Development of natural killer 1.1+ (NK1.1+) CD3+ (NK1.1+ T) cells was analyzed in zeta-associated protein 70 (ZAP-70) null (−/−) mice. Both NK1.1+ TCRαβ+ and NK1.1+TCRγδ+ cell populations were absent in the thymus and spleen. By contrast, the number of NK1.1+ CD3−cells was increased in these tissues. The NK1.1+CD3− thymocytes in ZAP-70−/− mice had surface phenotypes in common with NK or NK1.1+ T cells. However, some of them were discordant either with NK cells or with NK1.1+ T cells. The NK1.1+ CD3−cells produced interferon-γ upon stimulation with NK1.1 cross-linking in the presence of interleukin-2 and exhibited a substantial cytotoxicity against YAC-1 cells. Moreover, the generation of NK1.1+ T cells with invariant Vα14Jα281 chains was induced from the NK1.1+ CD3− thymocytes following stimulation with phorbol myristate acetate and ionomycin in a neonatal thymic organ culture. An introduction of TCRα and β transgenes to the ZAP-70−/− mice resulted in generation of an NK1.1+ TCRαβdim population, whereas no substantial CD4+ CD8− or CD4−CD8+ population that expressed the introduced TCRαβ was generated in the mainstream T lineage. These findings demonstrate that ZAP-70 kinase is indispensable for the development of NK1.1+ T cells and that the unique NK1.1+ CD3− thymocytes in ZAP-70−/− mice contain immediate precursors of NK1.1+ T cells.
Introduction
The natural killer 1.1+(NK1.1+) T-cell population is a T-cell subset that expresses NK markers, ie, NK1.1, and possesses unique biologic functions.1,2 It has been shown that the NK1.1+ T cells are selected by ligands on the bone marrow–derived cells in the thymus,3-5 although we have demonstrated that the thymic structure also plays an important role in the positive selection.6 The restriction molecule for most of the NK1.1+ T-cell population that expresses the canonical Vα14Jα281 has been revealed as CD1d or TL molecule.7-10 The CD1d molecule presents α-galactosylceramide originally derived from marine sponge to NK1.1+ T cells and activates them in the periphery.11 However, no α-galactosylceramide is present in mammals under normal conditions, and glycosyl-phosphatidylinositol (GPI) moiety of GPI-anchored antigens appears to be one of the natural ligands for CD1d-restricted NK1.1+ T cells.12,13 Although unique functions of the NK1.1+ T cells have been demonstrated,1,2 13-19 a number of questions remain unanswered on the developmental pathway in the thymus and periphery.
Previous studies20,21 demonstrated that T and NK cells were derived from a common precursor. Although NK1.1+ T cells may share the developmental pathway with the T and NK cells, it has not been clear where the NK1.1+ T cells branch off from this common pathway. In mouse, Ballas et al22 reported that NK cell function could be detected by gestational day 15 and that NK1.1+ CD4+ CD8+double-positive (DP) cells were demonstrable on day 15 to 16 prior to an appearance of NK1.1− DP thymocytes in the fetal thymic ontogeny. These authors suggested that the NK1.1+ DP cells that are transiently detected in the fetal thymus were the progenitors of NK1.1+ T cells. Meanwhile, the NK1.1− but not the NK1.1+ T-cell population that expressed both invariant Vα14Jα281 and Vβ8 T-cell receptor (TCR) and exhibited characteristics of NK1.1+ T cells were present in common γ chain–deficient mice.23 Similarly large granular lymphocytes with a phenotype of CD4− CD8−TCRγδ+ CD16+ NK1.1+CD45R+ were identified following cultivation of TCRγδ+ CD4− CD8−NK1.1− thymocytes and splenocytes with interleukin (IL)-2 alone.24 The latter 2 reports support that NK1.1+ TCR+ cells are generated from the NK1.1− TCR+ cell population that has already rearranged the respective TCR gene loci.
Another precursor candidate of NK1.1+ T cells may be NK1.1+ TCR− cell population. Sato et al25 demonstrated that the NK1.1+ surface CD3ε− population could differentiate into mature NK1.1+ T cells in the presence of IL-15, granulocyte-macrophage colony-stimulating factor (GM-CSF), and stromal cells in vitro. This report suggests that the expression of the NK1.1 molecule preceded that of TCR.
Recent studies with genetically engineered mice revealed that the development of NK1.1+ T cells was defective in IL-7−/−, IL-7R−/−, IL-2/15Rβ−/− ,26 and CD3ζ−/− mice.27 It was also shown with these engineered mice that development of mainstream T cells was defective. These findings are consistent with a postulate that the same or similar molecular mechanisms operate on the development of both NK1.1+ T cells and mainstream T cells. However, NK1.1+ T cells as well as TCRγδ+ cells but not mainstream T cells developed normally in the dominant negative mutant on the Ras/Raf/Mek/MAPK pathway.28 On the other hand, in T-cell factor-1–deficient mice,29 Fyn-deficient mice,30 and pre-Tα−/−mice,26 31 the development of NK1.1+ T cells was selectively abrogated, whereas only minimal defect was observed in mainstream T cells. These findings suggest that certain signaling pathways involved in generation of NK1.1+ T cells are different from those of mainstream T cells.
In the present study, we analyzed development of NK1.1+ T cells in zeta-associated protein (ZAP)-70−/− mice. It was shown that ZAP-70 tyrosine kinase was essential for the development of mainstream T cells but not for that of NK cells.32 Indeed, NK cells differentiated normally in the peripheral lymphoid tissues and retained the NK activity in ZAP-70−/− mice presumably because p72syk replaced ZAP-70 functions in the NK cell population. Herein, we demonstrate that development of NK1.1+ T cells is completely abrogated in ZAP-70−/− mice. Instead, a considerable population of NK1.1+ CD3− cells was detected in the thymus as well as in the spleen. Although the surface phenotype of the NK1.1+ CD3− population was quite unique, this population retained intact NK functions. Furthermore, it will be shown that generation of NK1.1+ T cells is induced in the NK1.1+ CD3− thymocytes following stimulation with phorbol myristate acetate (PMA) and ionomycin in vitro. A possible developmental pathway of the NK1.1+ T cells is discussed.
Materials and methods
Mice
ZAP-70−/− mice32 were provided by Dennis Y. Loh at the Department of Biology, Nippon Roche Research Center (Kamakura, Japan). These mice were backcrossed with C57BL/6 (B6) mice for several generations and maintained in the animal facility at the Institute for Genetic Medicine, Hokkaido University, in a specific pathogen-free condition. ZAP-70+/− and ZAP-70+/+ mice were used as controls for flow cytometric and functional analyses. ZAP-70−/−/DO10 TCR transgenic mice were prepared by crossing the ZAP-70−/− mice of B6 background with DO10 TCR transgenic mice33 of B10.D2 background. Progenies were screened for the mutant ZAP-70 allele and the TCR transgene. C57BL/6J-Rag-1tm1rag(RAG-1−/−), B6.PL-Thy1a/Cy (B6. Thy 1.1), and TCRα−/− and β−/− mice were purchased from the Jackson Laboratory (Bar Harbor, ME). All mice were used for experiments at the age of 14 to 20 weeks.
Antibodies and flow cytometry
Thymocytes and spleen cells were first incubated with unlabeled monoclonal antibody (mAb) 2.4G2 (anti-FcγR) to block nonspecific staining and were then stained with a combination of the following mAb conjugates: biotinylated (biotin)-CD1d (1B1), -CD5 (53-7.3), -CD16 (2.4G2), -CD24 (J11d), -CD25 (7D4), -CD34 (RAM34), -CD44 (IM7), -CD45RB (23G2), -CD62L (MEL-14), -CD69 (H1.2F3), -Ly49A (A1), -TCRγδ (GL3), -2B4, and -DX5; fluorescein isothiocyanate (FITC)-CD8 (53-6.7), -CD2 (RM2-5), -CD4 (RM4-5), -TCRαβ (H57-597), -CD117 (2B8), -CD90 (53-2.1), -CD3ε (145-2C11), -CD95 (Jo2), -Ly49C (5E6), and -NK1.1 (PK136); and phycoerythrin (PE)-NK1.1, -CD45R (RA3-6B2), and -CD122 (TM-β1) (all from Pharmingen, San Diego, CA). A clonotypic mAb for the transgenic TCR of DO10 mouse, KJ1-26,34 was purified from hybridoma supernatant with Hi-Trap Protein G (Amersham Pharmacia Biotech, Uppsala, Sweden) and biotinylated by incubating withN-hydroxy-succimide biotin (Pierce, Rockford, IL) in dimethyl sulfoxide solution. Streptavidin-FITC or –Red 670 (Gibco, Gaithersburg, MD) was used for biotin-mAb. Propidium iodide red fluorescent dye (Sigma Chemical, St. Louis, MO) was added to the cells immediately before analysis. Stained cells were analyzed with FACScan flow cytometer (Becton Dickinson, Mountain View, CA) using CellQuest software (Becton Dickinson).35
Preparation of NK1.1+ cells
Thymocyes were treated with anti-CD24 and anti-CD8 mAb, and splenocytes were treated with anti-CD24, CD8, and I-Ab(1E4)36 mAb at 4°C for 30 minutes. The cells were then washed twice and resuspended in magnetic beads coated with goat antirat immunoglobulin G (IgG) antibody (Miltenyi Biotec, Bergisch Gladbach, Germany) at 6°C for 15 minutes. Cells that had bound the antibody were depleted by magnetic-activated cell sorting (MACS) system with VarioMACS (Miltenyi Biotec). Thereafter, the CD24−, CD8−, and I-Ab− cells were stained with FITC–anti-TCRαβ mAb and PE–anti-NK1.1 mAb. The stained cells were sorted into NK1.1+ TCRαβ− cells with FACSVantage (Becton Dickinson). The sorted cells were further cultured for 4 days in the presence of recombinant human (rh)IL-2 (1000 U/mL; Pharmaceutical Research Division, Takeda Chemical Industries, Osaka, Japan) and used for functional analyses.
Cytokine enzyme-linked immunosorbent assay
To evaluate IL-4 production, either unsorted (1 × 106) or sorted (4 × 104) cells from the thymus and spleen were stimulated with immobilized anti-CD3ε mAb (145-2C11; Pharmingen) at 10 μg/mL in a total volume of 50 μL RPMI 1640 supplemented with 10% heat-inactivated fetal calf serum, 100 U/mL penicillin, 100 μg/mL streptomycin, and 5 × 10−5 M 2-mercaptoethanol in a 96-well flat-bottomed plate for 48 hours. IL-4 in the culture supernatants was quantitated with Cytoscreen Immunoassay kit for mouse IL-4 (BioSource International, Camarillo, CA) according to a manufacturer's protocol. To evaluate interferon (IFN)-γ production, cells were stimulated with immobilized anti-NK1.1 (PK136; Pharmingen) at 50 μg/mL in a total volume of 50 μL RPMI-1640 in the presence of rhIL-2 (1000 U/mL) for 48 hours. IFN-γ in the culture supernatants was quantitated with Cytoscreen Immunoassay kit for mouse IFN-γ (BioSource International).37
Assay for cytotoxic activity
Cytotoxic activities were evaluated as previously described.32 In brief, control and ZAP-70−/−mice were intraperitoneally administered 200 μg tilorone (2,7-bis[2-(diethylamino)ethoxy]-9H-fluoren-9-one; Sigma) per mouse 24 hours before collecting thymocytes and splenocytes. Total thymocytes or splenocytes depleted of red blood cells were cultured for 7 days in the presence of rhIL-2 (1000 U/mL). The harvested cells were used as effector cells and incubated with 51Cr-labeled YAC-1 or P815 cells (5 × 103) at indicated effector:target ratios for 4 hours.14 Then51Cr radioactivity released in the supernatant was quantified with γ-counter (Auto Gamma 5000; Packard, Canberra, Australia). Cytotoxicity was expressed as percent specific lysis, which was calculated as follows: percent specific lysis = [(experimental release − spontaneous release)/(maximum release − spontaneous release)] × 100. Spontaneous release and maximum release were obtained by incubating target cells alone or with 2N HCl solution, respectively.
Neonatal thymic organ culture
Thymic lobes were obtained from neonatal BALB/c, RAG-1−/−, or (B6 × B6.Thy1.1)F1 mice within 24 hours of birth and cultured for 5 to 7 days with medium containing 1.35 mM 2′-deoxyguanosine (dGuo; Nakaraitesque, Osaka, Japan) on the raft of a membrane filter (0.45 μm; Millipore, Bedford, MA) with a sterile sponge (Gelfoam; Pharmacia-Upjohn, Tokyo, Japan).38 The dGuo-treated thymic lobes were transferred to a Terasaki tissue culture plate (Becton Dickinson, Oxnard, CA) in a hanging-drop setup with a total volume of 20 μL. Then the sorted NK1.1− TCRαβ− or NK1.1+TCRαβ− thymocytes from ZAP-70−/− mice were seeded (about 5 × 103/lobe to 8 × 103/lobe) and cultured with the thymic lobes of BALB/c, RAG-1−/−, or (B6 × B6.Thy1.1)F1 mice in RPMI-1640–based medium in the presence of 1600 nM PMA (Sigma) and 130 nM ionomycin (Sigma).39 40 Five days later the thymic lobes were harvested and minced to obtain a single-cell suspension. Cells were stained with either PE–anti-NK1.1 mAb or PE-control IgG2a (Pharmingen) and FITC–anti-TCRαβ mAb and analyzed with FACScan as described above. The proportion of NK1.1+TCRαβ+ cells was calculated as follows: [percentage of NK1.1+ TCRαβ+ cells minus percentage of control IgG2a+ TCRαβ+ cells]. Statistical analysis was performed according to the Student ttest.
Detection of an invariant Vα chain and RAG-1 transcripts in induced NK1.1+ TCRαβ+ cells
Total RNA was extracted either from neonatal thymic lobes obtained from RAG-1−/− mice or those cultured with sorted NK1.1+ TCRαβ− cells from ZAP-70−/− thymi in the presence or absence of PMA plus ionomycin.35 Complementary DNA (cDNA) was synthesized using random hexanucleotide (Takara Shuzo, Ohtsu, Japan) and Moloney murine leukemia virus reverse transcriptase (SuperScript; Gibco) at 37°C for 1 hour in the presence of deoxyribonucleoside triphosphates and ribonuclease inhibitor (RNasin; Promega, Madison, WI). The cDNA products were used as templates in either ordinary or nested polymerase chain reactions (PCRs) for amplification of the following gene products with respective primer pairs (all from Hokkaido System Science, Sapporo, Japan): Vα14 Leader/C-rev1 (for first-round PCR), 5′-ATGAAAAAGCGCCTGAGTGCC-3′/5′-CAGGAGGATTCGGAGTCCCA-3′; Vα14/Jα281 (for nested PCR), 5-TAAGCACAGCACGTGCACAT-3′/5′-CAATCAGCTGAGTCCCAGCT-3′ 9,37,41; RAG-1 5′/RAG-1 3′ (for first-round PCR), 5′-CCAAGCTGCAGACATTCTAGCACTC-3′/5′-CAACATCTGCCTTCACGTCGATCC-3′; RAG-1 5′ nest/RAG-1 3′ nest (for nested PCR), 5′-CGAAGAAGCACAGAAGGAGAAGG-3′/5′-AAACGATTCCCACAGATGCGGC-3′; and EF-1α 5′/3′, 5′-CTGCTGAGATGGGAAAGGGCT-3′/5′-TTCAGGATAATCACCTGAGCA-3′.42Thermal cycling was performed with the following programs: 40 cycles of heat denaturation at 94°C for 1 minute, annealing at 53°C for Vα14 Leader/Cα-rev1, 52°C for Vα14/Jα281, or 50°C for EF-1α 5′/EF-1α 3′ for 1 minute, and elongation at 72°C for 2 minutes. PCR products were electrophoresed on either a 3.0% (for Vα14/Jα281 transcripts) or a 1% (for RAG-1 and EF-1α transcripts) agarose ethidium bromide gel according to the length of amplified bands.
Analysis of gene rearrangement of TCR Vβ chain from NK1.1+ TCRαβ− thymocytes in ZAP-70−/− mice
Genomic DNA was extracted from either B6 thymocytes, B6 ear skin, or NK1.1+ TCRαβ− thymocytes of ZAP-70−/− mice and subjected to a PCR-based analysis of gene rearrangements42 43 with slight modifications. PCR was performed using a primer pair of a coding region of Dβ2 (Dβ2-5′) and 3′-downstream region of Jβ2.7 (Jβ2-3′) to detect rearrangements of Dβ2 to Jβ2 cluster. Rearrangement of Vβ8 to Dβ2Jβ2 was examined by PCR with a primer pair of a coding region of Vβ8.2 (Vβ8-5′) and the same Jβ2 primer as described above. Sequences of PCR primers were: Dβ2-5′, 5′-GTAGGCACCTGTGGGGAAGAAACT-3′; Jβ2-3′, 5′-TGAGAGCTGTCTCCTACTATCGATT-3′; and Vβ8-5′, 5′-GCATGGGCTGAGGCTGATCCATTA-3′. PCR was performed according to a standard protocol with 2 units of AmpliTaq Gold DNA polymerase (Roche Molecular Systems, Branchburg, NJ) with 1 cycle of 8 minutes at 94°C and 25 cycles of 1 minute at 94°C, 2 minutes at 63°C, and 3 minutes at 72°C, followed by an extension step of 10 minutes at 72°C. Whole PCR products were extracted by phenol/chloroform and ethanol-precipitated and electrophoresed on 1% agarose gel in Tris-acetate-EDTA (TAE) buffer. Electrophoresed materials were then transferred to nitrocellulose membrane (Hybond-N+; Amersham, Little Chalfont, UK) and hybridized with a Jβ2.6 gene segment (5′-CAGCCCTTGCCCTGACTGATT-3′) probe labeled with 3′-oligo labeling and detection systems (ECL; Amersham) according to the manufacturer's protocol. Hybridization was performed at 37°C overnight in a 5 × standard saline citrate 0.02% sodium dodecyl sulfate–based hybridization solution and washed at room temperature with 5 × standard saline citrate 0.1% sodium dodecyl sulfate for 15 minutes once followed by a wash at 37°C for 15 minutes. Then, the membrane was washed several times with NaCl/Tris-based buffers and incubated with antifluorescein antibody conjugated with alkaline phosphatase at 4°C overnight with gentle shaking. The membrane was washed 4 times with 0.4 M NaCl, 0.1 M Tris-HCl, (pH 7.5), incubated with CDP-Star for 1 minute, and exposed to x-ray film (Hyperfilm; Amersham).
Results
Development of NK1.1+ T cells was arrested in ZAP-70−/− mice
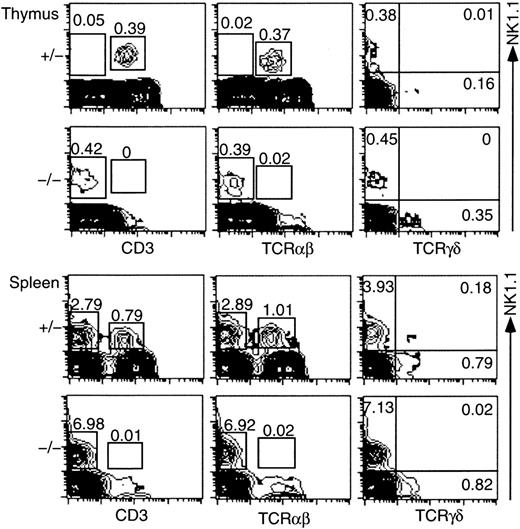
To examine development of NK1.1+ T cells in the ZAP-70−/− mice, we performed flow cytometric analysis of the thymocytes and spleen cells and compared the profiles with those of control mice (ZAP-70+/− or ZAP-70+/+ mice). Representative results are shown in Figure1. NK1.1+ CD3+ or NK1.1+ TCRαβ+ cells were totally absent in the thymus of ZAP-70−/− mice (0% or 0.02%, respectively). Instead, a substantial proportion of the NK1.1+ CD3− or NK1.1+TCRαβ− population was detected (0.42% or 0.39%, respectively). The NK1.1+ CD3− or NK1.1+ TCRαβ− population was rarely detectable in the thymus of control mice. When the TCRγδ+ cell population was compared, no NK1.1+ TCRγδ+ cells were detected in both ZAP-70−/− mice and control mice. However, a substantial proportion (0.35%) of TCRγδ+ cells was detected in the NK1.1− thymocytes of ZAP-70−/− mice but a quite low proportion in those of ZAP-70+/− mice. Similar results were obtained when spleen cells were analyzed. No NK1.1+ CD3+ or NK1.1+TCRαβ+ cell was detected in the spleen of ZAP-70−/− mice, whereas a markedly high proportion of NK1.1+ CD3− cells was demonstrated in the ZAP-70−/− spleen as compared with that of control mice (Figure 1).
NK1.1 expression on thymocytes and splenocytes from control (ZAP-70+/−) and ZAP-70−/− mice.
Thymocytes and splenocytes were stained with PE–anti-NK1.1 and FITC–anti-CD3, or FITC–anti-TCRαβ, or with PE–anti-NK1.1 and biotin–anti-TCRγδ/streptavidin-FITC. Expressions of NK1.1 (vertical) and CD3, TCRαβ, or TCRγδ (horizontal) are illustrated. Proportions of NK1.1+ CD3− and NK1.1+ CD3+ (left column), NK1.1+TCRαβ− and NK1.1+ TCRαβ+(middle column), and NK1.1+ TCRγδ−, NK1.1+ TCRγδ+, and NK1.1−TCRγδ+ (right column) cells are indicated in the figures. Results are representative of 6 independent experiments.
NK1.1 expression on thymocytes and splenocytes from control (ZAP-70+/−) and ZAP-70−/− mice.
Thymocytes and splenocytes were stained with PE–anti-NK1.1 and FITC–anti-CD3, or FITC–anti-TCRαβ, or with PE–anti-NK1.1 and biotin–anti-TCRγδ/streptavidin-FITC. Expressions of NK1.1 (vertical) and CD3, TCRαβ, or TCRγδ (horizontal) are illustrated. Proportions of NK1.1+ CD3− and NK1.1+ CD3+ (left column), NK1.1+TCRαβ− and NK1.1+ TCRαβ+(middle column), and NK1.1+ TCRγδ−, NK1.1+ TCRγδ+, and NK1.1−TCRγδ+ (right column) cells are indicated in the figures. Results are representative of 6 independent experiments.
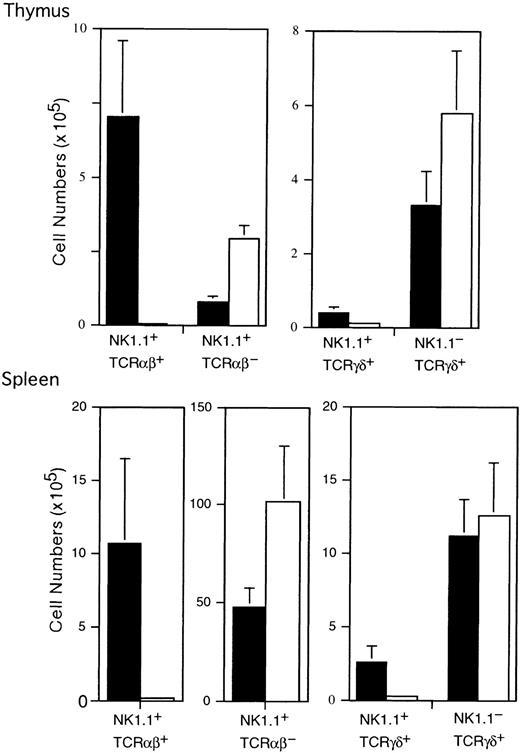
These differences shown in the flow cytometric profiles were again demonstrated when actual cell numbers were compared between ZAP-70−/− mice and control mice (Figure2). No significant difference in the mean cell numbers of the thymus and spleen was observed between ZAP-70−/− (n = 5) and control mice (n = 6) (thymus: 1.38 ± 0.26 × 108 in ZAP-70−/− mice and 1.33 ± 0.32 × 108 in control mice; spleen: 1.75 ± 0.44 × 108 in ZAP-70−/− and 1.58 ± 0.37 × 108 in control mice). However, the number (0.028 ± 0.056 × 105) of NK1.1+TCRαβ+ cells in ZAP-70−/− thymus was significantly smaller than that of control thymus (7.01 ± 2.56 × 105; P < .001). By contrast, the number of NK1.1+ TCRαβ−cells in ZAP-70−/− thymus was approximately 3- to 4-fold greater than that of control thymus (ZAP-70−/−, 2.94 ± 0.46 × 105; control mice, 0.80 ± 0.18 × 105; P < .005).
Cell number of NK1.1+TCRαβ+, NK1.1+ TCRαβ−, NK1.1+ TCRγδ+, and NK1.1−TCRγδ+ subpopulations in the thymus and spleen of control and ZAP-70−/− mice.
Mean cell numbers of each subpopulation in the thymus and spleen of control (▪, n = 6) and ZAP-70−/− (■, n = 5) mice were calculated and shown as means and SD.
Cell number of NK1.1+TCRαβ+, NK1.1+ TCRαβ−, NK1.1+ TCRγδ+, and NK1.1−TCRγδ+ subpopulations in the thymus and spleen of control and ZAP-70−/− mice.
Mean cell numbers of each subpopulation in the thymus and spleen of control (▪, n = 6) and ZAP-70−/− (■, n = 5) mice were calculated and shown as means and SD.
Although NK1.1+ TCRγδ+ cells were negligible in both control and ZAP-70−/− thymi with the flow cytometric analysis (Figure 1), the actual number of NK1.1+ TCRγδ+ cells in control mice was higher than that in ZAP-70−/− mice, as shown in Figure 2(control, 0.186 ± 0.074 × 105; ZAP-70−/−, 0.365 ± 0.163 × 105;P < .005). On the contrary, the mean cell number of the NK1.1− TCRγδ+ population was considerably higher in ZAP-70−/− thymus than that in control thymus (ZAP-70−/−, 5.79 ± 1.66 × 105; control, 3.29 ± 0.91 × 105; P < .05). These findings suggest that ZAP-70 is less influential to the development of TCRγδ+ cells than to that of TCRαβ+cells.
The marked decrease in the number of NK1.1+TCRαβ+ cells and significant increase of NK1.1+ TCRαβ− cells were also demonstrated in the spleen of ZAP-70−/− mice (Figure 2). The mean number of NK1.1+ TCRαβ+ cells in ZAP-70−/− mice was significantly smaller than that of control mice (ZAP-70−/−, 0.165 ± 0.176 × 105; control, 10.7 ± 5.75 × 105; P < .05), and the mean number of NK1.1+ TCRαβ− cells in ZAP-70−/− mice was significantly higher than that of control mice (ZAP-70−/−, 102 ± 28.6 × 105; control, 47.4 ± 9.90 × 105; P < .05). The small but substantial population of the NK1.1+TCRαβ− cells seen in control mice might be attributable to the presence of ordinary NK cells in the spleen. It was also noted in Figure 2 that the number of NK1.1+TCRγδ+ cells was considerably smaller in ZAP-70−/− spleen than that in normal spleen (ZAP-70−/−, 0.285 ± 0.193 × 105;control, 2.53 ± 1.09 × 105; P < .05), whereas the number of NK1.1− TCRγδ+ cells in ZAP-70−/− mice was almost the same as that of control mice (ZAP-70−/−, 12.6 ± 3.60 × 105; control, 11.1 ± 2.58 × 105; P = .282).
NK1.1+ CD3− thymocytes in ZAP-70−/− mice exhibited unique phenotype compared with either ordinary NK cells or NK1.1+ TCRαβ+T cells
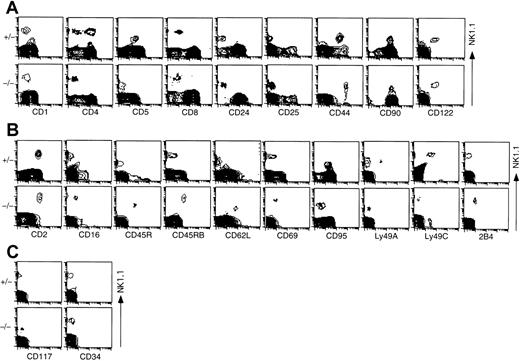
Next, to examine other surface phenotypes of NK1.1+CD3− cells detected in the thymus of ZAP-70−/− mice using various mAbs that react mainly to T cells (Figure 3A), mainly NK cells (Figure 3B), or stem cells (Figure 3C), whole thymocytes obtained from either control or ZAP-70−/− mice were analyzed with flow cytometry. As shown in Figure 3A, the NK1.1+population in control mice that corresponds mostly to the NK1.1+ T cells (Figure 1) was CD1low, CD4−/+, CD5+, CD8−, CD24−, CD25−, CD44+, CD90+, and CD122+ as reported in previous studies.1,2,14,37 44 As far as expressions of CD8, CD24, CD25, CD90, and CD122 molecules were concerned, NK1.1+TCRαβ− cells in ZAP-70−/− mice showed the same staining pattern as that of NK1.1+ T cells in control mice. However, these NK1.1+ TCRαβ−cells expressed neither CD4 nor CD5 molecules but showed higher CD44 (Figure 3A), CD2, and CD16 (Figure 3B) fluorescence intensity than NK1.1+ T cells in control mice. When markers for the precursor population were analyzed, both NK1.1+TCRαβ− thymocytes of ZAP-70−/− mice and the thymic NK1.1+ T cells of control mice were CD117− and CD34− (Figure 3C).
Phenotype of NK1.1+ thymocytes from control and ZAP-70−/− mice.
Thymocytes were stained with PE–anti-NK1.1 or FITC–anti-NK1.1 and various cell surface markers as described in “Materials and methods.” Expressions of NK1.1 (vertical) and other markers (horizontal) on thymocytes are illustrated: expressions of NK1.1 and T-cell surface markers (A), NK surface markers (B), and stem cell markers (C). Results are representative of 6 independent experiments.
Phenotype of NK1.1+ thymocytes from control and ZAP-70−/− mice.
Thymocytes were stained with PE–anti-NK1.1 or FITC–anti-NK1.1 and various cell surface markers as described in “Materials and methods.” Expressions of NK1.1 (vertical) and other markers (horizontal) on thymocytes are illustrated: expressions of NK1.1 and T-cell surface markers (A), NK surface markers (B), and stem cell markers (C). Results are representative of 6 independent experiments.
In addition, the NK1.1+ thymocytes in ZAP-70−/− mice were CD45R+, CD45RB+, CD62L+, CD69int, CD95−, Ly49A−, Ly49C−, and 2B4+, whereas the NK1.1+ T cells of control mice were CD2+, CD45R−, CD45RB−, CD62L−, CD69−/low, CD95+, Ly49A−/+, Ly49C−/+, and 2B4− (Figure 3B). These profiles of NK1.1+thymocytes in ZAP-70−/− were similar to those of ordinary NK cells in the peripheral lymphoid organs.
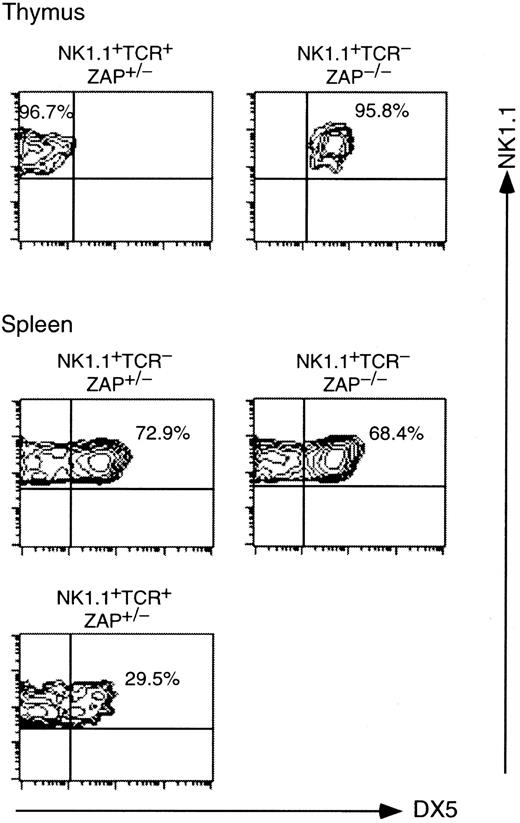
We then examined surface expression of a pan-NK marker, DX5,45 on NK1.1+ TCR− cells in the thymus and spleen of ZAP-70−/− mice and compared it with that of NK1.1+ cells in control mice. As shown in Figure 4, approximately 96% of NK1.1+ TCR− cells in ZAP-70−/−thymus expressed DX5, whereas most NK1.1+ TCR+cells expressed no DX5 in ZAP-70+/− thymus. In the ZAP-70+/− spleen, however, approximately 30% of NK1.1+ TCR+ cells expressed DX5. This finding suggests a phenotypic difference between NK1.1+ T cells in the thymus and those in spleen of normal mice. Almost the same proportions expressed DX5 in NK1.1+ TCR−cells of both in ZAP-70+/− and ZAP-70−/ −spleens.
DX5 expressions on NK1.1+ thymocytes and splenocytes from control (ZAP-70+/−) and ZAP-70−/− mice.
Thymocytes and splenocytes were stained with PE–anti-NK1.1, FITC–anti-TCRαβ, and biotinylated anti-DX5 followed by streptavidin–Red 670. NK1.1+ TCRαβ+ or NK1.1+ TCRαβ− cells in the thymus and spleen were electronically gated and analyzed for the expression of DX5 on FACScan. Dead cells were electronically gated out with propidium iodide staining. FACS profiles were shown in contour with NK1.1 versus DX5 staining.
DX5 expressions on NK1.1+ thymocytes and splenocytes from control (ZAP-70+/−) and ZAP-70−/− mice.
Thymocytes and splenocytes were stained with PE–anti-NK1.1, FITC–anti-TCRαβ, and biotinylated anti-DX5 followed by streptavidin–Red 670. NK1.1+ TCRαβ+ or NK1.1+ TCRαβ− cells in the thymus and spleen were electronically gated and analyzed for the expression of DX5 on FACScan. Dead cells were electronically gated out with propidium iodide staining. FACS profiles were shown in contour with NK1.1 versus DX5 staining.
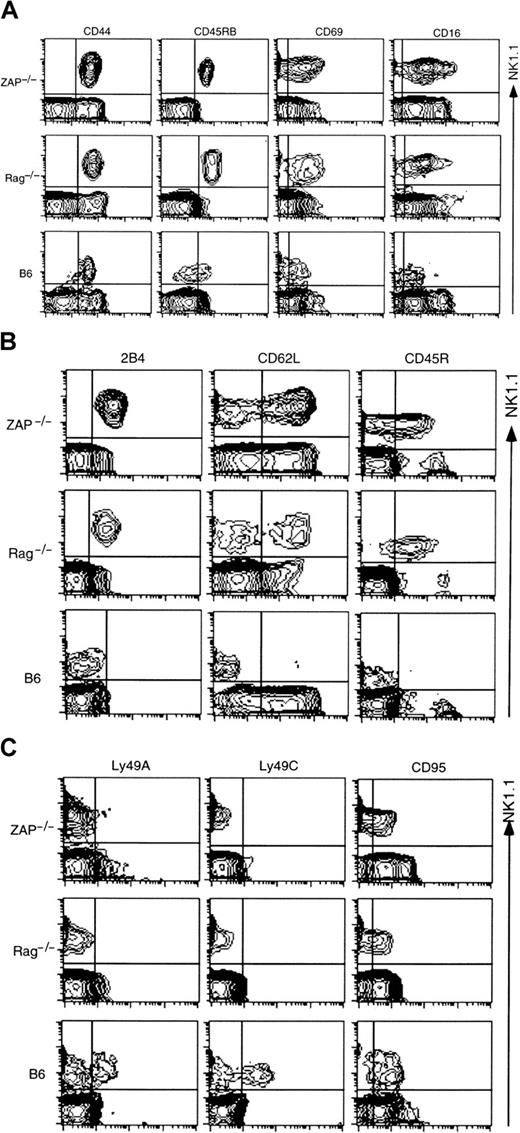
Then, to compare the surface phenotype of NK1.1+TCR− thymocytes in ZAP-70−/− mice with NK1.1+ TCR− cells of B6 and another NKT-deficient strain, RAG-1−/−, CD8−HSA− thymocytes of ZAP-70−/− mice, CD8− HSA− CD3− thymocytes from B6 mice, and whole thymocytes of RAG-1−/− mice were analyzed for the various surface markers (Figure5). These populations were enriched for the NK1.1+ CD3− cells and enabled us to compare the surface phenotypes more precisely. Figure 5A shows that CD44, CD45RB, CD69, and CD16 are more highly expressed on the NK1.1+ CD3− cells of ZAP-70−/−or RAG-1−/− mice than those from B6 mice. The higher expressions of these molecules on the cells of 2 NKT-defective mutants could not be explained with the cell sizes, because the NK1.1+ CD3− thymocytes from 3 kinds of mice showed almost the same values of forward light scatter. Figure 5B,C shows that 2B4, CD62L, or CD45R is expressed on NK1.1+CD3− thymocytes from ZAP-70−/− and RAG-1−/− mice but not on those of B6 mice. On the other hand, approximately 25% to 35% of ordinary NK cells of B6 mice were either Ly49A+ or Ly49C+.46 No expressions of Ly49A and Ly49C were detected on NK1.1+CD3− cells of ZAP-70−/− and RAG-1−/− mice. It was also noted that the expression of CD95 was lower on NK1.1+ CD3− thymocytes of ZAP-70−/− and RAG-1−/− than that of B6 mice.
Phenotype of NK1.1+CD3−thymocytes from ZAP-70−/−, RAG-1−/−, and B6.
Whole thymocytes (B6-RAG-1−/−), thymocytes enriched for CD8− HSA− population with MACS (ZAP-70−/−), and thymocytes enriched for CD8− HSA− with MACS and electronic gating for CD3− thymocytes (B6 mice) were stained with PE–anti-NK1.1 and various cell surface markers (grouped in panels A-C) as described in “Materials and methods.” Expressions of NK1.1 (vertical) and other markers (horizontal) on either enriched or whole thymocytes are indicated.
Phenotype of NK1.1+CD3−thymocytes from ZAP-70−/−, RAG-1−/−, and B6.
Whole thymocytes (B6-RAG-1−/−), thymocytes enriched for CD8− HSA− population with MACS (ZAP-70−/−), and thymocytes enriched for CD8− HSA− with MACS and electronic gating for CD3− thymocytes (B6 mice) were stained with PE–anti-NK1.1 and various cell surface markers (grouped in panels A-C) as described in “Materials and methods.” Expressions of NK1.1 (vertical) and other markers (horizontal) on either enriched or whole thymocytes are indicated.
These results are summarized in Table 1. This table demonstrates that the NK1.1+ CD3−cells in the thymus of ZAP-70−/− mice share largely common features with ordinary NK cells. However, the NK1.1+CD3− cells exhibit several distinct phenotypes that represent neither NK1.1+ T cells nor NK cells. In this table, it is of note that NK1.1+CD3− cells with surface phenotype similar to those of ZAP-70−/− mice are present in RAG-1−/− thymus.
Antigen expression on NK1.1+ thymocytes
| Antigen . | NK1.1+ CD3− cells in B6 . | NK1.1+ CD3+ cells in ZAP-70+/− . | NK1.1+ CD3− cells in ZAP-70−/− . | NK1.1+ CD3− cells in Rag−/− . |
|---|---|---|---|---|
| T cell markers | ||||
| TCRαβ | − | + | − | − |
| CD1 | − | low | low | ND |
| CD4 | − | + (50%) | − | ND |
| CD5 | − | + | − | − |
| CD8 | − | − | − | − |
| CD24 | − | − | − | ND |
| CD25 | − | − | − | ND |
| CD44 | + | + | ++ | ++ |
| CD90 | −/+ | + | + | + |
| CD122 | + | + | + | + |
| NK cell markers | ||||
| NKRP-1C | + | + | + | + |
| CD2 | + | + | + | + |
| CD16 | −/low | −/low | int | int |
| CD45R | − | − | int | int |
| CD45RB | +/low | − | + | + |
| CD62L | − | − | +/− | +/− |
| CD69 | −/low | −/low | int | int |
| CD95 | + | + | −/low | −/low |
| Ly49A | + (10%) | + (20%) | − | − |
| Ly49C | + (25%) | + (60%) | − | − |
| 2B4* | − | − | + | + |
| DX5 | + | −† | + | + |
| Stem cell markers | ||||
| CD34 | − | − | − | ND |
| CD117 | − | − | − | ND |
| Antigen . | NK1.1+ CD3− cells in B6 . | NK1.1+ CD3+ cells in ZAP-70+/− . | NK1.1+ CD3− cells in ZAP-70−/− . | NK1.1+ CD3− cells in Rag−/− . |
|---|---|---|---|---|
| T cell markers | ||||
| TCRαβ | − | + | − | − |
| CD1 | − | low | low | ND |
| CD4 | − | + (50%) | − | ND |
| CD5 | − | + | − | − |
| CD8 | − | − | − | − |
| CD24 | − | − | − | ND |
| CD25 | − | − | − | ND |
| CD44 | + | + | ++ | ++ |
| CD90 | −/+ | + | + | + |
| CD122 | + | + | + | + |
| NK cell markers | ||||
| NKRP-1C | + | + | + | + |
| CD2 | + | + | + | + |
| CD16 | −/low | −/low | int | int |
| CD45R | − | − | int | int |
| CD45RB | +/low | − | + | + |
| CD62L | − | − | +/− | +/− |
| CD69 | −/low | −/low | int | int |
| CD95 | + | + | −/low | −/low |
| Ly49A | + (10%) | + (20%) | − | − |
| Ly49C | + (25%) | + (60%) | − | − |
| 2B4* | − | − | + | + |
| DX5 | + | −† | + | + |
| Stem cell markers | ||||
| CD34 | − | − | − | ND |
| CD117 | − | − | − | ND |
Numbers in parentheses indicate the proportions of cells that express respective antigens; ND indicates not determined.
The expression of 2B4 is not observed on freshly isolated NK cells. However, 2B4 is expressed on cultured NK cells.47
Thirty percent of the cells were positive in spleen.
NK1.1+ CD3− thymocytes in ZAP-70−/− mice produced IFN-γ upon stimulation with NK1.1 cross-linking in the presence of IL-2
To examine functions of the NK1.1+TCRαβ− cells in the thymus of ZAP-70−/−mice, we analyzed the ability to produce cytokines. It has been shown that NK1.1+ T cells produce large amounts of IL-4 and IFN-γ shortly after stimulation with CD3 cross-linking.13,48 49 When thymocytes or splenocytes obtained from either control or ZAP-70−/− mice were stimulated with immobilized anti-CD3ε mAb for 48 hours, these cells from control ZAP-70+/− mice produced considerable amounts of IL-4 and IFN-γ. By contrast, neither IL-4 nor IFN-γ was produced from thymocytes or splenocytes of ZAP-70−/− mice (data not shown). This finding indicates again that NK1.1+ T cells are absent in the thymus and spleen of ZAP-70−/−mice and that TCRγδ+ cells present in these populations are unable to quickly respond to the CD3 cross-linking.
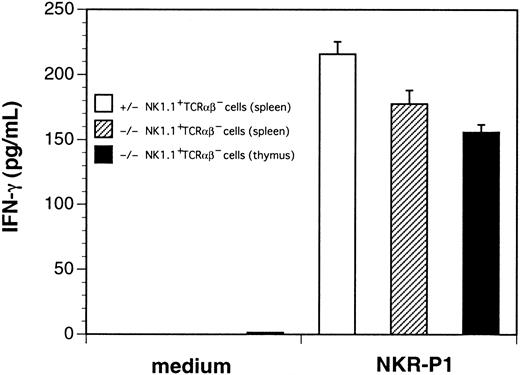
We then stimulated thymocytes from control or ZAP-70−/−mice with immobilized anti-NK1.1 mAb in the presence or absence of rhIL-2. Thymocytes from ZAP-70−/− mice produced substantial amounts of IFN-γ upon stimulation with NK1.1 cross-linking in the presence of rhIL-2 (data not shown). To quantify IFN-γ–producing ability of NK1.1+TCRαβ− cells, we sorted the NK1.1+TCRαβ− cells from thymi and spleens of ZAP-70−/− mice as well as from spleens of control mice. These cells were then stimulated with immobilized anti-NK1.1 mAb in the presence of rhIL-2 (Figure 6). The NK1.1+ TCRαβ− thymocytes and spleen cells of ZAP-70−/− mice produced considerable but slightly lower amounts of IFN-γ than that produced by the splenic NK cells of control mice.
Production of IFN-γ by thymocytes and splenocytes from control and ZAP-70−/− mice.
Sorted NK1.1+ TCRαβ− splenocytes from control mice (open bar), NK1.1+TCRαβ− splenocytes (shaded bar), and NK1.1+ TCRαβ− thymocytes (closed bar) from ZAP-70−/− mice were cultured in medium alone (medium) or with immobilized anti-NK1.1 mAb (NKR-P1) in the presence of rhIL-2 (1000 U/mL). Then, IFN-γ in the culture supernatants were quantified by enzyme-linked immunosorbent assay. Results are representative results of 3 separate experiments.
Production of IFN-γ by thymocytes and splenocytes from control and ZAP-70−/− mice.
Sorted NK1.1+ TCRαβ− splenocytes from control mice (open bar), NK1.1+TCRαβ− splenocytes (shaded bar), and NK1.1+ TCRαβ− thymocytes (closed bar) from ZAP-70−/− mice were cultured in medium alone (medium) or with immobilized anti-NK1.1 mAb (NKR-P1) in the presence of rhIL-2 (1000 U/mL). Then, IFN-γ in the culture supernatants were quantified by enzyme-linked immunosorbent assay. Results are representative results of 3 separate experiments.
NK1.1+ CD3− thymocytes in ZAP-70−/− mice showed an intact cytotoxic activity
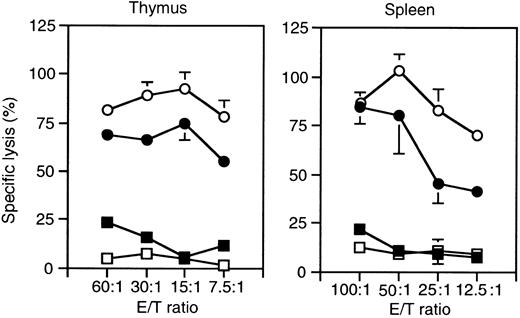
To examine cell-mediated functions of the NK1.1+TCRαβ− cells in ZAP-70−/− mice, cytotoxic activity was then analyzed. Thymocytes and splenocytes from either control or ZAP-70−/− mice that had been administered tilorone were cultured with rhIL-2 for 7 days.14 32 The harvested cells were then analyzed for the killing activity against 51Cr-labeled YAC-1 or P815 cells. As shown in Figure 7, both thymocytes and splenocytes from either control or ZAP-70−/− mice showed significant cytotoxicity against YAC-1 cells but not against P815 cells. The cytotoxicities seen in both thymocytes and spleen cells of ZAP-70−/− mice were consistently higher than those seen in control mice. This result was consistent with the increased proportions of NK1.1+ CD3− cells in the thymus and spleen of ZAP-70−/− mice as compared with those of control mice (Figure 1).
Cytotoxicity of thymocytes and splenocytes from control and ZAP-70−/− mice.
Thymocytes and splenocytes from control (●, ▪) and ZAP-70−/− (○, ■) mice administered tilorone 24 hours before collecting cells were cultured for 7 days in the presence of rhIL-2 (1000 U/mL). Cells were harvested and cultured with51Cr-labeled YAC-1 (●, ○) or P815 (▪, ■) cells at indicated effector:target ratios for 4 hours. Percent specific lysis was calculated as described in “Materials and methods.”
Cytotoxicity of thymocytes and splenocytes from control and ZAP-70−/− mice.
Thymocytes and splenocytes from control (●, ▪) and ZAP-70−/− (○, ■) mice administered tilorone 24 hours before collecting cells were cultured for 7 days in the presence of rhIL-2 (1000 U/mL). Cells were harvested and cultured with51Cr-labeled YAC-1 (●, ○) or P815 (▪, ■) cells at indicated effector:target ratios for 4 hours. Percent specific lysis was calculated as described in “Materials and methods.”
When the harvested cells from thymocyte cultures at day 7 were analyzed for NK1.1 and TCRαβ expressions with flow cytometry, almost 100% of cells were CD3− among NK1.1+ cells of ZAP-70−/− mice, whereas 40% and 60% of cells were CD3− and CD3+, respectively, among NK1.1+ cells of ZAP-70+/− mice, as reported previously.14 Similarly, when harvested spleen cells were analyzed, 100% of NK1.1+ cells were CD3− in ZAP-70−/− mice, whereas 70% and 30% of the NK1.1+ cells were CD3− and CD3+, respectively, in ZAP-70+/− mice (data not shown). These findings suggest that no proportional change of CD3− and CD3+ populations was induced during the culture with IL-2.
NK1.1+ TCRαβ− thymocytes had a germline configuration in TCR− β gene locus
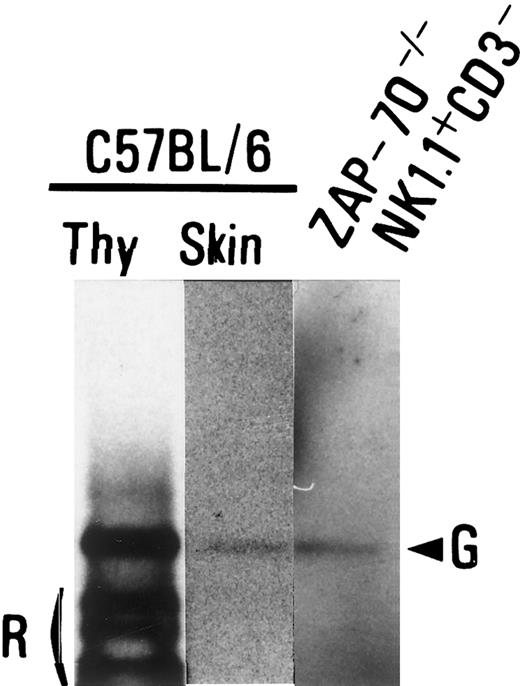
Flow cytometric analyses demonstrated that NK1.1+TCRαβ− cells expressed neither TCR nor CD3 molecules on the cell surface. To examine a configuration of TCR gene, the rearrangement of TCRβ gene in NK1.1+ CD3−cells obtained from the thymus of ZAP-70−/− mice and thymocytes and skin cells of C57BL/6 mice (control) was analyzed with a PCR-based technique. The rearrangement of Vβ8 to Dβ2Jβ2 was not detected in the TCRβ gene locus of the NK1.1+TCRαβ− cells from ZAP-70−/− mice (data not shown). In addition, we found no band being generated by Dβ2 to Jβ2 rearrangements in the NK1.1+ TCRαβ−cells of ZAP-70−/− mice (Figure8). Thus, it was demonstrated that the TCRβ gene locus of the NK1.1+ TCRαβ−cells from ZAP-70−/− mice was in a germline configuration.
Gene rearrangement on TCRβ loci in NK1.1+TCRαβ− thymocytes.
A PCR was performed with genomic DNA from B6 thymocytes (Thy; left lane), B6 ear skin (middle lane), or the NK1.1+CD3− thymocytes of ZAP-70−/− mice (right lane) with a primer pair of a coding region of Dβ2 (Dβ2-5′) and 3′-downstream region of Jβ2.7 (Jβ2-3′) to detect rearrangements of Dβ2 to Jβ2 cluster as described in “Materials and methods.” Germline bands (G) and a rearranged band (R) are indicated.
Gene rearrangement on TCRβ loci in NK1.1+TCRαβ− thymocytes.
A PCR was performed with genomic DNA from B6 thymocytes (Thy; left lane), B6 ear skin (middle lane), or the NK1.1+CD3− thymocytes of ZAP-70−/− mice (right lane) with a primer pair of a coding region of Dβ2 (Dβ2-5′) and 3′-downstream region of Jβ2.7 (Jβ2-3′) to detect rearrangements of Dβ2 to Jβ2 cluster as described in “Materials and methods.” Germline bands (G) and a rearranged band (R) are indicated.
Generation of NK1.1+ TCRαβ+ cells was induced from NK1.1+ TCRαβ− thymocytes of ZAP-70−/− mice in hanging-drop culture with PMA and ionomycin
To directly examine the developmental potential of NK1.1+ TCRαβ− thymocytes in ZAP-70−/− mice, a devised induction assay of NK1.1+ TCRαβ+ cells was performed. The NK1.1+ TCRαβ− cells were sorted from the thymocytes of ZAP-70−/− mice (Figure9A, left panel) and cultured with PMA and ionomycin in dGuo-treated neonatal thymi from BALB/c mice in a hanging-drop setup. As shown in Figure 9A (middle and right panels), a substantial proportion (3.03%) of NK1.1+TCRαβ+ cells was detected after 5 days of culture. BALB/c mice express no NK1.1 antigen. Thus, rearrangement of TCR genes appeared to be induced in the NK1.1+TCRαβ− population of ZAP-70−/− mice by an addition of PMA plus ionomycin. When NK1.1+TCRαβ− cells of ZAP-70−/− mice were cultured with thymi of RAG−/− mice in the presence of PMA plus ionomycin, small but substantial numbers of NK1.1+TCRαβ+ cells were induced (data not shown). No NK1.1− TCRαβ+ cells were detected in this setup. In addition, NK1.1+ TCRαβ+cells induced in the thymic organ culture of (B6 x B6.Thy1.1)F1 mice were Thy1.1− (data not shown). These findings again indicated that the induced NK1.1+ TCRαβ+ cells were derived from NK1.1+ TCRαβ− cells of ZAP-70−/− mice, and large NK1.1−TCRαβ+ populations seen in Figure 9A (middle and right panels) appeared to be derived from the dGuo-treated thymi of BALB/c mice.
Generation of NK1.1+ TCRαβ+cells from NK1.1+ CD3− thymocytes of ZAP-70−/− mice in neonatal thymic organ culture.
(A) A representative flow cytometric profile of inductive generation of the NK1.1+ TCRαβ+ cells in the dGuo-treated thymi in the presence of PMA plus ionomycin. The sorted NK1.1+ TCRαβ− cells for the culture were demonstrated in the square of the left panel. Proportions of sorted NK1.1+ TCRαβ− cells were 98.8% to 99.5%. Collected cells from cultures were stained with either PE-mouse IgG2a (isotype control of PK136)/FITC–anti-TCRαβ/propidium iodide (middle panel) or PE–anti-NK1.1/FITC–anti-TCRαβ/propidium iodide (right panel) and analyzed with FACScan. The proportions of NK1.1+ TCRαβ+ cells are indicated. (B) Mean proportion of NK1.1+ TCRαβ+ cells. The NK1.1+ CD3− (about 5 × 103/lobe to 8 × 103/lobe) were seeded to the dGuo-treated neonatal thymic lobes and cultured in hanging-drop setup in the absence (left column) or presence (right column) of PMA and ionomycin. Five days later, total cells were stained as described for panel A. The net proportion of induced NK1.1+ TCRαβ+ cells were calculated as follows: [percentage of NK1.1+TCRαβ+ cells minus percentage of control IgG2a+ TCRαβ+ cells]. The data indicate mean net proportion and SD.
Generation of NK1.1+ TCRαβ+cells from NK1.1+ CD3− thymocytes of ZAP-70−/− mice in neonatal thymic organ culture.
(A) A representative flow cytometric profile of inductive generation of the NK1.1+ TCRαβ+ cells in the dGuo-treated thymi in the presence of PMA plus ionomycin. The sorted NK1.1+ TCRαβ− cells for the culture were demonstrated in the square of the left panel. Proportions of sorted NK1.1+ TCRαβ− cells were 98.8% to 99.5%. Collected cells from cultures were stained with either PE-mouse IgG2a (isotype control of PK136)/FITC–anti-TCRαβ/propidium iodide (middle panel) or PE–anti-NK1.1/FITC–anti-TCRαβ/propidium iodide (right panel) and analyzed with FACScan. The proportions of NK1.1+ TCRαβ+ cells are indicated. (B) Mean proportion of NK1.1+ TCRαβ+ cells. The NK1.1+ CD3− (about 5 × 103/lobe to 8 × 103/lobe) were seeded to the dGuo-treated neonatal thymic lobes and cultured in hanging-drop setup in the absence (left column) or presence (right column) of PMA and ionomycin. Five days later, total cells were stained as described for panel A. The net proportion of induced NK1.1+ TCRαβ+ cells were calculated as follows: [percentage of NK1.1+TCRαβ+ cells minus percentage of control IgG2a+ TCRαβ+ cells]. The data indicate mean net proportion and SD.
Figure 9B summarizes 4 separate experiments with BALB/c thymi. Significant generation of NK1.1+ TCRαβ+cells was induced in the presence of PMA plus ionomycin as compared with those cultured in the absence of PMA plus ionomycin (P < .05). A small population of NK1.1+TCRαβ+ cells seen in the cultures without PMA plus ionomycin could not be explained.
When NK1.1− CD3− thymocytes from ZAP-70−/− mice were cultured in the hanging-drop setup, substantial proportions of both NK1.1+TCRαβ+ and NK1.1+ TCRαβ−cells were detected (data not shown). This finding suggests that the cells in the NK1.1− TCRαβ− population differentiate to NK1.1+ TCRαβ+ cells through NK1.1+ TCRαβ− stage.
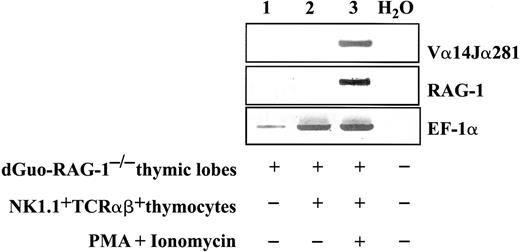
Invariant Vα14Jα281 and RAG-1 transcripts were detected in the induced NK1.1+ TCRαβ+ cells
We then analyzed TCRVα usage in NK1.1+TCRαβ+ cells that had been generated from NK1.1+ TCRαβ− cells of ZAP-70−/− mice following culture with the neonatal thymi in the presence of PMA plus ionomycin. In this experiment the neonatal thymi were obtained from RAG-1−/− mice of B6 background to exclude a possibility that Vα14Jα281 transcripts were derived from the thymi. Reverse transcriptase (RT)-PCR was then performed using total RNA extracted from the cultured NK1.1+TCRαβ− cells. As shown in Figure10 (lane 3), the Vα14Jα281 transcripts were detected in the thymic lobes in which sorted NK1.1+ TCRαβ− cells were cultured with PMA plus ionomycin. The Vα14Jα281 transcripts were detected neither in the culture of thymic rudiments alone nor in the NK1.1+TCRαβ− thymocytes cultured without PMA plus ionomycin (Figure 10, lanes 1,2). Thus, the NK1.1+TCRαβ+ cells induced from the NK1.1+TCRαβ− population by PMA plus ionomycin expressed Vα14Jα281 transcripts. When concomitant expressions of RAG-1 were examined in the organ cultures, RAG-1 expression was clearly detected in the culture where the band of invariant Vα was amplified (Figure10, lane 3). A very faint band was detected in the culture without PMA plus ionomycin (lane 2).
Detection of Vα14Jα281 transcripts with RT-PCR in inductive cultures.
Total RNA was extracted from the thymic lobes (RAG-1−/−in C57BL/6 background) alone (lane 1) or lobes with sorted NK1.1+ TCRαβ− cells in the presence (lane 3) or absence (lane 2) of PMA plus ionomycin, and RT-PCR was performed as described in “Materials and methods” with primer pairs Vα14 Leader/Cα-rev1 and Vα14/Jα281, RAG-1 5′/3′ and RAG-1 5′ nest/3′ nest, or EF-1α 5′/EF-1α 3′ for positive control. RT-PCR was also performed without RNA (lane 4) as control. Results are representative of 3 separate experiments.
Detection of Vα14Jα281 transcripts with RT-PCR in inductive cultures.
Total RNA was extracted from the thymic lobes (RAG-1−/−in C57BL/6 background) alone (lane 1) or lobes with sorted NK1.1+ TCRαβ− cells in the presence (lane 3) or absence (lane 2) of PMA plus ionomycin, and RT-PCR was performed as described in “Materials and methods” with primer pairs Vα14 Leader/Cα-rev1 and Vα14/Jα281, RAG-1 5′/3′ and RAG-1 5′ nest/3′ nest, or EF-1α 5′/EF-1α 3′ for positive control. RT-PCR was also performed without RNA (lane 4) as control. Results are representative of 3 separate experiments.
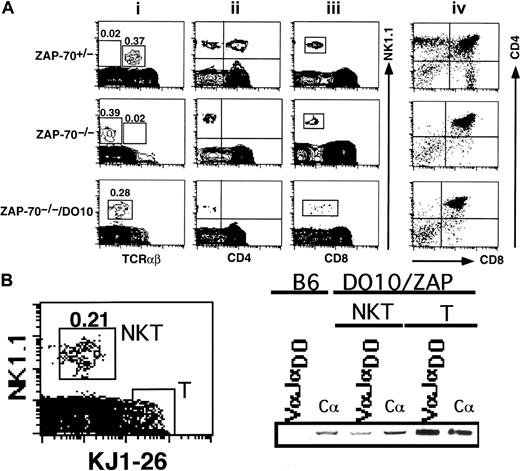
NK1.1+ TCRdim+ thymocytes were generated in ZAP-70−/−/DO10 TCR transgenic mice
The present results suggested that ZAP-70 deficiency was directly associated with lack of rearrangement of TCR genes in the NK1.1+ TCRαβ− population. We then asked whether an introduction of rearranged TCRα and β genes induced generation of a particular NK1.1+ TCRαβ+population in the ZAP-70−/− background. To examine this possibility, we crossed ZAP-70−/− mice with DO10 TCR transgenic mice and analyzed the generation of NK1.1+TCRαβ+ cells in the thymus of ZAP-70−/−/DO10 mice. As seen in Figure11A, the NK1.1+ thymocytes of ZAP-70−/−/DO10 mouse expressed a substantial level of TCRαβ molecules compared with those in ZAP-70−/−thymus. The level of TCRαβ expression, however, was low compared with that of an NK1.1+ TCRαβ+ population in ZAP-70+/− mice. When these thymocytes were stained with a clonotypic antibody, KJ1-26, it was shown that an NK1.1+KJ1-26dim population was present in the thymus (Figure11B). The total RNA was then extracted from either the sorted NK1.1+ KJ1-26dim or NK1.1−KJ1-26+ cells, reverse-transcribed, and PCR-amplified with specific primers for VαDO and JαDO. Figure11B shows that VαDOJαDO messages are present in both NKT and T-cell populations. Thus, it seemed that the lack of ZAP-70 showed no significant influence on the expression of rearranged TCR gene in the NK1.1+ CD3−population. The NK1.1+ TCRαβdim population expressed no CD4 but broader ranges of CD8 molecules (Figure 11A). Notably, Figure 11Aiv shows that development of the ordinary thymocyte was still arrested at the DP stage irrespective of the introduction of TCRα and β transgenes in ZAP-70−/−/DO10 mouse.
Detection of NK1.1+ TCRαβdim thymocytes in ZAP-70−/−/DO10 TCR transgenic mouse.
(A) Thymocytes obtained from ZAP-70+/−, ZAP-70−/−, or ZAP- 70−/−/DO10 mice were stained with PE–anti-NK1.1/FITC–anti-TCRαβ (i), PE–anti-NK1.1/FITC–anti-CD4 (ii), PE–anti-NK1.1/FITC–anti-CD8 (iii), and PE–anti-CD4/FITC–anti-CD8 (iv), and analyzed. Proportions of NK1.1+ TCRαβ+, NK1.1+TCRαβ− (upper and middle panels), or NK1.1+ TCRαβdim populations (lower panel) are indicated in the leftmost panels on the top of each squared region. Results are representative of 3 separate experiments. (B) Flow cytometric analysis and RT-PCR detection of transgenic TCR in NK1.1+ TCRαβdim thymocytes in DO10/ZAP-70−/− mice. Thymocytes of DO10/ZAP-70−/− mice were stained with PE–anti-NK1.1 antibody and biotinylated KJ1-26 followed by streptavidin-FITC. Dead cells were electronically gated out with propidium iodide staining. The NK1.1+ TCRαβdim thymocytes (NKT) and NK1.1− TCRαβ+ thymocytes (T) (demarcated with squares in the left panel) were sorted, and the expressions of DO10-specific TCRα chain (VαJαDO) and Cα were examined with RT-PCR.
Detection of NK1.1+ TCRαβdim thymocytes in ZAP-70−/−/DO10 TCR transgenic mouse.
(A) Thymocytes obtained from ZAP-70+/−, ZAP-70−/−, or ZAP- 70−/−/DO10 mice were stained with PE–anti-NK1.1/FITC–anti-TCRαβ (i), PE–anti-NK1.1/FITC–anti-CD4 (ii), PE–anti-NK1.1/FITC–anti-CD8 (iii), and PE–anti-CD4/FITC–anti-CD8 (iv), and analyzed. Proportions of NK1.1+ TCRαβ+, NK1.1+TCRαβ− (upper and middle panels), or NK1.1+ TCRαβdim populations (lower panel) are indicated in the leftmost panels on the top of each squared region. Results are representative of 3 separate experiments. (B) Flow cytometric analysis and RT-PCR detection of transgenic TCR in NK1.1+ TCRαβdim thymocytes in DO10/ZAP-70−/− mice. Thymocytes of DO10/ZAP-70−/− mice were stained with PE–anti-NK1.1 antibody and biotinylated KJ1-26 followed by streptavidin-FITC. Dead cells were electronically gated out with propidium iodide staining. The NK1.1+ TCRαβdim thymocytes (NKT) and NK1.1− TCRαβ+ thymocytes (T) (demarcated with squares in the left panel) were sorted, and the expressions of DO10-specific TCRα chain (VαJαDO) and Cα were examined with RT-PCR.
Discussion
It has been shown that ZAP-70 tyrosine kinase is essential for development of mainstream T cells but not for NK cells.32Targeted disruption of ZAP-70 led to complete blockade of the development of mature-type T cells both in the thymus and in the periphery. By contrast, the development of ordinary NK cells was intact in the ZAP-70−/− mice, although ZAP-70 was also expressed in the NK cells.50 Thus far, the lineage relationship among T, NK, and NK1.1+ T cells has been unclear.
In the present study, we examined development of the NK1.1+T cells in the ZAP-70−/− mice and found an arrested development of NK1.1+ CD3+ cells in both thymus and spleen. Neither NK1.1+ TCRγδ+ nor NK1.1+ TCRαβ+ cells were detected in the thymus and spleen. The absence of the NK1.1+TCRγδ+ population was not due to the defective development of the TCRγδ lineage T cells per se, because significantly large populations of NK1.1−TCRγδ+ cells were detected in the thymus and spleen of ZAP-70−/− mice as compared with those in control mice. It was reported that the NK1.1+ TCRγδ+ cell population was expanded in CD3ζ−/− mice.27Thus, the gene disruption of ζ chain and ZAP-70 led to different developmental defects in NK1.1+ TCRγδ+cells, even though ZAP-70 is located downstream of ζ chain. This difference should be pursued in further studies.
Interestingly, we found markedly increased numbers of NK1.1+ TCRαβ− cells in the thymus of ZAP-70−/− mice. The surface phenotype of the NK1.1+ TCRαβ− cells summarized in Table 1suggests that the NK1.1+ TCRαβ− cells belong to a unique sobpopulation different from ordinary NKT or NK cells. Approximately 25% to 35% of splenic NK cells express either Ly49A or Ly49C in the H-2b background.46However, the NK1.1+ TCRαβ− thymocytes of ZAP-70−/− mice (H-2b background) express neither Ly49A nor Ly49C. The expression of Ly49 on NK cells is developmentally regulated in a nonrandom manner.46 Thus, the present findings suggest that the NK1.1+TCRαβ− thymocytes are of distinct cell type or may belong to a precursor population of the NK1.1+ T cells. Indeed, we could show that the latter might be the case. The NK1.1+ TCRαβ− thymocytes stimulated by an addition of PMA plus ionomycin in the thymic organ culture developed into NK1.1+ TCRαβ+ cells.
Interestingly, the NK1.1+ CD3− thymocytes of B6-RAG-1−/− showed almost superimposable phenotypes to those of ZAP-70−/− mice. Thus, developmental defects that lead to TCR gene rearrangement may generate accumulation of cells of the same type. However, the TCR gene disruption on α or β locus exerted somehow differential influences on the development of NK1.1+ T cells. In the TCRα−/− mice, an accumulation of NK1.1+CD3− thymocytes was observed (data not shown). On the other hand, NK1.1+TCRγδ+ but not NK1.1+ CD3−thymocytes were detected in TCRβ−/− mice51(data not shown). Thus, it seems important to elucidate genes and signals vital on certain stages of the development of NK1.1+ T cells in further studies.
In normal B6 mice, a small population of NK1.1+CD3− thymocytes were detectable, and it was demonstrated that these NK1.1+D3− cells were phenotypically ordinary NK cells (Figure 5). This finding, however, does not exclude a possibility that some of these NK1.1+ thymocytes may correspond to a putative precursor population seen in ZAP-70−/− mice.
When the NK1.1+ TCRαβ− cells were analyzed in the spleen of ZAP-70−/− mice, the number of the NK1.1+ TCRαβ− cells also increased as compared with that of control mice. We reasoned that the putative precursor population for the NK1.1+ T cells that could not be readily distinguished from ordinary NK cells might also be present and both the precursor and ordinary NK cell populations were recognized with the increasing number of NK1.1+TCRαβ− cells in the spleen of ZAP-70−/−mice. Although expressions of CD45R, CD62L, and 2B4 molecules were relative characteristics of NK1.1+ TCRαβ−thymocytes in ZAP-70−/− mice (Figure 5), these molecules could be induced on the splenic NK cells upon activation. Thus far, no appropriate markers that can definitely distinguish NK cells and the precursor cells in the spleen are available. The precise populations that make up the NK1.1+ TCRαβ− cells in the spleen of ZAP-70−/− mice should be examined in further studies to clarify whether the NK1.1+TCRαβ− population in the spleen indeed contains the precursors of NK1.1+ T cells.
We demonstrated that substantial natural cytotoxicity and IFN-γ production upon stimulation via NKR-P1 molecules were demonstrated in the thymic NK1.1+ TCRαβ− cells of ZAP-70−/− mice. It was shown that a putative precursor population of NK cells in bone marrow expressed CD45R and NK1.1 molecules and exhibited cytotoxicity against YAC-1 target cells.52 Eberl and MacDonald53 reported that the same population in bone marrow with surface markers similar to NK1.1+ TCRαβ− thymocytes in ZAP-70−/− mice contained precursors for NK1.1+ T cells. Thus, it seems to us that the putative precursor population (NK1.1+ TCRαβ− cells) for either NK or NK1.1+ T cells first acquires NK functions.
The NK1.1+ TCRαβ− thymocytes in ZAP-70−/− mice showed a germline configuration in TCRβ gene. After 5 days of culture with PMA plus ionomycin, the NK1.1+ TCRαβ+ cells were detected and the canonical Vα14Jα281 transcripts were clearly demonstrated. Sato et al25 demonstrated that a pre-NKT cell population expressing NK1.1, TCRβ, pre-Tα, and RAG1/2 could differentiate into mature CD3+ Vα14+ NKT cells in the presence of IL-15, GM-CSF, and bone marrow–derived stromas. Because the TCRβ genes were not rearranged in the NK1.1+TCRαβ− thymocytes of ZAP-70−/− mice, these cells may be antecedents for the pre-NKT. However, these results appeared to be in discordance with the stepwise model proposed by DiSanto and Rodewald26 for the development of NK1.1+ T cells. Using γ chain−/− mice, these authors demonstrated that the NK1.1+ T cells differentiated from T cells that expressed Vα14 and Vβ8 chains. These precursor cells possessed characteristic profiles in cytokine productions but expressed no NK1.1 or Ly49 antigens. The difference may be attributable to the mice studied but should be elucidated in further investigation.
NK1.1+ TCRαβ− cells seen in ZAP-70−/− mice lacked both CD34 and CD117 expressions. It was reported that fetal thymic NK1.1+ CD117+and NK1.1− CD117+ cells at gestational day 15 were capable of generating mainstream T cells, NK1.1+ T cells, and NK cells, whereas NK1.1+ CD117−cells remained CD4− CD8− and committed exclusively to NK cells.54 Although the NK1.1+TCRαβ− cells in the thymus of ZAP-70−/−mice or pre-NKT cells reported by Sato et al25 resemble the NK1.1+ CD117− cells in the fetal thymus, the NK1.1+ TCRαβ− cells and pre-NKT cells could differentiate into the NK1.1+ T cells in the presence of PMA plus ionomycin or IL-15 plus GM-CSF in conjunction with stromal cells from either thymus or bone marrow, respectively.
The TCRβ gene loci of NK1.1+ TCRαβ−cells in ZAP-70−/− mice were in germline configuration. Because the disruption of ZAP-70 kinase led to no defect in TCR gene rearrangement in mainstream T cells,32 33 the mechanism underlying the lack of TCR expression on the NK1.1+TCRαβ− cells was unclear. In the last experiment, we showed that introduction of rearranged TCR genes to ZAP-70−/− mice resulted in generation of an NK1.1+ TCRαβdim population. Thus, disruption of ZAP-70−/− might influence on the TCRαβ rearrangement but not on the expression of the rearranged TCR genes. Perhaps the TCR rearrangement in NK1.1+ T-lineage cells is more ZAP-70–dependent than that in mainstream T cells, which cannot be compensated by Syk kinases.
We show herein a unique NK1.1+ CD3− cell population in the thymus of ZAP-70−/− mice, and this population may contain a precursor for NK1.1+ T cells and possesses intact NK cell functions. It seems that NK1.1+ T cells and NK cells share a critical pathway for their differentiation. Detailed single-cell–based analyses of the generation of NK1.1+ T cells from NK1.1+TCRαβ− thymocytes but not from minor contaminants in the dGuo-treated thymic lobes with PMA plus ionomycin are undertaken in our laboratory.
We wish to thank Dr D. Y. Loh for providing us with the ZAP-70−/− mouse, Drs T. Matsushita and M. Hosokawa for reagents, and the Pharmaceutical Research Division of Takeda Chemical Industries for IL-2. We also thank Ms Ryoko Hosohata and Ms Kaori Kohno for their secretarial assistance with the manuscript.
Supported by a Grant-in-Aid for Scientific Research (B, C) from the Ministry of Education, Science, Sports and Culture, Japan; a Research Grant for Immunology, Allergy and Organ Transplant and a Research Grant for Aging from the Ministry of Health and Welfare, Japan; Grants from the Hokkaido Foundation for the Promotion of Scientific and Industrial Technology; the Tomakomai East Hospital Foundation; the Nishimura Aging Fund; and the Itoh Foundation for the Promotion of Medical Sciences.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Kazunori Onoé, Division of Immunobiolgy, Institute for Genetic Medicine, Hokkaido University, Kita-15 Nishi-7, Kita-Ku, Sapporo 060-0815, Japan; e-mail: kazunori@imm.hokudai.ac.jp.

![Fig. 9. Generation of NK1.1+ TCRαβ+cells from NK1.1+ CD3− thymocytes of ZAP-70−/− mice in neonatal thymic organ culture. / (A) A representative flow cytometric profile of inductive generation of the NK1.1+ TCRαβ+ cells in the dGuo-treated thymi in the presence of PMA plus ionomycin. The sorted NK1.1+ TCRαβ− cells for the culture were demonstrated in the square of the left panel. Proportions of sorted NK1.1+ TCRαβ− cells were 98.8% to 99.5%. Collected cells from cultures were stained with either PE-mouse IgG2a (isotype control of PK136)/FITC–anti-TCRαβ/propidium iodide (middle panel) or PE–anti-NK1.1/FITC–anti-TCRαβ/propidium iodide (right panel) and analyzed with FACScan. The proportions of NK1.1+ TCRαβ+ cells are indicated. (B) Mean proportion of NK1.1+ TCRαβ+ cells. The NK1.1+ CD3− (about 5 × 103/lobe to 8 × 103/lobe) were seeded to the dGuo-treated neonatal thymic lobes and cultured in hanging-drop setup in the absence (left column) or presence (right column) of PMA and ionomycin. Five days later, total cells were stained as described for panel A. The net proportion of induced NK1.1+ TCRαβ+ cells were calculated as follows: [percentage of NK1.1+TCRαβ+ cells minus percentage of control IgG2a+ TCRαβ+ cells]. The data indicate mean net proportion and SD.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/97/6/10.1182_blood.v97.6.1765/6/m_h80610783009.jpeg?Expires=1769129756&Signature=r0KgnND5LYNwgRDs2sJ0LXS1GlH~dddb1ofY2Jw8jd8rzIS2kRI6wgiRS0iNvKKmfDAu0viazTKtJ9c15zPQMmDejDu2x1mRmobqULfZYTR7IkOl6ZBEFdhPXnDKvfIHJEIYO0tPy9jJNkwrqksBSnY2eKdUit0SqJTzIPFk4m5mo9Tu4imBWz5CyarrySlCjflF6x3htPOvJi2O2TJhlg5zQQkJsV6ZJzZu6Yk-fwuQjtovA0ovSOAuns~IIrx1bjFs3Od4i6wdm6WPBjCc3gI4Gd5p5DhB7UXUeAjeKLdeDia~Y6DUPlUf71fylQgGt-6etF-B-bf2g0OF-hERFg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal