Abstract
Leukostasis and tissue infiltration by leukemic cells are poorly understood life-threatening complications of acute leukemia. This study has tested the hypothesis that adhesion receptors and cytokines secreted by blast cells play central roles in these reactions. Immunophenotypic studies showed that acute myeloid leukemia (AML) cells (n = 78) of the M0 to M5 subtypes of the French-American-British Cooperative Group expressed various amounts of adhesion receptors, including CD11a, b, c/CD18, CD49d, e, f/CD29, CD54, sCD15, and L-selectin. The presence of functional adhesion receptors was evaluated using a nonstatic adhesion assay. The number of blast cells attached to unactivated endothelium increased by 7 to 31 times after a 6-hour exposure of endothelium to tumor necrosis factor (TNF)-α. Inhibition studies showed that multiple adhesion receptors—including L-selectin, E-selectin, VCAM-1, and CD11/CD18—were involved in blast cell adhesion to TNF-α–activated endothelium. Leukemic cells were then cocultured at 37°C on unactivated endothelial cell monolayers for time periods up to 24 hours. A time-dependent increase in the number of blasts attached to the endothelium and a concomitant induction of ICAM-1, VCAM-1, and E-selectin were observed. Additional experiments revealed that endothelial cell activation by leukemic myeloblasts was caused by cytokine secretion by blast cells, in particular TNF-α and IL-1β, and direct contacts between adhesion receptors expressed by blast cells and endothelial cells. Thus, leukemic cells have the ability to generate conditions that promote their own adhesion to vascular endothelium, a property that may have important implications for the pathophysiology of leukostasis and tissue infiltration by leukemic blast cells.
Introduction
The hyperleukocytosis that can be observed in acute myeloid leukemia (AML) may lead to leukostasis, a life-threatening complication caused by leukemic cell sludging in blood capillaries.1,2 In the absence of treatment, cellular hyperviscosity caused by extreme leukocyte count elevation may rapidly lead to multiple organ failure and death. The mechanisms at the origin of leukostasis are poorly understood. Size and stiffness of blast cells might play a role; leukostasis is more frequently observed in patients with AML or chronic myelocytic leukemia than in patients with acute lymphoblastic or chronic lymphocytic leukemia.1 However, additional factors may also be involved, as indicated by leukostasis development in the absence of hyperleukocytosis or by the frequent failure of therapeutic leukapheresis in controlling leukostasis.2-4
Although little is known about the molecular mechanisms that regulate myeloblast migration to tissues, adhesion receptors are likely to play an important role in this process.5,6 In vitro and in vivo observations have shown that the initial phase of normal leukocyte recruitment at site of inflammation is mediated by selectins that cooperate with integrins to mediate leukocyte rolling along vascular endothelium.7-12 During rolling on selectins, leukocytes are exposed to inflammatory mediators that induce integrin activation and facilitate integrin-mediated firm adhesion.13 Mention should be made that endothelium exposure to inflammatory cytokines such as tumor necrosis factor (TNF)-α, interleukin (IL)-1β, or IL-4 is required for the expression of endothelial adhesion receptors able to support leukocyte rolling. P-selectin is rapidly expressed by endothelial cells exposed to thrombin or histamine, whereas E-selectin and VCAM-1 expression are induced several hours after cytokine activation.10,11,13-18 P-selectin expression may also be promoted by a prolonged exposure to TNF-α.19 In vivo, the earliest phase of leukocyte rolling in rat postcapillary venules is mainly dependent on the interaction of P-selectin with P-selectin glycoprotein ligand-1 (PSGL-1), the major ligand of P-selectin on blood neutrophils, whereas L-selectin has a more important role during a later phase.20-23 Several observations have indicated that PSGL-1 is a common ligand for L- , P-, and E-selectin and that it supports L-selectin–dependent rolling of neutrophils on adherent neutrophils.24-27 This process may increase leukocyte recruitment at sites of inflammation.25,28 29
Adhesion studies performed under static conditions have shown that myeloblast adhesion to cytokine-activated endothelium is mediated by E-selectin, ICAM-1, and VCAM-1.6 L-selectin may play a role in initiating the attachment of L-selectin+ blast cells to cytokine-activated endothelium.30 In these latter investigations, endothelial cell preactivation by exogenous cytokine was required to observe blast cell adhesion. In the current study, we have investigated the mechanisms involved in the adhesion of AML blast cells to resting endothelium. We show that myeloblasts can activate endothelial cells and promote their own adhesion to the vascular wall by cytokine secretion and endothelial cell activation through endothelial cell adhesion receptors.
Materials and methods
Antibodies
Anti–L-selectin monoclonal antibodies (mAbs) LAM1-3 and LAM1-11, anti–E-selectin mAb (HAE-1), and anti-VCAM-1 mAbs HAE-2 and HAE-3 were generated as previously described.14 31Hybridomas producing mAbs TS1/22 (anti-CD11a), MY904 (anti-CD11b), TS1/18 (anti-CD18), and CSLEX-1 (anti-sLex) were obtained from the American Type Culture Collection (Manassas, VA). Anti–E-selectin mAb H18/7 (Fab′2 fragment) and anti–ICAM-1 mAb HU 5/3 (Fab′2 fragment) were gifts from F. W. Luscinskas (Brigham and Women's Hospital, Harvard Medical School, Boston, MA). Monoclonal antibodies were purified from hybridoma culture supernatants on protein A using the MAPP II kit (BioRad Laboratories, Glattbrugg, Switzerland). The following mAbs were obtained from the designated suppliers: AC1.2 (anti–P-selectin), SHCL-3 (anti-CD11c), NCAM16.2 (anti-CD56), and KPL1 (anti-PSGL-1) were from BD Biosciences, Heidelberg, Germany; HP2/1 (anti-CD49d), SAM1 (anti-CD49e), G0H3 (anti-CD49f), 84H10 (anti-CD54), and PL1 (anti-PSGL-1) were from Immunotech, Marseilles, France; 4B4 (anti-CD29) was from Coulter, Schlieren, Switzerland; hec-7 (anti-CD31) was from Endogen, Boston, MA); and G1 (anti–P-selectin) and SFF-2 (anti-CD44) were from Catalys AG, Wallisellen, Switzerland. Isotype-matched negative controls (Becton Dickinson, Basel, Switzerland), secondary goat anti-mouse immunoglobulin fluorescein isothiocyanate (FITC; Tago, Camarillo, CA), and neutralizing goat anti–TNF-α and anti–IL-1β antibody (R&D Systems, Abingdon, UK) were obtained from the designated manufacturers.
Patients and myeloblast immunophenotypic analysis
Heparinized peripheral blood or bone marrow samples were obtained from 78 newly diagnosed patients with AML. Each sample contained more than 90% blast cells. AML diagnosis was based on the criteria of the French-American-British (FAB) Cooperative Group and immunophenotypic studies.32-35 Blast cells, isolated by centrifugation on Ficoll-Hypaque, were used immediately or kept frozen until use. Cell viability, as assessed by trypan blue exclusion, was greater than 80% after 4 to 12 hours of blast cell culture in RPMI medium/10% fetal calf serum (FCS). Immunophenotypic analysis was performed using a large panel of directly FITC- or phycoerythrin-conjugated mAbs reacting with leukocyte differentiation antigens CD-2, -3, -4, -7, -10, -13, -14, -15, -19, -20, -22, -33, -34, -41, -61, glycophorin A, HLA-DR, TdT, or myeloperoxidase (antibodies were from Becton Dickinson, Basel, Switzerland; Immunotech-Coulter, Marseilles, France; DAKO, Glostrup, Denmark).30Unconjugated mAbs were detected by indirect immunofluorescence using an FITC-conjugated goat anti-mouse antibody (Fab′2 fragment) (Tago). Double-immunofluorescence analysis was performed with an Epics flow cytometer (Coulter Electronics, Hialeah, FL). Five thousand cells were analyzed for each sample.
Blast cell attachment assay to TNF-α activated-endothelial cell monolayers.
Myeloblast attachment to TNF-α–activated endothelial cells was assessed as previously described.14 30 Endothelial cells isolated from human umbilical cord (passages 2-4) were grown at confluence in Petri dishes coated with 0.5% gelatin (Sigma, St Louis, MO). Endothelial cell monolayers were cultured for 6 hours in medium containing 100 U/mL TNF-α, washed twice, and incubated for 15 minutes at 37°C with medium alone (RPMI 1640, 5% FCS) or medium containing 10 μg/mL adhesion-blocking mAb (anti–VCAM-1 mAb, HAE-2; anti–E-selectin mAb, H18/7) or control mAb (anti-HLA class I mAb, W6/32). Monoclonal antibodies were used at a concentration of 10 μg/mL. Endothelial cell monolayers were then washed twice before each attachment assay. In addition, blast cells were incubated for 15 minutes at 4°C with 350 μL medium alone or medium containing adhesion-blocking mAbs (anti-CD18 mAb, TS1/18; anti–L-selectin mAb, LAM1-3). Nonblocking anti–L-selectin mAb LAM1-11 was used as control mAb. Blast cells (2 × 106 blast cells from 14 patients) were then immediately added to human umbilical vein endothelial cells, and cell attachment assays were performed under horizontal rotation (72 rpm) for 10 minutes at 37°C. Nonadherent cells were discarded, and Petri dishes were placed in phosphate-buffered saline (PBS)–2% glutaraldehyde. After they were washed, adherent cells were counted in 4 to 8 microscopic fields (0.08 mm2/field). Results of adhesion studies were expressed as percentage of inhibition. Control studies were performed for each patient with blast cells suspended in medium alone.
Recruitment of blast cells on unactivated endothelial cell monolayers.
Leukemic blast cell recruitment on unactivated endothelial cells was assessed by coculturing endothelial cell monolayers in 21 cm2 Petri dishes with 8 × 106 blast cells in 6 mL medium for 0.75 hour, 3 hours, 6 hours, and 24 hours. Endothelial and blast cells were cultured in 199 medium containing 10% FCS (Myoclone Superplus; Gibco, Basel, Switzerland), 50 UI/mL porcine intestinal mucosa heparin (Leo Pharmaceutical Products, Ballrup, Denmark), 15 mM Hepes, 2 mM L-glutamine, penicillin, and streptomycin (all from Gibco). After coculture, Petri dishes were placed in PBS–2% glutaraldehyde. After overnight fixation, Petri dishes were washed and adherent blasts were counted.
Coculture of blast cells with endothelial cell monolayers
The ability of blast cells to induce endothelial cell adhesion receptor expression was evaluated by coculturing blast cells with confluent endothelial cell monolayers for various times in 25-cm2 plastic flasks. Cells (107 blast cells/flask) were cocultured in 7.5 mL 199 medium containing 10% FCS, heparin, Hepes, L-glutamine, penicillin, and streptomycin. After the removal of culture medium and nonadherent cells, adherent blast cells were detached from endothelial cell monolayers by gentle washing and processed for immunophenotypic analysis. In parallel, endothelial cells were detached from plastic flasks with PBS containing 5 mM EDTA, washed twice in RPMI medium/5% FCS, and stained with appropriate mAbs. Immunophenotypic analysis was performed by flow cytometry, as described above. Endothelial cell adhesion receptor expression was evaluated using non–cross-blocking FITC-labeled mAbs in experiments evaluating the effect of adhesion blocking mAbs on endothelial cell activation by blast cells. Results were compared to those obtained using endothelial cells grown in medium alone. For several experiments, culture media were collected after 3, 6, or 24 hours of culture, centrifuged, passed through a 0.2 μm filter (Millipore), and run on Detoxigel (Pierce, Oud-Beijerland, The Netherlands) to obtain endotoxin-free culture media. In some experiments, direct contact between leukemic blast cells and endothelial cells was prevented using a membrane with 0.4 μm pores (Transwell cell culture chamber; Costar, Badhoevedorp, The Netherlands). In 3 experiments, the percentage of apoptotic myeloblasts was assessed by cell staining with FITC-conjugated AnnexinV and propidium iodide using the AnnexinV kit (Immunotech).36The percentage of apoptotic cells did not significantly increase after 24 hours of coculture with endothelial cells (20% [t = 0 h] vs 21% after 24 hours of coculture; 11% vs 8% and 13% vs 16%).
Statistical analysis
Unpaired Student t tests or Mann-Whitney tests were used for group comparison. When 3 or more groups were compared, differences between treatments were evaluated by analysis of variance (ANOVA) and the Bonferroni multiple comparison test or the Kruskal-Wallis nonparametric ANOVA test. P < .05 was considered significant. Data are shown as mean ± 1 SD.
Results
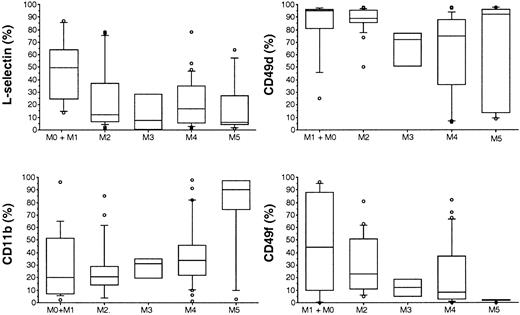
Expression of adhesion molecules by myeloblasts
The following FAB subtype distribution (subtype, number of patients) was observed among the 78 patients investigated in this study: M0, 3; M1, 10; M2, 24; M3, 4; M4, 30; M5, 7. Expression of the various adhesion molecules was heterogenous among the various FAB subtypes and also within each of them (Table1, Figure1). For example, there was a significant heterogeneity of L-selectin expression (P = .02; Figure 1and Table 1) with higher levels detected on M0 (median, 31%) or M1 (50%) blast cells and lower levels on M2 (12%), M3 (8%), M4 (17%), or M5 (6%) blast cells. Heterogeneity was also seen for CD11b expression (P = .05), with higher levels on M5 (median, 90%) blast cells than among other AML categories (M0, 3%; M1, 10%; M2, 22%; M3, 4%; M4, 29%; M5, 7%). Finally, there were significant variations in CD49d (M0, 96%; M1, 94%; M2, 89%; M3, 72%; M4, 75%; M5, 92%; P = .007) and in CD49f expression (M0, 44%; M1, 34%; M2, 23%; M3, 12%; M4, 8%; M5, 1%; P = .008) (Table 1, Figure 1). Blast cells from 15 patients with AML were analyzed for the expression of PSGL-1. A strong expression of this marker was observed in most cases (median, 95%; range, 32%-100%; n = 15).
Expression of adhesion receptors among 75 acute myeloid leukemic cell samples
| . | M0 + M1 . | M2 . | M3 . | M4 . | M5 . |
|---|---|---|---|---|---|
| L-selectin | 47 (11-84) | 12 (1-78) | 8 (0-42) | 17 (1-77) | 6 (1-64) |
| sCD15 | 67 (12-99) | 65 (6-95) | 41 (28-88) | 57 (3-94) | 61 (3-94) |
| CD11A | 88 (55-99) | 85 (10-99) | 70 (48-97) | 89 (33-99) | 97 (76-98) |
| CD11B | 20 (2-96) | 21 (3-85) | 31 (15-36) | 34 (1-98) | 90 (3-97) |
| CD11C | 19 (0-55) | 27 (4-97) | — | 20 (1-97) | 61 (0-85) |
| CD18 | 85 (24-98) | 77 (0-99) | 46 (40-95) | 79 (9-98) | 95 (15-98) |
| CD49D | 95 (25-97) | 89 (50-98) | 72 (43-79) | 75 (6-98) | 92 (9-98) |
| CD49E | 92 (9-99) | 92 (33-98) | 73 (42-98) | 83 (3-97) | 90 (1-98) |
| CD49F | 44 (0-96) | 23 (5-81) | 12 (2-21) | 8 (0-82) | 1 (0-2) |
| CD29 | 96 (9-99) | 93 (18-99) | 92 (64-99) | 93 (2-99) | 97 (11-99) |
| CD54 | 6 (0-76) | 23 (0-82) | 1 (0-25) | 21 (1-76) | 15 (0-30) |
| . | M0 + M1 . | M2 . | M3 . | M4 . | M5 . |
|---|---|---|---|---|---|
| L-selectin | 47 (11-84) | 12 (1-78) | 8 (0-42) | 17 (1-77) | 6 (1-64) |
| sCD15 | 67 (12-99) | 65 (6-95) | 41 (28-88) | 57 (3-94) | 61 (3-94) |
| CD11A | 88 (55-99) | 85 (10-99) | 70 (48-97) | 89 (33-99) | 97 (76-98) |
| CD11B | 20 (2-96) | 21 (3-85) | 31 (15-36) | 34 (1-98) | 90 (3-97) |
| CD11C | 19 (0-55) | 27 (4-97) | — | 20 (1-97) | 61 (0-85) |
| CD18 | 85 (24-98) | 77 (0-99) | 46 (40-95) | 79 (9-98) | 95 (15-98) |
| CD49D | 95 (25-97) | 89 (50-98) | 72 (43-79) | 75 (6-98) | 92 (9-98) |
| CD49E | 92 (9-99) | 92 (33-98) | 73 (42-98) | 83 (3-97) | 90 (1-98) |
| CD49F | 44 (0-96) | 23 (5-81) | 12 (2-21) | 8 (0-82) | 1 (0-2) |
| CD29 | 96 (9-99) | 93 (18-99) | 92 (64-99) | 93 (2-99) | 97 (11-99) |
| CD54 | 6 (0-76) | 23 (0-82) | 1 (0-25) | 21 (1-76) | 15 (0-30) |
Median (range) percentage expression is shown for each receptor.
Box plots of L-selectin, CD11b, CD49d, and CD49f expression by various AML FAB subtypes.
Boxes extend from the 25th to the 75th percentile. Lines within boxes indicate medians.
Box plots of L-selectin, CD11b, CD49d, and CD49f expression by various AML FAB subtypes.
Boxes extend from the 25th to the 75th percentile. Lines within boxes indicate medians.
Selectins and integrins mediate the attachment of blast cells to TNF-α–activated endothelial cells
The attachment of leukemic blast cells to human endothelium was evaluated using a nonstatic cell-binding assay.14,30 After 10 minutes of incubation at 37°C, only a few blasts were attached to unactivated endothelium (28 ± 26 cells/field; n = 14). In contrast, the exposure of endothelial cells to TNF-α (100 U/mL TNF-α for 6 hours at 37°C) induced a 7- to 31-fold increase in blast cell attachment (211 ± 80 cells/field; n = 14). The adhesive properties of L-selectin (CD62L), E-selectin (CD62E), β2-integrin (CD18), and VCAM-1 (CD106) were then evaluated by studying the adhesion of blast cells in the presence of the adhesion blocking mAbs LAM1-3 (anti-CD62L), H18/7 (anti-CD62E), HAE-2 (anti-CD106), and TS1/18 (anti-CD18). The assay duration was 10 minutes to limit L-selectin shedding observed with longer durations. In this low shear stress assay (approximately 0.7 dyne/cm2),37 blast cell attachment to TNF-α–activated endothelium was found to be dependent on L-selectin, E-selectin, β1 integrins, and β2-integrins (Table2). Anti–L-selectin mAb LAM1-3 inhibited a major part of L-selectin+ blast cell attachment to TNF-α–activated endothelium (50.7% ± 6.7%; n = 9;P < .001, Table 2). In contrast, preincubation of L-selectin− blast cells with LAM1-3 did not affect blast cell attachment (data no shown). E-selectin supported blast cell attachment of all blast cell suspensions that were tested and that contributed to a major part of blast cell attachment (57.0% ± 4.8%; n = 11; P < .001 Members of the superfamily of immunoglobulins, VCAM-1 and ICAM-1, were also found to support blast cell adhesion. Anti–VCAM-1 mAb HAE-2 inhibited blast cell attachment from 8 patients with AML by 66.7% ± 7.7% and anti-CD18 mAb TS1/18 by 49.0% ± 5.2% (n = 11). In 2 experiments, the anti–PSGL-1 mAb KPL1 inhibited blast cell adhesion by 52% and 66%, indicating an important role for PSGL-1 in recruiting blast cells at the surface of TNF-α–activated endothelium.
Inhibition of blast cell attachment to 6h-TNF-α-activated endothelium by adhesion-blocking mAbs
| mAbs . | % Inhibition blast cell attachment . | n . | P . | |
|---|---|---|---|---|
| Mean ± SEM . | Range . | |||
| LAM 1-11 (anti-L-selectin/CD62L) | 4.3 ± 2.0 | 0-14 | 8 | — |
| LAM1-3 (anti-L-selectin/CD62L) | 50.7 ± 6.7 | 34-95 | 9 | < .001 |
| TS1/18 (anti-CD18) | 49.0 ± 5.2 | 25-81 | 11 | < .001 |
| HAE-2 (anti-VCAM-1/CD106) | 66.7 ± 7.7 | 28-98 | 8 | < .001 |
| H18/7 (anti-E-selectin/CD62E) | 57.0 ± 4.8 | 31-89 | 11 | < .001 |
| mAbs . | % Inhibition blast cell attachment . | n . | P . | |
|---|---|---|---|---|
| Mean ± SEM . | Range . | |||
| LAM 1-11 (anti-L-selectin/CD62L) | 4.3 ± 2.0 | 0-14 | 8 | — |
| LAM1-3 (anti-L-selectin/CD62L) | 50.7 ± 6.7 | 34-95 | 9 | < .001 |
| TS1/18 (anti-CD18) | 49.0 ± 5.2 | 25-81 | 11 | < .001 |
| HAE-2 (anti-VCAM-1/CD106) | 66.7 ± 7.7 | 28-98 | 8 | < .001 |
| H18/7 (anti-E-selectin/CD62E) | 57.0 ± 4.8 | 31-89 | 11 | < .001 |
Myeloblasts progressively accumulate on unactivated endothelial cell monolayers
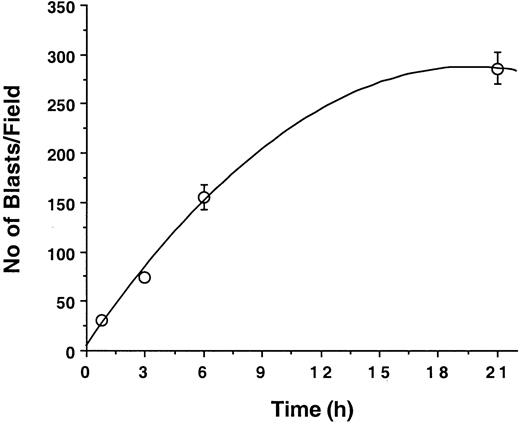
Because little blast cell adhesion to unactivated endothelium was detectable at 10 minutes, the effect of longer incubation times was examined. Leukemic blasts were allowed to adhere to unactivated endothelial cells under static conditions at 37°C for 0.75, 3, 6, or 24 hours; endothelial cell monolayers were then washed gently and adherent cells were counted. With incubation times of up to 24 hours, there was a strong positive nonlinear correlation (R2 = 0.996) between blast cell adhesion at endothelial cell surface and incubation time (Figure2). A possible explanation for this observation is that blast cells can activate endothelial cells, thereby inducing the expression of endothelial adhesion receptors such as ICAM-1, E-selectin, P-selectin, or VCAM-1.
Kinetics of leukemic blast cell adhesion to human vascular endothelium.
Blast cells (107 M5 AML) were cocultured with endothelial cell monolayers in 25-cm2 flasks. Numbers of adherent cells per field (0.08 mm2/field) counted at various time points are indicated by open circles. Results (mean ± SD) are representative of 3 similar experiments.
Kinetics of leukemic blast cell adhesion to human vascular endothelium.
Blast cells (107 M5 AML) were cocultured with endothelial cell monolayers in 25-cm2 flasks. Numbers of adherent cells per field (0.08 mm2/field) counted at various time points are indicated by open circles. Results (mean ± SD) are representative of 3 similar experiments.
Coculture of leukemic blast cells with unactivated endothelial cell monolayers induces endothelial E-selectin, P-selectin, ICAM-1, and VCAM-1 expression
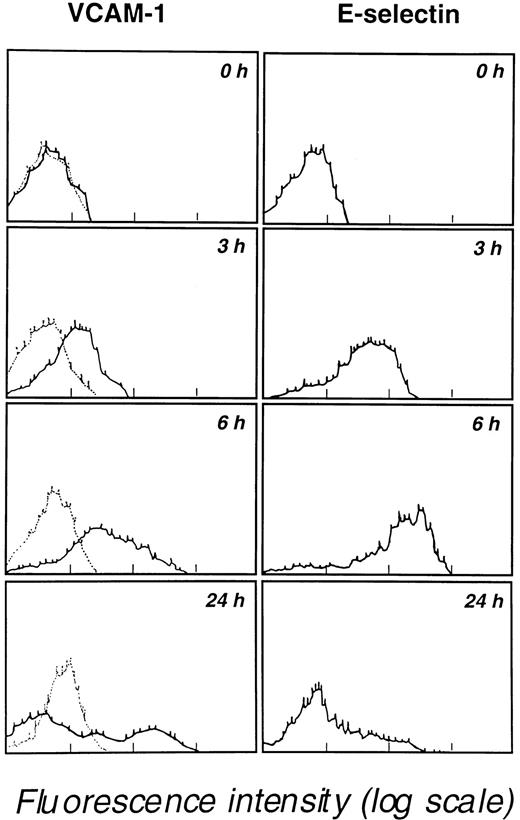
The ability of leukemic blasts to activate endothelial cells and to induce E-selectin, P-selectin, ICAM-1, and VCAM-1 expression was evaluated by coculturing blast cells with endothelial cell monolayers for 24 hours at 37°C. Immunostaining of endothelial cells with appropriate mAbs revealed the strong induction of ICAM-1, VCAM-1, E-selectin, and P-selectin expression but not of CD29 β1 integrin or CD31 (Table 3). At 24 hours, expression levels of ICAM-1 (CD54) and VCAM-1 (CD106E) were higher than levels seen for E-selectin (CD62E) or P-selectin (CD62P). VCAM-1 and E-selectin expression were then determined at various times (0, 3, 6, and 24 hours of coculture). A progressive increase in VCAM-1 expression was detected over the 24-hour coculture period; in contrast, E-selectin expression peaked at 6 hours and decreased afterward (Figure3). Importantly, in 2 experiments, the coculture of human neutrophils with endothelial cells did not induce the expression of P-selectin, E-selectin, and VCAM-1 and did not increase ICAM-1 expression after 3 hours, 6 hours, and 24 hours of coculture. These observations indicate that myeloblasts and neutrophils have distinct behaviors that may strongly affect cell recruitment at the endothelial cell surface.
Expression of endothelial adhesion receptors before (resting endothelium) and after 24 hours of blast cell coculture with endothelial cell monolayers at 37°C
| . | % Expression . | n . | P . | |
|---|---|---|---|---|
| Mean ± SEM . | Range . | |||
| CD54 | ||||
| Resting | 6 ± 1 | 0.5-22 | 14 | |
| Cocultured | 43 ± 5 | 4-78 | 14 | < .0001 |
| CD106 | ||||
| Resting | 6 ± 1 | 2-14 | 14 | |
| Cocultured | 34 ± 6 | 12-82 | 13 | .0003 |
| CD62E | ||||
| Resting | 3 ± 1 | 0-12 | 14 | |
| Cocultured | 27 ± 5 | 0-63 | 13 | .0002 |
| CD62P | ||||
| Resting | 4 ± 1 | 0-19 | 13 | |
| Cocultured | 22 ± 7 | 0-99 | 13 | .0019 |
| CD29 | ||||
| Resting | 87 ± 5 | 50-94 | 8 | |
| Cocultured | 91 ± 1 | 89-94 | 8 | .45 |
| CD31 | ||||
| Resting | 86 ± 5 | 37-99 | 11 | |
| Cocultured | 84 ± 3 | 63-98 | 10 | .79 |
| . | % Expression . | n . | P . | |
|---|---|---|---|---|
| Mean ± SEM . | Range . | |||
| CD54 | ||||
| Resting | 6 ± 1 | 0.5-22 | 14 | |
| Cocultured | 43 ± 5 | 4-78 | 14 | < .0001 |
| CD106 | ||||
| Resting | 6 ± 1 | 2-14 | 14 | |
| Cocultured | 34 ± 6 | 12-82 | 13 | .0003 |
| CD62E | ||||
| Resting | 3 ± 1 | 0-12 | 14 | |
| Cocultured | 27 ± 5 | 0-63 | 13 | .0002 |
| CD62P | ||||
| Resting | 4 ± 1 | 0-19 | 13 | |
| Cocultured | 22 ± 7 | 0-99 | 13 | .0019 |
| CD29 | ||||
| Resting | 87 ± 5 | 50-94 | 8 | |
| Cocultured | 91 ± 1 | 89-94 | 8 | .45 |
| CD31 | ||||
| Resting | 86 ± 5 | 37-99 | 11 | |
| Cocultured | 84 ± 3 | 63-98 | 10 | .79 |
Kinetics of VCAM-1 and E-selectin expression by endothelial cells cocultured in 25-cm2 flasks containing 107 M5 AML blast cells.
Endothelial cells, detached with 5 mM EDTA at the indicated times, were analyzed for VCAM-1 and E-selectin expression by flow cytometry. Results are representative of 3 similar experiments. Background staining obtained with an unreactive isotype-matched mAb is indicated by dotted lines in the left panels.
Kinetics of VCAM-1 and E-selectin expression by endothelial cells cocultured in 25-cm2 flasks containing 107 M5 AML blast cells.
Endothelial cells, detached with 5 mM EDTA at the indicated times, were analyzed for VCAM-1 and E-selectin expression by flow cytometry. Results are representative of 3 similar experiments. Background staining obtained with an unreactive isotype-matched mAb is indicated by dotted lines in the left panels.
Secretion by leukemic blast cells of factors inducing endothelial E-selectin, P-selectin, ICAM-1, and VCAM-1 expression
Similar kinetics of E-selectin and VCAM-1 expression were observed when endothelial cells were cocultured with leukemic blast cells or when they were activated with 100 U/mL TNF-α or 10 U/mL IL-1β. This observation suggests that activating cytokines might have been secreted by leukemic blast cells during the coculture period. We tested this hypothesis by culturing endothelial cell monolayers in the presence of conditioned medium obtained from blast cell and endothelial cell coculture. Strong expression of ICAM-1, VCAM-1, and E-selectin was detectable when endothelial cell monolayers were incubated for 24 hours at 37°C with conditioned medium obtained after 6 hours of coculture of leukemic blasts with unactivated endothelium. ICAM-1 expression at the surface of unactivated endothelial cells was 6% ± 1% and 43% ± 5% on endothelial cells exposed to coculture medium (n = 13; P = .0013). VCAM-1 expression was not detectable on unactivated endothelial cells and increased to 34% ± 6% on endothelial cells exposed to coculture medium (n = 13; P = .0001). E-selectin expression was not detectable on unactivated endothelial cells and 27% ± 5% on endothelial cells exposed to coculture medium (n = 14;P = .0001). The absence of VCAM-1 and E-selectin expression on unactivated endothelial cells was expected.14,38 39 Additional experiments were undertaken to test the possibility that endothelial cell receptor expression after endothelium exposure to coculture medium was related to the presence of endotoxin in conditioned medium. This possibility was rejected because similar levels of ICAM-1, VCAM-1, and E-selectin expression were observed using unprocessed coculture medium or coculture medium adsorbed 3 times on an endotoxin-removing affinity-gel column. Finally, the role of blast cell adhesion to endothelial cell monolayers in inducing ICAM-1 and VCAM-1 expression at the endothelial cell surface was examined by comparing the expression of these molecules after 20 hours of coculture of leukemic blast cells and endothelium in direct contact or separated by a membrane with 0.4-μm pores. After 6 hours of coculture at 37°C, weaker induction of ICAM-1 expression was observed when leukemic blast cells were separated from endothelial cell monolayers than when direct contact was allowed (67% ± 4% vs 77% ± 3%; n = 3; P = .007). A similar trend was detectable for VCAM-1 expression (26% ± 16% vs 39% ± 11%;n = 2).
Prevention of endothelial cell activation by anticytokine antibodies
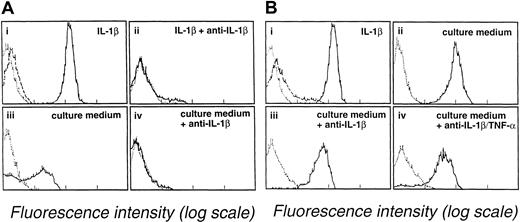
Several studies have shown that myeloblasts can secrete TNF-α or IL-1β. The involvement of these cytokines in coculture experiments presented here was evaluated using antibodies against TNF-α or IL-1β. Leukemic blast cell suspensions from 3 different patients (patient 1, M2 AML; patient 2, M0 AML; and patient 3, M4 AML) were treated with anti–IL-1β or anti–TNF-α antibodies. The effect of anti–IL-1β treatment was examined using E-selectin and ICAM-1 expression as criteria. E-selectin expression was 31% on endothelial cells incubated for 4 hours with the culture medium of a blast cell suspension (107 cells/mL) obtained from patient 1 with M2 AML (Figure 4A, lower left). In contrast, E-selectin expression was only 1% when blast cell culture medium was treated with anti–IL-1β (5 μg/mL) (Figure 4A, lower right). This treatment also reduced ICAM-1 expression. Similarly, the expression of ICAM-1 on endothelial cells was reduced from 38% in the presence of untreated blast cell culture medium from patient 1 to 15% in the presence of anti–IL-1β–treated conditioned medium (not illustrated). The effect of anti–TNF-α treatment was examined in subsequent experiments in which VCAM-1 and ICAM-1 expression were used as endothelial cell activation criteria. VCAM-1 and ICAM-1 expression were, respectively, 77% and 93% when endothelial cells were incubated for 14 hours with the culture medium of blast cells (107cells/mL) obtained from patient 2 with M0 AML. VCAM-1 and ICAM-1 expression were reduced to, respectively, 26% and 53% when blast cell culture medium was treated with anti–TNF-α (5 μg/mL) (not illustrated). Additional experiments were performed using a third culture medium prepared using a blast cell suspension from patient 3 with M4 AML. Anti–IL-1β treatment alone had only a weak effect in preventing E-selectin endothelial cell expression by this culture medium. E-selectin expression was 83% with anti–IL-1β-treated culture medium (Figure 4B, lower left) versus 95% with untreated culture medium (Figure 4B, upper right). A further small reduction in E-selectin expression to 76% was seen when anti–TNF-α antibody was added to the anti–IL-1β–treated culture medium (Figure 4B, lower right). Thus, although the results obtained with the first 2 culture media indicate that IL-1β and TNF-α can play a key role in promoting endothelial cell activation by blast cell supernatants, the data obtained with the third supernatant indicate that additional mechanisms are also involved in this reaction.
Anti–IL-1β and anti–TNF-α mAbs prevent endothelial cell activation by blast cell supernatants.
(Ai-ii) Endothelial cells were incubated with medium containing 10 U/mL IL-1β in the absence (i) or the presence (ii) of 5 μg/mL mAb anti-IL-1β. (Aiii-iv) Endothelial cells were incubated for 4 hours at 37°C with culture medium of M2 AML myeloblasts (patient 1) in the absence (iii) or the presence (iv) of 5 μg/mL mAb anti-IL-1β. Endothelial cells were analyzed for E-selectin expression by flow cytometry. (B) Partial inhibition of endothelial cell activation by anti–IL-1β and anti–TNF-α antibodies. (Bi-ii) Endothelial cells were incubated for 4 hours at 37°C with medium supplemented with 10 U/mL IL-1β (i) or with supernatant from M4 AML myeloblasts (ii). (Biii) Endothelial cells were incubated with the culture medium of M4 AML myeloblasts (patient 3) treated with anti–IL-1β (5 μg/mL). (Biv) endothelial cells were incubated with supernatant from M4 AML myeloblasts treated with anti–IL-1β (5 μg/mL) and anti–TNF-α (10 μg/mL). E-selectin expression by endothelial cells was evaluated by flow cytometry. Background staining by an unreactive isotype-matched mAb is indicated by the dotted line. Dashed lines show the expression of ICAM-1 by unactivated endothelium.
Anti–IL-1β and anti–TNF-α mAbs prevent endothelial cell activation by blast cell supernatants.
(Ai-ii) Endothelial cells were incubated with medium containing 10 U/mL IL-1β in the absence (i) or the presence (ii) of 5 μg/mL mAb anti-IL-1β. (Aiii-iv) Endothelial cells were incubated for 4 hours at 37°C with culture medium of M2 AML myeloblasts (patient 1) in the absence (iii) or the presence (iv) of 5 μg/mL mAb anti-IL-1β. Endothelial cells were analyzed for E-selectin expression by flow cytometry. (B) Partial inhibition of endothelial cell activation by anti–IL-1β and anti–TNF-α antibodies. (Bi-ii) Endothelial cells were incubated for 4 hours at 37°C with medium supplemented with 10 U/mL IL-1β (i) or with supernatant from M4 AML myeloblasts (ii). (Biii) Endothelial cells were incubated with the culture medium of M4 AML myeloblasts (patient 3) treated with anti–IL-1β (5 μg/mL). (Biv) endothelial cells were incubated with supernatant from M4 AML myeloblasts treated with anti–IL-1β (5 μg/mL) and anti–TNF-α (10 μg/mL). E-selectin expression by endothelial cells was evaluated by flow cytometry. Background staining by an unreactive isotype-matched mAb is indicated by the dotted line. Dashed lines show the expression of ICAM-1 by unactivated endothelium.
Inhibition of leukemic blast cell–mediated endothelial cell activation by adhesion-blocking monoclonal antibodies
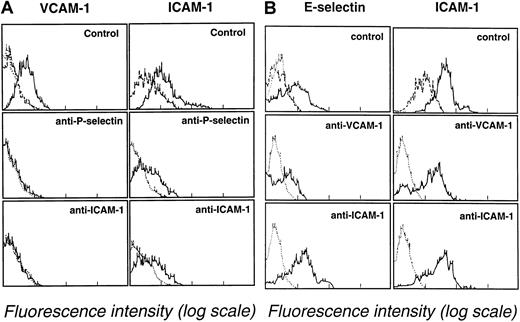
Additional experiments were performed to evaluate the role of blast cell adhesion in inducing endothelial cell adhesion receptor expression. Leukemic blast cells (107/mL) were cultured on endothelial cell monolayers in the presence of adhesion-blocking mAbs directed against VCAM-1, ICAM-1, E-selectin, or P-selectin (mAb concentration, 10 μg/mL). After blast cell–endothelial cell monolayer coculture for 6 hours at 37°C, VCAM-1, ICAM-1, E-selectin, and P-selectin expression were assessed by endothelial cell immunostaining using FITC-labeled non–cross-blocking mAbs. Leukemic blast cell suspensions were obtained from patient 4 (M2 AML) and patient 5 (M5 AML). Adhesion-blocking mAbs G1 (anti–P-selectin) and HU 5/3 (anti–ICAM-1) partially prevented the induction of the expression of VCAM-1 and ICAM-1 on endothelial cell monolayers cultured in the presence of leukemic blasts from patient 4. VCAM-1 and ICAM-1 expression after 6 hours of coculture were, respectively, 25% and 60% in the absence of mAbs (Figure5A, upper panels), 1% and 20% in the presence of the anti–P-selectin mAb G1 (Figure 5A, middle panels), and 1% and 26% in the presence of the anti–1 mAb HU 5/3 (Figure 5A, lower panels). However, ICAM-1 expression (55%) was not prevented by anti–VCAM-1 mAb HAE-2 (not illustrated). Similarly, ICAM-1 and VCAM-1 expression, which were 60% and 16% respectively, were not reduced by the presence of the anti–E-selectin mAb H18/7. The following pattern was observed with leukemic blasts from patient 5 with M5 AML. Anti–P-selectin mAb G1 and anti–ICAM-1 mAb HU 5/3 did not prevent VCAM-1 expression (not illustrated). However, anti–VCAM-1 mAb HAE-3 partially prevented E-selectin and ICAM-1 expression. Control experiments showed that E-selectin and ICAM-1 expression after 6 hours of blast cell–endothelial monolayer coculture were, respectively, 56% and 92% (Figure 5B, upper panels), 20% and 43% in presence of the anti–VCAM-1 mAb HAE-3 (Figure 5B, middle panels), and 50% and 68% in the presence of the anti–ICAM-1 mAb HU5/3 (Figure 5B, lower panels).
Inhibition of endothelial cell adhesion receptor expression by adhesion-blocking mAbs against P-selectin, ICAM-1, and VCAM-1.
(A) Effect of mAbs directed against P-selectin (G1, 10 μg/mL) and ICAM-1 (HU 5/3, 10 μg/mL) on VCAM-1 and ICAM-1 expression after 6 hours of endothelial cell coculture with M2 AML blasts (107blasts/25-cm2 flasks). Endothelial cells were analyzed for VCAM-1 and ICAM-1 expression by flow cytometry. (B) Effect of mAbs directed against and VCAM-1 (HAE-3, 10 μg/mL) or ICAM-1 (HU 5/3, 10 μg/mL) on E-selectin and ICAM-1 expression after 6 hours of endothelial cell coculture with M5 AML myeloblasts (107blasts/25-cm2 flasks). Endothelial cells were analyzed for E-selectin and ICAM-1 expression by flow cytometry. Dotted lines indicate background staining of endothelium labeled with an unreactive isotype-matched mAb. Dashed lines indicate expression of ICAM-1 by unactivated endothelium.
Inhibition of endothelial cell adhesion receptor expression by adhesion-blocking mAbs against P-selectin, ICAM-1, and VCAM-1.
(A) Effect of mAbs directed against P-selectin (G1, 10 μg/mL) and ICAM-1 (HU 5/3, 10 μg/mL) on VCAM-1 and ICAM-1 expression after 6 hours of endothelial cell coculture with M2 AML blasts (107blasts/25-cm2 flasks). Endothelial cells were analyzed for VCAM-1 and ICAM-1 expression by flow cytometry. (B) Effect of mAbs directed against and VCAM-1 (HAE-3, 10 μg/mL) or ICAM-1 (HU 5/3, 10 μg/mL) on E-selectin and ICAM-1 expression after 6 hours of endothelial cell coculture with M5 AML myeloblasts (107blasts/25-cm2 flasks). Endothelial cells were analyzed for E-selectin and ICAM-1 expression by flow cytometry. Dotted lines indicate background staining of endothelium labeled with an unreactive isotype-matched mAb. Dashed lines indicate expression of ICAM-1 by unactivated endothelium.
Discussion
Acute leukemias are heterogeneous regarding the origin of the neoplastic clone, cytogenetic abnormalities, clinical presentation, response to treatment, and biologic behavior.40Dissemination of blast cells in extramedullary sites and leukostasis are major concerns in the treatment of patients with AML.40-44 The study of myeloblast–endothelial cell interactions reported here may give new insights into the mechanisms of such complications. Observations made in this paper show that (1) the expression of adhesion receptors among AML M0 to M5 FAB subtypes is highly heterogeneous; (2) leukemic blast cell adhesion to TNF–α-activated endothelial cells is mediated by selectins, integrins, and members of the superfamily of immunoglobulins; (3) blast cells progressively accumulate on unactivated endothelium by inducing endothelial cell adhesion receptor expression including E-selectin, P-selectin, VCAM-1, and ICAM-1; and (4) secretion of cytokines by leukemic blast cells and adhesion of leukemic blast cells to endothelial cells by specific adhesion receptors play a major role in promoting blast cell recruitment. These results demonstrate that myeloblasts can promote their own adhesion to unactivated vascular endothelium, a reaction that might play a major role in the pathogenesis of leukostasis and tissue invasion.
Immunophenotypic analysis showed that the expression of adhesion receptors by blast cells was heterogenous among the various AML subtypes. Moreover, as shown in Table 1, the expression of adhesion molecules also varied widely within each subtype. Statistical analysis did not disclose any relation between the expression of CD15, CD11a, CD18, CD49e, CD29, CD54, and the various AML subtypes. In contrast, higher levels of L-selectin expression were detected at the surface of M0 and M1 AML than on more differentiated M2, M3, M4 or M5 AML blast cells (Figure 1). Higher levels of CD49f, a receptor for laminin, were also observed on M0 AML and M1 AML blast cells, whereas M5 AML blasts did not express this receptor. The opposite was seen with CD11b, a receptor for ICAM-1, ICAM-2, C3b, fibrinogen, and factor X, as the highest levels of expression of CD11b were observed on myelomonoblastic leukemia M4 AML and monoblastic leukemia M5 AML blast cells. In agreement with earlier studies, lower levels of CD11b expression were found on M0, M1, M2, and M3 AML blast cells.45,46 These differences in adhesion receptor expression may affect myeloblast trafficking and patient prognosis.42,47 A study by the Eastern Cooperative Oncology Group suggested that CD11b+AML may constitute a new leukemic syndrome with a poor response to chemotherapy and reduced survival.45 However, the impact of adhesion receptor expression on clinical presentation and response to therapy was not examined for the patients studied in this report. In future studies, correlations between the expression of these parameters and clinical presentation may be helpful to identify AML subgroups with unique trafficking behavior.
As previously reported for normal leukocytes,7-11,14,48myeloblast adhesion to TNF-α–activated endothelium under nonstatic conditions was dependent on L-selectin and E-selectin, β1, and β2 integrins and on members of the immunoglobulin superfamily ICAM-1 and VCAM-1 (Table 2). Observations made with normal leukocytes showing that selectins and members of the immunoglobulin superfamily function synergistically to mediate optimal blast cell attachment to inflamed endothelium may, therefore, also apply to myeloblasts. With nonmalignant cells, the various steps of leukocyte–endothelium adhesion were shown to be mediated through interdependent adhesion reactions. Selectins were functionally dominant during rolling whereas ICAM-1/β2 integrin played an important role in stabilizing selectin-mediated rolling.7 8
Inflammatory cytokines and chemokines play a major role in regulating leukocyte migration by inducing endothelial cell adhesion receptor expression and by modulating the affinity of leukocyte integrins during leukocyte rolling. As previously observed for normal leukocytes,14 myeloblasts did not attach to endothelium in a short-term (10-minute) nonstatic adhesion assay without prior endothelial cell activation by TNF-α. However, progressive recruitment of myeloblasts was observed when blast cells and endothelial cells were cocultured for several hours. During the first 6 hours of coculture, blast cell accumulation on endothelium was almost linear, but measures at 24 hours indicated the presence of a plateau (Figure 2). The kinetics of blast cell adhesion to cocultured endothelium was similar to the kinetics of normal leukocyte adhesion to TNF-α or IL-1β-activated endothelium. This observation suggests that myeloblasts may secrete TNF-α, IL-1β, or additional stimulating factors, thereby activating endothelial cells and promoting their own adhesion.
The possibility that factors secreted by blast cells could activate the endothelium was suggested by observations showing that endothelial cell exposure to supernatants of blast cell cultures induced E-selectin, P-selectin, VCAM-1, and ICAM-1 expression and that the addition of anti–TNF-α or anti–IL-1β antibodies to blast cell culture media prevented subsequent endothelial cell activation and endothelial cell adhesion receptor expression (Figure 4A-B). Myeloblasts have been shown to express TNF-α and IL-1β.49-51 In addition, several studies have indicated that TNF-α and IL-1β may play a key role in regulating myeloblast proliferation. TNF-α significantly increases blast cell proliferation in the presence of granulocyte macrophage–colony-stimulating factor (GM-CSF),52 whereas IL-1β supports the autocrine growth of AML blasts.53,54In addition to the autocrine loop, a paracrine loop supporting myeloblast proliferation is provided by the induction of GM-CSF, TNF-α, and IL-1β production by fibroblasts and endothelial cells.50,55-57 Finally, IL-1β may inhibit the programmed cell death of AML cells by activating nuclear transcription factor NF-κB, a pathway recently shown to be blocked by the arsenic compound phenyl arsine oxide.58 Induction of adhesion receptors at the surfaces of endothelial cells in direct contact with blast cells may constitute a mechanism favoring blast cell proliferation, thereby contributing to the rapid growth of AML cells in the vascular compartment and subsequent leukostasis and tissue invasion. Additional changes after endothelial cell activation (for example, loss of vascular integrity, modification of the endothelial phenotype from antithrombotic to prothrombotic, increased endothelial production of cytokines) may also contribute to activate the deleterious inflammatory reactions that can cause multiple organ failure.59 60
In the experiment illustrated in Figure 4B, endothelial cell activation by the cell culture supernatant was only partially inhibited by anti–IL-1β and anti–TNF-α, suggesting that endothelial cells could be activated by other factors. Various pathways are involved in endothelial cell activation.59 Activation of the complement system or generation of oxygen-reactive species plays an important role in the pathophysiology of several inflammatory diseases.61,62 Thrombin generation after tissue factor expression or cancer procoagulant secretion by blast cells may also contribute to activate endothelial cells.63-67 Finally, the generation of neutrophil membrane microparticles has been reported to activate endothelial cells.68 The extent to which these various mechanisms are involved in myeloblast-dependent endothelial cell activation will have to be determined.
Cell–cell contact generates intracellular signals that play an important role in regulating cell growth, survival, and locomotion. Binding of L-selectin to its ligand, GlyCAM-1, or binding of P-selectin to PSGL-1 increases leukocyte integrin function. Along with chemotactic stimuli, these activating signals may regulate the transition from leukocyte rolling to firm adhesion.69-73 Cross-talks between β1, β2, and β3integrins may play a more important role in regulating further steps of leukocyte migration.74-76 In addition, ligand binding to L-selectin, PSGL-1, β1, or β2 integrins can generate diverse intracellular signals that lead to the production of inflammatory mediators, including TNF-α, IL-1β, IL-8, and tissue factor.71,77-81 Intracellular signal transduction can also occur in activated endothelial cells after leukocyte adhesion. Transient increases in endothelial cell cytosolic free calcium and cytoskeleton rearrangement have been observed in this context.82 Endothelial selectins and VCAM-1 were shown to play key roles in the generation of endothelial intracellular signals.82 These cross-talks may also occur between myeloblasts and endothelial cells. Observations made in the current study suggest that blast cells could activate endothelial cells not only by secreting soluble factors but also by adhesive interactions with endothelial cells. In the experiment illustrated in Figure 5B, the inhibition of VCAM-1–mediated blast cell adhesion to endothelium abrogated E-selectin and significantly inhibited ICAM-1 expression. Thus, in this situation, VCAM-1 played a important role in regulating E-selectin and ICAM-1 expression, an observation concordant with results obtained in a study on blood neutrophils.82 The data on VCAM-1 and ICAM-1 expression shown in Figure 5A also indicate that P-selectin and ICAM-1 can play a role in endothelial cell activation. In addition, as reported for normal leukocytes, endothelial cell adhesion receptors may induce intracellular signals in blast cells that may stimulate the production of TNF-α or IL-1β; however, this possibility was not addressed in this study.
Current models of leukocyte adhesion to vascular endothelium propose that leukocytes roll on activated endothelial cells through selectins and integrins.7,8 Integrin activation by chemokines and selectins during rolling seems crucial for leukocyte arrest and firm adhesion.13 We show here that myeloblasts use integrins and selectins to attach to cytokine-activated endothelium and that blast cells can directly activate endothelial cells by secreting TNF-α, IL-1β, or additional activating factor cells and by adhering to endothelial cells. Thus, in contrast to neutrophils, myeloblasts have acquired the ability to create conditions necessary for their adhesion to vascular endothelium, migration to tissues, and proliferation. These observations increase our understanding of leukemic cell biology; importantly, they also suggest a therapeutic potential for cytokine inhibitors and adhesion receptor antagonists against leukemic cell adhesion, leukostasis, and leukemic tissue infiltration.
Supported by grant KFS-499.8-1997 from the Swiss Cancer Research Foundation and grant 32-54069.98 from the Swiss National Foundation for Scientific Research.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Olivier Spertini, Division of Hematology, BH 18-543, Centre Hospitalier Universitaire Vaudois, 1011 Lausanne, Switzerland; e-mail: olivier.spertini@chuv.hospvd.ch.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal