-, Smad3-, Smad4-, or a combination of Smad2 and Smad4 (Smad2/4)- or Smad3 and Smad4 (Smad3/4)-induced transcriptional activation of p3TP-Lux. Therefore, Tax inhibited TGF-β signaling by blocking the ability of the Smad2/Smad4 and Smad3/Smad4 complex to activate the transcription of TGF-β–responsive genes.
Tax inhibits Smad-induced responses to TGF-β.
Either pH2Rneo ([■], −Tax) or pH2R40M ([▪], +Tax), in combination with p3TP-Lux, was transfected into HepG2 cells, together with the indicated Smad constructs in the absence or the presence of 10 ng/mL TGF-β. Relative luciferase activities were measured in cell extracts, normalized to the renilla luciferase activity. Luciferase activity is presented as fold induction relative to the basal level measured in cells transfected with p3TP-Lux alone without treatment. Data represent the mean ± SD from 3 separate experiments.
Tax inhibits Smad-induced responses to TGF-β.
Either pH2Rneo ([■], −Tax) or pH2R40M ([▪], +Tax), in combination with p3TP-Lux, was transfected into HepG2 cells, together with the indicated Smad constructs in the absence or the presence of 10 ng/mL TGF-β. Relative luciferase activities were measured in cell extracts, normalized to the renilla luciferase activity. Luciferase activity is presented as fold induction relative to the basal level measured in cells transfected with p3TP-Lux alone without treatment. Data represent the mean ± SD from 3 separate experiments.
Tax inhibits transcriptional activation induced by the constitutively active TβRI
Smad2 and Smad3 are directly phosphorylated by the activated TβRI, associate with Smad4, and are subsequently translocated into the nucleus.28 29 To determine whether the activated TβRI-induced transcription is affected by Tax, we used TβRI-T204D (a constitutively active TGF-β type I kinase receptor). TβRI-T204D induced transcription, which is likely mediated by endogenous Smad proteins. On the other hand, elevation of luciferase activity by TβRI-WT (wild-type TGF-β type I receptor) did not occur with the p3TP-Lux construct because TβRI-WT could not signal in the absence of ligand. TβRI-T204D induced transcription was significantly reduced by Tax (Figure 4A).
TGF-β activation of Smad-responsive reporters is inhibited by Tax.
■, −Tax; ▪, +Tax. (A) Tax inhibits transcriptional activation induced by the constitutively active TβRI. Three micrograms pH2Rneo (−Tax) or pH2R40M (+Tax) was transfected with 5 μg TβRI-WT or TβRI-T204D into HepG2 cells. Luciferase activity derived from the cotransfected p3TP-Lux reporter construct is depicted. Luciferase activity is presented as fold induction relative to the basal level measured in cells transfected with p3TP-Lux alone. (B) TGF-β–dependent induction of the ARE is inhibited by Tax. HepG2 cells were transfected with the ARE-Lux reporter construct (1 μg), FAST-1 (1 μg), Smad2 (2 μg), Smad4 (2 μg), and 3 μg of either pH2Rneo (−Tax) or pH2R40M (+Tax), as indicated. Cells were incubated in the absence or presence of 10 ng/mL TGF-β, and the luciferase activity was analyzed. Luciferase activity is presented as in Figures 1and 3, except that the control was based on untreated Smad2/4.
TGF-β activation of Smad-responsive reporters is inhibited by Tax.
■, −Tax; ▪, +Tax. (A) Tax inhibits transcriptional activation induced by the constitutively active TβRI. Three micrograms pH2Rneo (−Tax) or pH2R40M (+Tax) was transfected with 5 μg TβRI-WT or TβRI-T204D into HepG2 cells. Luciferase activity derived from the cotransfected p3TP-Lux reporter construct is depicted. Luciferase activity is presented as fold induction relative to the basal level measured in cells transfected with p3TP-Lux alone. (B) TGF-β–dependent induction of the ARE is inhibited by Tax. HepG2 cells were transfected with the ARE-Lux reporter construct (1 μg), FAST-1 (1 μg), Smad2 (2 μg), Smad4 (2 μg), and 3 μg of either pH2Rneo (−Tax) or pH2R40M (+Tax), as indicated. Cells were incubated in the absence or presence of 10 ng/mL TGF-β, and the luciferase activity was analyzed. Luciferase activity is presented as in Figures 1and 3, except that the control was based on untreated Smad2/4.
TGF-β–dependent induction of the ARE is inhibited by Tax
In addition to analysis of the p3TP-Lux construct, we examined induction of the ARE construct, which contains Smad-responsive ARE sites from the Xenopus Mix.2 gene driving expression of a luciferase reporter in HepG2 cells. This ARE is stimulated by either TGF-β or activin signaling, which induces assembly of a DNA-binding complex that is composed of Smad2, Smad4, and a member of the FAST family of forkhead DNA-binding proteins. Because HepG2 cells do not have endogenous FAST activity, in the absence of overexpressed FAST, the ARE-Lux reporter construct was not stimulated by the overexpression of a combination of Smad2 and Smad4, either in the presence or absence of TGF-β (Figure 4B). In the presence of overexpressed FAST, the reporter gene activity was induced by TGF-β, which may be explained by an activation mediated by endogenous Smad2 and Smad4 proteins. Furthermore, the coexpression of Smad2, Smad4, and FAST resulted in an activation of the ARE-Lux construct, indicating that these 3 different proteins form a transcriptionally active complex. Enhancement of these transcription by TGF-β occurred. We investigated the effect of Tax on transcriptional activation of the ARE. Cotransfection of Tax markedly inhibited transcriptional activation of the ARE-Lux, mediated by a complex of Smad2, Smad4, and FAST (Figure 4B).
Lack of interaction of Tax with Smad proteins
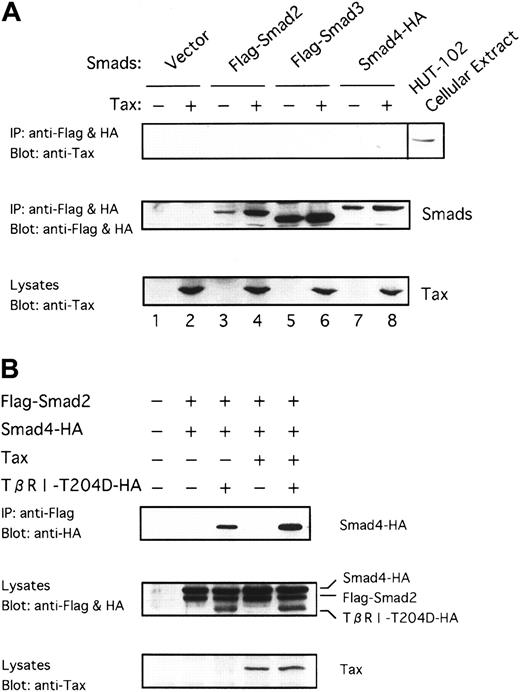
Because Tax was shown to associate with DNA-binding proteins in transactivation,59 60 it was suspected that Tax might physically interact with Smad proteins. To identify the target through which Tax represses TGF-β signaling, we examined whether Tax could interact with the Smad proteins. To this end, we transfected Smad2 and Smad3 tagged with the Flag peptide (Flag-Smad2 and Flag-Smad3) and Smad4 tagged with the HA peptide (Smad4-HA) into COS7 cells in the absence or the presence of Tax. Whole extracts from these cells were immunoprecipitated with the anti-Flag and anti-HA antibodies, and the precipitates were analyzed by immunoblotting with the anti-Tax antibody. As shown in Figure 5A, we could not observe that Tax was coimmunoprecipitated. Smad proteins were expressed efficiently along with Tax in the transfected cells, as can be seen by immunoblotting with the anti-Flag and anti-HA or the anti-Tax antibody (Figure 5A, middle and bottom, lanes 3-8). Whole cell extracts from COS7 cells transfected were also immunoprecipitated using anti-Tax. However, Smad proteins were not detected in the immunoprecipitates with anti-Tax (data not shown). These results suggest that Tax possibly antagonizes TGF-β signaling through an indirect mechanism that does not involve binding to Smad proteins.
Tax neither interacts with Smads nor inhibits receptor-dependent formation of heteromers containing Smads.
(A) Tax does not interact directly with Smad proteins. Tax was transfected into COS7 cells with the indicated Flag-tagged or HA-tagged Smad constructs. Cell extracts were subjected to immunoprecipitation (IP) with anti-Flag and anti-HA. This was followed by immunoblotting with anti-Tax antibody. As positive control, whole-cell lysates from HTLV-I–infected HUT-102 cells were blotted directly with anti-Tax (lane labeled as HUT-102 cellular extract) (top). Immunoprecipitates were blotted with anti-Flag and anti-HA to control for Smad expression (middle). Cell lysates were blotted with anti-Tax to control for Tax expression (bottom). (B) Tax does not inhibit receptor-dependent formation of heteromers containing Smad2 and Smad4. COS7 cells were transfected with the indicated combinations of Flag-Smad2, Smad4-HA, Tax, and TβRI-T204D-HA. The top panel shows the receptor-dependent formation of heteromers containing Smad2 and Smad4, and the lower 2 panels show the expression of each protein as indicated.
Tax neither interacts with Smads nor inhibits receptor-dependent formation of heteromers containing Smads.
(A) Tax does not interact directly with Smad proteins. Tax was transfected into COS7 cells with the indicated Flag-tagged or HA-tagged Smad constructs. Cell extracts were subjected to immunoprecipitation (IP) with anti-Flag and anti-HA. This was followed by immunoblotting with anti-Tax antibody. As positive control, whole-cell lysates from HTLV-I–infected HUT-102 cells were blotted directly with anti-Tax (lane labeled as HUT-102 cellular extract) (top). Immunoprecipitates were blotted with anti-Flag and anti-HA to control for Smad expression (middle). Cell lysates were blotted with anti-Tax to control for Tax expression (bottom). (B) Tax does not inhibit receptor-dependent formation of heteromers containing Smad2 and Smad4. COS7 cells were transfected with the indicated combinations of Flag-Smad2, Smad4-HA, Tax, and TβRI-T204D-HA. The top panel shows the receptor-dependent formation of heteromers containing Smad2 and Smad4, and the lower 2 panels show the expression of each protein as indicated.
Tax does not inhibit receptor-dependent formation of heteromers containing Smad2 and Smad4
Smad2 or Smad3 is directly phosphorylated by the activated TβRI, associates with Smad4, and is subsequently translocated into the nucleus.28 29 To determine which of these processes is affected by Tax, we first examined ligand-induced formation of heteromers containing Smad2 and Smad4. As shown in Figure 5B, Smad2 associates with Smad4 by the constitutively activated TβRI (TβRI-T204D) in the presence of Tax as strongly as it is in the absence of Tax, indicating that Tax does not inhibit receptor-dependent heteromers formation of Smad2 and Smad4. Tax did not immunoprecipitate with heteromers containing Smad2 and Smad4 (data not shown).
Tax mutant defective in CBP/p300 binding fails to repress the Smad-dependent transcriptional activation
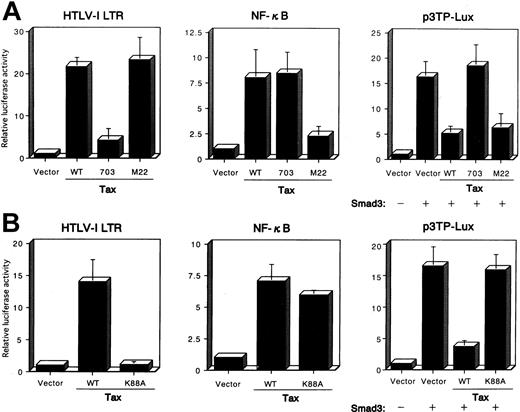
To analyze further the pathways through which Tax inhibited TGF-β signaling, we examined several previously characterized Tax mutants to see which failed to inhibit Smad transactivation. After an initial screen of multiple Tax mutants, we obtained data for 2 Tax mutants, Tax703 (M47), which contains amino acid substitutions at positions 319 and 320, and M22, which contains amino acid substitutions at positions 130 and 131.48 An HTLV-I LTR luciferase reporter that contains 3 unique CRE-containing 21-bp repeats was used to assay for the effects of Tax on the CREB pathway. HepG2 cells were transfected with the HTLV-I LTR-LUC or κB-LUC reporter plasmids, together with the control pHβAPr-1-neo or plasmid expressing the wild-type Tax or the Tax mutants M22 and Tax703. These studies demonstrated that wild-type Tax could activate gene expression from both the HTLV-I LTR and the NF-κB (Figure6A). In contrast, the Tax mutant Tax703 was defective in the activation of gene expression from the HTLV-I LTR but not the NF-κB, whereas the Tax mutant M22 activated gene expression from the HTLV-I LTR but not the NF-κB. The relative ability of each Tax mutant to repress the PAI-1 promoter luciferase was then compared in transient transfection assays in HepG2 cells. Both wild-type Tax and M22 were able to significantly repress transcription from the PAI-1 promoter (Figure 6A). In contrast, Tax mutant Tax703 failed to inhibit Smad function. These results indicate that Tax-mediated activation of CREB pathway is essential for the repression of Smad transactivation function. Interaction of Tax with CBP/p300 is essential for transactivation of the viral LTR.49,61Tax703 showed a decreased binding of CBP.62 To further demonstrate that Tax interaction with CBP/p300 was necessary for the repression of the PAI-1 promoter, we used a Tax mutant defective for CBP/p300 interaction.49 Tax K88A carries a single point mutation within the CBP/p300 binding domain, and this protein does not interact with the amino-terminal KIX domain of CBP/p300.49Tax K88A activated NF-κB but not HTLV-I LTR promoter activity, whereas wild-type Tax activated both promoter activities in HepG2 cells (Figure 6B). Using this mutant Tax, we analyzed the effect on the transactivation functions of Smad protein. As expected, Tax K88A failed to repress transcription from the PAI-1 promoter (Figure 6B), indicating that the CBP/p300 binding domain of Tax is involved in the suppression of Smad transactivation function. Taken together, our data demonstrate that Tax repression of the PAI-1 promoter activity correlates with the ability of Tax to interact with the coactivators CBP or p300.
Mutation of the Tax affects the Tax-mediated repression of the transactivation functions of Smad3.
(A) HepG2 cells were cotransfected with 10 ng HTLV-I LTR-LUC, 100 ng κB-LUC, or 100 ng p3TP-Lux reporter plasmids, together with 100 ng Smad3 expression plasmid, or 3 μg plasmid expressing wild-type Tax—pβMT-2Tax (WT), a mutant Tax, pβTax (703), or pβTax (M22). The luciferase assay was performed 24 hours later. (B) CBP/p300-binding domain in Tax is essential for the Tax-mediated repression of Smad3 transactivation functions. HepG2 cells were cotransfected, as in Figure6, panel A. All transfections were equalized for total DNA by addition of the empty vector. Luciferase activity is presented as fold induction relative to the basal level measured in cells transfected with the reporter plasmid alone. Data represent the mean ± SD from 3 separate experiments.
Mutation of the Tax affects the Tax-mediated repression of the transactivation functions of Smad3.
(A) HepG2 cells were cotransfected with 10 ng HTLV-I LTR-LUC, 100 ng κB-LUC, or 100 ng p3TP-Lux reporter plasmids, together with 100 ng Smad3 expression plasmid, or 3 μg plasmid expressing wild-type Tax—pβMT-2Tax (WT), a mutant Tax, pβTax (703), or pβTax (M22). The luciferase assay was performed 24 hours later. (B) CBP/p300-binding domain in Tax is essential for the Tax-mediated repression of Smad3 transactivation functions. HepG2 cells were cotransfected, as in Figure6, panel A. All transfections were equalized for total DNA by addition of the empty vector. Luciferase activity is presented as fold induction relative to the basal level measured in cells transfected with the reporter plasmid alone. Data represent the mean ± SD from 3 separate experiments.
Coexpression of CBP and p300 recovers repression of Smad3-mediated transactivation by Tax
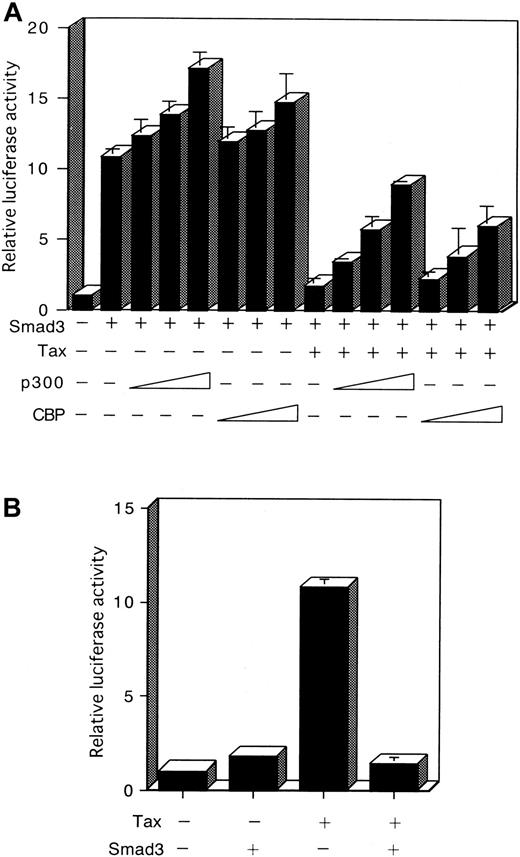
Recently, association of various Smads with the coactivators CBP and p300 for the potentiation of TGF-β–induced transcriptional activity has been demonstrated.42-46 Tax was also shown to bind to CBP and p300.62 63 The observations described above suggested that the suppression of Smad transactivation by Tax might occur through sequestration of a limiting pool of common transcriptional coactivators, such as CBP and p300, and thus may be reversed by the expression of additional amounts of these coactivators. First, we showed that cotransfection of HepG2 cells with Smad3 and with a CBP or p300 expression plasmid, together with the p3TP-Lux, led to an increase of Smad3 transcriptional activity (Figure7A). Next, a CBP or p300 expression plasmid was cotransfected with a p3TP-Lux reporter plasmid, together with Tax and Smad3 expression plasmids. As observed previously (Figure3), Tax inhibited Smad3 transcriptional activity in HepG2 cells (Figure7A). Significantly, coexpression of CBP or p300 reversed the inhibition of Smad3 by Tax (Figure 7A). These results confirm that CBP/p300 potentiated Smad3-dependent transcription. They also indicate these coactivators counter-inhibited the Tax trans-repressing effect on Smad3-dependent transcription.
Reciprocal repression between Tax and Smad3 is mediated through competition for CBP/p300.
(A) Repression of Smad3-mediated transactivation by Tax is recovered by p300 or CBP. HepG2 cells were transfected with 100 ng p3TP-Lux, 100 ng Smad3 expression plasmid, 3 μg Tax expression plasmid, and 0.1, 0.5, or 2 μg p300 or CBP expression plasmid. Cells were harvested 24 hours after transfection, and a luciferase assay was performed. Luciferase activity is presented as fold induction relative to the basal level measured in cells transfected with p3TP-Lux alone. (B) Reciprocal repression between Tax and Smad3. HepG2 cells were transfected with 10 ng HTLV-I LTR-LUC plasmid in combination with 3 μg of either the Tax or Smad3 expression plasmid. Results shown are expressed as the fold activation of luciferase activity of cells transfected with the LTR-LUC alone. Data represent the mean ± SD from 3 separate experiments.
Reciprocal repression between Tax and Smad3 is mediated through competition for CBP/p300.
(A) Repression of Smad3-mediated transactivation by Tax is recovered by p300 or CBP. HepG2 cells were transfected with 100 ng p3TP-Lux, 100 ng Smad3 expression plasmid, 3 μg Tax expression plasmid, and 0.1, 0.5, or 2 μg p300 or CBP expression plasmid. Cells were harvested 24 hours after transfection, and a luciferase assay was performed. Luciferase activity is presented as fold induction relative to the basal level measured in cells transfected with p3TP-Lux alone. (B) Reciprocal repression between Tax and Smad3. HepG2 cells were transfected with 10 ng HTLV-I LTR-LUC plasmid in combination with 3 μg of either the Tax or Smad3 expression plasmid. Results shown are expressed as the fold activation of luciferase activity of cells transfected with the LTR-LUC alone. Data represent the mean ± SD from 3 separate experiments.
Reciprocal repression between Tax and Smad3
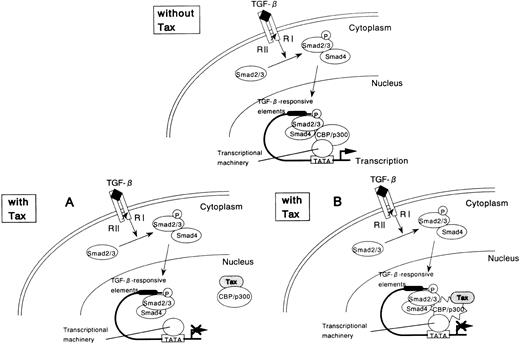
If repression of Smad3 by Tax occurs as a consequence of competition for CBP/p300, then overexpression of Smad3 should similarly repress Tax function. To test this possibility, we performed the reciprocal experiment using a reporter plasmid HTLV-I LTR-LUC. Cotransfection of the Smad3 expression plasmid repressed Tax transcriptional activation of the HTLV-I LTR (Figure 7B). The reciprocal repression observed with these 2 transcription factors shows that a cross-coupling mechanism is operating between Tax and Smad. Tax might compete with Smad in binding with CBP/p300, thereby repressing its transactivation function (Figure 8A). However, Tax has been shown to interact with the amino-terminal KIX domain, whereas the Smad proteins interact with a carboxy-terminal region of CBP/p300.62-64 Alternatively, either Tax or Smad3 directly interacts with CBP/p300, and this interaction leads to a change in conformation or stability of the complex comprising the other factor and CBP/p300 (Figure 8B). Taken together, these results indicate that the corepression of transcriptional activity by Tax and Smad is consistent with the sequestration of a limiting pool of CBP/p300.
Tax-mediated inhibition of TGF-β–induced and Smad-induced transcription: a model.
(A) Tax inhibits Smad-dependent transcription by competing with Smad for binding to the coactivator CBP/p300. (B) Tax binds to CBP/p300 and leads to a change in conformation or stability of the Smad-CBP/p300 complex, thereby repressing Smad transactivation function.
Tax-mediated inhibition of TGF-β–induced and Smad-induced transcription: a model.
(A) Tax inhibits Smad-dependent transcription by competing with Smad for binding to the coactivator CBP/p300. (B) Tax binds to CBP/p300 and leads to a change in conformation or stability of the Smad-CBP/p300 complex, thereby repressing Smad transactivation function.
Discussion
In the current study, we show that the HTLV-I Tax functions as a negative regulator in TGF-β signaling. We provide evidence to indicate that Tax can repress TGF-β–mediated growth inhibition in Mv1Lu cells. However, Mv1Lu mink lung epithelial cells are not the natural targets for HTLV-I infection. Therefore, we investigated whether Tax modified TGF-β signaling in T cells. As was observed in Mv1Lu cells, Tax represses growth-inhibitory signaling by TGF-β in CTLL-2, an IL-2–dependent T-cell line (data not shown). Various oncoproteins have been shown to interact with Smad proteins and directly block key steps in Smad signaling.65-68 However, Tax does not physically interact with Smad2, Smad3, and Smad4. Rather, it inactivates Smads through indirect interaction. These results are in contrast to what was found in E1A, Evi-1, SnoN, and Ski.65-68 Tax does not inhibit receptor-dependent formation of heteromers containing Smad2 and Smad4. Moreover, Tax does not change the DNA-binding activity and nuclear localization of Smads (data not shown). Thus, Tax-Smads interactions cannot account for the Tax-mediated inhibitory effect.
Tax is known to target cellular proteins, such as IκBα and IκBβ to the ubiquitin–proteasome degradation pathway.69,70However, Smads expression was not significantly affected when Smads and Tax were coexpressed in transient transfection assays, suggesting that Tax suppression of Smads transactivation potential and the consequent repression of the PAI-1 promoter are not direct consequences of a decrease in Smads at the protein level. Because both Tax and Smads use CBP/p300 to activate transcription, we considered direct coactivator competition as a conceivable mechanism for the observed Tax repression of Smad function in vivo. It is noteworthy that CBP/p300 protein is generally present at limiting concentrations within the cell nucleus, creating an environment of coactivator competition between transcription factors and providing an additional layer of regulated gene expression. Several recent studies suggest that a functional antagonism between transcription factors occurs as a consequence of direct competition for binding to common regions of CBP/p300.71,72 Consequently, we investigated whether the interaction of Tax with the transcriptional coactivators CBP/p300 is involved in the repression of the transcriptional activity of Smads. Importantly, a Tax mutant, K88A, defective in binding CBP/p300, could not repress the transactivation function of Smad, suggesting that the interaction of Tax with the coactivators might be necessary and sufficient to promote the inhibitory effect of this viral regulatory protein. Furthermore, CBP/p300 overexpression antagonizes the Tax trans-repressing effect. Because Smads and Tax have been previously reported to bind to distinct regions of CBP/p300,62-64this mechanism initially appeared unlikely. However, this competition does not require an identical or overlapping binding site on the coactivator and has been described for several cellular pathways, such as the nuclear receptor and AP-1, p53 and E2F, NF-κB and p53, NF-κB and nuclear receptor, Jak-Stat and AP-1 pathways.71-75Similarly, in the current study, we show that a cross-coupling mechanism is operating between Smad3 and Tax. Tax, when overexpressed, was found to repress the transcriptional activity of Smad3, whereas the overexpression of Smad3 led to the inhibition of Tax-mediated transcription. There might be other places on CBP/p300 where both Tax and Smads interact, and Tax may inactivate Smads by specifically competing for Smads–CBP/p300 interaction (Figure 8A). Alternatively, either Tax or Smad3 directly interacts with CBP/p300, and this interaction leads to a change in conformation or stability of the complex comprising the other factor and CBP/p300 (Figure 8B). To directly test these 2 hypotheses, it is under investigation whether Tax is able to inhibit Smads binding to CBP/p300 in vitro.
Although definitive involvement of Smad proteins in hematologic malignancies remains to be determined, defects in the TGF-β signaling pathways may contribute to the progression toward certain types of leukemias.65 By repressing TGF-β–induced growth inhibition, Tax may serve as a positive regulator of cell growth. Because Smad proteins are important tumor suppressors, the ability of high levels of Tax to repress TGF-β signaling could be responsible, at least partially, for the transforming activity of Tax.
We thank Dr M. Hatanaka for pH2R40M and pH2Rneo; Dr K. Matsumoto for pHβAPr-1-neo, pβMT-2Tax, pβTax703, and pβTaxM22; Dr J. Fujisawa for κB-Luc; Dr I. Futsuki for LTR-Luc; Dr J. Massague for p3TP-Lux; Dr X.-F. Wang for p15P113-Luc; Dr M. Abe for p800neoLuc; Dr M. Whitman for FAST-1 expression plasmid; Dr J. L. Wrana for Flag-Smad2, Flag-Smad3, Smad4-HA, TβRI-WT-HA, TβRI-T204D-HA, and ARE-Lux; and Dr K. Miyazono for CBP and p300 expression plasmids. We are very grateful to Dr K. Mitani and Dr M. Kurokawa for helpful discussions and advice. We also thank M. Yamamoto and M. Sasaki for excellent technical assistance.
Supported in part by a grant-in-aid for Scientific Research from the Japan Society for the Promotion of Science.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Naoki Mori, Department of Preventive Medicine and AIDS Research, Institute of Tropical Medicine, Nagasaki University, 1-12-4, Sakamoto, Nagasaki 852-8523, Japan; e-mail:n-mori@net.nagasaki-u.ac.jp.

![Fig. 2. Resistance of Tax-expressing cells to TGF-β treatment. / (A) Expression of Tax mRNA in stable Mv1Lu transfectants. A clone Neo-10 (lane 1) is a control line obtained from Mv1Lu cells transfected with pH2Rneo, followed by G418 selection. Clones Tax-3 (lane 2), Tax-10 (lane 3), and Tax-15 (lane 4) were established from cells transfected with pH2R40M. As a positive control, total cellular RNA from HTLV-I–infected HUT-102 cells (lane 5) was used. (B) Tax-expressing (clones Tax-3 [●], Tax-10 [○], and Tax-15 [▵]) and unmodified (clone Neo-10 [■]) Mv1Lu cells were treated with the indicated concentrations of TGF-β for 48 hours. Proliferation of cells was examined by MTT assay. Results are expressed as percentages of the values obtained from control cultures that did not receive TGF-β. Each experiment was performed at least 3 times, and representative results are shown.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/97/7/10.1182_blood.v97.7.2137/6/m_h80710845002.jpeg?Expires=1763557821&Signature=l7gw5oJ2prm2L-3jL8h~wh9DcGpyAP7uAbP5xV9CFDc89ThdihBfs1n9viVWeeQugCtutqhkZaKQdJqpX5M5yUrTZ-NDS7pr4jaY2zmbFtq6jYzLjHox3jampXXZM-qE9ORfN7d-hG6A1n9nPZTOiiR3W~pa20K7Ves-gyQ85c0Iq1W4RTY1rnxZI5G4QzmYyNtRXpGKV8u0sWShbICgpNdneuvVJjqS0CvvcFlLf8S1go40ss2KoJdOVLXnxBaIaEkr8SQ2x7ePxEfznPTRT1dNA6uHMRTB2VYmGSOzr8F7Un1BW4cPm5FBE--4HJKgHMSLGXijmJRs9EdFxhqY1Q__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 3. Tax inhibits Smad-induced responses to TGF-β. / Either pH2Rneo ([■], −Tax) or pH2R40M ([▪], +Tax), in combination with p3TP-Lux, was transfected into HepG2 cells, together with the indicated Smad constructs in the absence or the presence of 10 ng/mL TGF-β. Relative luciferase activities were measured in cell extracts, normalized to the renilla luciferase activity. Luciferase activity is presented as fold induction relative to the basal level measured in cells transfected with p3TP-Lux alone without treatment. Data represent the mean ± SD from 3 separate experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/97/7/10.1182_blood.v97.7.2137/6/m_h80710845003.jpeg?Expires=1763557821&Signature=3JWm7dmfXPyDB7AVr2g1WP4~pldG~V74JF52gShKfFPxmYd5H398dsAuHOaOIPoVdbteAz4woQgq5D34l-H3e4XhKfzZ9jJ5JzCva-lR2i7DPkBtNUI97DCyyWjLElp~tbWEO0pt8~S7WGCkPHwj8IGcXQjsrybAvDvUn2KcUecYnjxGN8eWrpWwhqnK8SUVQdH72t05mLbb6GetBvRs7rMIjn9mjhe~FTNDkfmtUuiAVBXX5INXMpRN8CX54jKQXxrmLWf39DpTj4iMeWup9zj-aaVI1O3YTD7ke4jgyQCZsdIP7CBUeiFjbjS-MDkKa7fyh5evt7H7LPsqt~1wgA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal