Abstract
Cyclin A1 is tissue-specifically expressed during spermatogenesis, but it is also highly expressed in acute myeloid leukemia (AML). Its pathogenetic role in AML and in the cell cycle of leukemic blasts is unknown. B-myb is essential for G1/S transition and has been shown to be phosphorylated by the cyclin A2/cdk2 complex. Here it is demonstrated that cyclin A1 interacts with the C-terminal portion of B-myb as shown by glutathione S-transferase (GST) precipitation. This interaction is confined to cyclin A1 because binding could not be detected between cyclin A2 and B-myb. Also, cdk2 was not pulled down by GST–B-myb from U937 lysates. In addition, co-immunoprecipitation of cyclin A1 and B-myb in leukemic cells evidenced protein interaction in vivo. Baculovirus-expressed cyclin A1/cdk2 complexes were able to phosphorylate human as well as murine B-myb in vitro. Tryptic phosphopeptide mapping revealed that cyclin A1/cdk2 complexes phosphorylated the C-terminal part of B-myb at several sites including threonine 447, 490, and 497 and serine 581. These phosphorylation sites have been demonstrated to be important for the enhancement of B-myb transcriptional activity. Further studies showed that cyclin A1 cooperated with B-myb to transactivate myb binding site containing promoters including the promoter of the human cyclin A1 gene. Taken together, the data suggest that cyclin A1 is a tissue-specific regulator of B-myb function and activates B-myb in leukemic blasts.
Introduction
Important differences in the cell cycle machinery are obviously necessary to direct processes as different as mitosis and meiosis. On the other hand, several cell cycle regulatory proteins (eg, retinoblastoma [Rb]) are expressed in meiotic cells as well as in cells going through the cell cycle of somatic cells.1Cyclin A1 has been demonstrated to be essential for spermatogenesis,2 and high-level expression in the healthy organism is restricted to pachytene spermatocytes undergoing meiosis.3 We reported previously that human cyclin A1 is also expressed in acute myeloid leukemia (AML) and in cell lines derived from leukemic blasts.4,5 In addition, cyclin A1 directly interacts with E2F-1 and the Rb family of proteins.6 However, the functions of cyclin A1 in the cell cycle of somatic cells and especially its role in the pathogenesis of AML have remained elusive.
B-myb plays an important role in the cell cycle of somatic cells, and its expression sharply increases at the G1/S transition.7It is ubiquitously expressed in proliferating somatic cells and is thought to be essential for G1/S progression. B-myb expression correlates with proliferation in human hematopoietic cells,8 and antisense oligonucleotides to B-myb inhibit proliferation of hematopoietic cell lines.9 B-myb was found to be strongly expressed in non-small cell lung cancer.10 In addition, high levels of B-myb are associated with increased bcl-2 expression and resistance to apoptosis.11 B-myb expression has been demonstrated in male germ cells.12,13 The exact stage of spermatogenesis where B-myb expression can be found is unclear. Whereas one group12 reported B-myb expression in spermatogonia, Sitzmann and colleagues13 provided evidence for B-myb expression during the first meiotic division.
The B-myb–mediated transactivation of mammalian promoters is cell-type specific14 and might depend on the B-myb phosphorylation status.15 The transactivation capacity of B-myb in transient transfections can be markedly enhanced by cotransfection of cyclin A2 (also known as cyclin A) and cyclin E.16 B-myb is phosphorylated in a cell cycle–dependent manner, and the cyclin A2/cdk2 and cyclin E/cdk2 complexes phosphorylate B-myb at several sites.17-19 Phosphorylation of B-myb is restricted to some cyclin/cdk complexes; for example, cyclin D1/cdk4 cannot phosphorylate B-myb.18 In addition, a B-myb/cyclin D1 complex strongly inhibits B-myb activity.20 Although cyclin A2/cdk2 complexes phosphorylate B-myb, a stable interaction between B-myb and cyclin A2 has not been detected. The regulatory role of cyclin A1 for B-myb is currently unknown.
Here we present data suggesting that cyclin A1 is an important partner of B-myb. Cyclin A1 and B-myb co-immunoprecipitate from leukemic cells in vivo, and cyclin A1 is able to interact directly with B-myb. Baculovirus-expressed human cyclin A1/cdk2 complexes can phosphorylate murine as well as human B-myb in vitro at several sites that have been shown to be functionally relevant. Phosphorylation is likely to be the mechanism by which cyclin A1 can activate B-myb to transactivate the human cyclin A1 promoter. Our findings suggest that cyclin A1 might be a tissue-specific regulator of B-myb function and that cyclin A1 activates B-myb function in leukemic blasts.
Materials and methods
Plasmids
The human cytomegalovirus (CMV)–B-myb expression plasmid, a gift of Dr Sala, has been described previously.16The myb-TK (thymidine kinase) plasmid, gift of Dr Klempnauer, consists of a minimal thymidine kinase promoter and several myb consensus binding sites driving expression of a luciferase reporter gene. The human glutathione S-transferase (GST)–B-myb plasmid was constructed by PFU–polymerase chain reaction of the CMV–B-myb plasmid using gene-specific primers encompassing restriction sites forBamHI and NotI. The PCR product was then cloned in frame into pGEX-2T. The murine GST–B-myb fusion plasmid and the generation of point mutations in predicted cdk2 phosphorylation sites have been described previously.18 GST fusion proteins encompassing the C-terminal portion of B-myb were constructed by either restriction digestion of the full-length plasmid (PvuII andXbaI) and religation or PFU-PCR amplification of the desired part of B-myb (base pair [bp] 819-2640) and subsequent cloning into pGEX-2T. The plasmid encoding the N-terminal portion of B-myb was constructed by restriction digestion of the full-length GST–B-myb plasmid with SalI and EcoRI, Klenow fill-in, and subsequent ligation. GST fusion proteins were expressed in BL21 cells and purified using glutathione sepharose beads as described.18 The cyclin A1 promoter (−190 to +145) luciferase reporter plasmid, the pCDNA3-cyclin A1 expression plasmid, and the baculovirus expression plasmids have been described previously.5,18 21
Co-immunoprecipitation and GST precipitation
Co-immunoprecipitation was carried out as described.6 The antibody against B-myb (Santa Cruz Biotechnology, Santa Cruz, CA) was purchased. GST fusion proteins were prepared as described above. The amount of either GST fusion proteins or GST alone was standardized on a protein gel stained with Coomassie blue prior to the precipitation. Lysates of U937 cells were prepared in RadioImmuno Precipitation Assay (RIPA) buffer; these lysates were incubated with GST fusion proteins for 2 hours at 4°C. Beads were washed 4 times in RIPA buffer, denatured in sodium dodecyl sulfate (SDS) sample buffer, and run on a 4% to 15% gradient gel. Western blot analysis was performed using antibodies against cyclin A1, cyclin A2, and cdk2 as described.6
In vitro kinase assays and tryptic phosphopeptide mapping
Cyclin A1 and cdk2 were baculovirus-expressed in Sf9 insect cells, and in vitro kinase assays were performed as described.6 Tryptic phosphopeptide mapping was performed essentially as described22 using the pH 1.9 electrophoresis buffer and the phosphochromatography buffer for ascending chromatography.
Cell lines and transfection
U937 and NB4 cells were grown in Roswell Park Memorial Institute (RPMI) medium plus 10% fetal calf serum (FCS) as described.23 CV-1 cells were cultured in Dulbecco modified essential medium (DMEM) supplemented with 10% FCS containing 100 U/mL penicillin and 100 μg/mL streptomycin. CV-1 cells were transfected in 60-mm plates using Superfect (Qiagen, Hilden, Germany) following the protocol of the manufacturer. A total of 6.5 μg DNA, which consisted of 0.5 μg luciferase reporter plasmid, 1 μg CMV–β-gal expression vector (or 20 ng pRL-SV40 expression vector), and where appropriate, 1 μg CMV–B-myb and pcDNA3/cyclin A1, was transfected. Empty expression vector was used to equalize the amount of DNA. Transfections were performed in duplicate, and β-galactosidase activity or renilla luciferase activity was used to standardize for transfection efficiency. Values present the mean ± SE of 3 independent experiments.
Expression of B-myb in male germ cells
The generation of transgenic mice expressing Enhanced Green Fluorescence Protein under control of the cyclin A1 promoter is described elsewhere.24 Frozen tissue sections from male testis were analyzed using a Zeiss confocal laser scanning microscope (Zeiss, Jena, Germany). The flow cytometric cell sorting of testis cells has been described previously.24 EGFP-expressing and EGFP-nonexpressing cell populations were sorted, and equal amounts of proteins (20 μg) were loaded on a 4% to 15% gradient gel. The anti–B-myb antibody mentioned above was used to analyze B-myb expression in both cell populations. To analyze whether the 2 strong bands detected by the anti–B-myb antibody represented differentially phosphorylated forms of B-myb, RIPA lysates from mouse testis were incubated with Protein Phosphatase 1 (New England Biolab, Beverly, MA) for 2 hours at room temperature before addition of the SDS buffer and loading of the gel.
Results
Cyclin A1/cdk2 complexes phosphorylate B-myb of human and murine origin in vitro
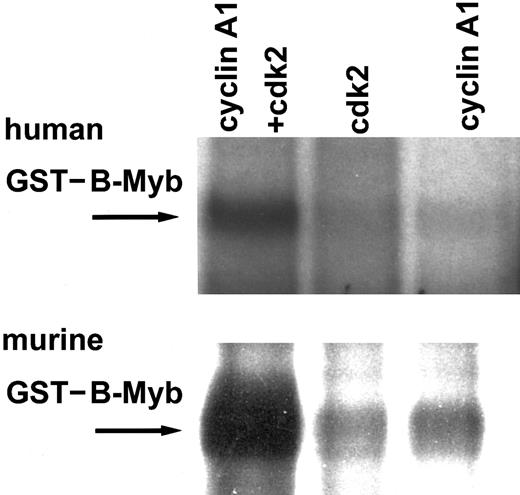
Cyclin E and cyclin A2 have previously been shown to activate B-myb in transient cotransfection assays and to direct cdk2 to phosphorylate B-myb. Other cyclins, such as cyclin B1 or D1, failed to activate or even inhibit B-myb.16 20 To study potential phosphorylation of B-myb by cyclin A1/cdk2, we expressed cyclin A1 and cdk2 in Sf9 insect cells using a baculovirus system. Strong phosphorylation in an in vitro kinase assay using phosphorous-32–adenosine 5′-triphosphate (32P-ATP) occurred only with lysates expressing cyclin A1 and cdk2 together, but not with either one expressed alone (Figure1). Both human and murine GST–B-myb became phosphorylated. These findings indicate that cyclin A1 can direct cdk2 to phosphorylate B-myb.
In vitro kinase assay for human GST–B-myb (full length) and murine GST–B-myb (aa 225-705).
GST fusion proteins were phosphorylated in vitro by cyclin A1 and cdk2 expressed in Sf9 lysates using 32P-ATP. Neither cyclin A1 nor cdk2 alone phosphorylated B-myb, but the combination of both led to strong phosphorylation of the murine as well as the human B-myb.
In vitro kinase assay for human GST–B-myb (full length) and murine GST–B-myb (aa 225-705).
GST fusion proteins were phosphorylated in vitro by cyclin A1 and cdk2 expressed in Sf9 lysates using 32P-ATP. Neither cyclin A1 nor cdk2 alone phosphorylated B-myb, but the combination of both led to strong phosphorylation of the murine as well as the human B-myb.
Tryptic phosphopeptide mapping
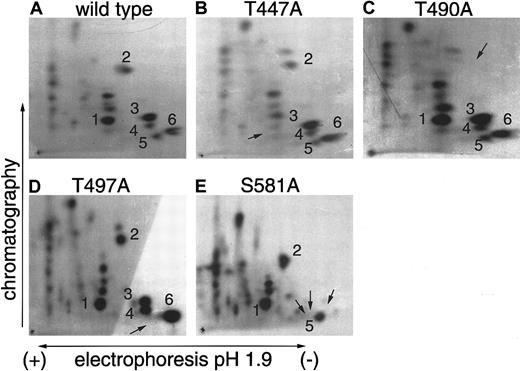
Several sites in the C-terminal portion of murine B-myb have previously been identified as targets for phosphorylation by cyclin A2/cdk2 complexes in vitro and in vivo.18,19 To analyze cyclin A1/cdk2 phosphorylation sites in the C-terminal portion of B-myb, we incubated the murine GST–B-myb fusion protein (amino acid [aa] 225-705) with 32P-ATP and cyclin A1/cdk2 expressed in insect cell lysates. The 32P-phosphorylated B-myb protein was run on a protein gel and excised after drying and autoradiography. Subsequent to purification and solubilizing, the protein was digested to completion using trypsin. The phosphopeptides were resolved in 2 dimensions on TLC plates and exposed to film for 2-6 days. More than 20 spots indicating phosphorylated peptides were observed on the film (Figure 2). B-myb contains more than 20 sites that possess the minimum specificity for cdk phosphorylation serine/threonine-proline (S/T-P). To identify specific sites of phosphorylation, we used GST–B-myb fusion proteins that harbored point mutations at potential target sites. Wild-type and mutant GST–B-myb fusion proteins were then phosphorylated in vitro by cyclin A1/cdk2, digested with trypsin, and analyzed by 2-dimensional phosphopeptide mapping. The mutations of threonine to alanine in aa 447 (447T>A), 490T>A, and 497T>A resulted in the loss of single spots on the TLC plate (Figures 2B-D). The mutation of 581S>A led to the loss of 3 spots. These results are similar to those obtained for cyclin A2/cdk2 complexes.18 These phosphorylation sites have also been identified as targets for cdk2 phosphorylation in vivo, and their mutation resulted in a loss of B-myb transcriptional activity.18 These findings indicate that cyclin A1/cdk2 can phosphorylate B-myb at functionally relevant amino acid residues.
Identification of cyclin A1/cdk2 phosphorylation sites in the C-terminal region of murine B-myb.
Wild-type or point-mutated B-myb fused to GST were phosphorylated in vitro by cyclin A1/cdk2 and subjected to 2-dimensional tryptic phosphopeptide mapping. (A) More than 20 different spots were identified when wild-type GST–B-myb was phosphorylated. Point mutations at potential phosphorylation sites were used to determine the identity of several of these spots. (B-D) Point mutations that changed the indicated threonine to an alanine (an amino acid that cannot be phosphorylated) led to the disappearance of single spots indicated by the arrow. (E) The mutation of S581 (serine to alanine) erased 3 radioactive spots. The reason for the absence of several spots is probably that S581 is located in a region of B-myb containing multiple basic amino acids, and trypsin cleavage after either arginine or lysine residues might lead to differently sized peptides containing the same S581.
Identification of cyclin A1/cdk2 phosphorylation sites in the C-terminal region of murine B-myb.
Wild-type or point-mutated B-myb fused to GST were phosphorylated in vitro by cyclin A1/cdk2 and subjected to 2-dimensional tryptic phosphopeptide mapping. (A) More than 20 different spots were identified when wild-type GST–B-myb was phosphorylated. Point mutations at potential phosphorylation sites were used to determine the identity of several of these spots. (B-D) Point mutations that changed the indicated threonine to an alanine (an amino acid that cannot be phosphorylated) led to the disappearance of single spots indicated by the arrow. (E) The mutation of S581 (serine to alanine) erased 3 radioactive spots. The reason for the absence of several spots is probably that S581 is located in a region of B-myb containing multiple basic amino acids, and trypsin cleavage after either arginine or lysine residues might lead to differently sized peptides containing the same S581.
Cyclin A1 activates B-myb to transactivate myb site-containing promoters
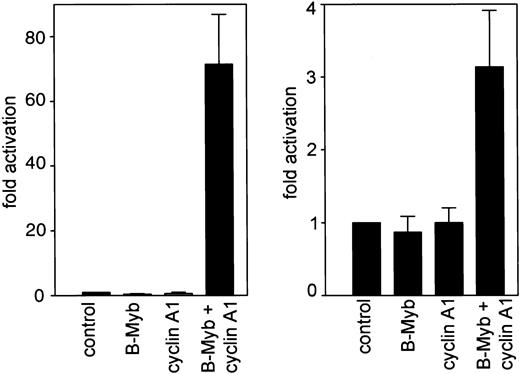
We investigated the functional relevance of the cyclin A1/B-myb interaction by analyzing the transcriptional activity of B-myb in the presence or absence of cyclin A1. Initially we used a construct containing a series of myb-consensus sites upstream of a minimal TK promoter. These experiments confirmed that neither B-myb nor cyclin A1 alone activated the promoter. However, co-expression of both genes led to more than a 60-fold induction of luciferase activity. These findings provide clear evidence that cyclin A1 can activate B-myb (Figure3). Previously we demonstrated that C-myb transactivates the human cyclin A1 promoter.23 Because cell cycle regulatory genes are often autoregulated (eg, theE2F gene), we analyzed whether B-myb either alone or activated by cyclin A1 could transactivate the human cyclin A1 promoter. A CMV–B-myb expression plasmid was cotransfected into CV-1 cells with a luciferase reporter containing −190 to +145 bp of the promoter of human cyclin A1. An empty expression vector was cotransfected with the luciferase construct as a control (Figure 3). The forced expression of B-myb alone did not transactivate the cyclin A1 promoter in CV-1 cells, but it reduced promoter activity by 20%. Also, cyclin A1 alone did not have any effect on promoter activity. However, B-myb and cyclin A1 together significantly activated the cyclin A1 promoter, leading to a 3-fold increase in promoter activity (Figure 3).
Cyclin A1 activates B-myb to transactivate myb-site containing promoters.
A luciferase construct containing 3 myb-consensus binding sites upstream of a minimal thymidine kinase promoter was transiently transfected into Cos cells (left side). Whereas no significant activation of this construct was detected by either cyclin A1 or B-myb transfection alone, a more than 60-fold increase in luciferase activity occurred upon cotransfection of cyclin A1 and B-myb expression vectors. In the right side of the figure, the human cyclin A1 promoter (bp −190 to +145) was analyzed for transactivation by B-myb alone or by the combination of cyclin A1 and B-myb. Cotransfection of B-myb alone decreased cyclin A1 promoter activity by an average of 20%. Cyclin A1 alone did not have a significant effect on cyclin A1 promoter activity. However, when cyclin A1 and B-myb were transfected together, cyclin A1 promoter activity was induced 3-fold.
Cyclin A1 activates B-myb to transactivate myb-site containing promoters.
A luciferase construct containing 3 myb-consensus binding sites upstream of a minimal thymidine kinase promoter was transiently transfected into Cos cells (left side). Whereas no significant activation of this construct was detected by either cyclin A1 or B-myb transfection alone, a more than 60-fold increase in luciferase activity occurred upon cotransfection of cyclin A1 and B-myb expression vectors. In the right side of the figure, the human cyclin A1 promoter (bp −190 to +145) was analyzed for transactivation by B-myb alone or by the combination of cyclin A1 and B-myb. Cotransfection of B-myb alone decreased cyclin A1 promoter activity by an average of 20%. Cyclin A1 alone did not have a significant effect on cyclin A1 promoter activity. However, when cyclin A1 and B-myb were transfected together, cyclin A1 promoter activity was induced 3-fold.
B-myb interacts with cyclin A1 in vivo
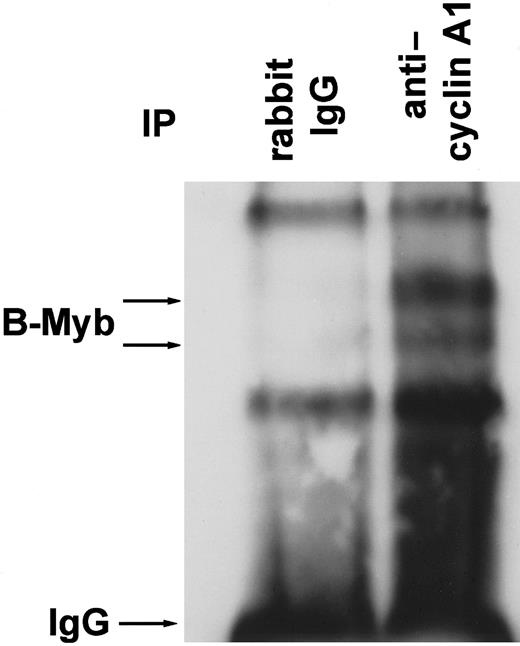
To analyze for an interaction between cyclin A1 and B-myb in vivo, RIPA buffer lysates from highly cyclin A1-expressing U937 leukemic cells were incubated with polyclonal anticyclin A1 antibody or with rabbit immunoglobulin G (IgG) as a control. Immunoprecipitation products were separated on a 4% to 15% gradient gel, and the blot was probed with anti–B-myb polyclonal antibody (Figure4). The anticyclin A1 antibody specifically co-immunoprecipitated B-myb, which appeared as 2 bands of approximately 95-105 kd. The 2 bands and their sizes were similar to those described by Sala and colleagues.16 Two non-specific bands were precipitated by control IgG as well as by the anticyclin A1 antibody.
B-myb co-immunoprecipitates with cyclin A1 in vivo.
U937 leukemic cells were lysed in RIPA buffer, and cyclin A1 was immunoprecipitated with rabbit polyclonal anticyclin A1 antibody. Polyclonal mouse IgG antibody served as a control. The Western blot was probed with anti–B-myb antibody. Besides the IgG band and 2 non-specific bands of similar strength in both lanes, 2 specific bands were seen that represent B-myb in its phosphorylated and non-phosphorylated forms (compare to Figure 7B).16
B-myb co-immunoprecipitates with cyclin A1 in vivo.
U937 leukemic cells were lysed in RIPA buffer, and cyclin A1 was immunoprecipitated with rabbit polyclonal anticyclin A1 antibody. Polyclonal mouse IgG antibody served as a control. The Western blot was probed with anti–B-myb antibody. Besides the IgG band and 2 non-specific bands of similar strength in both lanes, 2 specific bands were seen that represent B-myb in its phosphorylated and non-phosphorylated forms (compare to Figure 7B).16
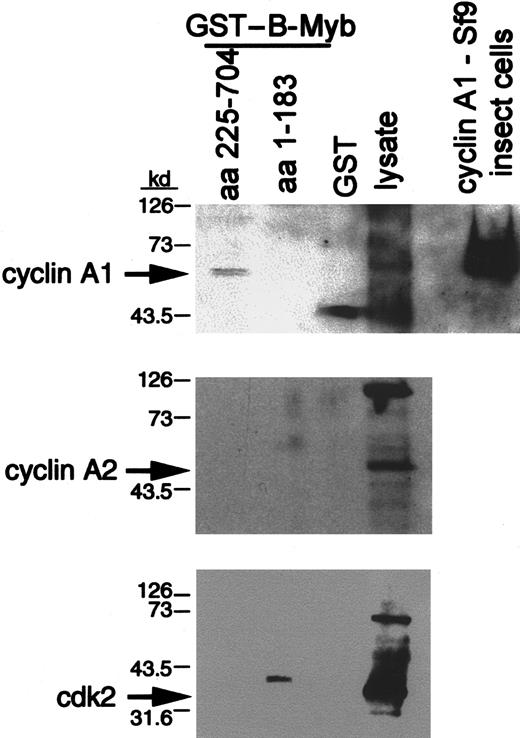
The C-terminus of B-myb interacts with cyclin A1 but not with cyclin A2
To confirm the interaction between cyclin A1 and B-myb, we used GST fusion proteins of B-myb to precipitate cyclin A1 from RIPA lysates of U937 cells (Figure 5). Two different fusion proteins were employed: one containing the murine C-terminal part (aa 225-705) of B-myb that encloses the region where cyclin A2/cdk2 complexes have been shown to phosphorylate B-myb and the other containing the N-terminal region (aa 1-184) of B-myb. GST coupled to glutathione sepharose beads was used as a negative control. The C-terminal fusion protein precipitated cyclin A1, as shown by a specific band (approximately 64 kd) of the same size as cyclin A1 from U937 lysate and recombinant protein from Sf9 insect cells (Figure 5). No bands were seen for the N-terminal portion of B-myb or the GST control. Interestingly, cyclin A2 was not pulled down with GST–B-myb (Figure 5). The antibody detected cyclin A2 in the U937 lysate, but there was no band detected in the lane of either one of the GST–B-myb fusion proteins, thereby providing evidence that cyclin A2 was not able to interact with B-myb in this assay. In addition, cdk2 was not precipitated by the C-terminus of GST–B-myb, indicating that the interaction between cyclin A1 and the C-terminus of B-myb might be independent of cdk2 (Figure 5).
The C-terminal portion of B-myb interacts with cyclin A1 in a GST precipitation assay.
Different portions of B-myb were fused in frame to GST (“Materials and methods”) and bound to glutathione beads. These were incubated with U937 lysates for 2 hours. SDS sample buffer was added to the extensively washed beads. Proteins were electrophoresed, blotted on nitrocellulose, and subjected to Western blotting with the indicated antibodies. Lysate (20 μL total) and cyclin A1 expressed in Sf9 insect cells (upper gel) were run in parallel. Overexposed films are shown for cyclin A2 and cdk2 to demonstrate that even prolonged exposure of the blots did not lead to the appearance of specific bands in any lane except for the lysate. The band in the aa 1-183 lane of the cdk2 Western blot runs slower than the band seen for cdk2.
The C-terminal portion of B-myb interacts with cyclin A1 in a GST precipitation assay.
Different portions of B-myb were fused in frame to GST (“Materials and methods”) and bound to glutathione beads. These were incubated with U937 lysates for 2 hours. SDS sample buffer was added to the extensively washed beads. Proteins were electrophoresed, blotted on nitrocellulose, and subjected to Western blotting with the indicated antibodies. Lysate (20 μL total) and cyclin A1 expressed in Sf9 insect cells (upper gel) were run in parallel. Overexposed films are shown for cyclin A2 and cdk2 to demonstrate that even prolonged exposure of the blots did not lead to the appearance of specific bands in any lane except for the lysate. The band in the aa 1-183 lane of the cdk2 Western blot runs slower than the band seen for cdk2.
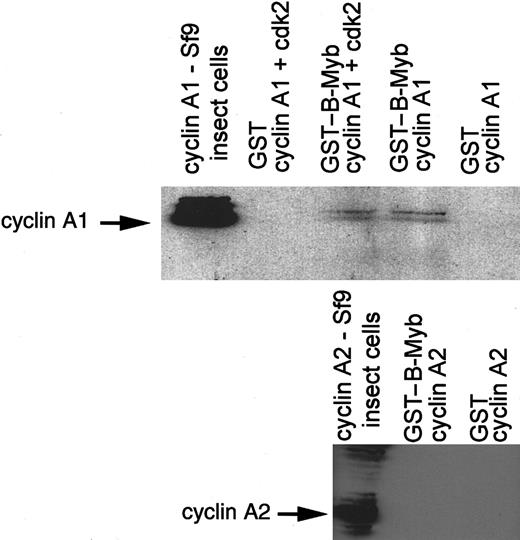
Binding of cyclin A1 to B-myb occurs independent of cdk2
To analyze whether cdk2 was required for the binding between B-myb and cyclin A1, we produced cyclin A1 and cdk2 as recombinant proteins in a baculovirus expression system in Sf9 cells. Approximately equal expression of cyclin A1 in both lysates and strong expression of cdk2 in the cyclin A1/cdk2 lysate was confirmed by Western blotting (data not shown). The GST–B-myb (aa 225-705) or GST alone was incubated with the baculovirus extracts. After extensive washing, proteins were separated on a gel, and Western blotting for cyclin A1 was performed. Cyclin A1 bound to GST–B-myb in the presence as well as in the absence of cdk2 (Figure 6). To confirm that cyclin A2 did not bind to B-myb even in the presence of high levels of cyclin A2 and cdk2, baculovirus-expressed cyclin A2, either alone or in combination with cdk2, was incubated with GST–B-myb (aa 225-705) (Figure 6). No binding could be detected, confirming the specificity of the cyclin A1 and B-myb binding.
Cyclin A1 and B-myb interaction is independent of cdk2.
Baculovirus-expressed cyclin A1 was incubated with GST or GST–B-myb (aa 225-704) in the presence or absence of baculovirus-expressed cdk2. The Western blot membrane was probed with anticyclin A1 antibody. The second blot shows that cyclin A2 and B-myb did not interact even when high levels of cyclin A2 and B-myb were present. Baculovirus-expressed cyclin A2 was incubated with GST–B-myb or GST alone. The Western blot was probed with anticyclin A2 antibody.
Cyclin A1 and B-myb interaction is independent of cdk2.
Baculovirus-expressed cyclin A1 was incubated with GST or GST–B-myb (aa 225-704) in the presence or absence of baculovirus-expressed cdk2. The Western blot membrane was probed with anticyclin A1 antibody. The second blot shows that cyclin A2 and B-myb did not interact even when high levels of cyclin A2 and B-myb were present. Baculovirus-expressed cyclin A2 was incubated with GST–B-myb or GST alone. The Western blot was probed with anticyclin A2 antibody.
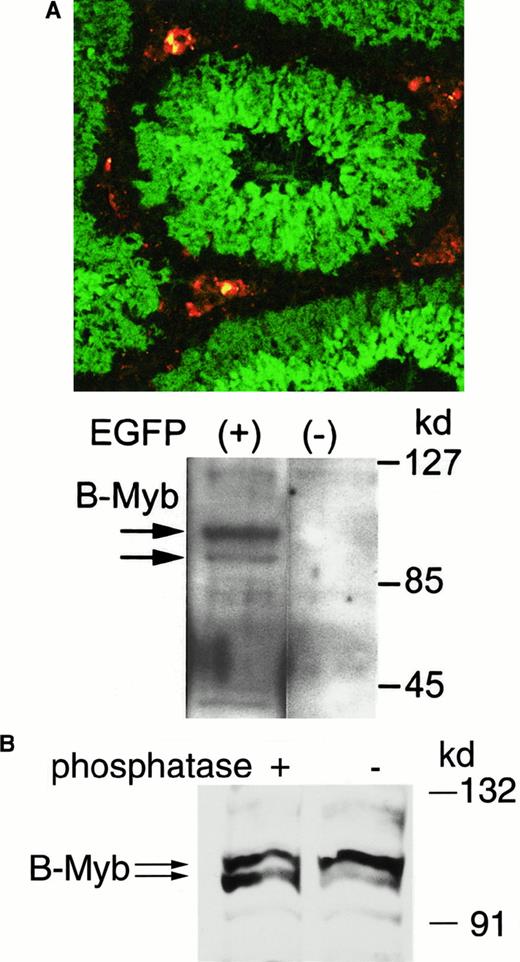
B-myb is expressed in cyclin A1 expressing male germ cells
The spatial organization of spermatogenesis leads to concentric layers of cells representing successive stages of male germ cell differentiation. These specific stages of male germ cell differentiation parallel the location of the cells within the seminiferous tubuli. Using cyclin A1 promoter-driven expression of EGFP in transgenic mice in vivo, we found that the human cyclin A1 promoter is strongly induced in spermatocytes undergoing their first meiotic division (Figure 7A). These EGFP-expressing cells also express endogenous murine cyclin A1, indicating the specificity of the transgenic promoter.24EGFP-expressing male germ cells from transgenic mice were sorted by flow cytometry, and protein lysates were prepared. Western blot analysis of 20 μg protein from these lysates revealed that B-myb expression was exclusively found in the EGFP-expressing cells (Figure7A). Similar to the human B-myb detected in U937 leukemic cells, B-myb protein presented as 2 distinct bands of sizes between 95 and 105 kd. To confirm that the 2 bands represented differentially phosphorylated forms of B-myb, protein lysates from murine testis were incubated with protein phosphatase before Western blotting. Phosphatase treatment led to a signal increase of the faster migrating band (Figure 7B). The increase was highly reproducible in different experiments and also occurred in U937 lysates (data not shown). These experiments show evidence that B-myb is phosphorylated during spermatogenesis.
Cyclin A1 and B-myb are localized in similar stages of spermatogenesis in meiotic male germ cells.
(A) Cyclin A1 expression was visualized as EGFP expression in the testes of mice that were transgenic for a cyclin A1 promoter-EGFP construct. The confocal laser microscopy image (top) shows EGFP expression in green and non-specific fluorescence in Leydig cells in red. The EGFP expression accurately reflects expression of cyclin A1 as determined previously by Western blotting of cells sorted by flow cytometry.23 To analyze B-myb expression in testis cells with and without cyclin A1 expression, EGFP-expressing testis cells were sorted and analyzed for B-myb expression using Western blot analysis (bottom). There was no B-myb expression found in cells not expressing EGFP, whereas expression could be found in cyclin A1 (EGFP)–expressing cells. (B) The 2 B-myb bands in Figure 7A were similar to the bands seen in human leukemic cells. To analyze whether the 2 bands represented differentially phosphorylated forms of B-myb, protein lysates from mouse testis were treated with Protein Phosphatase 1 before Western blotting. Phosphatase treatment led to an increase in the intensity of the faster migrating band, which is consistent with protein dephosphorylation.
Cyclin A1 and B-myb are localized in similar stages of spermatogenesis in meiotic male germ cells.
(A) Cyclin A1 expression was visualized as EGFP expression in the testes of mice that were transgenic for a cyclin A1 promoter-EGFP construct. The confocal laser microscopy image (top) shows EGFP expression in green and non-specific fluorescence in Leydig cells in red. The EGFP expression accurately reflects expression of cyclin A1 as determined previously by Western blotting of cells sorted by flow cytometry.23 To analyze B-myb expression in testis cells with and without cyclin A1 expression, EGFP-expressing testis cells were sorted and analyzed for B-myb expression using Western blot analysis (bottom). There was no B-myb expression found in cells not expressing EGFP, whereas expression could be found in cyclin A1 (EGFP)–expressing cells. (B) The 2 B-myb bands in Figure 7A were similar to the bands seen in human leukemic cells. To analyze whether the 2 bands represented differentially phosphorylated forms of B-myb, protein lysates from mouse testis were treated with Protein Phosphatase 1 before Western blotting. Phosphatase treatment led to an increase in the intensity of the faster migrating band, which is consistent with protein dephosphorylation.
Discussion
Most cyclins regulating S-phase progression and mitosis are ubiquitously expressed in proliferating cells. In contrast, expression of cyclin A1 is restricted to very few cell types.4,25Cyclin A1 does interact with E2F-1 and the Rb family of proteins,6 but the role of cyclin A1 in the cell cycle of somatic cells remained unknown. In this study we demonstrate that cyclin A1 and B-myb interact with each other in vivo and in vitro. This interaction involves the C-terminal region of B-myb and is specific for cyclin A1 because cyclin A2 did not bind to B-myb. Also, cyclin A1/cdk2 complexes phosphorylate murine B-myb at functionally relevant sites in the C-terminal region of B-myb. In addition, these proteins are expressed in the same cell types during spermatogenesis, and cyclin A1 can activate B-myb to transactivate myb site-containing genes including the human cyclin A1 promoter. The latter finding suggests a possible autoregulatory feedback loop.
B-myb is a member of the myb family of transcription factors. It is ubiquitously expressed and is thought to play an essential role in G1/S progression.7 Controversy existed over whether B-myb was actually an activator of transcription or a transcriptional repressor.14,26 Most of these issues have been resolved because studies have shown that transactivation by B-myb can be greatly enhanced by several cyclin family members.16 The activity of myb proteins is controlled by their C-terminal domain. For example, c-myb activity can be enhanced by C-terminal truncation.27In contrast to the negative regulatory function of the C-terminus in c-myb, the C-terminal portion of B-myb functions as an activator of transcription.28 So far, cyclin A2 and cyclin E have been the only cyclins shown to direct cdk2 to phosphorylate B-myb at several sites in its C-terminus.19 These cyclins are known to be essential for the cell cycle of somatic cells. In contrast, it is still unclear whether the tissue-specific cyclin A1 can play a role in cell cycle progression in mammalian cells. Our current study demonstrates that cyclin A1/cdk2 complexes are able to phosphorylate the C-terminus of B-myb at T447, T490, T497, and S581. These sites have been found to be phosphorylated in vivo, and mutations at these sites greatly reduced the transactivation potential of B-myb.18,19 Also, S581 appears to be involved in DNA binding of B-myb.19 In addition to the sites that we examined in our studies, 6 other sites have been described that can be phosphorylated by cyclin A2/cdk2 complexes.19 The functional relevance of these other sites is unclear because mutation of the 4 phosphorylation sites (T447, T490, T497, and S581) alone greatly reduced the activating properties of cyclin A2.18
Cyclin A2 did not bind to B-myb in our present study, which confirms previous results.18 In contrast, cyclin A1 binds to the C-terminal region of B-myb, but not to its DNA binding domain. This physical interaction does not require cdk2 because cdk2 was not pulled down along with cyclin A1 from U937 lysates. In addition, there was no observed enhancement of cyclin A1 binding to GST–B-myb when cdk2 was co-expressed in the baculovirus system. In contrast, phosphorylation of B-myb required co-expression of cyclin A1 and cdk2 (Figure 1). The finding that cyclin A1, but not cyclin A2, interacts with B-myb indicates that cyclin A1 might be an important partner for B-myb in cyclin A1–expressing tissues or that functional regulation of B-myb differs between cyclin A1 and cyclin A2. Cyclin A1 and B-myb are both expressed during spermatogenesis and hematopoiesis as well as in AML. In the current study we demonstrate the interaction of these proteins in leukemic cells in vivo as well as the expression in similar cell types during murine spermatogenesis. These data suggest that cyclin A1 might function by tissue-specifically altering B-myb function.
The appropriate timing of expression of cell cycle regulatory genes is crucial for the successful passage of the cell cycle. TheBMYB gene is strongly induced at the G1/S boundary by an E2F-mediated mechanism.29 Subsequently, the B-myb protein is phosphorylated and further activated. Expression of the cyclin A1 gene also rises after the cell enters S-phase. In the current study we found that B-myb, when activated by cyclin A1, transactivates the cyclin A1 promoter. This interesting finding suggests an autoregulatory feedback loop in which cyclin A1, through activation of B-myb activity, further increases transcription of its own gene. Thecis-reacting sites that are relevant for the transactivation of B-myb are currently unknown, but 2 mechanisms are likely to play a role. First, a myb site is located close to the transcriptional start site of the cyclin A1 promoter, and c-myb binds to this site as determined by electophoretic mobility shift assay.23Second, several Sp1 binding sites are located upstream of the cyclin A1 promoter. B-myb has been shown to transactivate its own promoter presumably through Sp1 sites.30 We have previously shown that the Sp1 sites of the cyclin A1 promoter are essential for cell cycle–regulated transcription of the cyclin A1 gene.22 An appealing hypothesis is that cell cycle regulation of cyclin A1 transcription might be influenced by B-myb, which alters Sp1 site-mediated activity.
Our data provide evidence that cyclin A1 interacts with B-myb in a functionally important way. These findings suggest that cyclin A1 does have a role in the cell cycle of somatic cells which express this cyclin. B-myb is important for the proliferation of normal hematopoietic cells and leukemic blasts, and cyclin A1 may be one of the co-activators that enable the transactivating ability of B-myb in these cell types. Cyclin A1 is essential for spermatogenesis, and murine males that have a homozygously deleted cyclin A1 gene are infertile.2 Currently, the target molecules of cyclin A1/cdk2, which are critical during spermatogenesis, are unknown.BMYB closely interacts with cyclin A1 and might be a potential target gene.
Taken together, our data establish that cyclin A1 interacts with B-myb in vivo and in vitro. Cyclin A1 is likely to be an important regulator of B-myb function in the cell cycle of normal hematopoietic cells and in leukemic blasts. Tissue-restricted expression as seen for cyclin A1 is rare for cell cycle regulators and suggests that cyclin A1 might be an interesting target gene for new therapeutic approaches in AML.
We are grateful to Dr A. Sala, Department of Molecular Pharmacology and Pathology, Consorzio Mario Negri Sud, Chieti, Italy; and Dr K. H. Klempnauer, Institute of Biochemistry, University of Münster, Germany, for providing expression vectors and reporter plasmids, respectively.
Supported by grants from the National Institutes of Health, Bethesda, MD; the U.S. Department of Defense, the Parker Hughes and C. and H. Koeffler Funds, the Lymphoma Foundation of America, the Horn Foundation to H.P.K.; and by grants from the Deutsche Forschungsgemeinschaft (Mu-1328/2-1), the Deutsche Krebshilfe (10-1539-Mü1), the Deutsche José-Carreras Leukemia Foundation and the Innovative Medizinische Forschung (IMF) Program at the University of Münster to C.M.; and by project grant SP2358/0101 from The Cancer Research Campaign to M.S. H.P.K. holds the Mark Goodson Chair in Oncology Research and is a member of the Jonsson Cancer Center.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Carsten Müller-Tidow, Department of Medicine, Hematology and Oncology, University of Münster, Domagkstr 3, Münster, Germany; e-mail: muellerc@uni-muenster.de.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal