Single-cell polymerase chain reaction (PCR) has been used as a tool to demonstrate clonality and B-cell origin of Reed-Sternberg (RS) cells in Hodgkin disease (HD). An analogous approach was used to investigate genomic imbalances in a (cyto)genetically poorly characterized subentity: lymphocyte predominance Hodgkin disease (LPHD). Nineteen cases of LPHD were selected for a comparative genomic hybridization (CGH) study. CGH was performed with degenerate oligonucleotide primed–PCR (DOP-PCR)–amplified DNA from 4-5 microdissected CD20+ malignant cells. All analyzed cases revealed a high number of genomic imbalances (average 10.8 per case), involving all chromosomes but the excluded 19, 22, and Y, indicating a high complexity of LPHD. The majority of detected aberrations were recurrent. Gain of 1, 2q, 3, 4q, 5q, 6, 8q, 11q, 12q, and X, and loss of chromosome 17 were identified in 36.8% to 68.4% of the analyzed cases. Some of them have also been found in non-Hodgkin lymphoma (NHL), and possibly represent secondary changes associated with disease progression. Gain of 2q, 4q, 5q, 6, 11q, however, are much more rarely observed in NHL and could be more specifically associated with LPHD. Particularly interesting is a frequent overrepresentation of chromosome arm 6q, a region usually deleted in NHL. Rearrangement of theBCL6 gene (3q27) demonstrated by cytogenetics and fluorescence in situ hybridization in 2 cases in this study suggests its contribution in pathogenesis of LPHD. In conclusion, the data show a consistent occurrence of genomic alterations in LPHD and highlight genomic regions that might be relevant for development and/or progression of this lymphoma entity.
Introduction
Hodgkin disease (HD), one of the most common malignant lymphomas in the western world, was first described by Thomas Hodgkin in 1832. The characteristic morphologic feature of HD is the cellular composition of the tumor tissue containing a small number (approximately 0.1% to 1%) of neoplastic Reed-Sternberg (RS) cells and a major population of nonneoplastic lymphocytes, histiocytes, neutrophils, fibroblasts, eosinophils, and plasma cells. On the basis of tumor cell morphology, immunophenotype, and the composition of the cellular background, 2 separate biologic entities, namely, lymphocyte predominance Hodgkin disease (LPHD) (or nodular paragranuloma) and classical HD have been recognized in the Revised European-American Lymphoma (REAL) classification.1 LPHD accounts for 5% of all HD cases. Typically, patients are 25- to 45-year-old men and usually present at an early clinical stage. More than 90% of patients with LPHD have a complete response to therapy, although recurrences frequently occur. In LPHD, a particular variant of RS cells, the “popcorn” or lymphocytic and histiocytic (L&H) cells, is found. In contrast to RS cells of classical HD, “popcorn” cells lack the expression of CD30 and CD15 but express CD45 and CD20. The expression of these markers and presence of nodular structures closely resembling lymphoid B follicles have suggested that “popcorn” cells represent neoplastic germinal center B cells.2-4
Because of the very low frequency of RS and “popcorn” cells in the tumor tissue and the lack of suitable methods for their purification and analysis, clonality and lineage derivation of neoplastic cells in HD have remained uncertain until recently. As soon as single-cell polymerase chain reaction (PCR)–based methods had been developed, clonal Ig rearrangement and consistent occurrence of somatic mutations within the variable-regions of Ig genes amplified from isolated RS and “popcorn” cells have been demonstrated in the majority of analyzed cases. These findings indicated a clonal germinal-center (GC) or post-GC B-cell origin of LPHD as well as of classical HD.5-11 On the other hand, polyclonal Ig rearrangements found in some HD cases12-14 led to the hypothesis of a sequential poly-/oligo-/monoclonal origin of HD, a matter that is still under discussion.15,16
Despite recent progress in HD research, genetic and molecular data are only scarcely available. There are intrinsic technical problems in obtaining metaphase chromosomes in HD (low number of RS cells, low proliferation index, infiltration of reactive cells), and therefore no more than 200 HD cases with abnormal karyotypes have been reported until now (reviewed by Falzetti et al17and Pedersen et al18). Despite the detection of recurrent chromosomal aberrations also demonstrated by interphase fluorescence in situ hybridization (FISH), by FISH combined with CD30 immunophenotyping of RS cells,19-21 and by comparative genomic hybridization (CGH),22,23 so far no specific cytogenetic marker(s) could be identified. Single RS-cell PCR analysis of CMYC, BCL2, and BCL1 suggested that these non-Hodgkin lymphoma (NHL)–specific genes are not involved in the pathogenesis of HD.24 25
Even less is known about genetic and molecular aberrations in the LP subtype of HD. To the best of our knowledge, only 9 LPHD cases with clonal chromosomal abnormalities have been reported.17,18 26-31 Five of these revealed hyperdiploid karyotypes and most showed complex chromosomal aberrations.
In this study, we investigated a series of 19 LPHD cases by using CGH. Because of the low number of neoplastic cells in LPHD, the analysis was performed on degenerate oligonucleotide primed–PCR (DOP-PCR)–amplified DNA obtained from microdissected CD20+“popcorn” cells. By using this approach, we found genomic imbalances in all cases, revealing the occurrence of recurrent chromosomal abnormalities in LPHD.
Patients, materials, and methods
Patients
Nineteen patients with lymphocyte predominance HD and available frozen material diagnosed at the Department of Pathology of the University of Hospital KULeuven were selected for this study and reviewed. Some relevant clinical features of these patients are summarized in Table 1.
Clinical, cytogenetic, and comparative genomic hybridization features of 19 patients with lymphocyte predominance Hodgkin disease
| Case . | Sex/age* . | Status† . | Clinical stage‡ . | Karyotype1-153 . | CGH1-153 Rev ish . |
|---|---|---|---|---|---|
| 1 | M/10 | PD | IIIA | ND | enh(1p32p12,3,4q,5p15q13,6q,8,10p12q23,12,20p,Xq22q26), dim(2,5q21q35,6p25p23,7,9,11q,13,16,17) |
| 2 | F/37 | PD | IA | ND | enh(1,2q,3,7,12q,16,18q11q21),dim(4q24q25,5q31q35,6,8p23q21, 10,11,13,20,21,Xp),amp(1q) |
| 3 | F/71 | PD | IA | 46,XX [11] | enh(5p13q35,20p),dim(11q) |
| 4 | M/30 | PD | IIA | NM | enh(3p14q13,5p14q12,6,9p,X),dim(1p,11q) |
| 5 | M/67 | PD | IIA | 46,XY [11] | enh(1,3p26p21,5q14q23,6p,7p,8p12q24,11p15q22,Xq) |
| 6 | M/25 | PD | IA | 46,XY [10] | enh(1p,3,6q,8,10,11,13,18q,X),dim(17p) |
| 7 | M/49 | PD | IA | 82-86,XXYY,4-7mar[inc2]/46,XY [4] | enh(3q,4q,11,Xq) |
| 8 | M/33 | PD | IIA | ND | enh(1q21q31,5p15q23,7,11,14q11q21,X),dim(8p,16,17,20q) |
| 9 | M/25 | PD | IA | 43,X,−Y,i(1)(q10),t(3;22)(q27;q12),−4,add(7)(q36), −10,−17,+mar[4]/46,XY[6] | enh(1q,5q,6,10q22q24,20p,Xq23q27),dim(1p,4,10p,17,Xp22p21) |
| 10 | M/33 | PD | IIA | NM | enh(1q,3,4q23q27,6,8q,11q,X),dim(16p,17) |
| 11a | M/441-155 | 1.R | II | ND | enh(2q21q34,3p,4q,5q14q23,6q,8q,11,12q,13,20p,X) |
| 11b | 2.R | IVA | ND | enh(2q21q34,3p,4q,5q14q23,6q,8q,11,12q,13,20p,X) | |
| 12 | M/44 | PD | IA | ND | enh(1q,2q24q34,3,4q12q25,5q,6p,8q,12q,X) |
| 13 | M/37 | R | IIA | ND | enh(2,5q12q23,6q12q24,9q,12q),dim(3,10p15q22,17) |
| 14 | M/27 | PD | NA | 46,XY [7] | enh(1,2,5q23q35,15,20,X),dim(4p16p15,6p,7p,8,18) |
| R | NA | 92,XX,YY,+X,−1,−2,?del(2)(q34),der(3)t(3;?;1) | |||
| (q27;?;q21)x2,add(4)(p15)x3,−5,+6,?der(6) | |||||
| del(6)(p21.3p21.2)del(6)(p23)x3,del(7)(p11)x2,−10, −10,−12,−13,−13,−14,−16,+17,−18,−18, −19[1]/46,XY[16]/92,XXYY[3] | |||||
| 15 | M/521-155 | R | IIA | NM | enh(1q,2,3,4,5q,7p15p11,10p15p13,12q12q15,13,18p,21), dim(1p,5p,6q23q27,7q31q36,9,10q,11q12q23,15,16p,17,18q), amp(13) |
| 16 | M/74 | PD | IIA | ND | enh(1p36q31,2q14q31,4q,5q,8q,X),dim(9q31q34,17,20q) |
| 17 | M/42 | PD | II | 42,X,−Y,del(1)(p12),+del(1)(p12),add(2)(p25),−11, −12,−13,−14 [1]/47,XY,+X [1]/46,XY [1] | enh(1q,2p,3,4q,6,8,11,12q,X) |
| 18 | M/28 | PD | IIIA | NM | enh(2p,4p15q22,Xp),dim(6p,8p,10p) |
| 19 | M/11 | PD | IIA | 46,XY [4] | enh(1p22q44,3p21p13,4q,5p,6,7p,8q21,9q13q22,12p13q12, 12q14q24,13,14q11q24,21,Xp),dim(2,5q13q35,10q22q26,11p, 15,16p,18p11q21,Xq21q28),amp(5p14,6q22,Xp21) |
| Case . | Sex/age* . | Status† . | Clinical stage‡ . | Karyotype1-153 . | CGH1-153 Rev ish . |
|---|---|---|---|---|---|
| 1 | M/10 | PD | IIIA | ND | enh(1p32p12,3,4q,5p15q13,6q,8,10p12q23,12,20p,Xq22q26), dim(2,5q21q35,6p25p23,7,9,11q,13,16,17) |
| 2 | F/37 | PD | IA | ND | enh(1,2q,3,7,12q,16,18q11q21),dim(4q24q25,5q31q35,6,8p23q21, 10,11,13,20,21,Xp),amp(1q) |
| 3 | F/71 | PD | IA | 46,XX [11] | enh(5p13q35,20p),dim(11q) |
| 4 | M/30 | PD | IIA | NM | enh(3p14q13,5p14q12,6,9p,X),dim(1p,11q) |
| 5 | M/67 | PD | IIA | 46,XY [11] | enh(1,3p26p21,5q14q23,6p,7p,8p12q24,11p15q22,Xq) |
| 6 | M/25 | PD | IA | 46,XY [10] | enh(1p,3,6q,8,10,11,13,18q,X),dim(17p) |
| 7 | M/49 | PD | IA | 82-86,XXYY,4-7mar[inc2]/46,XY [4] | enh(3q,4q,11,Xq) |
| 8 | M/33 | PD | IIA | ND | enh(1q21q31,5p15q23,7,11,14q11q21,X),dim(8p,16,17,20q) |
| 9 | M/25 | PD | IA | 43,X,−Y,i(1)(q10),t(3;22)(q27;q12),−4,add(7)(q36), −10,−17,+mar[4]/46,XY[6] | enh(1q,5q,6,10q22q24,20p,Xq23q27),dim(1p,4,10p,17,Xp22p21) |
| 10 | M/33 | PD | IIA | NM | enh(1q,3,4q23q27,6,8q,11q,X),dim(16p,17) |
| 11a | M/441-155 | 1.R | II | ND | enh(2q21q34,3p,4q,5q14q23,6q,8q,11,12q,13,20p,X) |
| 11b | 2.R | IVA | ND | enh(2q21q34,3p,4q,5q14q23,6q,8q,11,12q,13,20p,X) | |
| 12 | M/44 | PD | IA | ND | enh(1q,2q24q34,3,4q12q25,5q,6p,8q,12q,X) |
| 13 | M/37 | R | IIA | ND | enh(2,5q12q23,6q12q24,9q,12q),dim(3,10p15q22,17) |
| 14 | M/27 | PD | NA | 46,XY [7] | enh(1,2,5q23q35,15,20,X),dim(4p16p15,6p,7p,8,18) |
| R | NA | 92,XX,YY,+X,−1,−2,?del(2)(q34),der(3)t(3;?;1) | |||
| (q27;?;q21)x2,add(4)(p15)x3,−5,+6,?der(6) | |||||
| del(6)(p21.3p21.2)del(6)(p23)x3,del(7)(p11)x2,−10, −10,−12,−13,−13,−14,−16,+17,−18,−18, −19[1]/46,XY[16]/92,XXYY[3] | |||||
| 15 | M/521-155 | R | IIA | NM | enh(1q,2,3,4,5q,7p15p11,10p15p13,12q12q15,13,18p,21), dim(1p,5p,6q23q27,7q31q36,9,10q,11q12q23,15,16p,17,18q), amp(13) |
| 16 | M/74 | PD | IIA | ND | enh(1p36q31,2q14q31,4q,5q,8q,X),dim(9q31q34,17,20q) |
| 17 | M/42 | PD | II | 42,X,−Y,del(1)(p12),+del(1)(p12),add(2)(p25),−11, −12,−13,−14 [1]/47,XY,+X [1]/46,XY [1] | enh(1q,2p,3,4q,6,8,11,12q,X) |
| 18 | M/28 | PD | IIIA | NM | enh(2p,4p15q22,Xp),dim(6p,8p,10p) |
| 19 | M/11 | PD | IIA | 46,XY [4] | enh(1p22q44,3p21p13,4q,5p,6,7p,8q21,9q13q22,12p13q12, 12q14q24,13,14q11q24,21,Xp),dim(2,5q13q35,10q22q26,11p, 15,16p,18p11q21,Xq21q28),amp(5p14,6q22,Xp21) |
CGH indicates comparative genomic hybridization; Rev ish, reverse in situ hybridization; PD, primary diagnosis; ND, no data; R, relapse; NA, not available; NM, no mitosis.
Age at the time of primary diagnosis.
Status at the time of lymph node biopsy.
Clinical stage according to the Ann Arbor classification.
Cytogenetic and CGH results are described according to the ISCN nomenclature.
Death disease related.
Conventional cytogenetics
Cytogenetic analysis was performed after a short-term culture (24 hours) of lymph node cells in RPMI 1640 medium supplemented with 15% fetal calf serum, glutamine, and penicillin. After exposure to colcemid, cells were harvested according to the standard methods using hypotonic KCl solution and 3:1 methanol:acetic acid as a fixative. Metaphases were G-banded with Wright's stain. Karyotypes were described according to the International System for Human Cytogenetic Nomenclature (1995).32
Microdissection of tumor cells
Frozen sections of 8 μM were cut. For immunodetection, an alkaline phosphatase-conjugated ABC complex (Dakopatts, Glostrup, Denmark) was used subsequent to the application of the CD20 (L26) (Dako, Glostrup, Denmark) antibody. Slides were stained and pretreated for 60 minutes at 37°C with Collagenase H (1%, type II, Sigma-Aldrich, St Louis, MO) dissolved in phosphate-buffered saline (PBS) (pH 7.4). Single CD20+ L&H cells were microdissected and harvested under a microscope (Leica, Wetzlar, Germany). The manipulation procedures were performed with 2 3-dimensional hydraulic micromanipulators equipped with a joystick (Narishige, Tokyo, Japan). Manipulation tools consisted of a reception pipette and a manipulation knife prepared from glass capillaries. CD20+ cells were mobilized with the help of the manipulation knife and aspirated into the reception pipette. Four to 5 microdissected cells were collected in a PCR tube and resuspended in 7 μL of solution (26 mM Tris HCl, pH 9.5; 6.5 mM MgCl2) containing 2.3 μg proteinase K. The cells were digested overnight at 37°C, followed by boiling for 10 minutes to inactivate proteinase K.
DOP-PCR
The DOP-PCR was used to universally amplify tumor DNA and total normal DNA in a thermal cycler. The protocol used was modified from Kuukasjarvi et al.33 Four cycles were carried out in a 10-μL reaction mixture by adding 0.2 mM of each dNTP, 1 μM UNI primer and 10 units Thermo Sequenase DNA polymerase (Amersham, Cleveland, OH), followed by 25 cycles in a 50-μL reaction volume (adding 1 mM Tris HCl pH 8.3, 5 mM KCl, 0.16 mM dNTPs, 2.5 mM MgCl2, 1.2 μM UNI primer, 2.5 units AmpliTaq Polymerase [LD, PerkinElmer, Branchburg, NJ]). The DOP-PCR product of each microdissected case and normal DNA were purified by using the QIAquick PCR purification kit (Qiagen, Hilden, Germany).
Comparative genomic hybridization
CGH was performed according to methods described by Kallioniemi et al34 with minor modifications. Briefly, tumor DNA (PCR product) and normal DNA (PCR product) were labeled with direct fluorochrome-conjugated nucleotides by nick-translation with fluorescein-12-dUTP (green) and lissamine-5-dUTP (red) (NEN Life Science Products, Boston, MA), respectively. The size of the nick-translated fragments ranged from 300 to 3000 base pairs (bp). Equal amounts (500 ng) of labeled tumor and normal DNA were denatured together with 15 μg COT-1 DNA (GIBCO/BRL, Gaithersburg, MD), dissolved in hybridization mix (50% deionized formamide, 2 × standard saline–sodium citrate buffer (SSC), 10% dextran sulfate), and cohybridized to a denatured slide with normal metaphase chromosomes. After 72 hours of hybridization at 37°C, washes were performed to a stringency of 0.1 × SSC at 60°C. The slides were counterstained with 4,6-diamidino-2-phenylindole (DAPI) (blue) for chromosome identification.
The image analysis was performed using an epifluorescence microscope (DMRB, Leica) and image analysis software (QUIPS, Vysis, Downers Grove, IL). The ratio of fluorescein isothiocyanate/lissamine fluorescence intensities was calculated along each individual chromosome. Ratio values obtained from 10 metaphase spreads per case were averaged and the resulting profile was blotted next to the ideogram. Heterochromatic regions and the entire chromosome Y were excluded from analysis. Chromosomes 19 and 22 results were excluded from the final calculation because they are known to be critical in CGH.35 Because chromosomes 19 and 22 abnormalities were not observed in our control experiments, imbalances affecting these chromosomes found in some of our patients are shown in brackets in Figure1.
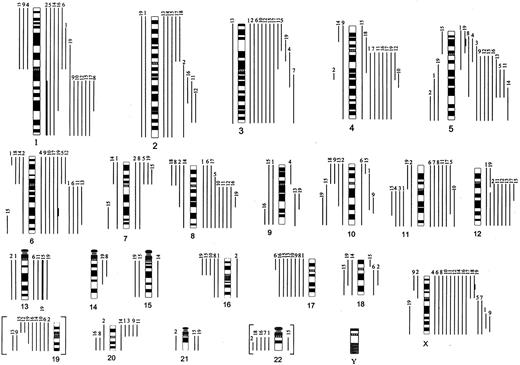
Summary of CGH results obtained in 19 cases of LPHD.
Chromosomal gains are shown on the right side of the ideogram and losses on the left side. High-level amplifications (average values more than 2.0) are shown in thick bars. The number on top of each line refers to the case number (Table 1). Chromosomal imbalances of critical regions of chromosomes 19 and 22 excluded from final calculation are shown here in brackets.
Summary of CGH results obtained in 19 cases of LPHD.
Chromosomal gains are shown on the right side of the ideogram and losses on the left side. High-level amplifications (average values more than 2.0) are shown in thick bars. The number on top of each line refers to the case number (Table 1). Chromosomal imbalances of critical regions of chromosomes 19 and 22 excluded from final calculation are shown here in brackets.
Control hybridization was performed with each CGH experiment and included hybridization of DNA from normal female on male chromosomes (overrepresentation of chromosome X being used as additional internal control).
By direct labeling, ratios above 1.15 and below 0.85 were considered to represent chromosomal gains and losses, respectively. These cut-off values are based on our results from control experiments that used 4 to 20 microdissected cells (data not shown). Overrepresentations were considered as high-level amplifications when the fluorescence ratio values exceeded 2.0.
The extension of imbalanced regions was assessed by comparison of the fluorescence ratio profiles with the corresponding regions in chromosome ideograms.
Control experiments and fluorescence in situ hybridization
To validate our approach (microdissection of 4 to 5 single “popcorn” cells, followed by DOP-PCR and CGH), various control experiments were performed.
To check whether the amplification of DNA by DOP-PCR influences CGH results, we performed the following investigations: (1) CGH on DOP-PCR–amplified DNA from normal total DNA (8 tests), and (2) CGH using DOP-PCR–amplified DNA from a microdissected area containing 10 to 15 normal cells from reactive lymph node biopsy cohybridized with DOP-PCR–amplified total DNA (3 tests). In both instances, no genomic imbalances were detected, and the green-to-red fluorescence ratios were always in the normal range in all analyzed chromosome arms, confirming the homogeneous amplification of genomic DNA by DOP-PCR.
To check whether CGH results obtained by using DOP-PCR–amplified tumor DNA are the same as CGH results obtained by using total tumor DNA (not amplified by PCR), 2 additional studies were performed. We selected 2 marginal zone B-cell lymphomas that were previously characterized by cytogenetics, conventional CGH, and FISH.36 In both cases, we microdissected small regions containing 10 to 15 neoplastic cells from frozen sections, amplified the tumor DNA as well as the normal DNA by DOP-PCR, and did CGH. The obtained CGH results were identical to the CGH results produced from total DNA (data not shown).
The reliability of our approach was finally also tested by performing CGH on: (1) 2 sets of 4 to 5 “popcorn” cells microdissected from different regions of the same lymph node biopsy (case 9), (2) DNA from 5 as well as 20 cells microdissected from the same lymph node in case 18, and (3) 2 sets of “popcorn” cells coming from 2 consecutive lymph node biopsy specimens taken at time of the first and second relapse (4 years later) in the same LPHD patient (case 11a and 11b). In the 3 experiments, 2 identical profiles of genomic imbalances were detected.
To confirm the CGH results, we performed FISH on frozen sections in 6 cases (1, 2, 4, 6, 9, and 10) with available frozen tissue. After CD20 immunostaining at least 20 CD20+ L&H cells were captured and positioned using a sensor control display (SCD) on a Zeiss Axioplan 2 (Zeiss, Jena, Germany) microscope. Sections were fixed as described by Weber-Matthiesen et al,19 and FISH was performed by using alpha-satellite probes for chromosomes 6 (D6Z1), 16 (D16Z1), and 17 (D17Z1) (all from Vysis, IL) and chromosomes 1 (puc 1.77), 3 (p3.5), and 7 (p7t1) (kindly provided by B. Beverloo, Rotterdam, The Netherlands). After FISH, the previously identified and captured CD20+ L&H cells were relocated and analyzed.
Cases 9 and 14 with available cytogenetic fixed cells were analyzed by using cos B5-2 for the BCL6 gene (kindly provided by T. Miki, Japan), puc 1.77 for centromere 1, and D6Z1 for centromere 6 in case 9 and cos B5-2, WCP1, and WCP3 (Vysis) in case 14. Experiments were carried out by using a previously described protocol.37
Results
Relevant clinical and cytogenetic data of the 19 LPHD cases selected for CGH studies are summarized in Table 1. The study comprised 17 males and 2 females ranging in age from 10 to 74 years (median, 37). All 18 patients with available clinical data showed involvement of peripheral lymph nodes. Patients were treated either with radiotherapy or chemotherapy, and for 16 (2 are still in therapy) complete remission was obtained. Three patients (3, 15, and 18) had a diffuse large B-cell lymphoma (DLBCL) developed after, respectively, 59, 248, and 126 months from the primary diagnosis of LPHD. Two patients (11 and 15) died of a disease-related cause after 202 and 248 months, respectively.
The material available for cytogenetic and CGH studies was from the time of primary diagnosis (16 cases) or was collected at the time of relapse (3 cases). Cytogenetic analysis was performed in 12 cases and metaphases were obtained in 8. Clonal chromosomal abnormalities, including t(3;22) (q27;q11), were identified in case 9 only. In the remaining cultures, either occasional polyploid cells with complex structural chromosomal aberrations (patient 7), cells with nonclonal chromosomal abnormalities (patient 17), or cells with a normal karyotype (patients 3, 5, 6, 14, and 19) were found. During preparation of this paper, a relapse occurred in patient 14, and cytogenetic analysis of the lymph node showed one abnormal metaphase with numerical and structural aberrations, including a t(3;?;1)(q27;?;q21) of 20 karyotyped cells. This metaphase was subjected to further FISH investigations.
CGH based on DOP-PCR–amplified genomic DNA from 4 to 5 microdissected CD20+ L&H cells was successfully performed in all 19 LPHD cases. The results are summarized in Figure 1, and an example is illustrated in Figure 2A,B. In this series of cases a total of 205 genetic imbalances were detected with an average of 10.8 imbalances per case (range from 3 to 22). Gains of chromosomal material were more frequent than losses (138 gains [7.3 per tumor] versus 67 losses [3.5 per tumor]). Imbalances were seen in all chromosomes but Y, which was not analyzed. Chromosomes 19 and 22 should be regarded critically and were finally excluded (discussed in “Patients, materials and methods”). Less involved were chromosomes 14, 15, and 21, showing 2 or 3 imbalances each, whereas all other chromosomes showed 6 or more changes. Gain/loss dislocations of short and long arm of single chromosomes found in cases 9 and 15 suggest the formation of isochromosomes.
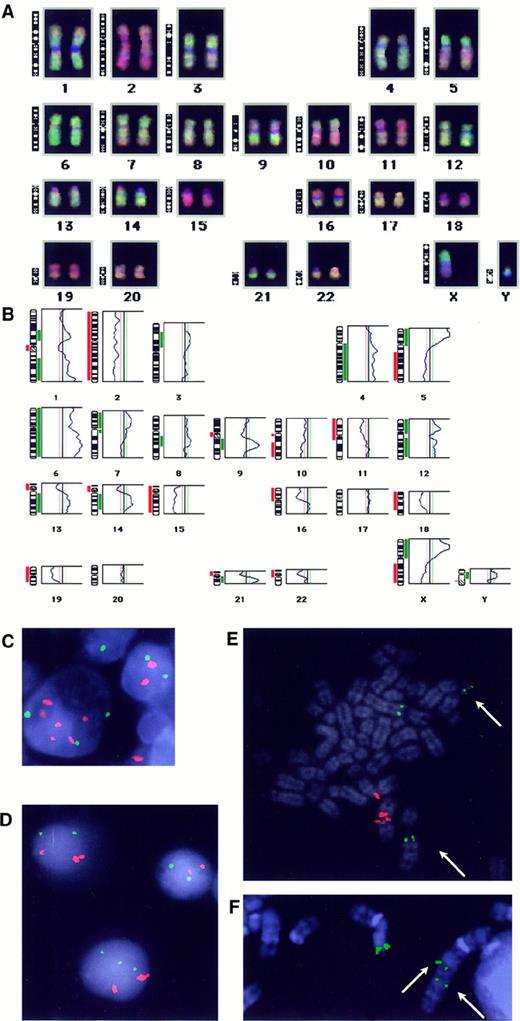
Examples of CGH and FISH results.
CGH-karyotype (A) and ratio profile (B) obtained in case 19. The vertical green line is the threshold for overrepresentations; the vertical left red line is the threshold for underrepresentation. Gains (green) and losses (red) are blotted next to the ideogram. Heterochromatic regions (on chromosomes 1, 9, 13, 14, 15, 21) and chromosomes 19, 22, and Y were excluded from analysis. (C) FISH with centromeric probes for chromosome 6 (green) and 3 (red) performed on a frozen section from case 6 that showed overrepresentation of chromosome 3. 3 green and 6 red signals found in the “popcorn” cell confirmed the CGH result and indicated the triploid status of the analyzed cell. (D) Interphase-FISH with the chromosome 1 centromeric probe (red) and the chromosome 6 centromeric probe (green) in case 9. Malignant cells were hallmarked by double red signal due to the idic(1)(q10) (panel E). Note presence of 2 or 3 green signals in malignant cells. (E) Metaphase-FISH with the B5-2 cosmid (green) for BCL6 and the chromosome 1 centromeric probe (red) performed in case 9. Arrows indicate the split cosB5-2 signal on both derivatives of t(3;22)(q27;q11). (F) Partial metaphase-FISH with the cosB5-2 probe (green) in case 14 showing the split signal on der(3) (arrows) and on normal chromosome 3.
Examples of CGH and FISH results.
CGH-karyotype (A) and ratio profile (B) obtained in case 19. The vertical green line is the threshold for overrepresentations; the vertical left red line is the threshold for underrepresentation. Gains (green) and losses (red) are blotted next to the ideogram. Heterochromatic regions (on chromosomes 1, 9, 13, 14, 15, 21) and chromosomes 19, 22, and Y were excluded from analysis. (C) FISH with centromeric probes for chromosome 6 (green) and 3 (red) performed on a frozen section from case 6 that showed overrepresentation of chromosome 3. 3 green and 6 red signals found in the “popcorn” cell confirmed the CGH result and indicated the triploid status of the analyzed cell. (D) Interphase-FISH with the chromosome 1 centromeric probe (red) and the chromosome 6 centromeric probe (green) in case 9. Malignant cells were hallmarked by double red signal due to the idic(1)(q10) (panel E). Note presence of 2 or 3 green signals in malignant cells. (E) Metaphase-FISH with the B5-2 cosmid (green) for BCL6 and the chromosome 1 centromeric probe (red) performed in case 9. Arrows indicate the split cosB5-2 signal on both derivatives of t(3;22)(q27;q11). (F) Partial metaphase-FISH with the cosB5-2 probe (green) in case 14 showing the split signal on der(3) (arrows) and on normal chromosome 3.
In the present series of LPHD, 3 cytogenetic subgroups could be constructed: a first group with mainly chromosomal gains (7 cases), a second with a balanced number of gains and losses (8 cases), and a third subgroup showing 17 and more genomic changes (4 cases) (Table 2). The 2 children (ages 10 and 11) were among the latter subgroup.
Cytogenetic subgroups constructed from comparative genomic hybridization results
| CGH/cytogenetic subgroups . | Case no. . | Recurrent gains (+) and losses (−) . | ||
|---|---|---|---|---|
| 80%-100% . | 50%-79% . | >33%-49% . | ||
| First | 5, 6, 7, 10, 11, 12, 17 | (+) 3p, 8q, 11q, Xq | (+) 1q, 3q, 4q, 6, 11p, Xp | (+) 2q, 5q, 8p, 12q, |
| 8 (4-11) gains/0 (0-2) losses | ||||
| n = 7 | ||||
| Second | 3, 4, 8, 9, 13, 14, 16, 18 | (+) 5q | (+) 1q, X | (+) 2, 5p, 6q, 20p |
| 5 (2-7) gains/3 (1-5) losses | (−) 17 | (−) 8p, 10p | ||
| n = 8 | ||||
| Third | 1, 2, 15, 19 | (+) 1, 3, 4q, 7p, 12q | (+) 2q, 5, 6q, 8q, 12p, 13, 21 | |
| ≥ 17 imbalances | (−) 5q, 10q, 11q, 16p | (−) 2, 6, 7q, 9, 11p, 13, 15, 17, 18q | ||
| n = 4 | ||||
| CGH/cytogenetic subgroups . | Case no. . | Recurrent gains (+) and losses (−) . | ||
|---|---|---|---|---|
| 80%-100% . | 50%-79% . | >33%-49% . | ||
| First | 5, 6, 7, 10, 11, 12, 17 | (+) 3p, 8q, 11q, Xq | (+) 1q, 3q, 4q, 6, 11p, Xp | (+) 2q, 5q, 8p, 12q, |
| 8 (4-11) gains/0 (0-2) losses | ||||
| n = 7 | ||||
| Second | 3, 4, 8, 9, 13, 14, 16, 18 | (+) 5q | (+) 1q, X | (+) 2, 5p, 6q, 20p |
| 5 (2-7) gains/3 (1-5) losses | (−) 17 | (−) 8p, 10p | ||
| n = 8 | ||||
| Third | 1, 2, 15, 19 | (+) 1, 3, 4q, 7p, 12q | (+) 2q, 5, 6q, 8q, 12p, 13, 21 | |
| ≥ 17 imbalances | (−) 5q, 10q, 11q, 16p | (−) 2, 6, 7q, 9, 11p, 13, 15, 17, 18q | ||
| n = 4 | ||||
CGH indicates comparative genomic hybridization.
In this series, a recurrent gain of genetic material from 18 chromosomes was observed. Genomic regions that were overrepresented in more than 36% of the studied cases included partial 1p (36.8%), 1q (57.9%), 2q (36.8%), 3p (52.6%), 3q (47.4%), 4q (47.4%), 5q (52.6%), 6p (36.8%), 6q (47.4%), 8q (47.4%), 11q (36.8%), 12q (42.1%), Xp (57.9%), and Xq (68.4%). The smallest commonly overrepresented regions involve 1p12p22, 1q21q31, 2q24q31, 3p13p21, 3q12q13, 4q12q25, 5q23, 6p, 6q12q24, 8q21, 11q12q22, 12q12, 12q14q15, Xp, and Xq23q26. High-level amplifications of the long arm of chromosome 1 and chromosome 13 were detected in cases 2 and 15, respectively. Case 19 showed amplifications of 5p14, 6q22, and Xp21 (Figure 2B).
Recurrent chromosomal losses of 17 chromosomes were detected. Most frequently lost were chromosome 17p/17 (42.1%/36.8%), followed by the loss of 11q (26.3%), 16p (26.3%), 6p (21.1%), 8p (21.1%), and 10p (21.1%). Losses of chromosomes 19 (42%) and 22q (26%) were recurrently seen; however, they were excluded from final calculation for reasons mentioned in “Patients, materials, and methods.”
The reliability of the CGH results was evaluated in 6 cases (1, 2, 4, 6, 9, and 10) by FISH performed on previously CD20 immunostained frozen sections. In these cases, centromeric probes for the imbalanced chromosomes 3 (pα3.5), 6 (D6Z1), 16 (D16Z1), and 17 (D17Z1) were applied together with a control probe for one of the unaffected chromosomes (1, 6, 7, 16, or 17). An example is shown in Figure 2C. In each case, 4 to 10 CD20+ “popcorn” cells could be evaluated. In case 2 with a loss of chromosome 6, 2 of 4 analyzed cells showed one cent-6 signal and 2 cent-17 signals, whereas 2 other cells showed 2 cent-6 signals and 4 cent-17 signals indicative for the diploid and tetraploid status of the analyzed cells, respectively. In the remaining cases with gain of chromosome 3 (patient 6) or 6 (patient 4) and loss of chromosome 16 (patient 1) or 17 (patients 9 and 10), the CGH genomic imbalances were also confirmed by FISH. The analyzed “popcorn” cells in cases 6, 4, and 1 showed to be diploid, triploid, or tetraploid, whereas in cases 9 and 10, all analyzed cells were diploid.
The reliability of CGH has also been proven by the finding of the same genomic imbalances detected in 2 consecutive lymph node biopsy specimens coming from the first and the second relapse (4 years later) in case 11 (11a and 11b; Table 1). These findings also indicated persistence of the malignant clone during the course of the disease. CGH results are also supported by the very recent cytogenetic data in case 14 obtained 7 years after the diagnosis of LPHD. The only abnormal polyploid cell identified in this material probably representing the malignant cell clone, showed similar imbalances as detected by CGH from tissue taken at the time of diagnosis (gain of X and 1q, and loss of 4p15, 6p, 7p11, and 18; Table 1).
CGH analysis performed in case 9 detected all genomic imbalances previously identified by cytogenetics [loss of 1p and gain of 1q due to the idic(1)(q10), monosomy 4 and 17, partial loss of chromosome 10 probably because of der(7)t(7;10)(q36;q11)]. In addition some other aberrations were found (gain of 5q, 6, 20p, partial Xq, and loss of partial Xp). To clarify some of these findings, we performed interphase-FISH analysis with centromeric probes for chromosomes 6 (gained) and 1 (control) on available cytogenetic fixed cells. Malignant cells were identified by 3 cent-1 signals, due to the idic(1)(q10). By this approach, we detected 2 subpopulations of malignant cells with either 2 or 3 cent-6 signals (Figure 2D). These results indicate that at least some of these imbalances not found by cytogenetics represent subclonal aberrations missed during the banding analysis. The karyotype of this case was also characterized by the t(3;22)(q27;q11). This translocation has been additionally analyzed by metaphase- and interphase-FISH by using a BCL6 probe. In the only abnormal metaphase found in the FISH experiment, the cosB5-2 probe hybridized with the normal chromosome 3, der(3) and der(22) indicating a rearrangement of the BCL6 gene (Figure 2E). This finding was confirmed by FISH analysis of 20 neoplastic interphase cells, which, as before, were identified by one normal and one double cent-1 signal because of the idic(1)(q10). Neither rearrangement ofBCL6 nor IgH/κ/λ was detected by Southern blot analysis performed in this case (data not shown), what can be explained by the low frequency of malignant cells in analyzed tissue. Analogous FISH study with cosB2-5 was performed in case 14 on the only abnormal cell identified by cytogenetics in the recent lymph node biopsy. Split of signals on the der(3)t(3;?;1)(q27;?;q21) (Figure 2F) indicated the BCL6 rearrangement due to the insertion of unknown material in the BCL6 region. This presumption was confirmed by a subsequent chromosome painting with WCP1 and WCP3 performed on the same metaphase.
Discussion
Genetic and molecular aspects of HD are still poorly understood and until now no (cyto)genetic marker(s) specific for HD could be identified. The use of molecular cytogenetic techniques in recent studies of HD, however, allowed difficulties caused by the rare occurrence and low proliferation index of RS cells to be overcome. With FISH combined with CD30 immunostaining, clonal occurrence of numerical chromosome changes in HD have been demonstrated.38 By this approach, however, only limited information restricted to the applied probes was obtained. Another molecular cytogenetic technique that allows the detection of genomic abnormalities in tumor cells without the need of dividing cells is CGH. However, it requires the presence of approximately 50% of neoplastic cells in the analyzed tumor lesion. To overcome this obstacle, in our CGH studies of LPHD we microdissected 4 to 5 single neoplastic cells and applied DOP-PCR for DNA amplification. This combined approach allowed us to study genomic imbalances in selected “popcorn” cells usually not contaminated with normal bystander cells. The reliability of this method was confirmed by several control experiments and additional investigations.
By using this strategy, we investigated 19 LPHD cases that showed typical histopathologic and clinical features of this entity. By CGH, genomic imbalances were detected in all cases, including those in which a normal karyotype was found by conventional cytogenetics. The high number of CGH-detected chromosomal imbalances per case (range from 3 to 22; average, 10.8) affecting all evaluated chromosomes suggests the occurrence of very complex chromosomal aberrations in most of the LPHD cases. No correlation between cytogenetic subgroups (Table 2) and clinical features could be established. The majority of detected genomic aberrations in LPHD was recurrent and appeared in a significant number of cases. The most frequent genomic imbalances found in 36.8% to 68.4% of the cases consisted of gains of chromosomes 1p, 1q, 2q, 3p, 3q, 4q, 5q, 6p, 6q, 8q, 11q, 12q, and X, and losses of chromosome 17/17p. Some of these, such as overrepresentation of 1q, 3q, 8q, 12q, X, and loss of 17p, have been frequently observed in various subtypes of NHL, and therefore may be regarded as secondary chromosomal changes associated with disease progression.39
We did, however, detect other recurrent imbalances such as gain of 2q, 4q, 5q, 6, and 11q, which are infrequent in lymphomas and other hematologic malignancies40,41 and therefore might be characteristic for LPHD. Particularly noteworthy is the frequent gain of 6q (47.4%), a chromosome arm typically deleted in NHL, in which 2 putative lymphoma-related tumor suppressor genes (TSGs) have been mapped.28,42,43 Our results suggest that chromosome 6q also carries gene(s) associated with the pathogenesis of LPHD. In this context, the finding of high-level amplification of the 6q22 region detected in case 19 is interesting. The only known candidate gene located in the amplified 6q22 region is the ROS1 oncogene, overexpressed in glioblastoma.44 Its association with hematologic malignancies, however, has not been demonstrated until now. The 9p23-24 amplification that was detected in the only LPHD case analyzed by CGH by Joos et al23 has not been found in our series of LPHD.
It is also noteworthy that the pattern of genomic imbalances found by us in LPHD is distinct from the patterns detected in classical HD cases by Ohshima et al22 and Joos et al23 This additionally illustrates a distinction of both subentities of HD.
Some other observations made in this study require additional comments. One of them is the high number of genomic imbalances per case (10.8). It contrasts with a relatively low number of genomic imbalances identified in other lymphoma subtypes (eg, average 2.5 imbalances per cell in marginal zone lymphoma [MZL],36 5.8 in mantle cell lymphoma [MCL],45 2.2 to 3 in DLBCL,46,47 and 4.4 in classical HD23). Therefore, our CGH results raise the question whether analyzed cells represented a monoclonal population characterized by a high number of genomic imbalances or a polyclonal/oligoclonal population with aberrations pooled together during the micromanipulation procedure. The latter possibility, however, becomes unlikely, as identical CGH profiles were found in 2 different samples from the same biopsy specimen (patient 9), in 5 as well as 20 cells from the same biopsy specimen (patient 18), and in samples derived from 2 consecutive lymph node biopsy specimens from the same patient (patient 11). Moreover, most of the genomic imbalances found at the time of diagnosis in case 14 have been confirmed by cytogenetic analysis performed 7 years later (14). Genomic complexity found in our series of LPHD seems to be a common feature of HD, as it was found in many published karyotypes of HD cases and HD-derived cell lines17,48 and in one of 2 very recently reported CGH studies of classical HD.22,23Thus, the high number of genetic imbalances in LPHD cases indicates that the clinical manifestation of this disorder is usually preceded by an accumulation of multiple genetic errors, including gains, losses, and amplifications of chromosomal material. According to a recent hypothesis, HD originates from B cells that have resided in a germinal center for an abnormally long period marked by a high load of acquired immunoglobulin variable heavy-chain gene mutations.11Simultaneously, an accumulation of complex genomic aberrations preceding the clinical manifestation of this disorder might occur.
Another comment concerns the frequent loss of the chromosome 17p/17 detected in 8/7 analyzed LPHD cases. Deletion of 17p has been traditionally associated with the involvement of p53, which is commonly inactivated in human tumors. Nonrandom loss of 17p was also observed in DLBCL,49-51 MCL,52 small lymphocytic lymphoma (SLL),53 and multiple myeloma.54 In contrast to solid tumors, however, mutations and/or deletions of p53/17p occur in a minor fraction of NHL cases, but if present, they usually hallmark a poor prognosis. Taking into consideration the indolent clinical course of the reported cases, a significantly higher incidence of 17/17p losses in LPHD in comparison with other NHL,55 and the absence of p53 immunostaining in studied LPHD cases,56it is possible that the 17/17p losses in LPHD did not target p53 but another gene located on chromosome 17. Such a putative tumor suppressor gene at 17p has been recently hypothesized by Phillips et al,57 Sankar et al,58 and Fioretos et al.59 Interestingly, lack of p53 mutations has been detected in 8 classical HD cases studied on single RS cells.60
High-level amplifications of genomic sequences have been identified in 3 cases in this study. One showed amplifications of 3 regions, including 5p14, 6q22, and Xp21. Two other LPHD cases revealed an amplification of 1q and chromosome 13. Interestingly, similar amplifications have been previously observed in DLBCL cases reported by Rao et al46 and Monni et al.47 In contrast to DLBCL and other NHL studied by CGH, neither 8q24/CMYC nor 18q21/BCL2 amplification have been detected in our series of LPHD.
To complement the CGH investigations, we performed additional FISH studies of the t(3;22)(q27;q11) and t(3;?;1)(q27;?;q21) identified by banding analysis in cases 9 and 14, respectively, that led to the demonstration of the BCL6 rearrangement. Chromosomal translocations involving the 3q27 region have been already recognized as B-NHL–related abnormalities occurring in approximately 40% of DLBCL and in 5% to 10% of follicular lymphomas.61,62 The t(3q27) results in a deregulation of the BCL6proto-oncogene, encoding a zinc finger transcription repressor either by the Ig gene clusters at 2p12, 14q32, and 22q11, or by a variety of other genomic loci.63-65 It has been shown that the BCL6 protein is expressed predominantly in normal GC B cells and related lymphomas, and is required for GC formation and function.60,66 Interestingly, LPHD cases consistently show expression of the BCL6 protein and lack expression of Syndecan-1 (a post-GC B-cell marker), reflecting their GC B-cell origin, whereas most of the classical HD cases reveal the post-GC phenotype (BCL6−/syn-1+).66,67 Our cytogenetic and FISH findings in cases 9 and 14 indicate that rearrangement of 3q27/BCL6 occurs recurrently in LPHD. A review of the literature showed 2 other LPHD cases with aBCL6 rearrangement68 or a t(3;22)(q27;q11).17 Altogether, these data indicate a nonrandom involvement of BCL6 in LPHD, support its GC origin, and suggest a relationship between this subtype of HD and DLBCL. The latter was already suggested by the simultaneous or consecutive development of DLBCL in 2% to 10% of LPHD cases. It is noteworthy that 3 of the patients reported here had DLBCL developed 5 to 20 years after the primary diagnosis of LPHD.
In summary, by using single microdissected cells–CGH, we studied genomic imbalances in 19 LPHD cases, the largest series of LPHD investigated by molecular cytogenetic techniques until now. The obtained results indicate a consistent occurrence of complex genomic aberrations in “popcorn” cells with a defined profile of recurrent chromosomal gains and losses. The high number of detected imbalances per case involving almost all chromosomes indicates a high complexity of LPHD karyotypes. Although some of the detected imbalances have been recurrently observed in different NHL subtypes, others, such as the gain of 2q, 4q, 5q, 6, and 11q, seem to be associated specifically with LPHD. A search for the involved genes located in these chromosomal regions can potentially shed light on the molecular pathogenesis of LPHD. Further studies are also required to determine an association between the 3q27/BCL6 rearrangement found in 2 of the presented cases and the development of LPHD.
We gratefully acknowledge Prof Benoit from the University of Gent, Dr de Ruysscher from Duffel, and Dr Uyttebroeck and Dr Thomas from the University of Leuven for providing clinical data of some of the patients. Thanks are due to Prof H. Van den Berghe for critically reading the manuscript. We thank E. van Dessel and U. Pluys for their excellent technical assistance and R. Logist for secretarial assistance.
Supported by grants G025298 and G011298 from the FWO (Fund for Scientific Research–Flanders [Belgium]). P.V. is a postdoctoral researcher of the FWO.
This text presents research results of the Belgian program of Interuniversity Poles of attraction initiated by the Belgian State, Prime Minister's Office, Science Policy Programming. The scientific responsibility is assumed by the authors.
A.H. and C.D.W.-P. share senior authorship.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Anne Hagemeijer, Center for Human Genetics–University of Leuven, Campus Gasthuisberg O&N6, Herestraat 49, B-3000 Leuven, Belgium; email: anne.hagemeijer@med.kuleuven.ac.be.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal