Abstract
Human Vα24NKT cells are activated by α-galactosylceramide (α-GalCer)-pulsed dendritic cells in a CD1d-dependent and a T-cell receptor–mediated manner. Here, we demonstrate that CD4+Vα24NKT cells derived from a patient with acute myeloid leukemia (AML) M4 are phenotypically similar to those of healthy donors and, in common with those derived from healthy donors, express tumor necrosis factor–related apoptosis-inducing ligand (TRAIL) when the cells are activated by α-GalCer–pulsed dendritic cells but not prior to activation. We also show that myeloid leukemia cells from patients with AML M4, but not from patients with AML M0 or M1, undergo apoptosis following culture with TRAIL-expressing autologous or allogeneic healthy donor Vα24NKT cells. Apoptosis of AML M4 leukemia cells from patient peripheral blood was almost completely blocked by a neutralizing monoclonal antibody against TRAIL, indicating that TRAIL on Vα24NKT cells is essential for the induction of apoptosis in AML M4 leukemia cells. A nonobese diabetic–severe combined immunodeficient human leukemia (AML M4) model showed that human activated CD4+Vα24NKT cells induced apoptosis of human leukemia cells in vivo. This is the first evidence that activated Vα24NKT cells express TRAIL and that TRAIL causes apoptosis of monocytic leukemia cells from patients with AML M4 in vitro and in vivo. Adoptive immune therapy with activated Vα24NKT cells, or other strategies to increase activated Vα24NKT cells in vivo, may be of benefit to patients with AML M4.
Introduction
Human Vα24NKT cells are a subpopulation of T cells, highly conserved between mice (Vα14NKT cells) and humans (Vα24NKT cells), which can be stimulated by α-galactocylceramide (α-GalCer, KRN7000) in a CD1d-dependent and a T-cell receptor (TCR)-mediated manner.1-3 Most human peripheral blood Vα24NKT cells have a double-negative (DN; CD4−CD8−) or CD4+ phenotype and coexpress CD161. Other surface markers of natural killer (NK) cells (CD16, CD56, and CD57) and immunoglobulin (Ig)-type NK receptors such as p58.1, p58.2, and p70 are not expressed. Expression of CD94, a lectin-type NK receptor, varies probably depending on Vα24NKT cell subpopulations.2-4 There is evidence for a role of these cells in preventing or controlling malignancy. Mice injected with α-GalCer were protected from experimentally induced tumors, and it is clear that Vα14NKT cells were responsible for these antitumor effects.5-7 Similar antitumor effects were seen when murine Vα14NKT cells or human Vα24NKT cells were adoptively transferred into tumor-bearing mice.8 We have recently shown in vitro susceptibility of human malignant cell lines to cytotoxic activity of Vα24NKT cells. U937 (a cell line with monocytic characteristics), THP-1 (monocytic leukemia cell line), and Molt-4 (T-cell lymphoma) were sensitive to the cytotoxic activity of DNVα24NKT cells, but neither K562 (chronic myelogeneous leukemia cell line) nor Daudi (Burkitt lymphoma cell line) were sensitive.9 Among tumor cell lines we have tested, U937 was the most susceptible to the cytotoxic activity of both DN and CD4+Vα24NKT cells.
These previous in vitro studies showing cytotoxic activity against human hematologic malignancies suggested that Vα24NKT cells, activated by α-GalCer, warranted further investigation for a possible immune therapeutic role in the treatment of acute myeloid leukemia (AML), particularly those of monocytic origin. AML encompasses a group of disorders characterized by clonal accumulation of immature and abnormal myeloid cells in the blood and marrow. Most adult patients with AML cannot be cured with current treatments involving maximum tolerated doses of chemotherapy; novel therapeutic strategies such as immune-based therapies are needed to sustain remission and increase the cure rate.
Although antitumor activity of Vα24NKT has been clearly demonstrated in vitro, the mechanisms for selective killing of malignant cells have not been defined to date. Activation of Vα24NKT cells is dependent on the presentation of α-GalCer by CD1d, but neither is essential for their cytotoxic activity against some malignant cells. It is still unknown how Vα24NKT cells kill malignant targets. Tumor necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL) is a recently identified type II integral membrane protein belonging to the TNF family that selectively induces apoptosis of various tumor cell lines.10-14 Lymphoid cells such as T cells and NK cells express TRAIL, and these TRAIL-expressing cells induce apoptotic cell death in various tumor cells.15-17The susceptibility to TRAIL-induced apoptosis may be related to the expression levels of multiple receptors on target cells. TRAIL binds to at least 4 receptors, death-inducing receptors (TRAIL-R1, and -R2) and non–death-inducing receptors (TRAIL-R3 and -R4), with similar affinities.18-22 The observation that U937 was sensitive to TRAIL-induced apoptosis, but that K562 and Daudi were resistant,17 is similar to the pattern of cytotoxic activity we observed with activated Vα24NKT cells.9 This led us to hypothesize that activation of Vα24NKT cells may induce the expression of TRAIL, which may be responsible for the observed killing activity against some malignant cells. We report here the expression of TRAIL by Vα24NKT cells and the resulting cytotoxic activity against human AML cells through TRAIL-induced apoptosis.
Patients, materials, and methods
Patient samples
Peripheral blood samples were collected from patients with AML with informed consent at the time of sample collection for diagnosis. Relative clinical and diagnostic laboratory data for the patients are shown in Table 1.
Clinical characteristics and cytogenetics of acute myeloid leukemia patients
| Patient . | Sex/age . | Sample . | Cytogenetics . | Blasts, % . | FAB . |
|---|---|---|---|---|---|
| 1 | M/60 | PB | 46, XY, del(12)(q15q22) | 82.20 | M4 |
| 2 | F/24 | PB | 49, XX, +8, inv(16)(q13q22), +21, +22 | 85.50 | M4 |
| 3 | M/59 | PB | 46, XX, no cytogenetic abnormalities | 86.00 | M4 |
| 4 | M/57 | PB | 46, XX, no cytogenetic abnormalities | 92.00 | M1 |
| 5 | M/34 | PB | 46, XX, multiple cytogenetic abnormalities | 80.00 | M0 |
| Patient . | Sex/age . | Sample . | Cytogenetics . | Blasts, % . | FAB . |
|---|---|---|---|---|---|
| 1 | M/60 | PB | 46, XY, del(12)(q15q22) | 82.20 | M4 |
| 2 | F/24 | PB | 49, XX, +8, inv(16)(q13q22), +21, +22 | 85.50 | M4 |
| 3 | M/59 | PB | 46, XX, no cytogenetic abnormalities | 86.00 | M4 |
| 4 | M/57 | PB | 46, XX, no cytogenetic abnormalities | 92.00 | M1 |
| 5 | M/34 | PB | 46, XX, multiple cytogenetic abnormalities | 80.00 | M0 |
FAB indicates French-American-British staging; PB, peripheral blood.
Antibodies for flow cytometry
The cell-surface phenotype was determined by single- or 2-color flow cytometry using Ortho Cytoron Absolute (Ortho Diagnostic Systems, Raritan, NJ). The fluorescein isothiocynate (FITC)- or phycoerythrin (PE)-conjugated monoclonal antibodies (mAbs) specific for CD1a (BL6), CD1b (M-T101), CD1c (L161), CD3 (UCHT1), CD4 (13B8.2), CD34 (581), CD8 (B9.1), Vα24 (C15), Vβ11 (C21), p58.1 (EB6), p58.2 (GL183), CD16 (3G8), CD56 (N901), CD94 (HP-3B1), p70 (NKB1) (DX9), NKRP1A (CD161) (DX12), and isotype-matched controls were obtained from Becton Dickinson (San Jose, CA), Immunotech (Marseille, France), and Pharmingen (San Diego, CA). The mAb against CD1d (42)23 was kindly provided by Dr S. Porcelli. The purified mAb specific for TRAIL (RIK-2) was prepared as described previously.15 Expression of TRAIL on Vα24NKT cells was determined by staining with RIK-2 followed by FITC-conjugated rat antimouse IgG1. Expression of TRAIL–R1-R4 on the target cells was determined by staining with anti-TRAIL–R1-R4 polyclonal antibodies (Dako, Kyoto, Japan) followed by FITC-conjugated rabbit antigoat immunoglobulins.
Preparation of dendritic cells
Human monocytes (purity of CD14+ cells: 98%) were isolated from peripheral blood mononuclear cells (PBMCs) from a patient with AML M4 and healthy donors (HDs) by magnetic-bead sorting using anti-CD14 mAb (MACS, Miltenyi Biotec, Bergisch Gladbach, Germany). Dendritic cells (DCs) were prepared by culturing the isolated monocytess for 5 days in AIM-V medium (Gibco, Rockville, MD) containing 10% fetal calf serum (Hyclone, Logan, UT) in the presence of granulocyte-macrophage colony-stimulating factor (400 U/mL) (Kirin Brewery, Gunma, Japan) and interleukin-4 (400 U/mL) (Becton Dickinson Labware, MA). DCs used in this study strongly expressed major histocompatibility complex class I, class II, CD80, CD83, CD1b, CD1c, and CD1d and significantly expressed CD86 and CD1a.
Separation of CD34+ cells from cord blood
Umbilical cord blood samples were obtained according to institutional guidelines. CD34+ cells were isolated from mononuclear cells by magnetic-bead sorting using anti-CD34 mAb (MACS). The purity of CD34+ cells determined by flow cytometry was 95%.
Establishment of CD4+Vα24NKT cell lines from Pt 1 with AML M4
T cells derived from Patient 1 (Pt 1) with AML M4 were cultured with autologous DCs in the presence of α-GalCer (KRN7000) (100 ng/mL) (Kirin Brewery) for 10 days. On day 10, CD4+Vα24+ T cells were separated using MACS with anti-Vα24TCR mAb and anti-CD4 mAb. The CD4+Vα24+ T cells obtained were restimulated weekly with irradiated α-GalCer–pulsed autologous DCs. Interleukin-2 (20 U/mL) (Boehringer Mannheim, Mannheim, Germany) was added to the cultures every 3 to 4 days. The surface phenotype of the CD4+Vα24+ T-cell line was assessed using flow cytometry after the cells were cultured for a total of 5 to 6 weeks.
Analysis of cytotoxicity
Cytotoxic activity of activated CD4+Vα24NKT cells was assessed against the tumor cell line U937, the NK-sensitive cell line K562, and the LAK-sensitive cell line Daudi as described previously.9 CD4+Vα24NKT cells were used as effector cells on day 3 after stimulation by α-GalCer–pulsed DCs. Briefly, cytotoxic activity was determined using a standard 4-hour51Cr release assay. 51Cr-labeled target cells (5 × 103 cells/well) were incubated at 37°C for 4 hours with effector cells at effector-target (E/T) ratios of 10:1 and 20:1. The percentage of specific 51Cr release was calculated as follows: (cpm experimental release − cpm spontaneous release)/(cpm maximal release − cpm spontaneous release) × 100. The ratio of spontaneous release to maximal release was less than 15% in all experiments.
Induction and detection of apoptosis
PBMCs derived from AML patients or cord blood CD34+cells (8 × 104 cells) were cultured with 8 × 104 activated CD4+Vα24NKT cell lines for 8 hours in a 48-well culture plate in AIM-V medium supplemented with 10% fetal calf serum. The target cells were then examined for cell death by propidium iodide (PI) uptake and early apoptosis by Annexin V (AV) binding using an Annexin V FITC kit (Immunotech), based on the report that early apoptosis can be determined by AV+PI− staining.24 Briefly, cells were stained with AV-FITC and PI according to the manufacturer's protocol and assessed using flow cytometry, gating the PBMCs or cord blood CD34+ cells according to the characteristic forward and side light scatters. CD34+ hematopoietic progenitor cells from cord blood were used as nonmalignant and early myeloid control target cells. Assays were performed in triplicate.
Blocking studies with mAbs
The effects of a neutralizing mAb against TRAIL on apoptosis of PBMCs from AML patients and cord blood CD34+ cells following coculture with activated CD4+Vα24NKT cells were assessed. The PBMCs or CD34+ cells (8 × 104) were cultured with activated CD4+Vα24NKT cells (8 × 104) in the presence of RIK-2 or isotype-matched control mouse IgG1 at 10 μg/mL. After a total culture period of 8 hours, target cell apoptosis was assessed by AV-FITC and PI staining as described above.
Assay for TRAIL–R1-R4 messenger RNA expression
To examine TRAIL-R1, -R2, -R3, and -R4 messenger RNA (mRNA) expression, total RNA was extracted from PBMCs from patients with AML and cord blood CD34+ cells by the isothiocyanate method using Trizol reagent (Gibco). The following gene-specific primer sequences for TRAIL-R1, -R2, -R3, and -R4 were used in polymerase chain reaction (PCR): TRAIL-R1 (forward: 5′-CTGAGCAACGCAGACTCGCTGTCCAC-3′; reverse: 5′-TCCAAGGACACGGCAGAGCCTGTGCCAT-3′), TRAIL-R2 (forward: 5′-GCCTCATGGACAATGAGATAAAGGTGGCT-3′; reverse: 5′-CCAAATCTCAAAGTACGCACAAAC-3′), TRAIL-R3 (forward: 5′-GAAGAATTTGGTGCCAATGCCACTG-3′; reverse: 5′-CTCTTGGACTTGGCTGGGAGATGTG-3′), and TRAIL-R4 (forward: 5′-CTTTTCCGGCGGCGTTCATGTCCTTC-3′; reverse: 5′-GTTTCTTCCAGGCTGCTTCCCTTTGTAG-3′), giving products of 506, 502, 612, and 453 base pairs, respectively. Multiple relative reverse transcriptase (RT)-PCR was performed using Quantum RNA 18S Internal Standards kit (Ambion, Austin, TX) to detect relative differences in TRAIL–R1-R4 mRNA levels. Quantification of PCR products were made by dividing the signal intensity obtained from TRAIL amplicon by the signal intensity obtained from internal standard 18S amplicon. This yielded a corrected relative value for the gene-specific product in each RNA sample. These values were compared among samples for an estimation of the relative expression of target RNA in the samples.
Transfer experiments of CD4+Vα24NKT cells into NOD SCID mice
Six nonobese diabetic (NOD) severe combined immunodeficient (SCID) mice (14-week-old males) obtained from Jackson Laboratories (Bar Harbor, ME) were used in this study (3 mice per group). Six mice were injected intraperitoneally (5 × 105 cells/mouse) with PBMCs from Pt 1 (AML M4). On day 5 following PBMC injection, 3 mice were intraperitoneally inoculated with activated or resting CD4+Vα24NKT cells derived from an HD (5 × 104 cells/mouse). Mice were killed 24 hours after the CD4+Vα24NKT cell inoculation. Peritoneal cells were harvested from each mouse. The apoptosis of patient-derived PBMCs in the peritoneal cells were analyzed as described above.
Statistical analysis
Values are expressed as mean ± SD. Student ttest was used, and P values less than .01 were considered statistically significant.
Results
Phenotype and function of Vα24NKT cell line derived from Pt 1 with AML M4
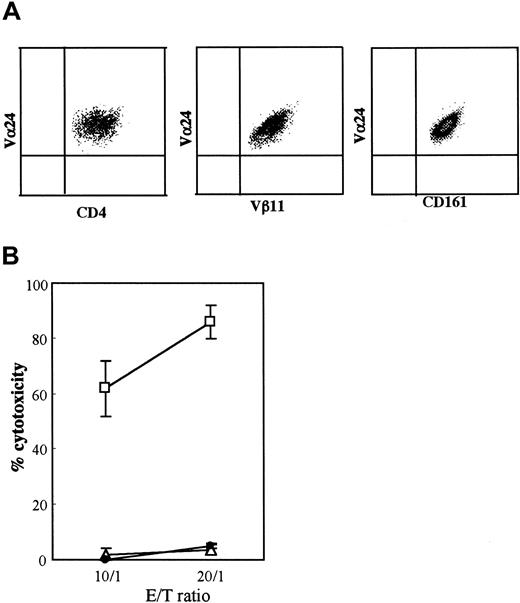
In this study, 2 CD4+Vα24NKT cell lines were used, one obtained from Pt 1 and the other from an HD. The CD4+Vα24+T cells from Pt 1 were Vβ11+ and NKRP1A+(CD161+), as shown in Figure1A. Neither of the CD4+Vα24NKT cell lines expressed other NK receptors (p58.1, p58.2, p72, and CD94) or NK markers (CD16 and CD56) (data not shown). The activated CD4+Vα24NKT cells from Pt 1 had marked cytotoxic activity against U937 but not against K562 or Daudi (Figure 1B). These results show that CD4+Vα24NKT cells derived from Pt 1 with AML are phenotypically and functionally similar to Vα24NKT cells derived from HDs as previously reported.3,25 The HD-derived CD4+Vα24NKT cells used in this study were also phenotypically and functionally similar to previously reported Vα24NKT cell lines derived from HDs3 25 (data not shown).
CD4+Vα24NKT cells derived from a patient with AML M4 were phenotypically and functionally similar to those from HDs.
(A) A representative immunophenotype of Vα24NKT cell line obtained from Pt 1 with AML M4. The Vα24NKT cells consistently express CD4, Vα24, Vβ11, and CD161. (B) Cytotoxic activity of activated CD4+Vα24NKT cells from Pt 1 against U937 (■), K562 (▵), and Daudi (●) target cells tested by the 4-hour51Cr release assay. CD4+Vα24NKT cells were used as effector cells on day 3 after stimulation by α-GalCer–pulsed DCs. The data indicate cytotoxicity at the E/T ratios of 10:1 and 20:1 using the 4-hour 51Cr release assay. The results are expressed as mean ± SD (n = 4) of the percentage cytotoxicity.
CD4+Vα24NKT cells derived from a patient with AML M4 were phenotypically and functionally similar to those from HDs.
(A) A representative immunophenotype of Vα24NKT cell line obtained from Pt 1 with AML M4. The Vα24NKT cells consistently express CD4, Vα24, Vβ11, and CD161. (B) Cytotoxic activity of activated CD4+Vα24NKT cells from Pt 1 against U937 (■), K562 (▵), and Daudi (●) target cells tested by the 4-hour51Cr release assay. CD4+Vα24NKT cells were used as effector cells on day 3 after stimulation by α-GalCer–pulsed DCs. The data indicate cytotoxicity at the E/T ratios of 10:1 and 20:1 using the 4-hour 51Cr release assay. The results are expressed as mean ± SD (n = 4) of the percentage cytotoxicity.
Expression of TRAIL on Vα24NKT cells
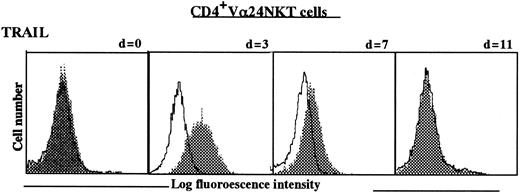
To evaluate correlation between expression of TRAIL and V24NKT cell cytotoxic activity, we assessed the expression level of TRAIL on CD4+Vα24NKT cells obtained from Pt 1 and HD before stimulation (day 0) and at various time intervals (days 3, 7, and 11) following stimulation by α-GalCer–pulsed autologous DCs. As shown in Figure 2, the expression of TRAIL on CD4+Vα24NKT cells from Pt 1 was dependent on the duration of stimulation, with highest expression being detected on days 3 to 7 following the stimulation. TRAIL expression returned to baseline levels by day 11 following stimulation. TRAIL expression on CD4+Vα24NKT cells derived from an HD was similarly dependent on stimulation by α-GalCer–pulsed DCs (data not shown). Restimulation with α-GalCer–pulsed DCs restored the expression of TRAIL to maximal levels. These results show that CD4+Vα24NKT cells express TRAIL only when the cells are activated by α-GalCer–pulsed DCs.
TRAIL expression on CD4+Vα24NKT cells before and at intervals following stimulation with α-GalCer–pulsed DCs.
Data are shown as histograms for staining with anti-TRAIL mAb (RIK-2) (shaded histograms) or with isotype-matched IgG1 control (open histograms) as determined by flow cytometry. Day 0 (d = 0) is before stimulation.
TRAIL expression on CD4+Vα24NKT cells before and at intervals following stimulation with α-GalCer–pulsed DCs.
Data are shown as histograms for staining with anti-TRAIL mAb (RIK-2) (shaded histograms) or with isotype-matched IgG1 control (open histograms) as determined by flow cytometry. Day 0 (d = 0) is before stimulation.
TRAIL-mediated apoptosis of AML M4 blasts following coculture with activated CD4+Vα24NKT cells
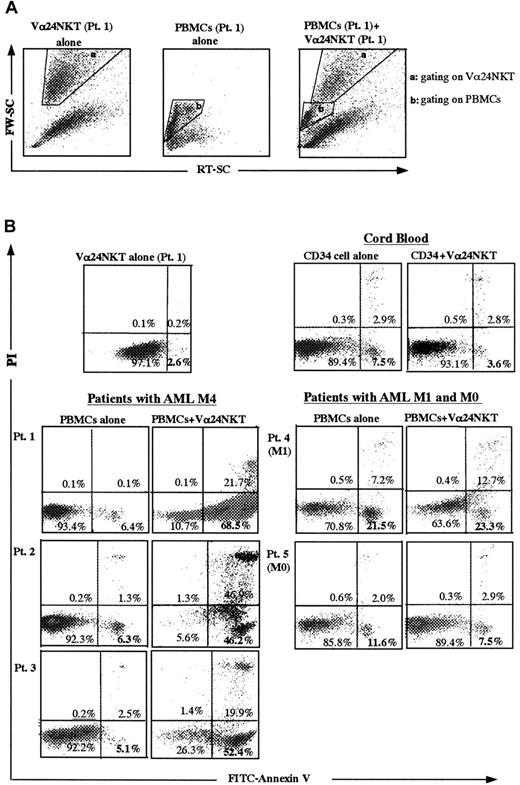
The above results were taken into account for testing the capacity of CD4+Vα24NKT cells to induce apoptotic cell death of malignant cells in PBMCs from patients with AML. Cord blood CD34+ cells were used as nonmalignant, early myeloid control cells. PBMCs from patients (Table 1) with AML M1 (Pt 4), AML M0 (Pt 5), and AML M4 (Pts 1, 2, and 3) were used as malignant targets. For the following apoptosis experiments, activated (TRAIL-expressing) CD4+Vα24NKT cells were used as effector cells on days 3 to 7 after stimulation because TRAIL expression levels were highest at this time (Figure 2). To evaluate apoptosis of target cells by flow cytometry, target cells were gated by their characteristic forward and side light scatter profiles as shown in Figure3A for the PBMCs from Pt 1. A similar gating procedure was used for assays using the other targets. As shown in Figure 3B, PBMCs from the patients with AML M4 had low rates of spontaneous death during the 8-hour culture period when cultured alone (6.4% from Pt 1, 6.3% from Pt 2, and 5.1% from Pt 3 by AV+PI− staining). Coculture of PBMCs from AML M4 Pt 1, Pt 2, and Pt 3 with activated CD4+Vα24NKT cells (from Pt 1) for 8 hours (cell ratio of 1:1) resulted in increased percentages of early apoptosis. AV+PI− cells increased to 68.5% (Pt 1), 46.2% (Pt 2), and 52.4% (Pt 3), respectively. The PBMCs from Pt 1 underwent apoptosis to a similar extent following culture with activated allogeneic CD4+Vα24NKT cells derived from an HD (data not shown). In contrast, coculture of PBMCs from Pt 4 (AML M1), Pt 5 (AML M0), and cord blood CD34+ cells with activated CD4+Vα24NKT cells (from Pt 1) did not significantly increase apoptosis (Figure 3B). In these experiments, spontaneous death of CD4+Vα24NKT cells was 2.6% as determined by AV+PI− staining (Figure 3B). The cytotoxic activity of activated Vα24NKT cells against malignant PBMCs from AML M4 patients was also verified by the conventional51Cr release assay. Specific lysis of PBMCs from Pt 1 with AML M4 during 4 hours of incubation with activated CD4+Vα24NKT cells was 45% ± 4% at an E/T ratio of 5:1 and 66% ± 5% at an E/T ratio of 10:1. The data were representative of the specific cytotoxicity of Vα24NKT cells against PBMCs derived from the other AML M4 patients with similar results. These cytotoxic activities were not blocked by anti-Vα24TCR mAb or anti-CD1d mAb, suggesting no involvement of the TCR/CD1d interaction in this killing (data not shown). In contrast, resting CD4+Vα24NKT cells did not have any cytotoxic activity against AML M4 PBMCs (data not shown).
Apoptotic cell death of PBMCs from AML patients and cord blood CD34+ cells following coculture with activated CD4+Vα24NKT cells derived from Pt 1.
Target cells were gated for the PBMCs from Pt 1 based on the forward light scatter (FW-SC) and the side light scatter (RT-SC) (A). A similar gating procedure was used for assays using the other targets. Early apoptotic cells are defined by the AV+PI−population in the dot plots for FITC-AV binding (FL 1 channel) and PI uptake (FL2 channel) (B). The data shown represent 1 of 3 experiments with similar results.
Apoptotic cell death of PBMCs from AML patients and cord blood CD34+ cells following coculture with activated CD4+Vα24NKT cells derived from Pt 1.
Target cells were gated for the PBMCs from Pt 1 based on the forward light scatter (FW-SC) and the side light scatter (RT-SC) (A). A similar gating procedure was used for assays using the other targets. Early apoptotic cells are defined by the AV+PI−population in the dot plots for FITC-AV binding (FL 1 channel) and PI uptake (FL2 channel) (B). The data shown represent 1 of 3 experiments with similar results.
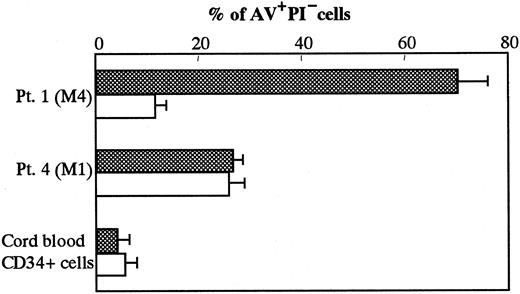
To determine whether the cytotoxic activity of activated CD4+Vα24NKT cells against the malignant PBMCs from AML M4 patients was associated with the TRAIL expression on CD4+Vα24NKT cells, we performed blocking experiments using a neutralizing anti-TRAIL mAb. The addition of 10 μg/mL anti-TRAILmAb (RIK-2) to the coculture of CD4+Vα24NKT cells (from Pt 1) and autologous malignant PBMCs inhibited 84% of early PBMC apoptosis (AV+PI− 70.1% with IgG1 isotype control versus 11.5% with anti-TRAIL mAb) (Figure4). An isotype-matched control IgG had no effects on the apoptosis of the PBMCs. In contrast, the anti-TRAIL mAb had no effect on apoptosis of the PBMCs from Pt 4 (M1) and cord blood CD34+ cells. These results indicated that activated CD4+Vα24NKT cells specifically kill the malignant PBMCs from patients with AML M4 through TRAIL-mediated apoptosis.
Effect of anti-TRAIL mAb on apoptosis of PBMCs from AML patients and cord blood CD34+ cells following coculture with activated CD4+Vα24NKT cells derived from Pt 1.
Early apoptosis of the PBMCs from Pt 1 (M4) and Pt 4 (M1) and cord blood CD34+ cells was defined by AV+PI− cells in the presence of anti-TRAIL mAb, RIK-2 (white column), or IgG1 isotype control (shadow column). The results are expressed as mean ± SD (n = 3) of the percentage of AV+PI− cells.
Effect of anti-TRAIL mAb on apoptosis of PBMCs from AML patients and cord blood CD34+ cells following coculture with activated CD4+Vα24NKT cells derived from Pt 1.
Early apoptosis of the PBMCs from Pt 1 (M4) and Pt 4 (M1) and cord blood CD34+ cells was defined by AV+PI− cells in the presence of anti-TRAIL mAb, RIK-2 (white column), or IgG1 isotype control (shadow column). The results are expressed as mean ± SD (n = 3) of the percentage of AV+PI− cells.
Expression of TRAIL receptors in AML blasts and cord blood CD34+ cells
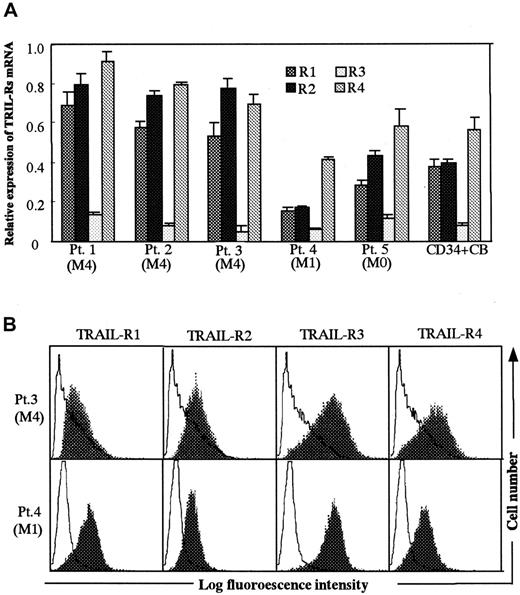
To evaluate whether TRAIL-mediated apoptosis is related to the levels of TRAIL-R expression in target cell population, we first assessed the relative expression levels of TRAILR1-R4 mRNAs in PBMCs from AML patients and cord blood CD34+ cells using multiple relative RT-PCR. The results are shown in Figure5A. The expression of TRAIL-R1 and -R2 mRNAs in PBMCs from AML M4 patients were significantly higher than in PBMCs from patients with AML M1 and M0 and cord blood CD34+cells. Expression of TRAIL-R3 mRNA was low and similar in all target cell populations. Expression of TRAIL-R4 mRNAs in PBMCs from AML M4 patients were higher than in PBMCs from patients with AML M1 and M0 and cord blood CD34+ cells, but these differences were not statistically significant. These results suggested that cellular susceptibility to TRAIL-mediated killing by activated Vα24NKT cells might be related to the expression levels of multiple TRAIL-Rs as assessed by the levels of mRNA expression. We also examined surface expression of TRAIL-R1-R4 using polyclonal antibodies against TRAIL-R1-R4 (Figure 5B). This flow cytometric analysis did not suggest a significant difference in relative expression levels of R1-R4 between AML M4 and M1 blasts as demonstrated by the mRNA analysis.
PBMCs from patients with AML M4.
(A) Relative expression levels of TRAIL-R1-R4 mRNA in PBMCs derived from AML patients and cord blood CD34+ cells. Relative TRAIL-R1-R4 mRNA expression was assessed by multiple relative RT-PCR as described in “Patients, materials, and methods.” The results are expressed as mean ± SD of 6 experiments. (B) Surface expression of TRAIL–R1-R4 on PBMCs derived from AML patients. Expression of TRAIL–R1-R4 on the PBMCs from Pt 1 (M4) and Pt 4 (M1) was assessed by flow cytometry after cell surface staining with polyclonal antibodies against TRAIL-R1, -R2, -R3, or -R4 (shaded histograms). Open histograms show the staining with control goat IgG.
PBMCs from patients with AML M4.
(A) Relative expression levels of TRAIL-R1-R4 mRNA in PBMCs derived from AML patients and cord blood CD34+ cells. Relative TRAIL-R1-R4 mRNA expression was assessed by multiple relative RT-PCR as described in “Patients, materials, and methods.” The results are expressed as mean ± SD of 6 experiments. (B) Surface expression of TRAIL–R1-R4 on PBMCs derived from AML patients. Expression of TRAIL–R1-R4 on the PBMCs from Pt 1 (M4) and Pt 4 (M1) was assessed by flow cytometry after cell surface staining with polyclonal antibodies against TRAIL-R1, -R2, -R3, or -R4 (shaded histograms). Open histograms show the staining with control goat IgG.
Apoptotic cell death of AML M4 blasts in NOD SCID mice after administration of activated CD4+Vα24NKT cells
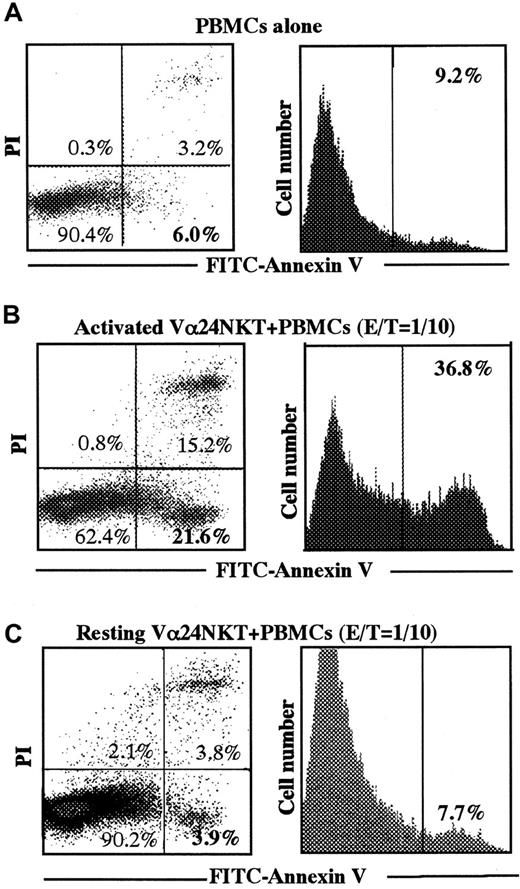
To evaluate the cytotoxic activity of CD4+Vα24NKT cells in vivo, we adoptively transferred activated CD4+Vα24NKT cells or resting CD4+Vα24NKT cells as a control into NOD SCID mice harboring leukemic blasts from a patient with AML M4. As shown in Figure6A, when only leukemic PBMCs from Pt 1 were intraperitoneally injected into NOD SCID mice, spontaneous apoptosis of the PBMCs was low (6% with AV+PI− cells and 9.2% total AV+cells). In contrast, leukemia-bearing NOD SCID mice receiving activated CD4+Vα24NKT cells had increased apoptosis of peritoneal leukemic PBMCs (21.6% with AV+PI− cells and 36.8% total AV+ cells), even at the low CD4+Vα24NKT cell–PBMC ratio of 1:10 (Figure 6B). Leukemia-bearing NOD SCID mice receiving resting CD4+Vα24NKT cells had no increase in apoptosis of peritoneal leukemic PBMCs (3.9% with AV+PI−cells and 7.7% total AV+ cells) (Figure 6C). The data show 1 representative experiment out of the 3 experiments with similar results.
Apoptosis of AML M4 blasts in NOD SCID mice after adoptive transfer of activated CD4+Vα24NKT cells.
PBMCs from Pt 1 (AML M4) were intraperitoneally injected into 6 mice at 5 × 105 cells/mouse. On day 5 after PBMC injection, 5 × 104 CD4+Vα24NKT cells/mouse were intraperitoneally inoculated into 3 of the 6 mice. Mice were killed 24 hours after inoculation of the CD4+Vα24NKT cells, and peritoneal cells were harvested from each mouse. Apoptosis of AML PBMCs from Pt 1 in the peritoneal cells was assessed as described in Figure3. Apoptosis of PBMCs when only the PBMCs were injected into NOD SCID mice (A). Apoptosis of the PBMCs when activated CD4+Vα24NKT cells (B) or resting CD4+Vα24NKT cells (C) were transferred. The data are representative of 3 experiments with similar results.
Apoptosis of AML M4 blasts in NOD SCID mice after adoptive transfer of activated CD4+Vα24NKT cells.
PBMCs from Pt 1 (AML M4) were intraperitoneally injected into 6 mice at 5 × 105 cells/mouse. On day 5 after PBMC injection, 5 × 104 CD4+Vα24NKT cells/mouse were intraperitoneally inoculated into 3 of the 6 mice. Mice were killed 24 hours after inoculation of the CD4+Vα24NKT cells, and peritoneal cells were harvested from each mouse. Apoptosis of AML PBMCs from Pt 1 in the peritoneal cells was assessed as described in Figure3. Apoptosis of PBMCs when only the PBMCs were injected into NOD SCID mice (A). Apoptosis of the PBMCs when activated CD4+Vα24NKT cells (B) or resting CD4+Vα24NKT cells (C) were transferred. The data are representative of 3 experiments with similar results.
Discussion
In this study, we demonstrated that CD4+Vα24NKT cells derived from a patient with AML M4 were phenotypically and functionally similar to those from HDs25 (Figure 1). We also showed that CD4+Vα24NKT cells derived from the patient with AML and HDs express TRAIL, but only when the cells were activated by α-GalCer–pulsed DCs (Figure 2 and data not shown). Furthermore, malignant PBMCs from patients with AML M4, but not those from patients with AML M1 or AML M0, underwent apoptosis during coculture with activated CD4+Vα24NKT cells (Figure 3), which was almost completely blocked by a neutralizing mAb against TRAIL (Figure 4). These results provide the first evidence that activated Vα24NKT cells express TRAIL and can cause depletion of leukemia cells through TRAIL-mediated apoptosis.
It is well established that cytotoxic T lymphocytes (CTLs) kill target cells via 2 major effector pathways: perforin- or FasL-mediated pathways.26-28 Additional effector mechanisms for CTL cytotoxicity, such as TNFα- and TRAIL-mediated cytotoxic pathways, also exist.15,29-31 As regards cytotoxicity by Vα24NKT cells, we previously demonstrated that Vα24NKT cells had cytoplasmic perforin and killed U937 mainly through a perforin-mediated pathway.9 25 Vα24NKT cells also express CD95L (FasL) and can induce apoptosis in Fas-expressing cells through Fas-FasL interactions (unpublished observations, 1998). The observations described here indicate that TRAIL is an additional mechanism through which Vα24NKT cells can kill malignant target cells.
TRAIL is of particular interest because it has been reported to induce apoptosis in a wide range of transformed cell lines but not in normal cells.12,13 In the present study, we demonstrated that primary leukemia cells in the peripheral blood of AML M4 patients are also susceptible to TRAIL-induced apoptosis (Figure 3). Vα24NKT cell cytotoxicity against these cells was abrogated by a neutralizing anti-TRAIL mAb (Figure 4). In contrast, nonmalignant early hemopoietic cells (cord blood CD34+ cells) (Figure 3), phytohemagglutinin-stimulated T cells, transformed B cells, and fresh monocytes were not susceptible to Vα24NKT cell cytotoxicity.9 The only nonmalignant target cells observed to be sensitive to Vα24NKT cell cytotoxicity were DCs,32but this was not blocked by the anti-TRAIL mAb (our unpublished data, 2000), suggesting that TRAIL–TRAIL-R interactions are not involved in this killing against nonmalignant targets. TRAIL-mediated Vα24NKT cell cytotoxicity therefore appears to be preferentially directed to malignant cells.
Constitutive expression of TRAIL mRNA has been reported in most normal tissues and cell types.17 Consequently it was postulated that regulation of TRAIL-induced apoptosis occurs at the level of TRAIL receptors (TRAIL-Rs). In the PBMCs from 3 patients with AML M4 that showed high sensitivity to the TRAIL-mediated Vα24NKT cell cytotoxicity, higher expression levels of mRNA for both death-inducing receptors, TRAIL-R1 and -R2, were found than in cord blood CD34+ cells and PBMCs from AML M0 and M1 patients that were not susceptible to Vα24NKT cell-induced apoptosis (Figure 5).
TRAIL-R1 and TRAIL-R2 have cytoplasmic domains containing a death domain and appear to be responsible for TRAIL-induced apoptotic cell death in various tumor cells. In contrast, TRAIL-R3 lacks a cytoplasmic domain and may function as a decoy receptor, competing with the death-inducing TRAIL-Rs for TRAIL binding. Low levels of TRAIL-R3 compared with TRAIL-R1 and -R2 could explain the TRAIL-mediated apoptosis observed in the patients with AML M4. Although these explanations are plausible and consistent with our mRNA data, RT-PCR analysis of TRAIL-R mRNA expression levels in a panel of human tumor cell lines showed poor correlation between TRAIL resistance and TRAIL-R3 and -R4 mRNA expression.17 This suggests more complex regulation of the cellular susceptibility to TRAIL-mediated apoptosis than simply relative expression levels of the 4 receptors. Our flow cytometry results, showing only minor differences in cell surface TRAIL-R expression between the target cell populations analyzed, are in keeping with this notion. This may be due to post-transcriptional regulation and differential subcellular localization of TRAIL-R1-R4 as recently reported.33
AMLs comprise a heterogeneous group of disorders that differ in etiology, pathogenesis, natural history, and prognosis. Cytotoxic T cell lines specific for a range of AML targets have been generated34 and might be of clinical benefit in some patients. Unfortunately, immune therapeutic strategies aimed at tumor-specific protein antigens are intrinsically difficult for AML because of the need to identify the peptide sequence for CTL targets for each patient from a large range of candidate targets. In vivo generation of CTLs by vaccination with antigen-pulsed DCs may also be limited as a result of immune suppression following intensive therapy. Time is frequently limited in leukemia patients with relapsed disease. In contrast to antigen-specific CTLs, Vα24NKT cell lines can be reliably and rapidly established with simple in vitro culture methods. We have confirmed that Vα24NKT cell lines can be established from patients with active AML (Figure 1 and data not shown) with similar efficiency to HDs and that more than 100-fold expansion of Vα24NKT cells can be obtained within a week of in vitro culture with α-GalCer–pulsed DCs.
The potential therapeutic implication of this work is substantial. Strategies aimed at expanding and activating Vα24NKT cells, either in vivo or in vitro with subsequent adoptive transfer, can be applied clinically, allowing selective killing of malignant cells through TRAIL-mediated apoptosis. Our present results indicate that this type of therapy may be applicable to patients with AML M4 and other TRAIL-sensitive malignancies, particularly those resistant to standard therapies.
We are particularly grateful to Drs Toshio Yabe and Tsuyoshi Takahashi for helpful suggestions and discussions.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Mie Nieda, Dept of Research, The Japanese Red Cross Central Blood Center, Hiroo 4-1-31, Shibuya-ku, Tokyo 150-0012, Japan; e-mail: nieda@cbc.jrc.or.jp.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal