Abstract
Dimerization defects of von Willebrand factor (vWF) protomers underlie von Willebrand disease (vWD) type 2A, subtype IID (vWD 2A/IID), and corresponding mutations have been identified at the 3′ end of the vWF gene in exon 52. This study identified and expressed 2 additional mutations in this region, a homozygous defect in a patient with vWD type 3 (C2754W) and a heterozygous frameshift mutation (8566delC) in a patient with vWD type 2A, subtype IIE. Both mutations involve cysteine residues that we propose are possibly essential for dimerization. To prove this hypothesis, transient recombinant expression of each of the 2 mutations introduced in the carboxy-terminal vWF fragment II and in the complete vWF complementary DNA, respectively, were carried out in COS-7 cells and compared with expression of vWD 2A/IID mutation C2773R and the wild-type (WT) sequence in COS-7 cells. Recombinant WT vWF fragment II assembled correctly into a dimer, whereas recombinant mutant fragments were monomeric. Homozygous expression of recombinant mutant full-length vWF resulted in additional dimers, probably through disulfide bonding at the amino-terminal multimerization site, whereas recombinant WT vWF correctly assembled into multimers. Coexpression of recombinant mutant and recombinant WT vWF reproduced the multimer patterns observed in heterozygous individuals. Our results suggest that a common defect of vWF biosynthesis—lack of vWF dimerization—may cause diverse types and subtypes of vWD. We also confirmed previous studies that found that disulfide bonding at the vWF amino-terminal is independent of dimerization at the vWF carboxy-terminal.
Introduction
Von Willebrand disease (vWD) is a heterogeneous hereditary bleeding disorder caused by diverse molecular defects of von Willebrand factor (vWF) that often correlate with the observed phenotype. Although deletions, nonsense mutations, and frameshift mutations are responsible for most cases of vWD type 3 (vWD 3),1-3 because of the virtual absence of vWF protein in the patient's plasma and platelets, some mutations alter particular functions of vWF. Functions affected by these mutations include the binding to platelet glycoprotein Ib in vWD type 2B4 and the factor VIII (FVIII) binding activity of vWF in vWD type 2N.5 Several vWF defects are consequential to impaired posttranslation biosynthesis, including defective dimerization of vWF monomers in vWD 2A, subtype IID (vWD 2A/IID),6 defective multimerization in vWD 2A, subtype IIC (vWD 2A/IIC),7,8and defective intracellular transport and secretion of intermediate and high-molecular-weight multimers (HMWM) in vWD 2A, subtype IIA (vWD 2A/IIA; mutation group 1).9 Mutations associated with vWD 2A/IIA (group 2) lead to enhanced proteolysis of vWF multimers in plasma9 by a specific metalloprotease.10 The vWD subtypes IIA, IIC, IID, IIE, IIF, IIG, and IIH, have been combined to form a common type, vWD type 2A, which comprises all variants lacking HMWM in plasma.11The single exception is type 2B, in which the absence of plasma HMWM reflects an enhanced binding of HMWM to glycoprotein Ib followed by clearance from the circulation.4
We previously demonstrated that the underlying cause of the vWD 2A/IID phenotype is a heterozygous dimerization defect at the carboxy-terminal of vWF.6 We here report on the expression and characterization of 2 additional mutations, which are located in the same amino-terminal dimerization domain of vWF. One mutation leads to vWD 2A, subtype IIE (vWD 2A/IIE), which is characterized by a mild to moderate bleeding tendency, decreased large vWF multimers, and decreased proteolysis of vWF.12 The other mutation results in vWD 3. Both mutations involve cysteine residues that may be essential for dimerization.
Patients, materials, and methods
Patients
Patient 0103 with vWD 2A/IIE had a history of easy bruising, epistaxis, heavy menorrhagia, and heavy bleeding after the birth of her first child. Her parents, siblings, and child are not affected. Patient 0203 was given a diagnosis in early childhood of a severe bleeding tendency suggesting vWD 3. She is the only affected family member. Patient 0303 was given a diagnosis of vWD 2A/IID, as we reported previously.6 For comparison, her hemostasis variables are shown in Table 1. All patients or their parents were informed about the experimental nature of this study and gave their consent to participation according to the principles of the Declaration of Helsinki.
Hemostasis variables in the patients (boldface) and their relatives
| Proband . | Bleeding time (min) [< 6] . | FVIII:C (U/mL) [> 0.6] . | vWF:Ag (U/mL) [> 0.5] . | vWF:Ag (plt) (U/109 plt) [0.1-0.5] . | RC (U/mL) [> 0.5] . | vWF:CBA (U/mL) [0.8-2.0 × vWF:Ag] . |
|---|---|---|---|---|---|---|
| 0101 (f) | — | — | 1.24 | — | — | 1.09 |
| 0102 (m) | — | — | 0.62 | — | — | 0.59 |
| 0103 | > 12 | 0.06 | 0.07 | 0.06 | — | < 0.01 |
| 0104 (s) | — | — | 0.9 | — | — | 0.93 |
| 0105 (b) | — | — | 0.86 | — | — | 0.9 |
| 0201 (f) | 5 | 0.54 | 0.33 | — | 0.38 | — |
| 0202 (m) | 3 | 0.55 | 0.38 | — | 0.43 | — |
| 0203 | > 20 | 0.12 | < 0.05 | < 0.05 | < 0.05 | — |
| 0303 | > 20 | 0.91 | 1.19 | — | 0.73 | 0.07 |
| Proband . | Bleeding time (min) [< 6] . | FVIII:C (U/mL) [> 0.6] . | vWF:Ag (U/mL) [> 0.5] . | vWF:Ag (plt) (U/109 plt) [0.1-0.5] . | RC (U/mL) [> 0.5] . | vWF:CBA (U/mL) [0.8-2.0 × vWF:Ag] . |
|---|---|---|---|---|---|---|
| 0101 (f) | — | — | 1.24 | — | — | 1.09 |
| 0102 (m) | — | — | 0.62 | — | — | 0.59 |
| 0103 | > 12 | 0.06 | 0.07 | 0.06 | — | < 0.01 |
| 0104 (s) | — | — | 0.9 | — | — | 0.93 |
| 0105 (b) | — | — | 0.86 | — | — | 0.9 |
| 0201 (f) | 5 | 0.54 | 0.33 | — | 0.38 | — |
| 0202 (m) | 3 | 0.55 | 0.38 | — | 0.43 | — |
| 0203 | > 20 | 0.12 | < 0.05 | < 0.05 | < 0.05 | — |
| 0303 | > 20 | 0.91 | 1.19 | — | 0.73 | 0.07 |
The patient with von Willebrand disease (vWD) type 2A, subtype IIE (0103), is from family 01, the patient with vWD type 3 (0203) is from family 02, and 0303 is the patient with vWD 2A, subtype IID. Normal results for each variable are in square brackets. Values for von Willebrand factor: collagen-binding assay (vWF:CBA) are relative to the von Willebrand factor: antigen (vWF:Ag) measured in the same sample. FVIII:C indicates factor VIII coagulant activity; plt, platelet; RC, ristocetin cofactor; f, father of patient; m, mother of patient; s, sister of patient; and b, brother of patient.
Diagnosis of vWD
Conventional diagnostic tests were carried out in a reference laboratory (of U.B.) with the patients' plasma (0.011 M trisodium citrate) and platelets. The leukocyte buffy coat was separated from whole citrated blood after differential centrifugation, then stored at −20°C for subsequent DNA extraction. The following standard variables were obtained for each patient: bleeding time,13FVIII coagulant activity (FVIII:C),14 vWF: antigen (vWF:Ag),15 and ristocetin cofactor16 or vWF:collagen-binding assay (vWF:CBA).17 Multimer analysis was performed in sodium dodecyl sulfate (SDS)–agarose electrophoresis gels18 with luminescent visualization.19Recombinant forms of vWF with mutations specific to each patient were generated and tested for FVIII binding capacity.20
Mutation screening
Nucleotides of the vWF complementary DNA (cDNA) sequence are numbered from the major transcription cap site (+1) located 250 nucleotides upstream of the first nucleotide in the ATG initiation codon. Amino acid residues are numbered from the ATG initiation codon (residue 1) to the carboxy-terminal lysine (residue 2813) of pre-pro-vWF. Correspondence with residues in the mature vWF sequence is obtained by subtracting 763 from the residue number of pre-pro-vWF.
High-molecular-weight genomic DNA was prepared from frozen buffy coats by using standard techniques21 and used in amplification of vWF gene sequences by polymerase chain reaction (PCR).22 PCR was done in a Trio-Thermoblock device (Biometra, Göttingen, Germany) using reagents and Taq polymerase from Gibco BRL (Gaithersburg, MD). Mutation screening was carried out through amplification of single exons flanked by sufficient intron sequences to also allow detection of splice-site mutations.6 Primers that differed from the pseudogenic sequences were designed to avoid amplification of the pseudogene.23 DNA from a patient with a complete homozygous deletion of the vWF gene served as a negative control. PCR was combined with analysis of single-strand conformation polymorphisms (SSCP)24 and heteroduplex analysis25 of exons 2 through 52, as described previously.6 The aberrant PCR products were sequenced by using phosphorus 33–labeled dideoxynucleoside triphosphate terminators (Amersham, Braunschweig, Germany). The mutations were confirmed by sequencing both strands. In the case of the frameshift mutation 8566delC in the patient with vWD 2A/IIE, the individual alleles were cloned into pCR-Script Amp SK(+) (Stratagene, Heidelberg, Germany) before sequencing, and this was followed by amplification in Epicurian XL1-Blue MRF′ Kan supercompetent cells according to the manufacturer's instructions. Mutation screening in this patient was further extended by sequencing all coding exons together with respective flanking intron regions. Haplotypes were established by analyzing published intragenic polymorphisms26 (and http://mmg2.im.med.umich.edu/vWF) and SSCP detected during mutation screening.
Expression studies
Posttranslational processing of vWF monomers includes dimerization by disulfide bonding at the cysteine-rich carboxy-terminal and polymerization to multimers of different size at the amino-terminal. Because it was shown that disulfide bonding at the amino-terminal is independent of dimerization at the carboxy-terminal,27 we hypothesized that recombinant expression of a dimerization defect by using full-length vWF cDNA would nevertheless result in the formation of dimers by disulfide bonding at the intact amino-terminal. Following that strategy, we expressed the carboxy-terminal proteolytic vWF fragment II to assess the isolated dimerization process as previously described6 and compared the results with those obtained by expression of full-length vWF cDNA. Both mutations were compared with the wild type (WT) and the previously described vWD 2A/IID defect.6
Recombinant expression of the carboxy-terminal vWF fragment II
In vitro mutagenesis of the plasmid pJB6, containing the DNA sequence coding for vWF fragment II, was carried out as described previously.6 Deviating from the originally described transfer into baculovirus, we used the mammalian expression vector pRc cytomegalovirus (pRc.CMV; Invitrogen, Leek, The Netherlands) for transfection of COS-7 cells for all mutants, including the vWD 2A/IID defect and the WT. The mutant and WT inserts were cut withXhoI, and the cohesive ends were blunted by T4 polymerase. Subsequently, the inserts were digested with NotI. pRc.CMV was cut with HindIII, and the cohesive ends were blunted by T4 polymerase. The vector was digested with NotI, and this was followed by ligation with the insert.
In the case of the vWD 2A/IIE mutant, the 3′ untranslated region (UTR) of pJB6 did not extend far enough to include the novel stop codon, which was 34 codons further downstream. Therefore, 2 PCR fragments overlapping at 65 base pairs, one including the 5′ end of the pJB6 insert and the vWD 2A/IIE frameshift mutation 8566delC (template, pJB6; sense, M13 reverse primer; antisense, 5′-TGG TAC ACA ACA GAG CCA TTG G-3′; and annealing temperature, 48°C) and the other including the 3′ UTR down to nucleotide 38/1071 of the published sequence28(template, genomic DNA; sense, 5′-TCT CCG ACA CGG ACG GAG CCC-3′; antisense, 5′-ATT GCGGCCGC TCA GAA GGG CAC AAG AGC AGA AC-3′ [underlining indicates NotI site]; and annealing temperature, 60°C) were joined by a third PCR (sense, M13 reverse primer; antisense, 5′-ATT GCGGCCGC TCA GAA GGG CAC AAG AGC AGA AC-3′ [underlining indicates NotI site]; and annealing temperature, 48°C). This combined PCR product was digested withXhoI and NotI and cloned into pBS/KS(−) (Stratagene). The resulting plasmid, designated pTO1, was transfected into Epicurian XL1-Blue MRF′ Kan supercompetent cells. Individual clones were sequenced completely to exclude involuntarily introduced mutations. PTO1 was digested with XhoI, and the cohesive ends were filled in by T4 polymerase. The sequence was then digested with NotI.
The expression vector pRc.CMV was digested with HindIII, and the cohesive ends were filled in by T4 polymerase. The vector was subsequently digested with NotI, and this was followed by ligation with the insert of pTO1. After the correct ligation was confirmed by sequencing, the plasmids were cloned into Epicurian XL1-Blue MRF′ Kan. Plasmids were purified by using the Endofree Plasmid Maxi Kit (Qiagen, Hilden, Germany). COS-7 cells (4 × 105; DSMZ, Braunschweig, Germany) were transiently transfected with mutant and WT fragment II DNA (10 μg each) by using the Lipofectamine Plus Kit (Life Technologies, Paisley, United Kingdom). The cells, adapted to serum-free medium (high-glucose Dulbecco modified Eagle medium and 2% Ultroser G; Life Technologies), were grown for 60 hours. vWF product was secreted in the medium and analyzed by SDS-electrophoresis in 6% polyacrylamide gels25 without prior concentration under nonreducing conditions and after reduction by dithiothreitol (DTT; 25 mM). Electrophoretic bands were visualized by using luminescent immunoblotting.19
Recombinant homozygous expression of full-length vWF cDNA
The origin of the full-length vWF cDNA was the plasmid vWF-PMT229 (67122; American Type Culture Collection). The vWF cDNA insert was obtained by EcoRI digestion and transferred into the EcoRI site of pBS/KSY(−), a modified pBS/KS(−) vector with an additional NotI site and lacking the BamHI and XhoI sites (a kind gift of Y. Hashimoto). After NotI digestion, the insert was transferred into the NotI site of the expression vector pcDNA3.1 Hygro(−) (Invitrogen). The resulting plasmid was designated vWF-pcDNA3.1. This vector has an EcoRV site that was used together with an vWF intragenic EcoRV site to cut out a small 3′ portion of the vWF gene. Mutant 3′ fragments were then produced by PCR using the 2 different mutant pJB6 vectors and the mutant pTO1 vector as templates (sense primer, 5′-GAA GAG GGT CAC AGG CTG CC-3′; antisense primer, M13-20; and annealing temperature, 48°C). The PCR products were digested with SacI, and this was followed by filling in the cohesive ends and subsequent digestion with EcoRV. The resulting inserts were ligated to the large 5′ EcoRV-digested vWF-pcDNA3.1 fragment. The ligation sites and the PCR-amplified fragments were sequenced to exclude involuntarily introduced mutations and to select constructs with the correct orientation.
Epicurian XL1-Blue MRF′ Kan supercompetent cells were transformed with mutant or WT vWF-pcDNA3.1. Plasmids were purified by using the Endofree Plasmid Maxi Kit (Qiagen). COS-7 cells (4 × 105) were transiently transfected with 10 μg mutant or WT full-length vWF cDNA by using the method described above. The serum-free medium was concentrated in Centricon tubes to one tenth of the original volume before further analysis in a high-resolution SDS–agarose gel18 under nonreducing and reducing conditions. Electrophoretic bands were visualized by using luminescent immunoblotting.19
Recombinant coexpression of WT and mutant full-length vWF cDNA
The same methods were used for cotransfection with WT and mutant full-length vWF cDNA (10 μg of each). To compensate for a possible lower translation efficiency of mutant sequences, 20 μg of a 1:1 proportion of WT to mutant plasmid; 1:3 proportions for all 3 mutants; and 1:9, 1:19, and 1:39 proportions for recombinant vWF 2A/IIE were used. The resulting recombinant vWF proteins were analyzed by multimer analysis in a medium-resolution SDS–agarose gel.18 19
Protein truncation assay
As the consequence of a frameshift mutation, the 8566delC product was predicted to be extended because of disruption of the original stop codon and the presence of a newly generated stop codon located 34 codons further downstream. This elongated product was demonstrated through a unique application of an in vitro protein truncation assay (Boehringer Mannheim, Germany) originally designed for detecting truncating nonsense or frameshift mutations.30Following the supplier's recommendations, we amplified a portion of the 3′ cDNA end, starting with the first codon of exon 48 (codon 2630) by using the sense primer 5′-gga tcc taa tac gac tca cta tag gaa cag acc acc ATG GGTTACAAGGAAGAAAATAACACAGGTGAATGTTGTGGG-3′ and the antisense vector primer M13-20. (Nucleotides of the sense primer, written in lowercase letters, represent the sequence for a T7 promotor and a Kozak consensus sequence; uppercase letters represent the coding sequence, with the vWF-specific sequence underlined.) The annealing temperature was 48°C. As templates, we used pJB6 for the WT sequence and pTO1 for the mutant sequence. In translating, we expected a peptide of 184 amino acids for the WT sequence and a peptide of 218 amino acids for the mutant sequence.
Results
Diagnosis of vWD 2A/IIE and vWD 3
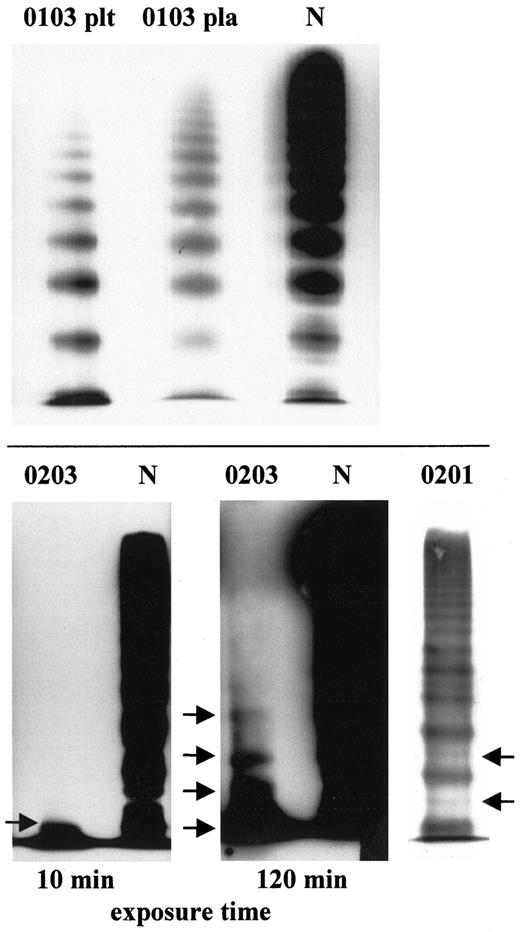
Hemostasis variables in patients and their relatives are shown in Table 1. The diagnosis of vWD 2A/IIE in patient 0103 was based on multimer analysis of vWF, which found absence of the triplet structure and absence of HMWM from both plasma and platelets. The fastest migrating electrophoretic band was not pronounced12(Figure 1), which is in contrast to findings in vWD 2A/IIC. The markedly reduced vWF:Ag levels in the patient and the low-normal vWF:Ag levels in the patient's mother suggest compound heterozygosity for a qualitative and quantitative defect.
Multimer analysis of vWF in patient 0103 with vWD 2A/IIE and patient 0203 with vWD 3.
(Top) Characteristic for vWD 2A/IIE are the lack of HMWM, the lack of a triplet structure (indicating decreased proteolysis), and the presence of the defect in both the patient's plasma (pla) and platelets (plt). (Bottom) Multimer analysis of vWF derived from platelets from the patient with vWD 3 (0203) compared with a normal (N) control. Bands corresponding to low-molecular-weight vWF (arrows) appeared only after prolonged exposure of the film, indicating a very low concentration of vWF. The 0201 indicates vWF multimer pattern of the heterozygous father's plasma. Arrows point to extra intervening bands between triplets.
Multimer analysis of vWF in patient 0103 with vWD 2A/IIE and patient 0203 with vWD 3.
(Top) Characteristic for vWD 2A/IIE are the lack of HMWM, the lack of a triplet structure (indicating decreased proteolysis), and the presence of the defect in both the patient's plasma (pla) and platelets (plt). (Bottom) Multimer analysis of vWF derived from platelets from the patient with vWD 3 (0203) compared with a normal (N) control. Bands corresponding to low-molecular-weight vWF (arrows) appeared only after prolonged exposure of the film, indicating a very low concentration of vWF. The 0201 indicates vWF multimer pattern of the heterozygous father's plasma. Arrows point to extra intervening bands between triplets.
In the patient with vWD 3, vWF:Ag levels in both plasma and platelets were below the detection limit of the enzyme-linked immunosorbent assay used. The results of a multimer analysis were consistent with vWD 3. After obtaining knowledge of the underlying mutation, we repeated the multimer analysis of the patient's platelets with higher sensitivity and detected low-molecular-weight multimers. Levels of vWF:Ag were decreased in both the patient's parents (Table 1), and multimer analysis revealed faint extra intervening bands between individual multimers (Figure 1).
Identification of candidate mutations
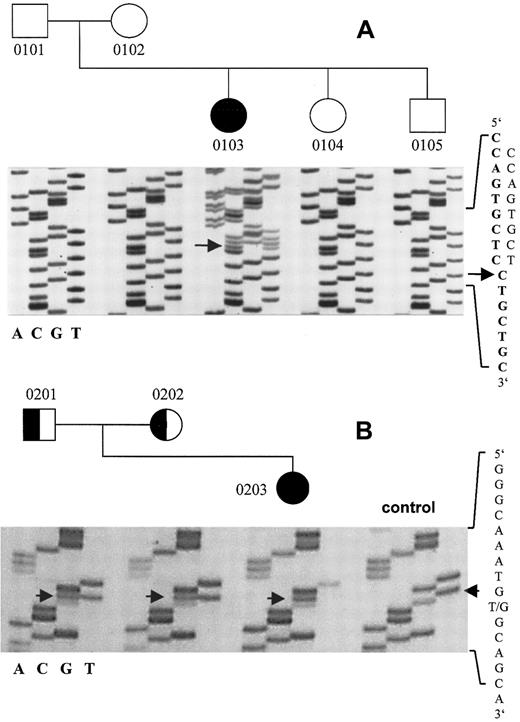
In the patient with vWD 2A/IIE, but not in her parents, we identified a novel heterozygous mutation of the vWF gene31that consisted of a single base deletion (8566delC) in exon 52 (Figure2). The resulting frameshift predicted a vWF monomer that was 34 amino acids longer than the WT. The first amino acid affected by the frameshift was cysteine 2773, which is mutated in vWD 2A/IID.6 Intragenic vWF marker alleles analyzed in the patient and her parents confirmed paternity, thereby suggesting a de novo mutation. Markedly decreased vWF:Ag in the patient (0.07 U/mL) and low-normal vWF:Ag levels in her mother (0.62 U/mL) suggested an additional quantitative defect. However, although we sequenced all 51 coding exons and flanking intron regions of the vWF gene, we did not identify additional mutations.
Direct sequencing of the vWD 2A/IIE frameshift mutation 8566delC and the vWD 3 transversion 8512T>G.
(A) The mutation 8566delC in the translated region of exon 52 of the vWF gene predicts an aberrant and elongated translation product and is present only in the patient, not in either of her parents. The WT sequence is in boldface type. (B) The patient 0203 is homozygous for the transversion 8512T>G, which predicts the amino acid substitution C2754W; both parents are heterozygous. Numbers correspond to those in Table 1.
Direct sequencing of the vWD 2A/IIE frameshift mutation 8566delC and the vWD 3 transversion 8512T>G.
(A) The mutation 8566delC in the translated region of exon 52 of the vWF gene predicts an aberrant and elongated translation product and is present only in the patient, not in either of her parents. The WT sequence is in boldface type. (B) The patient 0203 is homozygous for the transversion 8512T>G, which predicts the amino acid substitution C2754W; both parents are heterozygous. Numbers correspond to those in Table 1.
Protein truncation assay
The vWD 2A/IIE frameshift mutation 8566delC predicted an elongated translation product, which the results of the in vitro protein truncation assay confirmed. The predicted WT product was a peptide of 184 amino acids (including the first ATG), whereas the predicted mutant product corresponded to a peptide of 218 amino acids (Figure3). Along with this elongation, the mutation predicted the loss of 6 cysteines from the WT amino acid sequence and the generation of 7 cysteines at other positions.
Protein truncation assay.
In vitro expression of the 3′ fragments of the normal and the mutant vWF gene demonstrated an elongated translation product of 218 amino acids that was caused by the vWD 2A/IIE frameshift mutation 8566delC.
Protein truncation assay.
In vitro expression of the 3′ fragments of the normal and the mutant vWF gene demonstrated an elongated translation product of 218 amino acids that was caused by the vWD 2A/IIE frameshift mutation 8566delC.
Recombinant expression of the carboxy-terminal vWF fragment II
Recombinant expression of the carboxy-terminal of vWF fragment II in COS-7 cells resulted in monomeric fragments for all 3 recombinant mutant peptides. Dimerized product was observed only with the recombinant WT peptide and could be reduced to a monomer by DTT (Figure4).
Recombinant expression of the isolated carboxy-terminal vWF fragment II in COS-7 cells.
Fragment II harbors the dimerization site of vWF. SDS–polyacrylamide gel electrophoresis (6%) showed monomeric bands of the recombinant mutant fragments and a band corresponding to a dimer of the WT fragment, which was reduced to a monomer by DTT (bottom).
Recombinant expression of the isolated carboxy-terminal vWF fragment II in COS-7 cells.
Fragment II harbors the dimerization site of vWF. SDS–polyacrylamide gel electrophoresis (6%) showed monomeric bands of the recombinant mutant fragments and a band corresponding to a dimer of the WT fragment, which was reduced to a monomer by DTT (bottom).
Recombinant homozygous expression of full-length vWF cDNA
Expression of the recombinant mutant full-length vWF in COS-7 cells resulted in vWF monomers and dimers for all 3 mutations. Additionally, fainter bands in the range of tetramers were observed. A complete set of multimers was observed in the recombinant WT vWF (Figure 5A). Under reducing conditions, the WT and mutant vWF proteins had a single band of monomer size. Relative to a plasma pool, vWF:Ag of recombinant WT vWF was 20.7%, that of recombinant C2754W vWF was 4.9%, that of recombinant C2773R vWF was 14.6%, and that of recombinant 8566delC vWF was 1.8%. The FVIII binding activity of recombinant WT vWF was 90%, and that of the mutant molecules C2754W, 8566delC, and C2773R, was 80%, 85%, and 90%, respectively. In contrast, vWF:CBA was 80% in the recombinant WT vWF but below the limit of detection in all 3 mutants.
Homozygous recombinant expression of full-length mutant and WT vWF and coexpression of WT and mutant full-length vWF.
(A) High-resolution SDS–agarose gel electrophoresis under nonreducing conditions identified a majority of bands of monomer (m) and dimer (d) size of mutant vWF compared with fully multimerized recombinant WT vWF. Fainter bands in the range of tetramers were also observed in the mutants. Mutant and WT recombinant vWF under reducing conditions are shown as monomers (right). (B) Medium-resolution SDS–agarose gel electrophoresis of recombinant WT and mutants coexpressed vWF. Two different proportions of WT to mutant transfection plasmid concentrations were used (1:1 and 1:3). Arrows indicate extra intervening bands between triplets observed in the multimer pattern of the vWD 2A/IID mutation and the vWD 3 mutation but not in the pattern of the vWD 2A/IIE mutation. In combination with the reduced HMWM, the multimer phenotype of the patients' plasma is reproduced exactly. The intervening bands and the relative decrease in HMWM were more pronounced in the 1:3 transfection experiment.
Homozygous recombinant expression of full-length mutant and WT vWF and coexpression of WT and mutant full-length vWF.
(A) High-resolution SDS–agarose gel electrophoresis under nonreducing conditions identified a majority of bands of monomer (m) and dimer (d) size of mutant vWF compared with fully multimerized recombinant WT vWF. Fainter bands in the range of tetramers were also observed in the mutants. Mutant and WT recombinant vWF under reducing conditions are shown as monomers (right). (B) Medium-resolution SDS–agarose gel electrophoresis of recombinant WT and mutants coexpressed vWF. Two different proportions of WT to mutant transfection plasmid concentrations were used (1:1 and 1:3). Arrows indicate extra intervening bands between triplets observed in the multimer pattern of the vWD 2A/IID mutation and the vWD 3 mutation but not in the pattern of the vWD 2A/IIE mutation. In combination with the reduced HMWM, the multimer phenotype of the patients' plasma is reproduced exactly. The intervening bands and the relative decrease in HMWM were more pronounced in the 1:3 transfection experiment.
Recombinant coexpression of WT and mutant full-length vWF cDNA
In an attempt to replicate the characteristic patterns of multimers from the plasma of heterozygous individuals, the coexpression of WT and mutant full-length vWF were carried out with use of several different proportions between WT and mutant plasmids for transfection. The characteristic vWD 2A/IID multimer pattern—a decrease in large multimers and an extra central band between individual multimers—was replicated by using a ratio of 10 μg WT to 10 μg mutant plasmid. A ratio of 5 μg WT and 15 μg mutant plasmid replicated the heterozygous phenotype of the vWD 3 mutation (Figure 5B). Heterozygous expression of the vWD 2A/IIE mutation by using different proportions of WT to mutant plasmid resulted in a relative decrease in larger multimers and a lack of ultralarge multimers compared with the WT results. However, intervening bands were not detected, even at the lowest ratio (1:39) of WT to mutant plasmid concentration (Figures6 and 7).
Coexpression of recombinant full-length WT and vWF 2A/IIE at different proportions of WT to mutant vWF.
Only the ultralarge multimers (arrows) were absent in the 2A/IIE transfection experiment, especially at the 1:39 proportion of WT to mutant DNA. Relative to recombinant WT vWF, concentrations of the larger multimers were decreased. Intervening bands like those observed in the other mutants were not present.
Coexpression of recombinant full-length WT and vWF 2A/IIE at different proportions of WT to mutant vWF.
Only the ultralarge multimers (arrows) were absent in the 2A/IIE transfection experiment, especially at the 1:39 proportion of WT to mutant DNA. Relative to recombinant WT vWF, concentrations of the larger multimers were decreased. Intervening bands like those observed in the other mutants were not present.
Densitometric evaluation of the multimer pattern of recombinant vWF.
Recombinant WT vWF was compared with the product from the coexpression of WT and 2A/IIE vWF in the proportion of 1:39. Ultralarge multimers were absent (arrows), and there was a relative decrease in large multimers in the multimer pattern of the coexpressed vWF.
Densitometric evaluation of the multimer pattern of recombinant vWF.
Recombinant WT vWF was compared with the product from the coexpression of WT and 2A/IIE vWF in the proportion of 1:39. Ultralarge multimers were absent (arrows), and there was a relative decrease in large multimers in the multimer pattern of the coexpressed vWF.
Discussion
We explored the posttranslational processing of vWF monomers into dimers and multimers in detail by using recombinant expression of mutant and WT vWF. Intermolecular bonding of normal vWF is provided first by the dimerization site at the vWF carboxy-terminal and then by the multimerization site at the amino-terminal. Dimerization at the carboxy-terminal of normal vWF monomers in the endoplasmic reticulum (ER) appears to be quantitative, and only dimers are expected to be further transported to the Golgi and post-Golgi region where the multimerization process occurs.32 33 Consequently, normal multimers consist only of even numbers of subunits (Figure8).
Hypothetical mechanism of the effects of dimerization defects on vWF multimer assembly.
In heterozygous individuals with either the vWD 2A/IID or vWD 3 mutation, normal vWF (symbols with shaded and black half circles) is released from the ER in the form of dimers, and aberrant vWF (symbols with shaded and white half circles) is released in the form of monomers. The symbols' black half circles represent an intact dimerization site, whereas their shaded half circles represent the amino-terminal multimerization site. White half circles represent the defective dimerization site in mutant vWF monomers. On inclusion of aberrant monomers during the ongoing multimerization process in the Golgi and post-Golgi region, further polymerization is blocked, resulting in the observed lack of HMWM. Apposition of an aberrant monomer at only one site results in a multimer with an odd number of individual subunits, represented by the extra intervening bands (marked by odd numbers) between individual multimers. The original multimer pattern shown on the right is from the coexpression product of recombinant mutant 2A/IID with recombinant WT vWF shown in Figure 5B.
Hypothetical mechanism of the effects of dimerization defects on vWF multimer assembly.
In heterozygous individuals with either the vWD 2A/IID or vWD 3 mutation, normal vWF (symbols with shaded and black half circles) is released from the ER in the form of dimers, and aberrant vWF (symbols with shaded and white half circles) is released in the form of monomers. The symbols' black half circles represent an intact dimerization site, whereas their shaded half circles represent the amino-terminal multimerization site. White half circles represent the defective dimerization site in mutant vWF monomers. On inclusion of aberrant monomers during the ongoing multimerization process in the Golgi and post-Golgi region, further polymerization is blocked, resulting in the observed lack of HMWM. Apposition of an aberrant monomer at only one site results in a multimer with an odd number of individual subunits, represented by the extra intervening bands (marked by odd numbers) between individual multimers. The original multimer pattern shown on the right is from the coexpression product of recombinant mutant 2A/IID with recombinant WT vWF shown in Figure 5B.
Dimerization and intermolecular bonding at the vWF amino-terminal are processes that can occur independently of each other.26Thus, in the case of a dimerization defect at the carboxy-terminal, we would nevertheless expect the formation of dimers by disulfide bonding at the amino-terminal. To assess the effects of the mutations at the carboxy-terminal on the dimerization process of vWF monomers and on the intermolecular disulfide bonding at the amino-terminals of vWF dimers to multimers, we first expressed only the carboxy-terminal fragment II that harbors the dimerization site. All 3 mutations in exon 52 of the vWF gene affected cysteine residues and severely impaired dimerization of vWF monomers, as demonstrated by the synthesis of only monomeric mutated fragment II compared with the dimeric WT (Figure 4).
We then did 2 different transfection experiments with mutant and WT full-length vWF cDNA. First, to mimic a homozygous mutation, we used only mutant vWF cDNA for transfection, and second, to reproduce the heterozygous state, we conducted cotransfection studies of mutant and WT vWF. In spite of the dimerization defect, the homozygous expression of mutant full-length vWF resulted in synthesis of dimeric protein, suggesting that the disulfide bonding at the amino-terminal of the mutant vWF was intact. This finding confirms a previous report that amino-terminal intersubunit disulfide bonding occurs independently of dimerization at the carboxy-terminal.26 We also observed small amounts of tetramers in all mutants studied (Figure 5A), a result that suggests the presence of a functionally incomplete block of dimerization, possibly based on the additional carboxy-terminal cysteine residues involved in intersubunit bonding; however, we did not observe analogous findings in expression studies of the carboxy-terminal fragment II. As expected, expression of full-length WT vWF resulted in synthesis of the complete set of multimers. Using the same seeding concentration of 4 × 105 COS-7 cells in each of the transfection experiments, we obtained different yields of vWF:Ag for mutant and WT vWF. The lowest values were for the 8566delC mutation and the vWD 3 mutation C2754W, and a concentration close to that for recombinant WT vWF was obtained for the vWD 2A/IID mutation C2773R.
Functional studies of the recombinant mutant dimeric vWF revealed severely impaired vWF:CBA but normal FVIII binding activity. Correspondingly, in the patient with vWD 2A/IID, normal vWF:Ag levels correlated with normal FVIII:C values, whereas in the patients with vWD 2A/IIE or vWD 3, low or undetectable vWF:Ag levels corresponded with low FVIII:C values (Table 1).
Coexpression of WT and mutant vWF should theoretically lead to the formation of normal dimers and aberrant monomers in the ER. If both dimers and monomers were subsequently transported to the Golgi compartment, in the second step, further intermolecular disulfide bonding at the vWF amino-terminal could result in the formation of either dimers (made up of aberrant monomers), trimers (made up of one aberrant monomer and a normal dimer), or normal tetramers (consisting of 2 normal dimers). In the third step, aberrant dimers cannot further multimerize because they only carry the defect dimerization domain at both terminals, whereas aberrant trimers can bind to either an aberrant monomer or a normal dimer forming aberrant tetramers and aberrant pentamers. The normal tetramers can further expand to aberrant pentamers, normal hexamers, and so forth (Figure 8). Thus, along with the formation of multimers with an even number of subunits, it is possible that multimers with an odd number of subunits are formed.
The vWF multimer patterns from heterozygous relatives of the patients with vWD 3 and, in an even more pronounced fashion, heterozygous patients with vWD 2A/IID, showed characteristic intervening bands that are clearly distinct from the bands contributing to the usual triplet structure of normal vWF multimers.6 34 This phenotype was replicated through coexpression of recombinant WT and mutant full-length vWF (Figure 5B). Our results provide evidence that these intervening bands represent multimers with an odd number of subunits. The absence of HMWM in the patient with vWD 2A/IID may be explained on a statistical basis by an increased probability that aberrant C2773R monomers—which are synthesized in a comparable concentration as WT monomers—are incorporated in the polymeric structure of the larger multimers. Analogous to a termination reaction, aberrant monomers bind to the growing multimers only by means of the intact amino-terminal binding site, thereby preventing further growth of multimers (Figure8). In contrast, HMWM were present in plasma of the heterozygous relatives of the patient with vWD 3. This could be explained by the observed lower expression of the recombinant mutant C2754W compared with the WT allele and a corresponding preponderance of normal subunits. Subsequently, by increasing the proportion of mutant to WT DNA for transfection, thereby compensating for lower expression of the mutant allele, we observed a relative decrease in HMWM, as in the recombinant IID vWF (Figure 5B).
Neither plasma vWF from the patient with vWD 2A/IIE nor the respective recombinant vWF from the coexpression experiment had intervening bands to indicate multimers with odd numbers of subunits. The reason for this is not clear. However, the 8566delC mutation differs from the 2 other defects described in this study in its consequences, which are rather complex. The frameshift completely alters subsequent codons, substitutes 6 cysteines with 7 others at aberrant positions, and predicts an elongated translation product. Thus, along with elimination of C2773, the cysteine that is mutated in the patient with vWD 2A/IID, there is elimination of cysteines at positions 2774, 2788, 2804, 2806, and 2811, of which C2811 could also be part of an intermolecular disulfide bond (although its mutation to alanine permitted formation of dimers in recombinant expression experiments).35In addition, the generation of 7 new cysteines in the predicted aberrant sequence could give rise to alternative intramolecular disulfide bonds contributing to the unique vWD 2A/IIE phenotype in comparison to the other dimerization defects. It is possible that the resulting mutant pro-peptide could also influence the subsequent multimerization process at the amino-terminal, which would further impair the binding of aberrant monomers and formation of multimers with odd numbers of subunits.
Another reason for the lack of intervening bands could be a very low expression of the second allele. Normal multimers derived from this allele would not be abundant and could be readily saturated at both amino-terminal sites with the more abundant mutant 8566delC monomers, resulting in a preponderance of multimers with an even number of subunits. The electrophoretic pattern of homozygous recombinant 8566delC vWF did not differ much from those of the 2 other homozygously expressed vWD defects, but in contrast to those defects, only small amounts of WT DNA were sufficient to rescue most of the normal phenotype. This was demonstrated through transfection with different proportions of WT to 8566delC mutant DNA (Figures 6 and 7). Even transfection proportions of 1 part WT and 39 parts mutant DNA did not completely replicate the multimer pattern observed in patient 0103, and the only similarities to the in vivo phenotype were absence of ultralarge multimers normally observed in WT vWF expression experiments and a relative decrease in the concentration of large multimers compared with WT vWF (Figures 6 and 7). These findings suggest that expression of vWF derived from the patient's second putative vWD allele was very low, albeit not completely absent. We sequenced the complete coding region and flanking introns of the vWF gene without detecting a second mutation. Therefore, we do not have experimental data that explain the lack of intervening bands in this particular patient with vWD 2A/IIE.
The shared underlying defect of vWF dimerization is an interesting feature of the manifestation of different vWD types and subtypes. In the case of the homozygous missense mutation C2754W, the defect may even result in the phenotype of severe vWD 3 clinically and with regard to vWF laboratory findings. Minute amounts of vWF were detected only in the patient's platelets by high-sensitivity luminescent multimer analysis (Figure 1). Possibly, in contrast to the situation in the circulation, from which vWF is cleared by proteolysis and consumption, platelets might be regarded as a protective environment for vWF. Compared with plasma, the higher concentration of mutant vWF in COS-7 cells might reflect the nonphysiologic conditions of in vitro protein expression in heterologous cells, eg, absence of the vWF-specific metalloprotease in the cell-culture medium (unpublished data).
A study of the possible role of the vWF carboxy-terminal cysteine residues in the dimerization process was done by Katsumi et al.35 The results suggested that C2771, C2773, and C2811 are the only cysteines that could form the intersubunit bonds between vWF monomers in the cystine knot-like (CK) domain at the vWF carboxy-terminal, a common motive found in the dimerization region of several proteins. One of these cysteines, C2773, is mutated in our patient with vWD 2A/IID, whereas C2771 was found to be mutated in another patient with vWD 2A/IID.36 Furthermore, in our patient with vWD 2A/IIE, C2773 was the first amino acid predicted to be altered by the underlying frameshift mutation. Thus, our data obtained by studying patients are in perfect agreement with the results of a biochemical study of the cysteine residues directly involved in vWF dimerization.35 According to that study, C2754, the cysteine mutated in our patient with vWD 3, is probably not directly involved in intersubunit binding but rather is involved in an intramolecular disulfide bond.35 Nevertheless, the mutation C2754W clearly results in a dimerization defect. Interestingly, C2754 is bound to C2803 and both cysteines contribute to the CK consensus sequence of vWF. A homozygous mutation at C2803 (C2803R) has been reported.37 Its consequences are similar to those of C2754W and comprise the presence of only dimers, both in plasma and in recombinant mutant vWF in the homozygous patient, and the presence of characteristic intervening bands, suggesting multimers with an odd number of individual subunits in heterozygous individuals.
Our results suggest that the 3 mutations involving cysteines in the CK domain at the vWF carboxy-terminal cause severe defects of dimerization in different types of vWD. The mutation C2773R eliminates an intermolecular disulfide bond in the patient with vWD 2A/IID, and C2754W eliminates an intramolecular bond as part of the vWF CK consensus sequence in the patient with vWD 3. The mutation 8566delC (vWD 2A/IIE) involves C2773 and several other cysteines that may be important for dimerization in the vWF CK domain.
Supported by the Deutsche Forschungsgemeinschaft, grants DFG Schn 325/4-1 and DFG Schn 325/4-2.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Reinhard Schneppenheim, University Children's Hospital Hamburg, Department of Pediatric Hematology and Oncology, D-20246 Hamburg, Germany; e-mail: schneppenheim@uke.uni-hamburg.de.







This feature is available to Subscribers Only
Sign In or Create an Account Close Modal