Human immunodeficiency virus (HIV)-infection is characterized by loss of CD4+ T cells associated with high levels of immune activation, T-cell proliferation, and lymphocyte apoptosis. To investigate the role of intrinsic perturbations of cell-cycle control in the immunopathogenesis of acquired immunodeficiency syndrome (AIDS), we studied the expression of cell-cycle–dependent proteins in lymphocytes from HIV-infected patients. Cyclin B1 expression, Nucleolar Organizer Regions (NORs) number, and NORs area of distribution were all consistently increased in HIV-infected patients, but returned to normal after effective antiretroviral therapy, suggesting that viral replication is directly implicated in the genesis of the observed changes. Analysis of cyclin B1 intracellular turnover showed that the increased cyclin B1 expression is (1) caused by defective degradation in the presence of normal rates of synthesis, and (2) is temporally associated with decreased levels of ubiquitination. After in vitro activation of lymphocytes from healthy individuals, cyclin B1 and cdc25 expression and ubiquitination, p34 cdc2 activity, NORs morphology, and C23/nucleolin localization showed a 72- to 96-hour cyclic pattern that led to a biologic state similar to baseline. On the contrary, complex but consistent changes of the same indices followed activation of T lymphocytes from HIV-infected patients, resulting in a 5-fold increase in apoptosis. Overall, our data indicate that a profound dysregulation of cell-cycle control is present in lymphocytes from HIV-infected patients. This finding may provide a novel biologic link between immune activation, accelerated lymphocyte turnover, and increased apoptosis during HIV infection.
Introduction
Human immunodeficiency virus (HIV)-infection induces a progressive depletion of CD4+ T lymphocytes and high susceptibility to opportunistic infections.1 The mechanisms of HIV-induced CD4+ T-cell loss include the virus-mediated killing of infected cells as well as the death of uninfected bystander cells.1,2 Among the indirect mechanisms that have been implicated in the lymphocyte depletion of patients with acquired immunodeficiency syndrome (AIDS), increased level of apoptosis may be significant. In HIV-infected patients, increased susceptibility to apoptotic cell death has been shown in CD4+ and CD8+ T lymphocytes and appears to be correlated with the general state of immune activation.3-6Interestingly, both increased propensity to apoptosis and overall levels of immune activation are reversed by the institution of effective anti-HIV therapy.7,8 It is therefore likely that an abnormal relationship between T-cell activation/proliferation and occurrence of apoptosis may play a significant role in the lymphocyte depletion in the HIV-infected patient.9 10
Lymphocyte activation in response to extrinsic signals ultimately results in either progression through the cell cycle, or activation of proapoptotic pathway(s).11,12 Although influenced by many factors, the fate of activated T cells is eventually determined by intracellular concentration, and/or subcellular localization of cyclins, a family of regulatory molecules that activate specific cyclin-dependent kinases (CDKs) and regulate the progression through cell cycle.13,14 Cyclin B1 is minimally expressed in resting cells (G0) but on mitogenic activation, progressively accumulates, reaching a peak during late G1 and S phases. When the intracellular concentration of cyclin B1 reaches a critical threshold, binding and activation of the p34 cdc2 kinase occurs, with consequent phosphorylation of target proteins.13,14 The p34 cdc2 is concurrently activated via dephosphorylation by cdc25, a cell-cycle–dependent phosphatase,15 whose intracellular concentration is regulated via ubiquitination.16 The complex of cyclin B1, cdc25, and activated p34 cdc2 is called mitosis promoting factor (MPF), maturation promoting factor, or anaphase promoting complex, and its proper function is a key step required to successfully complete the mitotic division.11-14 After initiation of mitosis, cyclin B1 is no longer required, and its down-regulation is completed by the time the cell enters anaphase.13,14 Degradation of both cyclin B1 and cdc25 is dependent on the ubiquitin-proteasome pathway and results in the complete inactivation of p34 cdc2 kinase, which in turn allows the cell to reenter the G0 phase.16-20 Cyclin B1 expression and p34 cdc2 activation at inappropriate times are associated with serious perturbations of the cell cycle and increased frequency of apoptosis.21 22
In a previous study,23 we have shown that cyclin B1 expression is increased in lymphocytes from HIV-infected patients, resulting in inappropriate p34 cdc2 activation. Interestingly, both anomalies are reverted when patients are treated with antiretroviral therapy (ART). The possibility that cells overexpress cyclin B1 simply because they are committed to entering G1 is not consistent with a metabolic and biochemical profile (proline uptake, ornitine decarboxylase [ODC] activity, interleukin-2 recepton [IL-2R] expression, and protein synthesis) typical of a G0 state.23 This finding is in agreement with studies showing that, during HIV infection, only a small portion of lymphocytes is undergoing active proliferation,24 25 and suggests that the dysregulation of cyclin B1 expression and p34 cdc2 activation during HIV infection is a more complex phenomenon.
Nucleolar organizer regions (NORs) are chromosomal regions in which ribosomal genes are encoded. The structural component of NORs is mainly constituted by 2 multifunctional proteins: the C23 or nucleolin, 110 kd,26,27 and the B23 or nucleophosmin, 39 kd.28-30 NOR-associated proteins, whose abundance,31-33 turnover,34-40 and localization41-44 are closely linked to ribosomal gene activity, are usually called “AgNOR” because of their argyrophilic property. Their pattern of expression has been studied in normal and neoplastic tissues,45,46 with the aim of assessing cell kinetics parameters such as the percentage of cycling cells and time of cell replication. Cells in G0 are characterized by diploid DNA content and the presence of a single AgNOR dot.47 In a diploid cell, a 2-fold increase in total amount of AgNOR proteins signals the transition from G0 to G1.31-33,47 During further progression in the cell cycle, more complex modifications in the number, localization, and quaternary structure of AgNOR proteins take place. These modifications are assessed by computerized image analysis of silver stained nuclei, which show patterns of nucleolar AgNOR segregation and aggregation that characterize each phase of the cell cycle.47
Here we report results of a further analysis of the cell-cycle dysregulation, which occurs during HIV infection, focusing on (1) intracellular turnover of cyclin B1, p34 cdc2, and cdc25, (2) AgNOR number and area of distribution, and (3) expression and cellular localization of C23/nucleolin.
Patients, materials, and methods
Patient population
Twenty HIV-infected patients were included in this study. Thirteen patients underwent antiretroviral therapy comprising 2 reverse transcriptase (RT) inhibitors and one protease inhibitor. Twenty uninfected individuals were used as controls. After obtaining informed consent, blood samples were collected, and HIV viremia measured by branched DNA (bDNA) technique (Quantiplex, Chiron, Emeryville, CA).
Lymphocytes
Immunologic phenotyping was performed on FACScaliber (Becton Dickinson, San Jose, CA) after direct staining with the following monoclonal antibodies: antihuman CD4-FITC, antihuman CD8-PE (Becton Dickinson). For the in vitro activation studies, peripheral blood lymphocytes (PBLs) were cultured in 10% fetal calf serum (FCS) RPMI at initial density of 106 cells per milliliter. Concanavalin A (ConA) was added (5 ng/mL), and cells were monitored for ODC activity, proline uptake, IL-2 production, and activity of cell machinery for protein and DNA synthesis (data not shown). All cell-cycle–related metabolic parameters were measured as previously detailed.23 49
Cell ploidy and apoptosis
Cells were stained with propidium iodide (PI) and flow cytometry was used to determine DNA content, lymphocyte distribution in the cell cycle, and percentage of apoptotic cells.49Apoptotic cells were also identified by immunohistochemical staining (Apop Tag Kit, Oncor, Gaithersburg, MD), and the apoptotic fraction was quantified by image analysis (data not shown).
AgNOR staining
AgNOR staining was performed as previously described.50 Briefly, lymphocytes were washed with phosphate-buffered saline (PBS), suspended in 95% alcohol, and transferred to a cover glass. After alcohol evaporation, cover glasses were stained (2 parts 50% AgNO3 aqueous solution and 1 part 2% gelatin in 1% formic acid) for 12 minutes in the dark. AgNORs appeared as black intranuclear dots, and their number per cell was evaluated in at least 500 cells. AgNOR area per cell was measured by using Image-Pro Plus software (Media Cybernetics, Silver Spring, MD). After definition of the gray threshold corresponding to the AgNOR alone, the AgNOR area was measured automatically.
Cyclin B1 expression
Cyclin B1 expression was measured by Western blot (mAbs from Santa Cruz Biotechnology Inc, Santa Cruz, CA), and the bands analyzed with SigmaGel (Handel Scientific Co, San Rafael, CA). The numerical values, on a scale from 0 to 250, indicate the absolute area of the band, ie, the total calibrated pixel intensity values of each band. Two to 5 replicates were performed for each sample. In all measurements of cyclin B1 concentration, internal controls were always performed, including lysing equal cell numbers, loading into each lane equal volumes of equal protein concentrations (15 μg per lane), and after the electrophoresis by performing Coomassie staining. If different protein concentrations were observed at this time, the whole procedure was repeated, and the initial loading protein concentration was adjusted, based on the actin band.
Cyclin B1 synthesis and degradation
Cyclin B1 synthesis was measured as follows: ConA-activated lymphocytes were labeled for 4 hours with35S-methionine in RPMI (1.85 MBq/mL [50 μCi/mL]), followed by incubation with excess of methionine. The rate of cyclin B1 synthesis was calculated as radioactive content associated with the cyclin B1 band immediately after the pulse phase, whereas cyclic B1 degradation was calculated as percentage of radioactivity associated with the band after 20-, 40-, and 90-minute chase. Electrophoresis and autoradiography of 35S methionine–labeled proteins were performed as follows: Each lane received the same number of cells, and in parallel, we measured the rate of general protein synthesis by [3H] leucine incorporation.
Protein ubiquitination
Cells were treated with SDS in the presence of protease inhibitors as previously described.51,52 After removal of cell debris, protein was quantified by the Lowry method53and SDS-PAGE carried out by using a minigel apparatus (Bio-Rad, Hercules, CA).54 Samples were boiled for 5 minutes in buffer containing 2% mercaptoethanol. Coomassie blue R-250 (Sigma) was the stain used. Molecular mass standards used were 200, 116, 97, 66, 45, 31, and 21 kd from Bio-Rad. Gels were electroblotted,55 blots were incubated with rabbit antiubiquitin antibody (Sigma; 1:200) and then goat antirabbit IgG-HRP (1:3000, Bio-Rad). Enhanced chemiluminescence was the detection system (Amersham, Uppsala, Sweden). Each lane received the protein content of 1.5 × 105 cells. For each sample, quantification of conjugated ubiquitin pools was determined in parallel by a solid-phase immunochemical method.56 Samples and ubiquitin-conjugate standards were dot-blotted onto a polyvinylidenefluoride (PVDF) membrane and probed sequentially with antiubiquitin antibody and goat antirabbit IgG-HRP conjugate. After autoradiography, films were quantified by densitometry by using SigmaGel. The numerical values indicate the absolute area of the band, ie, the total calibrated pixel intensity values of each band. Finally, in the immunoblots for cyclin B1 expression the identification of bands of protein ubiquitination was performed after removal of the anticyclin B1 mAb with stripping buffer.51 52 In these experiments, ubiquitin-reactive bands were then analyzed in the region corresponding to the already detected cyclin B and to the expected molecular weight(s) of ubiquitinated cyclin B. After autoradiography, films were quantified by densitometry as described previously.
Confocal microscopy
Peripheral blood mononuclear cells were fixed on slides (Labtech, Campbell, CA) by using 4% paraformaldehyde (PFA), 15 minutes. Cells were then permeabilized with 0.5% Triton X-100 and washed with PBS. Unconjugated mouse anti-C23 antibody (Santa Cruz Biotechnology sc-8031) was added (1/100, 45 minutes at 37°C). After washes, FITC-conjugated GAM-Ig (Sigma, St Louis, MO) was added (1/200 dilution). After washes, propidium iodine (PI) was added at 5 μg/mL plus RNase at 200 μg/mL, 30 minutes. After washes, Moviol was finally added and slides were covered by a coverslip. Confocal microscopy was from Leika (Wetzlar, Germany), with a ×63-zoom 1.6 objective.
Statistical analysis
A 2-tailed, 2-sample Student t test was used to calculate the P value for differences in means of AgNOR number, AgNOR area of distribution, and cyclin B expression between HIV-infected patients and uninfected controls. To evaluate the correlation between lymphocyte AgNOR number and cyclin B1 expression in the same group(s) of patients, a linear regression analysis was performed.
Results
Cell-cycle distribution of lymphocytes from HIV-infected patients as assessed by cyclin B1 expression, AgNORs dot distribution, and DNA content
To assess the cell-cycle distribution of PBLs from HIV-infected patients and controls, we investigated, in parallel, the DNA content, as detected by PI staining, intracellular cyclin B1 concentration, as measured by Western blot, and AgNOR number and area, as detected by image analysis. Figure 1shows selected experiments performed on 20 HIV-infected individuals and 20 healthy controls. All measurements were conducted in HIV-infected individuals before the initiation of ART, with an average viral copy number of 41 000 per milliliter.
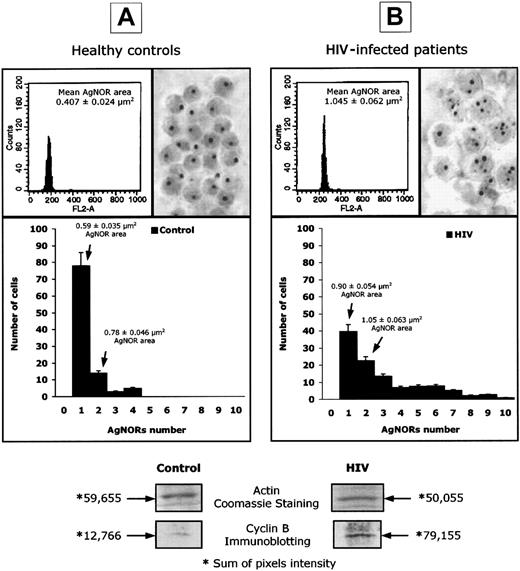
Abnormal expression of AgNORs and cyclin B1 in lymphocytes from HIV-infected patients.
DNA content, assessed by PI staining, AgNOR number and area of distribution, and cyclin B1 intracellular concentration, expressed as pixel intensity of protein bands isolated by Western blot, in selected experiments performed on peripheral blood lymphocytes isolated from healthy controls (panel A) and HIV-infected patients (panel B). The numeric results represent the mean of 20 patients and 20 uninfected controls.
Abnormal expression of AgNORs and cyclin B1 in lymphocytes from HIV-infected patients.
DNA content, assessed by PI staining, AgNOR number and area of distribution, and cyclin B1 intracellular concentration, expressed as pixel intensity of protein bands isolated by Western blot, in selected experiments performed on peripheral blood lymphocytes isolated from healthy controls (panel A) and HIV-infected patients (panel B). The numeric results represent the mean of 20 patients and 20 uninfected controls.
Flow cytometric analysis of DNA content indicated that the vast majority of PBLs from both HIV-infected and uninfected donors are diploid and located within the G0-G1 phases of the cell cycle (Figure1). On the contrary, the intracellular concentration of cyclin B1 was 79 155 pixels per densitometric area in HIV-infected patients and 12 766 in healthy individuals (Figure 1, P < .001), confirming our previous observations on cyclin B1 overexpression in PBLs from HIV-infected patients.23 The number and area of distribution of AgNOR dots were also markedly different between HIV-infected patients and controls (Figure 1). In healthy individuals, more than 80% of cells displayed a single AgNOR dot (mean area per cell = 0.59 μm2), and 12% of cells displayed 2 AgNOR dots (mean area per cell = 0.78 μm2). In all tested controls, the mean number of dots per cell was lower than 1.5 and the mean AgNOR area per cell was below 0.73 μm2. In HIV positive patients, the number of AgNORs was more heterogeneous with a significant percentage of cells (more than 30%), with more than 3 dots and a mean number of dots per cell, ranging from 1.35 to 4.1 in different patients. Consistent with this finding, in PBLs from HIV-infected patients, the AgNOR area per cell ranged from 0.72 to 1.84 μm2 (Figure 1). It is of note that cyclin B overexpression and increased AgNOR number and area of distribution were observed in both CD4+ and CD8+ T lymphocytes when selected experiments were performed by using sorted subpopulations (data not shown). Interestingly, in all performed experiments, a direct correlation was found between the level of cyclin B1 expression and the number and area of distribution of AgNORs (data not shown).
Overall, these results confirm the presence of a complex perturbation of cell-cycle control in PBLs from HIV-infected patients with active viral replication. Indeed, although high expression of cyclin B1 and AgNORs seemed to indicate a massive commitment to the G1/S transition, the analysis of DNA content and the in vitro biochemical profile (proline uptake, ODC activity, IL-2 receptor expression, total protein synthesis) suggested that most cells were in a G0-like state (data not shown).
Antiretroviral therapy normalizes cyclin B1 expression and AgNORs number and area of distribution
Several immunologic abnormalities described in lymphocytes from HIV-infected patients can be corrected by an effective ART.7 8
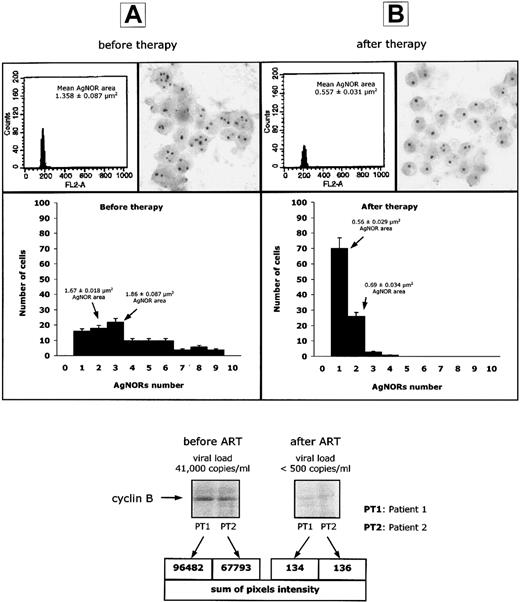
To assess the effect of ART on the cell-cycle abnormalities described in Figure 1, we have performed a comparative analysis of (1) DNA content, (2) AgNOR dot number per cell, and (3) cyclin B1 intracellular concentration, on peripheral blood lymphocytes from 12 HIV-infected patients with active viral replication, before and 2 to 3 weeks after the initiation of antiretroviral therapy. At the latter time, HIV viremia was below 500 copies/mL in all patients. As shown in Figure2, the fall in viremia was associated with a significant (P < .01) decline of the AgNOR dot numbers per lymphocyte and a significant (P < .01) decrease of cyclin B1 expression, so that both parameters returned to levels similar to those observed in uninfected individuals. Of note, ART did not induce any change in the DNA content of PBLs from HIV-infected patients (Figure 2). These results indicate that an effective ART induces the repopulation of peripheral blood by lymphocytes that express cyclin B1 and AgNORs at normal levels.
Normalization of AgNORs and cyclin B1 expression after ART.
DNA content, AgNOR number and area of AgNOR distribution, and cyclin B1 intracellular concentration in selected experiments performed on peripheral blood lymphocytes isolated from 12 HIV-infected patients, before (panel A) and 15 to 21 days after (panel B) initiation of antiretroviral therapy.
Normalization of AgNORs and cyclin B1 expression after ART.
DNA content, AgNOR number and area of AgNOR distribution, and cyclin B1 intracellular concentration in selected experiments performed on peripheral blood lymphocytes isolated from 12 HIV-infected patients, before (panel A) and 15 to 21 days after (panel B) initiation of antiretroviral therapy.
Interestingly, when cyclin B1 expression and AgNOR number and area of distribution were studied in 5 HIV-infected individuals defined as long-term nonprogressors (ie, with near normal CD4+ T-cell counts and viremia less than 500 copies per millilter), we observed values comparable with those of uninfected controls (data not shown). Overall, those observations underscore the role of HIV replication in inducing cell-cycle dysregulation as well as the prompt ability of lymphocytes to resume a normal cell-cycle homeostasis once the stimulus has been removed.
Cyclin B1 accumulates in lymphocytes from HIV-infected patients because of defective degradation
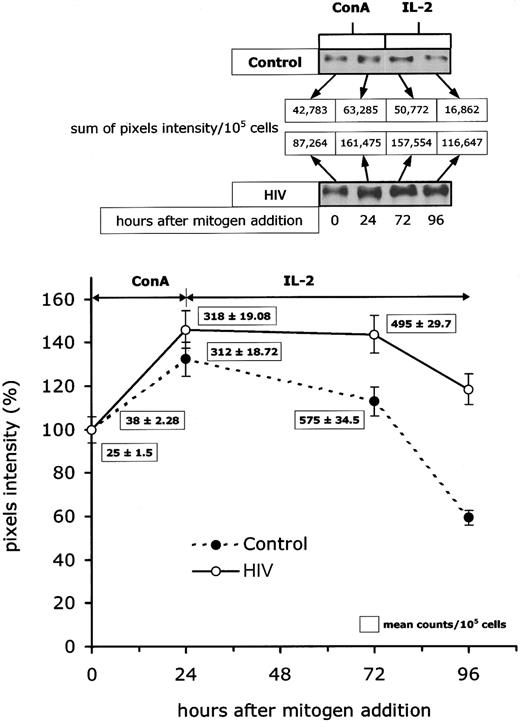
Detection of abnormally high levels of cyclin B1 may result either from excessive synthesis, defects in degradation or a combination of the 2. To define the cause of cyclin B1 overexpression, we have determined (1) the rate of cyclin B1 accumulation, (2) the initial velocity of cyclin B1 synthesis, and (3) the rate of cyclin B1 degradation, after T-cell activation in vitro. This study was performed on PBLs from HIV-infected patients before initiation of ART (average viremia of 41 000 copies per millilter), and PBLs from healthy donors were used as controls. In all experiments, PBLs were activated with ConA, and 24 hours later IL-2 was added to the medium. Figure3 shows that, in PBLs isolated from HIV-infected patients, the activation-induced cyclin B1 expression was consistently higher than in controls. However, when densitometric values were normalized to 100 and plotted against the time of culture (Figure 3), no major difference was observed in the rate of postactivation cyclin B1 accumulation between 0 and 24 hours (G1/S transition), indicating that cells from patients and controls synthesize cyclin B1 at a similar rate. However, after IL-2 addition (ie, G2/M phase), cyclin B1 cell content rapidly decreased in lymphocytes from controls, whereas it remained relatively high in cells from patients. The fact that the intracellular levels of cyclin B1 failed to decrease after the G2/M transition suggested that the abnormal expression of cyclin B1 in lymphocytes from HIV-infected patients is due to decreased degradation rather than increased synthesis.
Intracellular turnover of cyclin B1 in lymphocytes from HIV-infected patients.
Kinetic analysis of intracellular concentration of cyclin B1 (expressed as percentage of the baseline pixel intensity) on in vitro mitogen activation was performed in peripheral blood lymphocytes from 20 HIV-infected patients and 20 controls. The top panel reports Western blots from selected experiments in which the cyclin B1 overtime cellular expression was sequentially studied after lymphocyte activation. The curve in the bottom panel describes results of a representative experiment in which sequential cyclin B expression in one HIV-infected patient and one control were analyzed in the same Western blot experiment (3 measurements per patient per time point). The numeric results in the box represent the mean levels of initial cyclin B synthesis as studied at time 0, 24, and 72 hours after activation in the same representative experiment.
Intracellular turnover of cyclin B1 in lymphocytes from HIV-infected patients.
Kinetic analysis of intracellular concentration of cyclin B1 (expressed as percentage of the baseline pixel intensity) on in vitro mitogen activation was performed in peripheral blood lymphocytes from 20 HIV-infected patients and 20 controls. The top panel reports Western blots from selected experiments in which the cyclin B1 overtime cellular expression was sequentially studied after lymphocyte activation. The curve in the bottom panel describes results of a representative experiment in which sequential cyclin B expression in one HIV-infected patient and one control were analyzed in the same Western blot experiment (3 measurements per patient per time point). The numeric results in the box represent the mean levels of initial cyclin B synthesis as studied at time 0, 24, and 72 hours after activation in the same representative experiment.
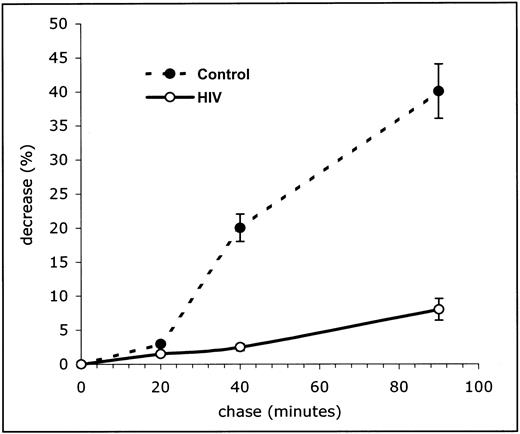
To confirm this hypothesis, we studied the kinetics of degradation of newly synthesized cyclin B1 by using pulse and chase experiments. Cells were incubated with 35S-methionine for 4 hours (between 68 and 72 hours of culture) to radioactively label the newly synthesized proteins, and then washed and resuspended in a large excess of nonradioactive methionine. At any given time points, the amount of methionine incorporated into newly synthesized cyclin B1 during the labeling phase was calculated as described below. Figure4 shows the mean counts of radioactivity from experiments performed in 20 HIV-infected individuals and 20 controls. The rate of cyclin B1 degradation was markedly reduced in PBLs from HIV-infected patients: After a 90-minute chase, PBLs from patients lost only 8% of their cyclin B1-linked radioactivity, whereas 40% was lost in controls (P < .01). Overall, these results indicate that the difference in cyclin B1 intracellular concentration between HIV-infected patients and controls is due to a reduced degradation of a molecule that is synthesized at very similar initial rates.
Impaired cyclin B1 degradation in lymphocytes from HIV-infected patients.
Kinetic analysis of degradation of the newly synthesized cyclin B1 in peripheral blood lymphocytes from HIV-infected patients and controls. In these experiments cells were pulse labeled for 4 hours with35S-methionine after 24 hours in vitro activation with ConA. Cyclic B degradation was calculated as a percentage of radioactivity associated with the protein band after a 20-, 40-, and 90-minute chase period. The curves in the top panel and the numeric results in the bottom panel represent the mean of 20 patients and 20 uninfected controls.
Impaired cyclin B1 degradation in lymphocytes from HIV-infected patients.
Kinetic analysis of degradation of the newly synthesized cyclin B1 in peripheral blood lymphocytes from HIV-infected patients and controls. In these experiments cells were pulse labeled for 4 hours with35S-methionine after 24 hours in vitro activation with ConA. Cyclic B degradation was calculated as a percentage of radioactivity associated with the protein band after a 20-, 40-, and 90-minute chase period. The curves in the top panel and the numeric results in the bottom panel represent the mean of 20 patients and 20 uninfected controls.
Decreased cyclin B1 degradation is associated with a defect in cyclin B ubiquitination
Decreased cyclin B1 degradation can be caused by inappropriate phosphorylation, binding to p53, and/or reduced degradation via the ubiquitin proteasome pathway.46,57Impaired function of the ubiquitin/proteasome pathway of protein degradation constitutes an attractive hypothesis to investigate, because it has been shown that this intracellular compartment can be functionally impaired in clinical conditions associated with an accelerated cell activation and replication.58
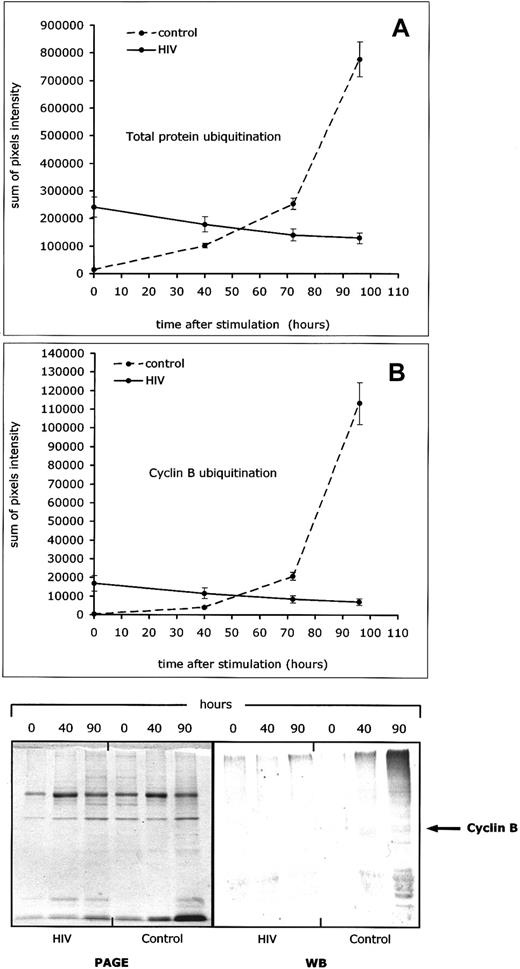
To assess the function of the ubiquitin pathway of protein degradation in PBLs from HIV-infected patients, we studied the ubiquitination of both cyclin B1 and total proteins at various time points after mitogen activation. All measures were performed in cells activated with ConA and IL-2, with the 35S-methionine administered as a 4-hour pulse. As shown in Figure 5, ConA/IL-2 activation induced a sharp increase of total protein ubiquitination in lymphocytes from controls and not in lymphocytes from HIV-infected individuals (Figure 5A). When cyclin B1 ubiquitination was studied under the same experimental conditions, lymphocytes from HIV-infected patients showed significantly lower rates of ubiquitinated cyclin B1 than controls (Figure 5B).
Defective cyclin B1- and total protein-ubiquitination in lymphocytes from HIV-infected patients.
Protein and cyclin B ubiquitination were studied after in vitro activation (ConA + IL-2) of peripheral blood lymphocytes from 20 HIV-infected patients and 20 controls, and measured at different time points (0, 40, 70, and 90 hours). (A) Level of total protein ubiquitination. (B) Level of cyclin B1 ubiquitination. Both curves show results of a representative experiment in which sequential cyclin B and protein ubiquitination in lymphocytes from one HIV-infected patient and one control were analyzed in the same Western blot experiment (3 measurements per patient per time point). The bottom part of the figure shows pictures of 2 representative gels, one for total protein ubiquitination and one for cyclin B ubiquitination, as sequentially determined at 40 and 90 hours after activation in one HIV-infected patient and one control.
Defective cyclin B1- and total protein-ubiquitination in lymphocytes from HIV-infected patients.
Protein and cyclin B ubiquitination were studied after in vitro activation (ConA + IL-2) of peripheral blood lymphocytes from 20 HIV-infected patients and 20 controls, and measured at different time points (0, 40, 70, and 90 hours). (A) Level of total protein ubiquitination. (B) Level of cyclin B1 ubiquitination. Both curves show results of a representative experiment in which sequential cyclin B and protein ubiquitination in lymphocytes from one HIV-infected patient and one control were analyzed in the same Western blot experiment (3 measurements per patient per time point). The bottom part of the figure shows pictures of 2 representative gels, one for total protein ubiquitination and one for cyclin B ubiquitination, as sequentially determined at 40 and 90 hours after activation in one HIV-infected patient and one control.
Overall, these results suggest that PBLs from HIV-infected patients may have a general defect of protein degradation, which involves the ubiquitin-proteasome pathway, and could be responsible for the abnormally low levels of cyclin B1 ubiquitination that are consistently observed under these experimental conditions.
Abnormal expression and localization of cell-cycle–dependent proteins is followed by increased levels of activation-induced apoptosis
Lymphocyte activation and proliferation involves the sequential expression and degradation of numerous proteins, whose proper intracellular concentration, localization, and activity are needed to complete the task of cell division. Inappropriate regulation of cell-cycle–dependent proteins is a known cause of apoptosis.21 22
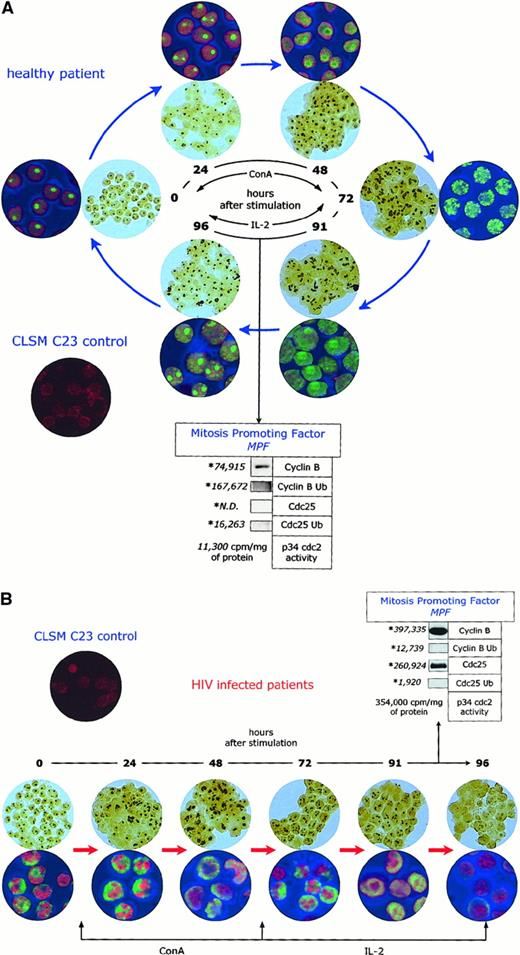
To assess the overall function of cell-cycle–dependent proteins in lymphocytes from HIV-infected patients, we have used a combined approach, by simultaneously assessing the functional status of the maturation promoting factor (ie, cyclin B1 and cdc25 expression and ubiquitination by Western blot, and p34 cdc2 activity by H1 histone phosphorylation), the AgNOR morphology (by image analysis), and C23/nucleolin subcellular localization (by confocal microscopy), after in vitro activation with ConA and IL-2. As shown in Figure6A, lymphocytes from uninfected controls show a cyclic and ordered pattern of expression, localization, and ubiquitination of cell-cycle–dependent proteins. This pattern consists of: (1) increased AgNOR number and area of distribution, which is parallelled by an increased area of distribution of C23/nucleolin; (2) increased expression and then ubiquitination of cyclin B1 and cdc25; and (3) temporary increase of p34 cdc2 activity. The whole process is completed between 90 and 96 hours after the initial stimulation and results in the presence of cells that have a biochemical and morphologic profile similar to that observed before ConA/IL-2 activation. Although the process is not 100% effective, the rate of apoptotic cell death (measured either as the number of cells with DNA content less than 2n or the number of cells permeable to PI) was relatively small (average 8.9%).
Abnormal intracellular kinetics of cell-cycle–dependent proteins in lymphocytes from HIV- infected patients.
AgNOR pattern and C23/nucleolin subcellular localization after in vitro activation of lymphocytes from healthy controls (panel A) and HIV-infected individuals (panel B). The indexes of the functional status of the maturation promoting factor (cyclin B expression and ubiquitination, cdc25 expression and ubiquitination, and p34 cdc2 activity) are relative to time 90 to 96 hours after activation.
Abnormal intracellular kinetics of cell-cycle–dependent proteins in lymphocytes from HIV- infected patients.
AgNOR pattern and C23/nucleolin subcellular localization after in vitro activation of lymphocytes from healthy controls (panel A) and HIV-infected individuals (panel B). The indexes of the functional status of the maturation promoting factor (cyclin B expression and ubiquitination, cdc25 expression and ubiquitination, and p34 cdc2 activity) are relative to time 90 to 96 hours after activation.
On the contrary, when lymphocytes from HIV-infected individuals were activated under similar experimental conditions (Figure 6B), a profound perturbation of the normal pattern of the expression, localization, and ubiquitination of cell-cycle–dependent proteins was observed. Most notably, in a large percentage of cells, the number and area of distribution of AgNORs, as well as the area of distribution of C23/nucleolin, never returned to normal. Moreover, in many of these cells, AgNORs and C23/nucleolin-positive areas in confocal microscopy disappeared completely, giving the peculiar appearance of an empty nucleolus. Interestingly, the intracellular levels of C23/nucleolin were similar between HIV-infected patients and controls (data not shown), indicating that the loss of the AgNOR structure is related to an abnormal cellular localization of nucleolin rather than to the absence of this protein. In this system, the expression of cyclin B1 and cdc25 increased over time without being accompanied by a physiologic level of ubiquitination, which led to an abnormal and inappropriate level of p34 cdc2 activity. As a result of this profound dysregulation of the cell-cycle control, high levels of apoptotic cell death (mean 43.0%) were observed 96 hours after activation. It is of note that under these experimental conditions no overt cytopathic effect was observed, no HIV-p24 antigen was detectable in the medium, and cells undergoing apoptosis were equally distributed among CD4+ and CD8+ T lymphocyte subpopulations (data not shown), suggesting that the observed increase in cell death was not a mere consequence of HIV replication occurring in in vitro–activated CD4+ T cells.
Overall, these results show that in vitro activation of peripheral blood lymphocytes from HIV-infected individuals induces a major perturbation in the intracellular turnover of cell-cycle–dependent proteins, which is followed by levels of apoptotic cell death that are about 5-fold higher than in healthy individuals.
Discussion
The profound immunodeficiency that follows HIV infections has a complex pathogenesis, in which the direct, ie, virus-mediated, killing of HIV-infected CD4 cells appears to be associated with conspicuous loss of uninfected bystander cells. Numerous studies have tried to explain this unexpected cell loss, mostly focusing on the consequences of an exaggerated immune activation and/or an increased T-cell turnover. It is of note, however, that contrasting results have been published on the overall level of in vivo cell proliferation during HIV infection, the turnover rate of different T-cell subsets, and the pathogenic mechanisms of these kinetic abnormalities.9,10,24,25,59,60 The pathophysiologic relationship between increased T-cell activation/turnover and increased level of apoptosis, known to be a consistent feature in T lymphocytes from HIV-infected patients,3-6 is also uncertain. Although events directly related to immune activation can induce apoptosis of uninfected cells in the setting of HIV infection, ie, CD95/CD95L up-regulation, increased tumor necrosis factor (TNF) and γIFN production, and others,61-65 it is still unclear whether there is an intrinsic biologic link between accelerated T-cell proliferation and exaggerated levels of apoptosis. In this perspective, it should be remembered that a dysregulation of cell-cycle control has the potential to induce apoptosis independently of specific environmental stimuli.21 22
In a previous study, we have shown that the intracellular content of cyclin B1 is increased in PBLs of HIV-infected patients.23To understand whether the abnormal cyclin B1 expression merely reflects the presence of a large number of cycling lymphocytes in the peripheral blood or, alternatively, indicates a more complex perturbation of cell-cycle control, we have now assessed the position in cycle of lymphocytes by determining the level of cyclin B1 expression in parallel with DNA content and pattern of distribution of AgNORs. The use of the AgNOR technique for diagnostic and prognostic purpose in oncology and hematology has been recently reviewed.31,32,45 AgNORs accumulate in cells when duplication time decreases,32 and therefore the finding of increased AgNOR number or area of distribution indicates that cells are actively proliferating.32 66 To our knowledge, this technique has never been used to study T-cell turnover and cell-cycle control in HIV infection.
In healthy individuals, most lymphocytes contain only one AgNOR dot and express low levels of cyclin B1. Consistent with these findings, PBLs from uninfected individuals are diploid and metabolically inactive, thus confirming their G0 state. In PBLs from HIV-infected patients, the majority of cells are also diploid and metabolically inactive; however, cyclin B1 is significantly overexpressed, and many cells show more than one AgNOR dot with a significantly increased area of distribution. Once patients are treated with anti-HIV therapy, AgNOR distribution and cyclin B1 expression return to normal levels, thus indicating that the mechanism(s) responsible for the cell-cycle perturbation of PBLs from HIV-infected patients is related to the presence of high levels of viral replication. This hypothesis is further confirmed by the fact that AgNOR distribution and cyclin B1 expression are normal in HIV-infected individuals who spontaneously harbor very low levels of viral replication, ie, long-term nonprogressors.
When translated in terms of cell cycle, these results suggest that active HIV replication is associated with the presence of large numbers of circulating lymphocytes showing a G0-like metabolic profile associated with a G1/S-like level of cyclin B1 and NORs expression. This discrepancy cannot be explained only as the result of increased number of activated cells (ie, committed to G1), because in this case at least some of the biochemical markers of the G0 to G1 transition should show levels that are compatible with a G1 state. Interestingly, the lack of increase in circulating cells that are unequivocally committed to G1 is consistent with recent data indicating that, during HIV infection, only a small portion of peripheral blood lymphocytes is undergoing active proliferation.24 25
The presence of an abnormal cyclin B1 intracellular concentration in cells with a G0-like metabolic profile can be due to excessive or premature synthesis, and/or reduced or delayed degradation. As shown in Figures 3 to 5, we provide evidence that a significant decrease in cyclin B1 degradation is present in PBLs from HIV-infected patients, whereas the rates of cyclin B1 synthesis are comparable to those of normal PBLs. The presence of a defect in cyclin B1 degradation is supported by 4 different measurements: rate of intracellular accumulation, rate of synthesis, rate of degradation as measured in pulse and chase experiments, and level of ubiquitination. The fact that total protein ubiquitination is also decreased in PBLs from HIV-infected patients suggests the presence of a more general defect of the ubiquitin pathway of protein degradation.
Several mechanisms can be hypothesized to explain the failure of the protein degradation machinery in lymphocytes from HIV-infected patients, including the presence of specific metabolic defects in the ubiquitination pathway(s) induced by a specific HIV gene product(s), and/or a defect in the ubiquitination pathway(s) that would be intrinsically related to the abnormal proliferative history of lymphocytes isolated from HIV-infected patients. This latter condition would be caused by either direct ubiquitin loss, lack of ubiquitination enzymes, or saturation of an otherwise normal ubiquitination system, possibly due to the shortened time available to lymphocytes to complete their anabolic activity in the context of an accelerated rate of cell turnover. In any case, the imbalance between synthesis and degradation of cyclin B1 constitutes a serious perturbation of the normal sequence of cell cycle-related events that could ultimately result in a strong proapoptotic signal.58 67
The presence of a major defect of cell-cycle control in lymphocytes from HIV-infected individuals was confirmed by the comparative and integrated analysis of cyclin B1 and cdc25 expression and ubiquitination, p34 cdc2 activity, AgNOR number and area of distribution, and C23/nucleolin subcellular localization after in vitro activation with ConA/IL-2. This series of experiments indicated that the progressive accumulation of nonubiquitinated cyclin B1 and cdc25 was temporally associated not only with inappropriate activation of p34, but also with progressive disruption of the nucleolar structure and, ultimately, with the presence of high levels of apoptosis. Interestingly, the disruption of AgNORs and the absence of C23/nucleolin in the nuclei is associated with normal cellular levels of C23/nucleolin. Because C23/nucleolin is known to be a target of p34-mediated phosphorylation,68 it is possible that the abnormal p34 cdc2 activity observed in these conditions causes an excess of phosphorylated C23/nucleolin that cannot properly associate in a functional NOR structure. Taken as a whole, our results indicate that HIV infection induces the presence of circulating lymphocytes, both CD4+ and CD8+, with impaired control of the sequential expression and degradation of cell-cycle–dependent proteins. The effects of this impaired control are consistently observed in freshly isolated lymphocytes and can be reproduced in vitro after ConA/IL-2 activation, resulting in abnormally high levels of apoptosis.
At present, we ignore the final molecular mechanism(s) causing the abnormal regulation of cell-cycle–dependent proteins that follows in vitro activation of lymphocytes from HIV-infected patients. The possibility that this perturbation is a direct effect of an activation-induced burst of HIV replication in CD4 T cells is highly unlikely because in our experimental conditions (1) viral cytopathic effect was not observed, (2) p24 antigen was not detected in the medium, and (3) the proliferation-induced apoptosis was found at similar rates in CD4+ and CD8+ T lymphocytes. On the other hand, it is tempting to speculate that the perturbed kinetics of cell-cycle–dependent proteins observed after in vitro activation of lymphocytes might be an intrinsic biologic consequence of the abnormal in vivo levels of cyclin B1 and AgNORs, which in turn may be related to the high levels of overall lymphocyte activation occurring during HIV-infection. According to this hypothesis, high levels of T-cell activation/proliferation, mostly occurring in lymphoid organs, would induce a functional saturation of the ubiquitin-mediated pathway of protein degradation, thus affecting the capability of lymphocytes to readjust the intracellular concentration, function, and/or localization of cyclin B, cdc25, p34 cdc2, and C23/nucleolin. If these proliferating lymphocytes can no longer maintain the sequential expression of these key regulatory proteins, phenomena of cell death may occur, as commonly described in lymph node-derived, uninfected CD4+ and CD8+ T lymphocytes from HIV-infected patients. In this scenario, lymphocytes that have exited the lymph node and are found in the peripheral blood would show high levels of “undegraded” cyclin B1 and AgNORs as a memory of the recent rounds of replication, despite a metabolic profile that is now typical of a G0 phase. These cells are viable but more susceptible to a “cell cycle-related” apoptosis in case of further in vivo or in vitro activation. Interestingly, when anti-HIV therapy decreases HIV replication and overall immune activation, cells will slow their proliferation rate and recover the protein degradation machinery and the overall control of the expression and function of cell-cycle–dependent proteins. Because of the reduced cell turnover, lymphocytes that are now leaving lymphoid organs to join the circulating pool have not experienced rapid rounds of replication in their recent past, thus translating into normal levels of “undegraded” cyclin B1 and AgNORs. In this regard, an important point to investigate in further experiments would be the role of cell-cycle perturbations in “naive” and “memory” subpopulations of T cells. Although difficult to perform for technical reasons, this analysis might provide important insights into the role of the previous proliferative history of a given T lymphocyte in inducing the abnormal expression of cell-cycle–dependent proteins in vivo and the increased susceptibility to apoptosis after in vitro activation. The possibility that the abnormal intracellular turnover of cyclin B is related to the abnormal in vivo lymphocyte turnover typical of HIV-infection also raises the possibility that other diseases with chronic immune activation may be accompanied by similar perturbation of the intracellular turnover of cell-cycle–dependent proteins. At present we have data neither to support nor to exclude this possibility. However, it should be noted that in the case of HIV-infection, whose specific feature is the progressive loss of both infected and uninfected T lymphocytes, the presence of the described cell-cycle perturbations carries a higher potential to be a pathogenic factor of significant relevance in vivo.
In conclusion, our studies indicate that a perturbation of the normal cell-cycle control is present in lymphocytes from HIV-infected patients with active viral replication. This perturbation can be observed in freshly isolated peripheral blood T lymphocytes as an abnormal intracellular content of cyclin B1 and AgNORs, and becomes more evident after in vitro activation as a complex but consistent dysregulation of the intracellular turnover and subcellular localization of several phase-dependent proteins (ie, cyclin B1, p34 cdc2, cdc25, and nucleolin), which ultimately results in a 5-fold increase of the number of apoptotic cells. These findings might represent a novel biologic link among high levels of immune activation, accelerated T-cell turnover, and increased apoptosis occurring in the setting of HIV infection.
This work has been supported by grants 30B.65 (to G.P.) and 30B52 (to M.M.) from the Programma nazionale di Ricerca sull'AIDS, Istituto Superiore di Sanita', Rome, Italy. The authors wish to thank Drs Mark B. Feinberg, Stephen G. Emerson, Jeffrey Goldsmith, George Xiaowei Xu, and Rebecca L. Elstrom for the helpful discussion.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Guido Silvestri, Dept of Pathology and Laboratory Medicine, 6th Founders, Hospital of the University of Pennsylvania, 3400 Spruce St, Philadelphia, PA 19104; email:gsilvest@mail.med.upenn.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal