Abstract
To assess the relationship between venous thrombosis and plasma glucosylceramide (GlcCer) or phosphatidylethanolamine (PE), plasma levels of GlcCer and PE were determined for 70 venous thrombosis patients referred for evaluation and 70 healthy blood donors. The mean GlcCer level, but not the PE level, was lower in patients versus controls (4.9 vs 6.5 μg/mL [P = .0007] and 66 vs 71 μg/mL [P = .48], respectively). As a measure of relative risk, the odds ratio for deep vein thrombosis in subjects with GlcCer levels below the 10th percentile of controls was 5.7 (95% CI, 2.3-14). To assess the influence of glycolipids on anticoagulant response to activated protein C (APC):protein S in modified prothrombin time assays, the effects of depleting endogenous plasma GlcCer by glucocerebrosidase treatment or of adding exogenous purified GlcCer or other neutral glycolipids to plasma were tested. Glucocerebrosidase treatment reduced plasma sensitivity to APC:protein S in parallel with GlcCer reduction. Exogenously added GlcCer and the homologous Glc-containing globotriaosylceramide (Gb3Cer), but not galactosylceramide, dose-dependently prolonged clotting times of normal plasma in the presence, but not absence, of APC:protein S, which suggests that GlcCer or Gb3Cer can enhance protein C pathway anticoagulant activity. In studies using purified proteins, inactivation of factor Va by APC:protein S was enhanced by GlcCer alone and by GlcCer in multicomponent vesicles containing phosphatidylserine and phosphatidylcholine. These results suggest that the neutral glycolipids GlcCer and Gb3Cer may directly contribute to the anticoagulant activity of the protein C pathway and that deficiency of plasma GlcCer may be a risk factor for venous thrombosis.
Introduction
Poor anticoagulant response to activated protein C (APC), termed APC resistance, is detected in 20% to 50% of patients with venous thrombosis,1 and it can be present in patients with normal factor V genotype2-4, with the factor V polymorphism, Arg506Gln,5-7 or with a variety of acquired conditions, eg, oral contraceptive use8 or auto-antibody against APC.9 APC resistance is also associated with increased risk of ischemic stroke in subjects with normal Arg506-factor V.10,11 Severe deficiency of protein C or protein S causes life-threatening thrombosis.12-15 Thus, the protein C pathway provides a major physiologic anticoagulant mechanism for down-regulation of thrombin generation in which APC proteolytically inactivates factors Va and VIIIa.16
Blood coagulation reactions and the anticoagulant protein C pathway are stimulated by phospholipid membrane surfaces.17 However, procoagulant and anticoagulant complexes may be differentially affected by different membrane phospholipid components.18-20 Because we found that high density lipoprotein (HDL) exhibits anticoagulant cofactor activity for APC:protein S,21 we decided to further evaluate the influence of plasma lipids and lipoproteins on the protein C pathway.
Plasma lipoproteins contain glycolipids as well as phospholipids.22-24 Glycolipids can play critical roles as bioregulators of a variety of processes such as cell proliferation, cell mobility, and apoptosis.23 Glycolipid molecules present their highly varied saccharide residues on the surface of lipoprotein particles as well as on cell surfaces, thereby exposing saccharides to interactions with other cells, antibodies, bacterial toxins, and viral envelope proteins.23 Several hundred glycolipids are known. However, the relevance of glycolipids to the blood coagulation system is currently unknown. Studies of the relationship between glycolipids and risks of venous thrombosis or of the influence of glycolipids on the APC:protein S anticoagulant pathway have not been reported.
This paper presents studies of the relationship between venous thrombosis and plasma levels of glucosylceramide (GlcCer) (glucocerebroside) and phosphatidylethanolamine (PE). Studies presented here show that a remarkable percentage of venous thrombosis patients have low plasma GlcCer levels and, furthermore, that GlcCer and globotriaosylceramide (Gb3Cer) enhance the anticoagulant action of APC:protein S both in plasma and in purified reaction mixtures containing factor Va. Consequently, these studies suggest that GlcCer deficiency is a potential risk factor for venous thrombosis and give rise to the novel broad concept that neutral glycolipids may contribute to regulation of thrombin generation, blood coagulation, and thrombosis.
Patients, materials, and methods
Patient and control groups
The study population consisted of 70 Caucasian patients with venous thromboembolism (VTE) and 70 Caucasian controls (50 of each from the University of Vienna, Austria, and 20 of each from the Mayo Clinic, Rochester, MN), and all subjects gave informed consent. The baseline characteristics for patients from the 2 medical centers were similar. Patients from the 2 centers had a similar prevalence of features typical of patients with venous thrombophilia including 20% to 26% prevalence of Q506–factor V and 10% to 15% prevalence of prothrombin 20210A. For all patients the diagnosis of VTE was confirmed by objective methods (phlebography, compression duplex ultrasonography, pulmonary computerized tomography, or perfusion-ventilation lung scan). The site of the first diagnosed VTE was deep vein thrombosis (DVT) of the leg in 40 patients, DVT and pulmonary embolism in 14 patients, pulmonary embolism without evidence of DVT in 11 patients, DVT of the arm in 3 patients, and cerebral vein thrombosis in 2 patients. Blood samples were collected at least 3 months after an episode of thrombosis. The first VTE event occurred spontaneously in 43 (61%) of 70 patients. These “spontaneous” cases included 4 women receiving oral contraceptives, one woman with previous breast cancer, and one man with previously resected prostate cancer at the time of thrombosis. At the time of blood sampling, but not at the time of thrombosis, 2 women were taking oral contraceptives or hormone replacement therapy, and one woman was taking tamoxifen. Because several patients from the Mayo Clinic were receiving anticoagulant therapy or had evidence of antiphospholipid antibody syndrome at the time of blood sample collection, we report coagulation studies only for the patients from the University of Vienna without lupus anticoagulant and for the controls (n = 50 each).
The control group comprised 70 healthy individuals (41 women and 29 men) without a history of VTE or arterial thromboembolism who had a mean age of 42 ± 14 years (range, 19-85 years old). These healthy laboratory blood donors were routine volunteers who were not taking oral contraceptives or estrogen replacement therapy. For an additional determination of normal GlcCer levels, a panel was collected at The Scripps Research Institute, La Jolla, CA, from 40 healthy adult donors (20 male and 20 female) with a median age of 35.8 ± 7.1 years (range, 19-56 years). Healthy subjects gave informed consent for studies approved by appropriate institutional review. Blood samples were obtained from routine venipuncture after an overnight fast, then mixed with 0.129 M sodium citrate (vol:vol, 9:1). Plasma was prepared by centrifugation at 2000g for 20 minutes at room temperature and then stored at −80°C prior to analysis.
Lipid analysis
Plasma lipids were extracted twice from citrated plasma with 4 volumes of chloroform and methanol at a concentration of 2:1, vol/vol.25 A Waters high-performance liquid chromatography (HPLC) system (Waters Corp, Milford, MA) coupled to a Sedex-55 evaporative light-scattering detector (ELSD) system (Sedere, Vitry sur Seine, France) was used for GlcCer analysis as previously described for quantitation of cardiolipin in plasma, unless otherwise noted.25 A μ-Porosil column (300 × 3.9 mm) was used with isocratic chloroform, methanol, 0.5% trifluoroacetic acid (TFA), and water in a concentration of 79:19:1:1. Under this condition, purified reference GlcCer from human spleen (Sigma Chemical Co, St Louis, MO) showed one peak eluting at 4.1 minutes (data not shown). The identity of the putative GlcCer peak observed for plasma extracts on the μ-Porosil column was confirmed. This confirmation was based on having the same retention time at 4.1 minutes as reference GlcCer and on observing the predicted increase in peak area when purified GlcCer was added to plasma lipid extracts prior to HPLC analysis. Furthermore, the plasma-derived lipid obtained from the HPLC peak eluting at 4.1 minutes comigrated with reference GlcCer on thin-layer chromatography (TLC) when using the following as developing solvents (concentrations given as vol/vol): (1) chloroform, methanol, hexane, and acetic acid at 50:10:30:5; (2) chloroform, methanol, and ammonium hydroxide at 65:25:4; or (3) chloroform, methanol, and water at 60:30:5.
Material from the GlcCer HPLC peak eluting at 4.1 minutes showed one spot on TLC corresponding to purified GlcCer, and the spot, as well as reference GlcCer, were stained by the α-napthol sugar test.26 When the following lipids were applied to this μ-Porosil HPLC column, the GlcCer peak at 4.1 minutes was separated from peaks for all of these lipids: other plasma glycolipids, such as lactosylceramide (LacCer) (Matreya, Inc, Pleasant Gap, PA), Gb3Cer, and globotetraosylceramide (Gb4Cer) (Sigma); phospholipids, such as phosphatidylcholine (PC), lysophosphatidylcholine, PE, sphingomyelin, phosphatidylserine (PS), phosphatidylinositol, cardiolipin, phosphatidylglycerol, and phosphatidic acid; cholesterol; or free fatty acids. To quantify GlcCer, a calibration curve was made by analyzing varying amounts of unfiltered reference GlcCer (Sigma) using HPLC as previously described for standardization of cardiolipin,25 and the standard curve was fitted by the following equation, which covered the range 0.1-2.0 μg GlcCer: area = 2.37 × 106 × (GlcCer [μg])1.73 (data not shown). Each plasma lipid extract sample was filtered prior to HPLC analysis using Millex Fg (0.2 μm) (Millipore, Bedford, MA).
Digestion of plasma GlcCer by glucocerebrosidase
Endogenous plasma GlcCer was hydrolyzed by 2 different glucocerebrosidases (β-glucosidase) (Sigma): recombinant glucocerebrosidase (specific activity, 1.35 U/mg) or almond-derived glucocerebrosidase (specific activity, 2.85 U/mg), which are relatively specific hydrolases for GlcCer. Plasma was incubated with varying concentrations of glucocerebrosidase for 3 minutes, and then lipid was extracted from plasma aliquots using chloroform and methanol for GlcCer determination by HPLC. In these studies, determining hydrolysis of GlcCer by glucocerebrosidase, the concentration of PE, which was not hydrolyzed by glucocerebrosidase, was used as internal standard for lipid recovery. In parallel, prothrombin time (PT) assays (see below) were performed using aliquots of glucocerebrosidase-treated plasma.
Anticoagulant response to APC:protein S
Modified dilute PT-based assays were performed as previously described.21 Briefly, 7.5 μL test plasma was mixed with fibrinogen (final concentration, 0.6 mg/mL) and APC (final concentration, 8.7 nM) plus protein S (final concentration, 28 nM) or buffer and incubated for 3 minutes at 37°C for a total volume of 100 μL. Clotting times were measured after addition of 50 μL recombinant human tissue factor (Innovin; Dade, Miami, FL) diluted 1:40 in Tris (tris[hydroxymethyl] aminomethane)–buffered saline containing 0.5% bovine serum albumin (TBSA) and 30 mM calcium dichloride (CaCl2). Baseline clotting times without addition of APC:protein S were also determined. For studies of the effects of glycolipid vesicles or glucocerebrosidase treatment on clotting assays, a 1:64 dilution of Innovin was used in the presence or absence of APC and protein S. In the study of the effect of glucocerebrosidase, 13 nM APC and 42 nM protein S were employed.
Preparation of glycolipid vesicles
To prepare extruded glycolipid vesicles, dried glycolipids—GlcCer, Gb3Cer, galactosylceramide (GalCer) (Sigma), and LacCer—were suspended at 100 μg/mL in tris buffered saline (TBS) at room temperature, vortexed 5 minutes, and passed through a 0.22-μm filter (Millipore Corporation, Bedford, MA). Concentrations of glycolipids recovered after filtration were determined by HPLC analysis as described above.
Large unilamellar multicomponent vesicles were prepared generally as described.27 Appropriate aliquots of PC, PS, and GlcCer were mixed and dried under nitrogen. The lipid mixture was hydrated at a concentration of 6.25 mM lipid in TBS and freeze-thawed 10 times using liquid nitrogen, and the solution of vesicles was passed 15 times through a 0.22-μm filter. Concentrations of glycolipid recovered after filtration were determined by HPLC analysis as described above. PC concentration was determined by an enzymatic colorimetric method (Phospholipids B kit; Wako Chemical USA, Inc, Richmond, VA).
Factor Va inactivation assay
To study the time course of factor Va inactivation by APC:protein S, mixtures containing final concentrations of 21.5 μM GlcCer vesicles, 21.5 μM control PC vesicles, or buffer and final concentrations of 6 nM APC, 18 nM protein S, and 1.5 nM factor Va with 5 mM CaCl2 were incubated at 37°C. Aliquots were withdrawn at various times, and the reaction was quenched by adding ethylenediamine tetraacetic acid (EDTA) prior to determination of residual factor Va activity. For the various lipid mixtures (wt/wt)—PC alone, 90% PC/10% GlcCer, 90% PC/10% PS, and 80% PC/10% PS/10% GlcCer—vesicles at varying concentrations were incubated with final concentrations of 1 nM APC, 18 nM protein S, and 1.5 nM factor Va for 3 minutes at 37°C, and then the reaction was quenched by adding EDTA. The residual factor Va activity was quantitated using PT clotting assays and standard log-log calibration curves of clotting time versus factor Va concentration generated using purified factor Va and factor V–deficient plasma.21
Statistical analysis
Statistical analyses were performed using Prism 3.0 software (Graph Pad Software, Inc, San Diego, CA) to determine correlations using the 2-tailed Pearson test with 95% CI. Mean and SD values for various plasma lipid levels were derived from distributions based on logarithm transformation of observed values. The SD between 2 groups was determined using the Student t test (unpaired, 2-tailed with 95% CI) (Prism 3.0), and the difference was considered significant when P ≤ .05.
Results
Plasma GlcCer and PE in patients with DVT
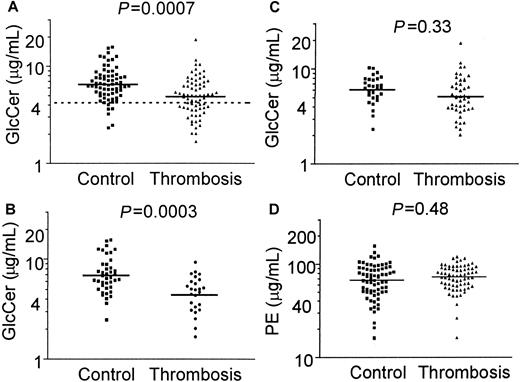
A method to quantitate both GlcCer and PE in plasma extracts using HPLC was developed and validated as described in “Patients, materials, and methods.” When GlcCer and PE in fasting plasma lipid extracts from 70 patients with DVT and 70 controls were quantitated and when logarithm-transformed data were analyzed as the mean (± SD), the plasma GlcCer level was 4.89 (3.1-7.1) μg/mL in patients compared with 6.54 μg/mL (4.4-9.7) in controls (Figure1A), and the difference was statistically significant (P = .0007). As a measure of relative risk, the odds ratio for DVT in subjects with GlcCer levels below the 10th percentile of controls was 5.7 (95% CI, 2.3-14).
Plasma GlcCer levels are low in patients with venous thrombosis.
Plasma GlcCer and PE were quantitated using HPLC for 70 patients with venous thrombosis and 70 controls. Solid lines indicate mean values from analysis of the distribution of log-transformed data, and the dashed line in panel A indicates the 10th percentile value for controls. (A) GlcCer levels in 70 patients with venous thrombosis and 70 controls (P = .0007); (B) GlcCer levels for patients and controls who are less than 45 years old (P = .0003); (C) GlcCer levels for patients and controls who are at least 45 years old (P = .33); and (D) PE levels for 70 patients with venous thrombosis and 70 controls (P = .48).
Plasma GlcCer levels are low in patients with venous thrombosis.
Plasma GlcCer and PE were quantitated using HPLC for 70 patients with venous thrombosis and 70 controls. Solid lines indicate mean values from analysis of the distribution of log-transformed data, and the dashed line in panel A indicates the 10th percentile value for controls. (A) GlcCer levels in 70 patients with venous thrombosis and 70 controls (P = .0007); (B) GlcCer levels for patients and controls who are less than 45 years old (P = .0003); (C) GlcCer levels for patients and controls who are at least 45 years old (P = .33); and (D) PE levels for 70 patients with venous thrombosis and 70 controls (P = .48).
In contrast to GlcCer, there was no significant difference between patients and controls for the mean ± SD plasma PE levels (P = .48): 70.6 μg/mL (50-100) for patients and 65.5 μg/mL (42-102) for controls (Figure 1D). As previously determined25 for another healthy control panel of 40 blood donors from The Scripps Research Institute, the mean level of GlcCer was 6.9 μg/mL (range, 4.2-14.7 μg/mL), and the mean level of PE was 66 μg/mL (range, 16.1-156 μg/mL). These values do not differ significantly from the values of our 70 controls (Figures 1A,D) from the University of Vienna and the Mayo Clinic (P = .71 andP = .31, respectively). Moreover, the mean plasma GlcCer value reported here for each control group is comparable to previously reported normal values,28 thus validating our quantification of plasma GlcCer. The influence of gender on plasma GlcCer levels was analyzed. There were no statistically significant differences between mean values of plasma GlcCer for male or female controls (P = .54) or for male and female patients (P = .67) (data not shown), thereby showing no influence of sex on plasma GlcCer levels. A slight influence of age on plasma GlcCer level was noted for the entire group of 140 subjects (P = .014, r2 = 0.043) and for the 70 controls (P = .038, r2 = 0.062), but not for the 70 patients (P = .78) (data not shown). This small influence of age was insufficient to alter the statistically significant differences between patients and controls.
Patients were separated into those experiencing thrombosis at less than 44 years of age and those who were at least 45 years old. When each group of patients was compared with the corresponding controls, the mean GlcCer level was significantly lower for younger thrombosis patients (4.4 μg/mL) compared with controls (6.9 μg/mL) (Figure 1B) (P = .0003), but not for older thrombosis patients (5.2 μg/mL) compared with controls (6.1 μg/mL) (Figure 1C) (P = .33). When patients and controls who were less than 45 years old were analyzed, the odds ratio for DVT for GlcCer levels below the 10th percentile value of controls was 5.2 (95% CI, 1.4-19.4). This odds ratio of 5.2 was similar to that of 5.7 for the overall group of 70 thrombosis patients compared with 70 controls. For the groups of people aged 44 years or younger, the mean ages of patients and controls at the time of plasma sampling were 34.4 and 32.4 years old, respectively, and were not significantly different (P = .25). Comparison of the GlcCer values for patients below the 10th percentile value of controls who were at least 45 years old gave an odds ratio for venous thrombosis of 1.87 (95% CI, 0.45-7.7) (P = .33) (Figure 1C). Although statistical significance for a difference in GlcCer between the older patients and controls was lacking, it may be noteworthy that 7 (17%) of 45 patients had low GlcCer values below 3.3 μg/mL compared with 2 (6%) of 29 controls, which suggests that plasma GlcCer deficiency also occurs in older patients with venous thrombosis. For each of the 2 age groups above or below 45 years old, the mean PE values were not statistically significantly different between patients with venous thrombosis and controls (P = .57 and 0.15, respectively) (data not shown).
Glucocerebrosidase effect on blood coagulation and anticoagulant response to APC:protein S
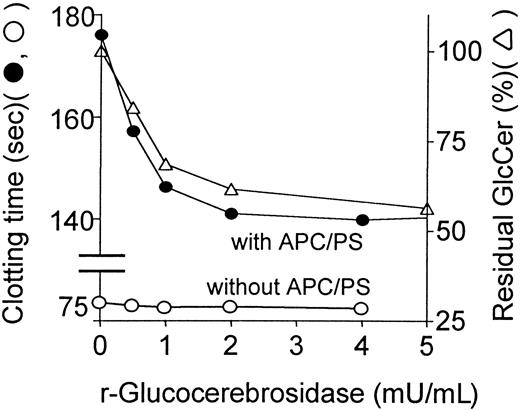
To assess the direct influence of GlcCer in plasma on procoagulant reactions and on anticoagulant activities of APC:protein S, endogenous GlcCer in plasma was hydrolyzed by varying amounts of recombinant glucocerebrosidase. In parallel, glucocerebrosidase-treated plasma was analyzed for its coagulation properties and for loss of GlcCer. Recombinant glucocerebrosidase decreased the concentration of plasma GlcCer by approximately 50% in a dose-dependent fashion (Figure2). The anticoagulant response to APC:protein S, as reflected by prolongation of observed clotting times (Figure 2), was reduced in parallel to reduction of endogenous GlcCer concentrations. Similar results showing parallel decrease of plasma GlcCer and decreased response to APC:protein S were obtained in similar experiments using purified almond-derived glucocerebrosidase added to plasma (data not shown), proving that this effect was independent of the source of glucocerebrosidase. PE levels were not affected by this enzyme (plus or minus 10%), showing that significant phospholipid hydrolysis had not occurred. Interestingly, in the absence of APC:protein S, recombinant glucocerebrosidase did not affect the baseline clotting times (Figure 2). Thus, depletion of endogenous GlcCer by specific hydrolytic enzymes is associated with decreased anticoagulant response to APC:protein S.
Reduction of endogenous plasma GlcCer by recombinant glucocerebrosidase is associated with reduced anticoagulant response to APC:protein S.
Following hydrolysis of endogenous plasma GlcCer for 3 minutes due to addition of recombinant glucocerebrosidase to plasma, the anticoagulant response to APC:protein S was immediately assayed, and using parallel aliquots, residual plasma GlcCer levels (right ordinate, ▵) were determined by HPLC analysis as described in “Patients, materials, and methods.” The clotting times (left ordinate) observed in the presence (●) and absence (○) of APC:protein S are shown. The plasma GlcCer level prior to glucocerebrosidase treatment was defined as 100%.
Reduction of endogenous plasma GlcCer by recombinant glucocerebrosidase is associated with reduced anticoagulant response to APC:protein S.
Following hydrolysis of endogenous plasma GlcCer for 3 minutes due to addition of recombinant glucocerebrosidase to plasma, the anticoagulant response to APC:protein S was immediately assayed, and using parallel aliquots, residual plasma GlcCer levels (right ordinate, ▵) were determined by HPLC analysis as described in “Patients, materials, and methods.” The clotting times (left ordinate) observed in the presence (●) and absence (○) of APC:protein S are shown. The plasma GlcCer level prior to glucocerebrosidase treatment was defined as 100%.
Influence of exogenous glycolipids on anticoagulant response of plasma to APC:protein S
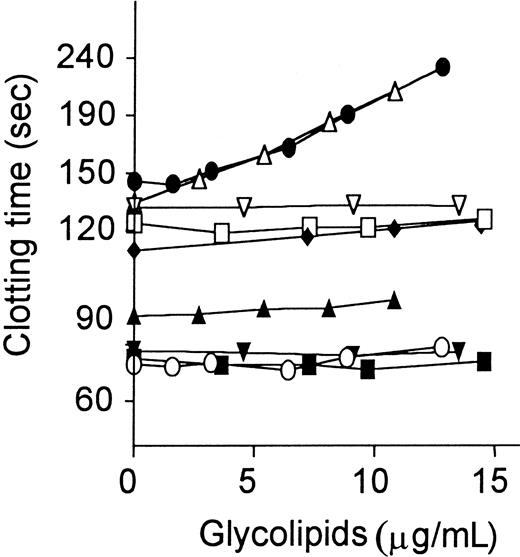
Several purified glycolipids, including GlcCer, LacCer, Gb3Cer, and GalCer, in vesicles were added to plasma, which was then assayed as described.21 When GlcCer was added to plasma in the presence of APC:protein S, it caused dose-dependent marked prolongation of the modified PT assay (Figure 3). Similar effects of GlcCer causing clotting time prolongation in the presence, but not absence, of APC:protein S were observed in factor Xa–1-stage assays performed without addition of exogenous phospholipids (data not shown). When APC alone was added without protein S, addition of GlcCer caused much less clotting time prolongation compared with APC plus protein S (Figure 3). When protein S alone was added in the absence of APC, GlcCer had no effect on clotting times (data not shown). Gb3Cer, a trisaccharide-containing glycolipid in which the Glc unit of GlcCer is extended by two Gal units, had activity very similar to GlcCer (Figure 3). However, GalCer, which simply has Gal in place of Glc, did not enhance APC:protein S action and had no effect on baseline clotting times (Figure 3). LacCer, a disaccharide-containing glycolipid, also had no effect on the anticoagulant response. Neither 0-100 μg/mL D-Glc alone nor ceramide alone affected APC:protein S anticoagulant response (data not shown). Thus, the anticoagulant enhancement of APC action by neutral glycolipids was stereo-specific for the saccharide moieties.
Anticoagulant response of plasma to APC:protein S is enhanced by addition of GlcCer and Gb3Cer.
Glycolipids were added to normal plasma aliquots, which were then assayed using the dilute modified PT assay as described21in the presence and absence of APC:protein S. Clotting times are shown for the presence of APC:protein S (● indicates GlcCer; ▵, Gb3Cer; ■, GalCer; and ▿, LacCer) and for baseline controls (without APC or protein S added) (○ indicates GlcCer; ▴, Gb3Cer; ▪, GalCer; and ▾, LacCer). For GlcCer addition, clotting times are also shown for addition of APC alone (♦ indicates GlcCer).
Anticoagulant response of plasma to APC:protein S is enhanced by addition of GlcCer and Gb3Cer.
Glycolipids were added to normal plasma aliquots, which were then assayed using the dilute modified PT assay as described21in the presence and absence of APC:protein S. Clotting times are shown for the presence of APC:protein S (● indicates GlcCer; ▵, Gb3Cer; ■, GalCer; and ▿, LacCer) and for baseline controls (without APC or protein S added) (○ indicates GlcCer; ▴, Gb3Cer; ▪, GalCer; and ▾, LacCer). For GlcCer addition, clotting times are also shown for addition of APC alone (♦ indicates GlcCer).
Correlation of anticoagulant response to APC:protein S with plasma GlcCer levels
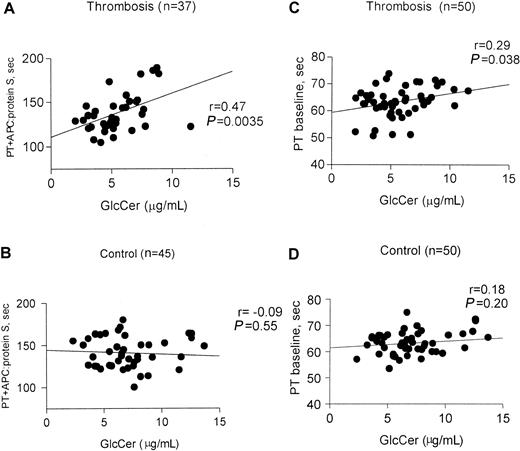
To assess the correlation between plasma GlcCer levels and procoagulant reactions or anticoagulant response, clotting assays using diluted tissue factor were performed21 in the presence and absence of APC and protein S. For this analysis, plasmas from subjects known to carry Q506–factor V were excluded because of the significant influence of this polymorphism on anticoagulant response to APC. The anticoagulant response, as reflected by the clotting time in the presence of APC:protein S, was correlated with GlcCer levels for thrombosis patients (P = .0035, r = 0.45, n = 37) (Figure 4A), but not for controls (P = .55, n = 45) (Figure 4B). Further analysis of subgroups showed that this anticoagulant response correlated with GlcCer for male patients with thrombosis (P = .0025, r = 0.65, n = 19) but not for female patients with thrombosis (P = .21, r = 0.31, n = 18) (data not shown). Analysis was done to correlate between baseline clotting times for thrombosis patients (P = .038, r = 0.29, n = 50) (Figure 4C) and controls (P = .18, r = 0.20, n = 50) (Figure4D).
Correlation of anticoagulant response to APC:protein S with plasma GlcCer levels in patients with venous thrombosis.
Fasting plasma samples from 50 patients with venous thrombosis and 50 controls were assayed using modified PT clotting assays21in the presence and absence of added APC:protein S. Baseline PT values were determined in the absence of added APC:protein S. Parameters from analysis of the correlation between the observed clotting times and GlcCer are shown for each subgroup. (A) PT in the presence of added APC:protein S for patients with venous thrombosis (excluding those with Q506–factor V); (B) PT in the presence of added APC:protein S for controls (excluding those with Q506–factor V); (C) baseline PT for thrombosis patients; and (D) baseline PT for control subjects.
Correlation of anticoagulant response to APC:protein S with plasma GlcCer levels in patients with venous thrombosis.
Fasting plasma samples from 50 patients with venous thrombosis and 50 controls were assayed using modified PT clotting assays21in the presence and absence of added APC:protein S. Baseline PT values were determined in the absence of added APC:protein S. Parameters from analysis of the correlation between the observed clotting times and GlcCer are shown for each subgroup. (A) PT in the presence of added APC:protein S for patients with venous thrombosis (excluding those with Q506–factor V); (B) PT in the presence of added APC:protein S for controls (excluding those with Q506–factor V); (C) baseline PT for thrombosis patients; and (D) baseline PT for control subjects.
Direct effect of GlcCer on purified factor Va inactivation
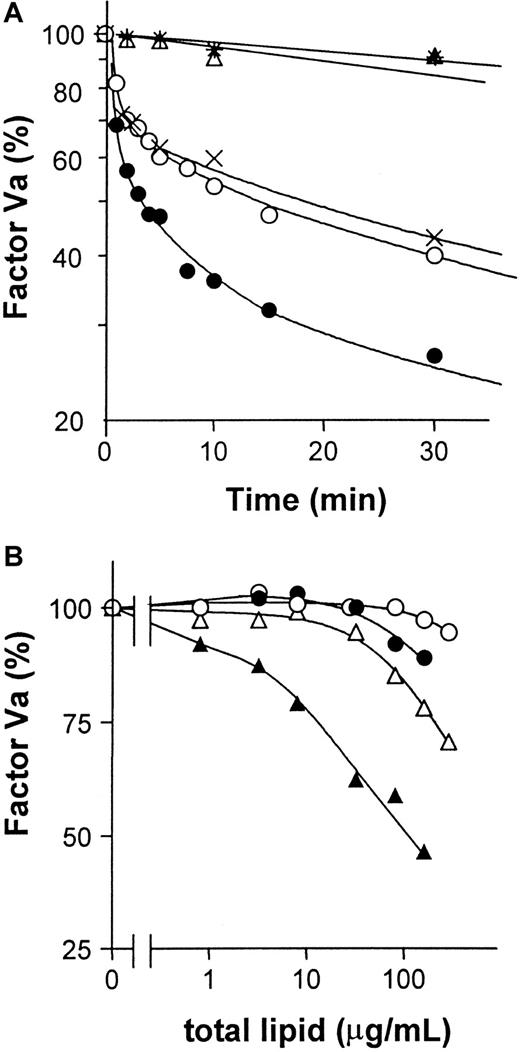
To test whether GlcCer vesicles have a direct effect on factor Va inactivation by APC:protein S in purified system, purified proteins were used to study the time course of factor Va inactivation. Factor Va inactivation by APC:protein S was enhanced by GlcCer vesicles compared with PC vesicles or buffer alone (Figure5A), indicating that GlcCer vesicles can directly enhance factor Va inactivation. Experiments were also performed to characterize the anticoagulant cofactor potency of GlcCer incorporated into multicomponent phospholipid vesicles. When such vesicles containing 10% GlcCer were used to stimulate inactivation of purified factor Va by APC:protein S, PC/GlcCer vesicles were more active than PC vesicles, but less active than PC/PS vesicles (Figure 5B). Incorporation of 10% GlcCer into 80% PC/10% PS vesicles enhanced factor Va inactivation compared with 90% PC/10% PS vesicles (Figure 5B). The apparent potency of these tricomponent GlcCer/PC/PS vesicles was approximately an order of magnitude greater than PC/PS vesicles (Figure 5B). Thus, GlcCer can significantly directly enhance inactivation of purified factor Va by APC:protein S.
GlcCer enhances factor Va inactivation by APC:protein S.
Purified factor Va was incubated with APC:protein S and varying lipids to allow factor Va inactivation for times indicated, and then residual factor Va activity was determined using clotting assays as described in “Patients, materials, and methods.” (A) Time course for 1.5 nM factor Va inactivation by final concentrations of 6 nM APC and 18 nM protein S in the presence of 5 mM CaCl2 and GlcCer (●) or PC vesicles (21.5 μM) (○), or buffer (×) at 37°C. Controls without APC:protein S included GlcCer (▵) or PC vesicles (*). The factor Va activity observed at 0 time was defined as 100%. (B) For studies using multicomponent vesicles, various concentrations of PC (○), PC/GlcCer (●), PC/PS (▵), and PC/PS/GlcCer (▴) vesicles were incubated with final concentrations of 1 nM APC, 18 nM protein S, and 1.5 nM factor Va for 3 minutes at 37°C, and then residual factor Va was determined. The factor Va activity observed with no lipid vesicles added was defined as 100%.
GlcCer enhances factor Va inactivation by APC:protein S.
Purified factor Va was incubated with APC:protein S and varying lipids to allow factor Va inactivation for times indicated, and then residual factor Va activity was determined using clotting assays as described in “Patients, materials, and methods.” (A) Time course for 1.5 nM factor Va inactivation by final concentrations of 6 nM APC and 18 nM protein S in the presence of 5 mM CaCl2 and GlcCer (●) or PC vesicles (21.5 μM) (○), or buffer (×) at 37°C. Controls without APC:protein S included GlcCer (▵) or PC vesicles (*). The factor Va activity observed at 0 time was defined as 100%. (B) For studies using multicomponent vesicles, various concentrations of PC (○), PC/GlcCer (●), PC/PS (▵), and PC/PS/GlcCer (▴) vesicles were incubated with final concentrations of 1 nM APC, 18 nM protein S, and 1.5 nM factor Va for 3 minutes at 37°C, and then residual factor Va was determined. The factor Va activity observed with no lipid vesicles added was defined as 100%.
Characteristics of patients with DVT
| Characteristics . | Total (n = 70) . |
|---|---|
| Age, mean ± SD y | |
| Current (range, y) | 50.1 ± 14.4 |
| (21-79) | |
| At first VTE episode, | 45.5 ± 14.2 |
| Other characteristics, n (%) | |
| Gender (female) | 35 (50) |
| VTE | |
| Recurrent | 18 (26) |
| Spontaneous | 43 (61.4) |
| Recent surgery | 4 (5.7) |
| Recent trauma | 8 (16) |
| Malignant neoplasm | |
| Active | 3 (4.3) |
| Previous | 2 (2.8) |
| Leg paresis | 1 (1.4) |
| Prolonged travel | 4 (5.7) |
| Oral contraceptive | 4 (5.7) |
| Tamoxifen use | 1 (1.4) |
| Other diseases | |
| Puerperium | 1 (1.4) |
| Inflammatory bowel disease | 2 (2.9) |
| Bechet disease | 1 (1.4) |
| Characteristics . | Total (n = 70) . |
|---|---|
| Age, mean ± SD y | |
| Current (range, y) | 50.1 ± 14.4 |
| (21-79) | |
| At first VTE episode, | 45.5 ± 14.2 |
| Other characteristics, n (%) | |
| Gender (female) | 35 (50) |
| VTE | |
| Recurrent | 18 (26) |
| Spontaneous | 43 (61.4) |
| Recent surgery | 4 (5.7) |
| Recent trauma | 8 (16) |
| Malignant neoplasm | |
| Active | 3 (4.3) |
| Previous | 2 (2.8) |
| Leg paresis | 1 (1.4) |
| Prolonged travel | 4 (5.7) |
| Oral contraceptive | 4 (5.7) |
| Tamoxifen use | 1 (1.4) |
| Other diseases | |
| Puerperium | 1 (1.4) |
| Inflammatory bowel disease | 2 (2.9) |
| Bechet disease | 1 (1.4) |
Discussion
Based on our interest in the influence of plasma lipids and lipoproteins on the antithrombotic protein C pathway,18-21,25 we established HPLC-based methods to quantitate various plasma phospholipids including cardiolipin, PS, and PE.25 The HPLC methodology used for PE analysis also permitted quantitation of the neutral glycosphingolipid GlcCer. When the influence of GlcCer on anticoagulant response to APC:protein S was studied, we found that depletion of endogenous GlcCer by addition of glucocerebrosidase to normal pooled plasma proportionately reduces sensitivity to APC:protein S (Figure 2), while addition of exogenous GlcCer to normal plasma dose-dependently enhances the anticoagulant response without affecting baseline clotting time (Figure 3). Thus, plasma GlcCer appeared to act like an anticoagulant cofactor for APC:protein S, leading us to hypothesize that GlcCer would influence anticoagulant activity by enhancing APC:protein S in vivo and that GlcCer deficiency may predispose to thrombosis.
To test the hypothesis that plasma GlcCer deficiency is a marker or risk factor for thrombosis, plasma GlcCer and PE mean levels of 70 patients with DVT were compared to those of 70 healthy controls. This comparison showed a remarkably lower mean level of GlcCer, but not of PE, in patients compared with controls (P = .0007 andP = .48, respectively). Plasma GlcCer levels did not differ by gender in patients or in controls. As a measure of relative risk, the odds ratio for DVT in subjects with low plasma GlcCer levels below the 10th percentile of controls was 5.7 (95% CI, 2.3-14). For those patients under age 45 in whom hereditary risk factors might play a more easily identifiable role, the difference between plasma GlcCer values for patients and controls was remarkable (P = .0003), and the odds ratio for venous thrombosis for subjects with GlcCer below the 10th percentile of controls was 5.2 (95% CI, 1.40-19.4). These observations suggest that plasma GlcCer deficiency may be a marker or a risk factor for venous thrombosis and that younger age may be an important factor for low plasma GlcCer associated with venous thrombosis.
In suggesting that low plasma GlcCer could be a potential risk factor rather than simply a marker for venous thrombosis, we note that experiments here demonstrate biological plausibility for the ability of GlcCer to directly enhance the anticoagulant action of APC:protein S in both the plasma milieu and in reaction mixtures containing purified clotting factors. In normal plasma, depletion of endogenous GlcCer by enzymatic treatment proportionately decreased GlcCer levels and anticoagulant response to APC:protein S, and furthermore, when purified GlcCer was added to plasma, it dose-dependently enhanced this anticoagulant response. In studies using purified proteins, GlcCer directly enhanced inactivation of purified factor Va by APC:protein S. The direct anticoagulant effects of GlcCer were apparent for vesicles containing GlcCer in the absence and presence of PS.
The ability of glycolipids to enhance APC:protein S anticoagulant activity in plasma demonstrated specificity for the conformation and composition of the saccharide moieties. Anticoagulant activity appeared to be, at least in part, specific for D-Glc linked covalently to Cer because neither D-Glc nor ceramide alone was anticoagulantly active while GlcCer was very active and because GalCer was also relatively inactive. The effects of extending the saccharide unit attached to Cer were complex because GlcCer (Glcβ1-Cer) and Gb3Cer (Galα1,4Galβ1,4Glcβ1-Cer) were approximately equally active, whereas the disaccharide-containing glycolipid, LacCer (Galβ1,4Glcβ1-Cer), showed much lower activity than the highly active glycolipids containing either a monosaccharide or a trisaccharide.
The major plasma neutral glycolipids that are associated with each major class of plasma lipoproteins, very low-density lipoprotein (VLDL), low-density lipoprotein (LDL), and HDL, include GlcCer, LacCer, Gb3Cer, and Gb4Cer and have plasma levels of approximately 10, 5.5, 2.1, and 2.8 μM, respectively.28 Their biological activities are partially determined by their sugar head groups and partially by their lipid moieties. LacCer accumulation in atherosclerotic lesions is caused by oxidized LDL and is associated with atherosclerosis via stimulation of smooth muscle cell proliferation by superoxide generation.29Interestingly, in contrast to LacCer, neither GlcCer nor Gb3Cer supports this atherogenic reaction.29 We speculate that GlcCer and Gb3Cer could suppress thrombin generation which contributes to development of atherothrombosis by enhancing the APC:protein S anticoagulation system. Notably, LacCer does not possess this anticoagulant property. Thus, from these perspectives, LacCer may be considered an atherogenic glycolipid, while GlcCer and Gb3Cer may be anti-atherothrombogenic glycolipids. Moreover, this specific activity of oxidized LDL on selective accumulation of LacCer provides another case, in addition to that presented here, where a biologic activity of these 3 neutral glycolipids, each containing Glc linked to Cer by β1-linkage, is different for LacCer compared with GlcCer and Gb3Cer.
How does GlcCer directly enhance the anticoagulant action of APC:protein S? APC cleaves the heavy chain of factor Va at R506 and then R306 and perhaps R679,30 and protein S and phospholipids are known to accelerate factor Va inactivation by selectively promoting the otherwise slow phospholipid-dependent cleavage at R306.31 In purified protein systems, GlcCer might directly accelerate cleavage at R306, R506, or R679 in factor Va by APC in the presence of protein S by binding to one or more of these proteins. In the plasma milieu there are additional potential mechanisms for GlcCer and its analogues. Protein S can abrogate the ability of factor Xa to protect factor Va from APC cleavage,32 and factor Va residues 493-506 provide binding sites for protein S and factor Xa.33 It is also possible that GlcCer might enhance the ability of protein S to compete better with factor Xa for binding factor Va. Cleavage of factor V at R506 augments the anticoagulant cofactor activity of factor V for APC-dependent inactivation of factor VIIIa, and GlcCer might affect these anticoagulant reactions.34 Moreover, it is possible that GlcCer associates with another plasma component that itself directly enhances APC:protein S anticoagulant activity, in which case the anticoagulant effect of GlcCer on APC:protein S would be an indirect one. Extensive studies are needed to define the mechanisms of action of GlcCer as an anticoagulant cofactor.
When data were analyzed for correlations between plasma GlcCer levels and anticoagulant response to APC:protein S, several patterns were observed (Figure 4). For all patients with venous thrombosis and normal factor V genotype, ie, those who did not carry the APC-resistant Q506–factor V, the anticoagulant response to APC:protein S correlated with GlcCer levels. However, analysis of patient subgroups showed that this correlation was significant only for males, and there was no statistically significant correlation for controls. The variability of these correlations is puzzling because in vitro adjustments of plasma levels of GlcCer in pooled normal plasma affect the anticoagulant response to APC:protein S.
Because GlcCer is a minor lipid component of all classes of plasma lipoproteins, it is possible that one or more minor apolipoproteins also play functional roles in the expression of APC:protein S anticoagulant activity in conjunction with plasma GlcCer. If so, one wonders what are the effects on plasma lipoproteins of the in vitro manipulations that alter GlcCer levels. Because the metabolism of GlcCer is linked to that of other plasma neutral glycolipids, eg, Gb3Cer, which could also affect anticoagulant response, correlations might preferably be sought between anticoagulant response and the combined plasma levels of GlcCer and Gb3Cer. Finally, plasma GlcCer low levels might reflect defects in cellular GlcCer availability that relate to cell-dependent antithrombotic mechanisms which affect risks of thrombosis.
Our observations that two neutral glycolipids, GlcCer and Gb3Cer, can influence the anticoagulant action of APC:protein S and that low plasma GlcCer is a potential risk factor for venous thrombosis provide the surprising implication that neutral glycolipids may influence blood coagulation reactions.17 It is widely recognized that activation of the blood coagulation system is enhanced by charged phospholipid membrane surfaces as is the expression of the anticoagulant protein C pathway. However, procoagulant and anticoagulant complexes may be differently affected by different membrane phospholipid components or lipoproteins. For instance, the anionic phospholipid phosphatidylserine very effectively enhances prothrombin activation by factor Xa and factor Va complexes. VLDL can potentially support prothrombinase activity,35-37 whereas HDL, particularly HDL2 (H.D., J.A.F., and J.H.G., unpublished data, May 2000), can act as an anticoagulant cofactor by enhancing protein S–dependent APC activity.21 Thus, the blood coagulation pathways and the counterbalancing protein C anticoagulant system can be differentially modulated by various lipids and lipoproteins. Studies presented here raise the possibility that any number of the several hundred glycolipids might contribute to up-regulation or down-regulation of thrombin generation.
The ability of GlcCer and Gb3Cer to modulate plasma sensitivity to APC:protein S may be clinically relevant. Pregnancy and oral contraceptive usage reduce the anticoagulant response to APC.8,38,39 Estradiol lowers GlcCer synthase, the primary enzyme that synthesizes GlcCer, and it also raises glucosidase activity,40 suggesting that plasma GlcCer levels might be reduced by pregnancy or oral contraceptive use. Some anticancer drugs are also known to reduce GlcCer synthesis,41,42 and tamoxifen decreases GlcCer synthesis in cultured cells.42Because tamoxifen use is also associated with increased risk of venous thrombosis,43 lowered GlcCer may reduce the sensitivity of tamoxifen-treated patients to APC:protein S and thereby increase risk of venous thrombosis.
The importance of vascular bed–specific signals and molecules as risk factors for vascular bed–specific thrombosis has been recently highlighted.44 Given the possibility of vascular bed–specific expression of any number of several hundred glycolipids, it is tempting to speculate that tissue bed–specific glycolipids may modulate both procoagulant and anticoagulant reactions. For example, negatively charged sulfatides can exert both procoagulant and anticoagulant activities.45 46Thus, there are a number of intriguing implications of the observation that the neutral glycolipids GlcCer and Gb3Cer enhance APC:protein S anticoagulant response and that a low level of plasma GlcCer appears to be a potential risk factor for venous thrombosis.
We are grateful to Professor Ernest Beutler and the Stein Endowment Fund (La Jolla, CA) for support of the HPLC/ELSD system and to Ms Aimie Goto Ogawa and Ms Young Mee Lee for skillful technical assistance. We appreciate the support and encouragement of this project by Professor Klaus Lechner at the University of Vienna, Austria.
Supported in part by grants RO1HL21544 and R37HL52246 from the National Institutes of Health, Bethesda, MD; a grant from the Stein Endowment Fund; and a postdoctoral fellowship from the American Heart Association, Western States Affiliate.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
John H. Griffin, Dept of Molecular and Experimental Medicine, The Scripps Research Institute, MEM180, 10550 North Torrey Pines Rd, La Jolla, CA 92037; e-mail:jgriffin@scripps.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal