Multiple myeloma (MM) is a B-cell malignancy. The monoclonal immunoglobulin, secreted by myeloma plasma cells, carries unique antigenic determinants (idiotype [Id]) that can be regarded as a tumor-specific antigen. Id-based immunotherapy has been explored in myeloma patients for the purpose of enhancing or inducing Id-specific immune responses that might lead to tumor destruction. However, despite some evidence obtained from mouse plasmacytoma models, it is still unclear whether Id-specific immunity may play a role in the regulation of tumor cells in MM. In the current study, using dendritic cells (DCs) as antigen-presenting cells, autologous Id-specific cytotoxic T lymphocyte (CTL) lines containing both CD4+ and CD8+ T cells were generated from myeloma patients. The results show that Id-specific CTLs not only recognized and lysed autologous Id-pulsed DCs but also significantly killed the autologous primary myeloma cells. The cytotoxicity against the primary tumor cells was major histocompatibility complex (MHC) class I– and, to a lesser extent, class II–restricted, indicating that myeloma cells could process Id protein and present Id peptides in the context of their surface MHC molecules. Furthermore, the CTLs lysed the target cells mainly through the perforin-mediated pathway because Concanamycin A, but not Brefeldin A—the selective inhibitors for perforin- or Fas-mediated pathways—abrogated the cytolytic activity of the cells. These CTLs secreted predominantly interferon-γ and tumor necrosis factor-α on antigen stimulation, indicating that they belong to the type-1 T-cell subsets. Taken together, these findings represent the first demonstration that Id-specific CTLs are able to lyse autologous tumor cells in MM and, thus, provide a rationale for Id-based immunotherapy in the disease.
Introduction
Multiple myeloma (MM) is a B-cell malignancy that accounts for 1% of all cancers and 10% of all hematologic malignancies. With conventional chemotherapy, complete remission rates have not exceeded 5%, and median survival has not been extended beyond 3 years.1,2 High-dose chemotherapy followed by stem cell support results in long-term, disease-free survival in one third of myeloma patients.3 However, relapse of the underlying disease remains the primary cause of treatment failure.4Therefore, novel alternative therapeutic strategies aiming at improved outcome in these patients are much needed.
Tumor vaccines based either on immunization against specific tumor antigens or on adoptive transfer of ex vivo-generated lymphocytes, with high levels of specific reactivity against tumor antigens, represent attractive approaches.5-8 In MM, the idiotype (Id) determinant of the paraprotein can be regarded as a tumor-specific marker and has been used for immunotherapy of MM.9-14Although modest and transient, Id-specific immune responses can be induced in 25% to 100% of these patients. However, clinical responses, defined by a significant reduction in M components, have been observed in few patients after Id vaccination.11 13Thus, the role of Id-specific immune responses in the regulation of tumor cell growth is poorly understood, and, more important, whether Id-specific cytotoxic T lymphocytes (CTLs) lyse the primary myeloma cells has not been demonstrated in MM. It is still unclear whether the induced/enhanced Id-specific immune response after immunization is relevant to or responsible for the clinical response observed in patients.
The objectives of this study were to generate Id-specific CTLs from myeloma patients and to examine the efficacy of these T cells in lysis of their target cells, including Id-pulsed dendritic cells (DCs) and autologous primary myeloma cells. Id-specific CTLs were generated by using autologous adherent peripheral blood mononuclear cell-derived DCs as antigen-presenting cells (APCs). We show for the first time that Id-pulsed DCs reproducibly led to successful in vitro generation of Id-specific CTLs capable of killing the autologous primary myeloma cells.
Materials and methods
Isolation of idiotype protein
Affinity chromatography column prepared with anti–human IgA (α-chain specific) monoclonal antibody-conjugated agarose (A2691; Sigma, St Louis, MO) was used to isolate and purify IgA Id protein from serum obtained from a patient with IgA myeloma. A Hi-Trap affinity column packed with 1 mL Protein-G Sepharose High Performance (both Amersham Pharmacia Biotech AB, Uppsala, Sweden) was used to isolate and purify IgG Id protein from the serum of another patient with IgG myeloma. The protein purity was determined by sodium dodecyl sulfate–polyacrylamide gel electrophoresis. Isoelectric focusing estimated that more than 90% of the purified IgG or IgA was monoclonal.
Isolation of primary myeloma plasma cells
Fresh bone marrow aspirates from 2 patients with advanced MM were collected, and bone marrow mononuclear cells (BMMCs) were separated using Ficoll-Hypaque (Amersham Pharmacia Biotech AB) density centrifugation. The BMMCs from the 2 patients contained more than 95% CD138+CD38++ myeloma plasma cells, determined by flow cytometry analysis. Aliquots of BMMCs were cryopreserved in −80°C until use.
Generation of dendritic cells
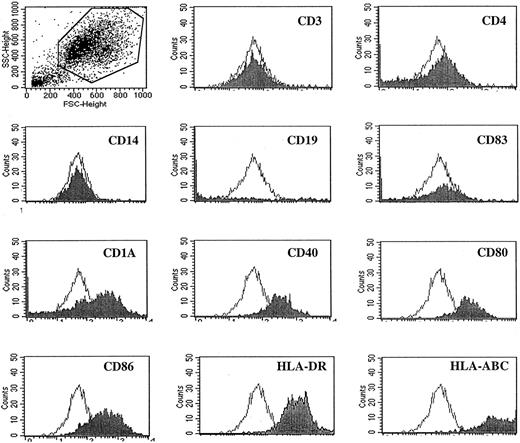
Peripheral blood mononuclear cells (PBMCs) from the same 2 patients were isolated from leukapheresis-collected blood cells using Ficoll-Hypaque (Amersham Pharmacia Biotech AB) gradient centrifugation. Aliquots of PBMCs were cryopreserved in −80°C until use. The cryopreserved PBMCs were recovered and resuspended at 5 × 106 cells/mL in RPMI 1640 supplemented with 10% fetal calf serum, 1 mM glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin (referred to as complete medium) (Gibco, Grand Island, NY). PBMCs were incubated in a humidified incubator at 37°C in 5% CO2 for 2 hours to allow cell adhesion. Nonadherent cells were removed by gentle washes. The adherent cells were then cultured in complete medium supplemented with GM-CSF (100 ng/mL) and IL-4 (100 ng/mL; both from Schering-Plough Research Institute, Kenilworth, NJ) for 7 days, with further addition of cytokines on days 3 and 5. During the culture, the Id or an allogeneic paraprotein (both at 50 μg/mL) was added on days 1 and 5 to pulse the cells. After 7 days of culture, cells were harvested and phenotypically characterized to ensure they met the typical phenotype of immature DCs—that is, CD3−, CD4−, CD14−, CD19−, CD83−, CD1a+, CD40+, CD80+, CD86+, HLA-DR+, HLA-ABC+.15 16
Generation of idiotype-specific cytotoxic C lymphocytes
CTLs were elicited from autologous PBMCs using repeated stimulation by DCs pulsed with myeloma Id. Washed PBMCs (2 × 106/mL) were resuspended in complete medium containing recombinant IL-2 (30 IU/mL; Chiron, Emeryville, CA) and IL-7 (5 ng/mL; R&D Systems, Minneapolis, MN), and Id-pulsed autologous DCs (2-5 × 105/mL) were added to primary cultures. The cells were incubated in 50-mL tissue culture flasks at 37°C in 5% CO2/95% air. Responder cells were restimulated with Id-pulsed autologous DCs every 2 weeks, and the cultures were fed every 5 days with fresh medium containing recombinant IL-2 and IL-7. After 3 to 4 cycles of antigen stimulation and selection, T-cell lines were established and cells were expanded in complete medium containing rIL-2 (30 IU/mL) and IL-7 (5 ng/mL) for 2 weeks and then subjected to functional tests.
Proliferation assays
T cells (5 × 104/100 μL/well) were seeded into 96-well flat-bottom tissue culture plates. DCs pulsed with the autologous Id, the allogeneic paraprotein from the other patient, or an isotype-matched Id protein from other patients were added to cultures in different concentrations, and cultures were incubated at 37°C in 5% CO2/95% air for 4 days. T-cell proliferation was measured using overnight incubation with [3H]-thymidine (0.5 μCi/well; Amersham Pharmacia Biotech, Piscataway, NJ). Results from triplicate cultures are given as the arithmetic means.
Cytotoxicity assays
Target cells included autologous DCs pulsed with the autologous Id, the allogeneic paraprotein from the other patient and an isotype-matched Id protein, thawed autologous or allogeneic primary myeloma cells from the other patient, and Ficoll-Hypaque centrifuged to get rid of dead cells. Cells were labeled with sodium chromium Cr 51 chromate for 1 hour, and 1 × 104 target cells/well were mixed with various numbers of effector cells in a standard 4-hour cytotoxicity assay using 96-well round-bottom plates. All assays were performed at least in triplicate. Results are shown as mean percentage51Cr release calculated as follows: [(sample counts − spontaneous counts)/(maximum counts − spontaneous counts)] ×100. Spontaneous release was less than 25% of the maximum [51Cr] uptake.
To determine whether the cytolytic activity was restricted by major histocompatibility complex (MHC) class I or II molecules, antibodies against human leukocyte antigen (HLA)-ABC (W6/32; Serotec, Oxford, United Kingdom) or HLA-DR (B8.12.2; Immunotech, Marseilles, France) and an isotopic control mouse IgG (Immunotech) at 10 μg/mL were added to the cultures at the initiation of the assay.
Inhibition of perforin- and Fas-mediated pathways of cytolysis
Concanamycin A (CMA; Sigma), an inhibitor of vacuolar type H+-adenosine triphosphate (ATPase), or Brefeldin A (Sigma) was used as a selective inhibitor of perforin-mediated and Fas-mediated cytotoxicity,17 respectively. Effector cells were pretreated with 100 nmol/L CMA or 10 μmol/L Brefeldin A for 2 hours and assayed for cytotoxicity in the presence of the drugs.
Enzyme-linked immunospot assay
The detailed methods of the enzyme-linked immunospot (ELISPOT) assay for the enumeration of antigen-specific, cytokine-secreting cells have been described previously.18 19 Briefly, plates (Millititer-HAM; Millipore, Bedford, MA) were coated with mouse monoclonal anti–human interferon γ (IFN-γ) (1 μg/mL), IL-4 (2.5 μg/mL), or tumor necrosis factor (TNF)-α (2.5.μg/mL) (R&D Systems). Cultured T cells (104 cells/well) were added and incubated with Id-pulsed DCs for 36 hours at 37°C. Cells incubated without target cells or with phytohemagglutinin (10 μg/mL; Sigma) were used as controls. After incubation, cells were detached from the plates by washing, and polyclonal anti–human IFN-γ (250 ng/mL), IL-4 (1 μg/mL) (R&D System), or TNF-α (4 μg/mL; Serotec) was added and incubated for 2 hours. The spots were developed by sequential incubation with biotinylated antigoat (1/500; IFN-α and IL-4) or antirabbit (1/100; TNF-α) antibodies (Vector Labs, Burlingame, CA), followed by peroxidase staining, using the substrate 3-amino-9-ethyl-carbazol (Sigma). Spots corresponding to the cytokine-secreting cells were enumerated under a dissection microscope (Stemi SV6; Zeiss, Jena, Germany). All samples were run in duplicate. Data are expressed as the mean number of cytokine-secreting cells/104 T cells.
Immunophenotyping
Immunophenotyping was carried out on all cultured cells using a fluorescence-activated cell scan (Facscan; Becton Dickinson, San Jose, CA) and analyzed by Lysis II program. Briefly, cells were first washed twice in washing buffer (phosphate-buffered saline + 2% bovine serum albumin and 0.5% sodium azide). Phycoerythrin- or fluorescein isothiocyanate-conjugated monoclonal antibodies (Immunotech) at the manufacturer's recommended concentrations were added to each cell pellet, and the cells were incubated on ice for 30 minutes before they were washed 3 times and resuspended in phosphate-buffered saline ready for analysis. Controls consisted of cells stained with irrelevant mouse IgG antibodies.
Results
Idiotype-pulsed dendritic cells induce idiotype-specific T-cell responses in vitro
To enhance the immunogenicity of Id, we used DCs as APCs. DCs were successfully generated from adherent cells of PBMCs after 7 days of culture and confirmed by immunophenotyping showing that the cells were CD3−, CD4−, CD14−, CD19−, CD83−, CD1a+, CD40+, CD80+, CD86+, HLA-DR+, and HLA-ABC+ (Figure1).
Flow cytometry analysis.
Histograms with closed areas depict DCs expressing different surface markers. Histograms with open areas depict staining with isotype-matched control antibody. Gate was set on the big cell population. These results are representative of at least 6 independent experiments.
Flow cytometry analysis.
Histograms with closed areas depict DCs expressing different surface markers. Histograms with open areas depict staining with isotype-matched control antibody. Gate was set on the big cell population. These results are representative of at least 6 independent experiments.
We initially examined the immune response to myeloma Id in primary cultures. No significant proliferative response was observed in any of the repeated experiments using either freshly recovered PBMC or rIL-2 (50 IU/mL) pretreated PBMCs (data not shown). However, after repeated rounds of T-cell stimulation with Id-pulsed DCs, Id-specific T-cell lines were generated and propagated from PBMCs from both myeloma patients. Id-specific proliferative responses were observed when T cells were rechallenged with the autologous Id-pulsed DCs (Figure2A-B). No proliferative response was noted when T cells were restimulated with autologous DCs pulsed with the allogeneic or isotype-matched Id proteins. These results suggest that autologous T cells could recognize immune epitope(s) within the myeloma Id proteins, which had been taken up, processed, and presented by DCs.
Proliferative responses.
Proliferative response (cpm) of CTL lines generated from patients 1 (A) and 2 (B), in response to DCs pulsed with the autologous Id (●), the allogeneic Id from the other patient (○), or an isotype-matched (▵) Id protein. (C) Inhibition by antibodies against MHC class I and class II or an isotypic control IgG on Id-induced proliferation of CTLs from patients 1 (▪) and 2 (■). These results are representative of 3 independent experiments.
Proliferative responses.
Proliferative response (cpm) of CTL lines generated from patients 1 (A) and 2 (B), in response to DCs pulsed with the autologous Id (●), the allogeneic Id from the other patient (○), or an isotype-matched (▵) Id protein. (C) Inhibition by antibodies against MHC class I and class II or an isotypic control IgG on Id-induced proliferation of CTLs from patients 1 (▪) and 2 (■). These results are representative of 3 independent experiments.
The T-cell proliferative responses observed in both patients were blocked partially by monomorphic monoclonal antibody to HLA class I and II molecules (Figure 2C). In patient 1, the blocking was observed predominantly with the HLA class I antibody, whereas in patient 2, the blocking was observed mainly with the HLA class II antibody. A combination of these 2 antibodies produced a synergistic inhibition of the proliferative response (data not shown). These results indicate that the recognition of Id T-cell epitopes was mediated through MHC class I and II molecules. This was supported by flow cytometry analysis showing that the Id-specific T-cell lines consisted of approximately 45% CD8+ and 55% CD4+ T cells.
Cytolytic activity of the T cells against idiotype-pulsed dendritic cells
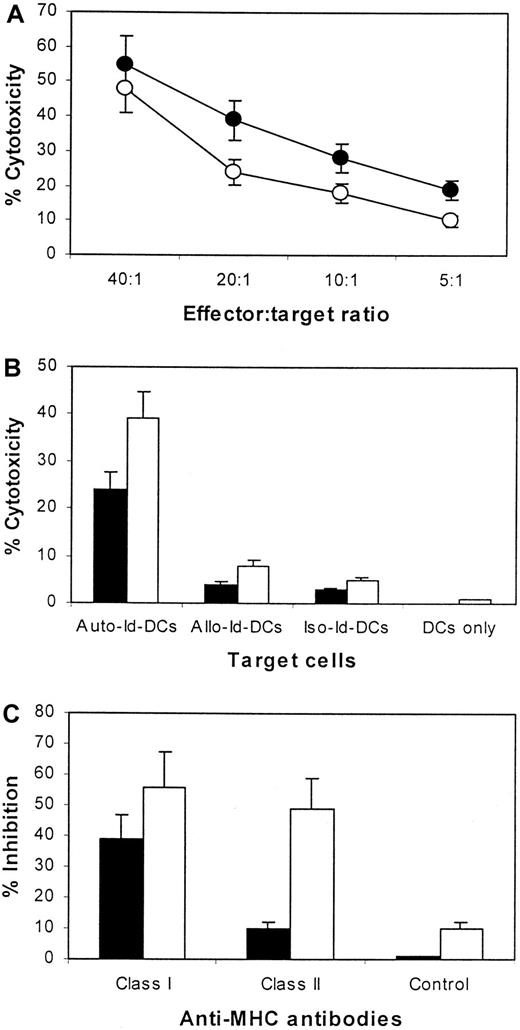
To test whether an Id-specific CTL response could be effectively induced with Id-pulsed DCs, we next assessed the cytotoxic activity of Id-specific T cells in vitro. As target cells, autologous DCs and DCs pulsed with autologous, allogeneic, or isotype-matched Id proteins were used. As shown in Figure 3A, Id-specific CTL lines lysed the autologous DCs pulsed with the autologous Id. No significant killing was observed in autologous DCs without antigen pulsing or in autologous DCs pulsed with the allogeneic or isotype-matched Id proteins (Figure 3B). The results indicate that the T cells were able to mediate Id-specific cytotoxicity.
Cytotoxicity of CTLs against DCs.
(A) Cytotoxicity of CTL lines generated from patients 1 (●) and 2 (○) against DCs pulsed with the autologous Id at different effector–target (E:T) ratios. (B) Comparison of the cytotoxicity of CTLs from patients 1 (▪) and 2 (■) against DCs pulsed with the autologous Id (Auto-Id-DCs), the allogeneic Id protein from the other patient (Allo-Id-DCs), an isotype-matched Id protein (Iso-Id-DCs), or unpulsed DCs (DCs only). The E:T ratio was 20:1. (C) Inhibition by antibodies against MHC class I and class II or an isotypic control IgG on the cytotoxicity of CTLs from patients 1 (▪) and 2 (■). Data are expressed as the mean ± SEM of 4 independent experiments.
Cytotoxicity of CTLs against DCs.
(A) Cytotoxicity of CTL lines generated from patients 1 (●) and 2 (○) against DCs pulsed with the autologous Id at different effector–target (E:T) ratios. (B) Comparison of the cytotoxicity of CTLs from patients 1 (▪) and 2 (■) against DCs pulsed with the autologous Id (Auto-Id-DCs), the allogeneic Id protein from the other patient (Allo-Id-DCs), an isotype-matched Id protein (Iso-Id-DCs), or unpulsed DCs (DCs only). The E:T ratio was 20:1. (C) Inhibition by antibodies against MHC class I and class II or an isotypic control IgG on the cytotoxicity of CTLs from patients 1 (▪) and 2 (■). Data are expressed as the mean ± SEM of 4 independent experiments.
To determine MHC restriction of these Id-specific CTL lines, we studied the inhibitory effect of anti-HLA antibodies on their cytolysis activity. Blocking MHC class I (HLA-ABC) or class II (HLA-DR) molecules of the target cells resulted in an inhibition of the cytotoxicity of the Id-specific CTL against Id-pulsed DCs (Figure 3C). The result further confirmed that the immune epitopes within myeloma Id were presented in association with MHC class I and class II molecules of the patients.
Idiotype-specific cytotoxic T lymphocytes lyse primary myeloma plasma cells in vitro
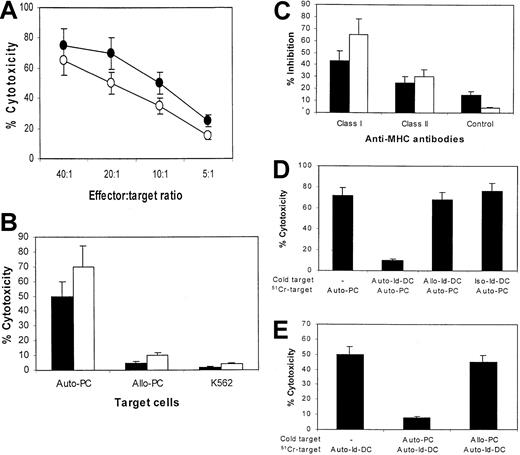
To evaluate whether the Id-specific immune response is relevant to a clinical antimyeloma effect, we examined whether Id-specific CTLs lysed autologous primary myeloma cells. Using the CTL lines generated from the patients as effector cells, significant killing of the autologous myeloma cells was observed (Figure4A). No killing was noted when the allogeneic primary myeloma cells from the other patient or when the natural killer-sensitive target cells K562 were used as target cells (Figure 4B). Blocking of HLA-ABC and, to a lesser extent, HLA-DR molecules of the target cells inhibited the cytotoxicity of the CTLs (Figure 4C). Thus, the results demonstrate that Id-specific CTLs are capable of mediating MHC-restricted cytotoxicity against the autologous primary myeloma cells and suggest that myeloma cells express, on their surfaces, T-cell epitopes derived from Id proteins in the context of MHC molecules.
Cytotoxicity of CTLs against primary tumor cells.
(A) Cytotoxicity of CTL lines generated from patients 1 (●) and 2 (○) against the autologous primary myeloma plasma cells at different E:T ratios. (B) Comparison of cytotoxicity of CTL lines generated from patients 1 (▪) and 2 (■) against the autologous primary myeloma plasma cells (Auto-PC) or the allogeneic myeloma plasma cells from the other patient (Allo-PC), or K562. E:T ratio was 20:1. (C) Inhibition by antibodies against MHC class I and class II or an isotypic control IgG on the cytotoxicity of CTLs from patients 1 (▪) and 2 (■). Data are expressed as the mean ± SEM of 3 independent experiments. Cold target inhibition assay using CTLs from patient 2 at an E:T ratio of 20:1, showing that (D) preincubation of CTLs with autologous Id-pulsed DCs (Auto-Id-DC) inhibited killing of labeled autologous primary cells (Auto-PC) and (E) preincubating with autologous primary cells inhibited the killing of labeled DCs pulsed with autologous Id proteins. Data are expressed as the mean ± SEM of 2 independent experiments.
Cytotoxicity of CTLs against primary tumor cells.
(A) Cytotoxicity of CTL lines generated from patients 1 (●) and 2 (○) against the autologous primary myeloma plasma cells at different E:T ratios. (B) Comparison of cytotoxicity of CTL lines generated from patients 1 (▪) and 2 (■) against the autologous primary myeloma plasma cells (Auto-PC) or the allogeneic myeloma plasma cells from the other patient (Allo-PC), or K562. E:T ratio was 20:1. (C) Inhibition by antibodies against MHC class I and class II or an isotypic control IgG on the cytotoxicity of CTLs from patients 1 (▪) and 2 (■). Data are expressed as the mean ± SEM of 3 independent experiments. Cold target inhibition assay using CTLs from patient 2 at an E:T ratio of 20:1, showing that (D) preincubation of CTLs with autologous Id-pulsed DCs (Auto-Id-DC) inhibited killing of labeled autologous primary cells (Auto-PC) and (E) preincubating with autologous primary cells inhibited the killing of labeled DCs pulsed with autologous Id proteins. Data are expressed as the mean ± SEM of 2 independent experiments.
To examine whether the same T cells mediated the killing of both the Id-pulsed DCs and primary myeloma cells, a cold target inhibition assay was performed. In these experiments, CTLs were first incubated for 2 hours at 37°C with cold, unlabeled autologous DCs pulsed with the Id or the allogeneic Id proteins or with autologous primary tumor cells. As shown in Figure 4D, preincubation of CTLs with the autologous DCs pulsed with the Id, but not the allogeneic or isotype-matched Id proteins, led to diminished killing of the autologous51Cr-labeled primary tumor cells. On the other hand, incubation of CTLs with autologous, but not allogeneic, primary tumor cells first resulted in reduced lysis of the autologous Id-pulsed DCs (Figure 4E). These results indicate that the same T cells were responsible for the killing of both target cells.
Idiotype-specific cytotoxic T lymphocyte response is mediated by the perforin exocytosis pathway
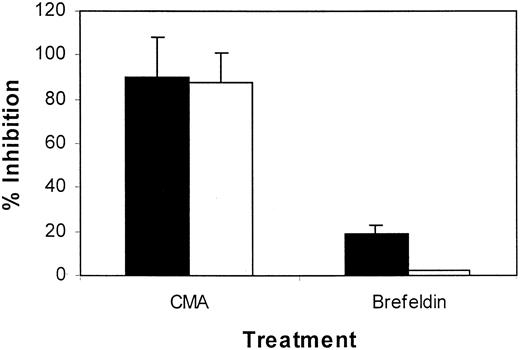
To investigate whether the cytotoxic function of Id-specific CTLs was mediated by the FasL/Fas or perforin systems, we examined the effect of CMA and Brefeldin A, the selected inhibitors of perforin-mediated and Fas-mediated cytotoxicity,17respectively. Treatment of the CTL lines with Brefeldin A induced less than 20% inhibition in the lytic activity of Id-specific CTLs (Figure5), indicating that the Fas/FasL system was not the main pathway of cytotoxicity mediated by our Id-specific CTLs. This result was supported by flow cytometry analysis showing low or absent FasL expression by the T cells (data not shown). In contrast, when we studied the inhibition of cytotoxicity by pretreatment with CMA, the cytolytic activity was almost completely abrogated (Figure 5). Treatment of the CTLs with Brefeldin A or CMA did not induce apoptosis in the cells (data not shown). Taken together, these results indicate that the cytotoxic function of Id-specific CTLs was mediated mainly by the perforin-dependent pathway.
Inhibition of CTL-mediated cytotoxicity against Id-pulsed DCs by the treatment of T cells with CMA or Brefeldin A.
CTLs tested are generated from patients 1 (▪) and 2 (■). Data are expressed as the mean ± SEM of 3 independent experiments.
Inhibition of CTL-mediated cytotoxicity against Id-pulsed DCs by the treatment of T cells with CMA or Brefeldin A.
CTLs tested are generated from patients 1 (▪) and 2 (■). Data are expressed as the mean ± SEM of 3 independent experiments.
Cytokine production
To characterize cytokine-secretion profile and its corresponding subsets of T cells, an ELISPOT assay was used to detect cytokine secretion. As exemplified by the experiments depicted in Figure6A-B, a small number of IFN-γ secreting cells was detected in the unstimulated T cells. After rechallenge with Id-pulsed autologous DCs, elevated numbers of IFN-γ– and TNF-α–secreting cells were demonstrated. Levels of IL-4 secreting cells were extremely low or undetectable. Incubation with DCs only, or with DCs pulsed with the allogeneic Id protein from the other patient, did not significantly affect the number of cytokine-secreting cells. These results were reproduced in the repeated experiments and indicate that the effector cells were of the type-1 T cells.20 21
ELISPOT assay.
ELISPOT assay showing the number of cytokine-secreting cells per 104 CTLs generated from patients 1 (A) and 2 (B), in medium or induced by DCs pulsed with autologous Id (Auto-Id-DCs), allogeneic Id (Allo-Id-DCs), or unpulsed DCs (DCs). Cytokines examined are IFN-γ (▪), TNF-α (■), and IL-4 ( ).
).
ELISPOT assay.
ELISPOT assay showing the number of cytokine-secreting cells per 104 CTLs generated from patients 1 (A) and 2 (B), in medium or induced by DCs pulsed with autologous Id (Auto-Id-DCs), allogeneic Id (Allo-Id-DCs), or unpulsed DCs (DCs). Cytokines examined are IFN-γ (▪), TNF-α (■), and IL-4 ( ).
).
Discussion
In this study, by using Id-pulsed DCs as APCs, we have generated autologous Id-specific CTL lines from patients with MM. These Id-specific CTLs were able to recognize and lyse not only autologous Id-pulsed DCs but also autologous primary myeloma plasma cells, justifying the exploration of Id-based immunotherapy in MM.
Results observed in this study indicate that myeloma Id contains T-cell epitopes recognized by cells mediating proliferative and cytotoxic responses in an MHC-restricted manner. Both the proliferative and the cytotoxic responses of the CTLs generated from 2 patients were restricted by MHC class I and class II molecules, and the CTLs contained CD4+ and CD8+ T cells. The observation is in agreement with some previous studies showing that the Id produced by malignant B cells contains tumor-specific protein sequences that stimulated CD4+ and CD8+ T cells.23-29 Furthermore, the cytokine release by the CTLs was analyzed by the ELISPOT assay. The production of a significant amount of IFN-γ and TNF-α was observed when the CTLs were restimulated with Id-pulsed DCs. IL-4–secreting cells were extremely low or undetectable. Thus, these results indicate that the Id-specific CTL lines contained type-1 CD4+ T-helper cell (Th1) and CD8+ CTL subsets,20 21 and both subsets may play important roles in the antimyeloma effects.
Plasma cells represent the terminal differentiation stage of B-cell development. In MM, plasma cells constitute at least 10%, and can even reach more than 90%, of the total bone marrow cell count.22 One important aspect is whether the myeloma plasma cells can be recognized and regulated by tumor-specific T cells. Our previous study has shown that freshly isolated primary myeloma cells can activate T cells; they stimulate alloreactive T cells and present recall antigens to autologous T cells,30suggesting that myeloma cells can act as APCs. The results of the current study demonstrate that Id-specific CTLs lyse the autologous plasma cells in an MHC-restricted manner, indicating that the tumor cells can process Id protein and present Id peptides in the context of MHC molecules on the cell surfaces. Taken together, these findings indicate that myeloma plasma cells express tumor antigens on their surfaces and are susceptible to tumor-specific, T cell-mediated lysis.
Because we used CTL lines in the current study that might have contained T cells with different specificity, it was important to examine whether the same T cells lysed both Id-pulsed DCs and primary tumor cells. As shown by the results of cold target inhibition experiments in Figure 4, it is clear that preincubation of the CTLs with Id-pulsed DCs inhibited the killing of primary tumor cells and vice versa. Thus, we can conclude that the same T cells mediated the killing. This is an important issue because it indicates that by using the same Id proteins to vaccinate patients, the generation of a strong CTL response against the Id proteins could lead to tumor destruction. Our results also demonstrate that the immune response may be directly against the idiotypic determinants because the T cells responded only to the autologous Id protein, not to the allogeneic or isotype-matched Id proteins. Furthermore, we and others11,28 29 have shown that T-cell responses induced by Id proteins also were against synthetic peptides corresponding to the complementarity-determining region I-III of the Id proteins.
CTL recognition of target cells through their T-cell receptors activates 2 distinct mechanisms of cell lysis.31,32 The first is granule exocytosis mediated by the pore-forming perforin and granzyme A and B. The second involves interaction between the Fas ligand on effector cells and Fas molecules expressed on the target cells. In the current study, the cells appeared to lyse the target cells mainly through the perforin-mediated pathway because CMA, the selective blocking agent, induced 90% inhibition of cytotoxicity. Brefeldin A, a selective inhibitor of the Fas-mediated pathway, resulted in less than 20% inhibition. Hence, these findings are in agreement with our previous study showing that myeloma-reactive allospecific T cells also lysed target cells through the perforin pathway33 and are of special importance in view of published results on Fas expression on myeloma cells. Landowski et al34 have shown that Fas antigen point mutation was detected in 10% of patients' bone marrow samples. The mutations were located in the cytoplasmic region involved in the transduction of an apoptotic signal and, thus, render the cells resistant to Fas-induced apoptosis. Furthermore, they found that myeloma cells induced to be drug resistant also became resistant to Fas-mediated apoptosis.35 Thus, the use of CTLs that are cytotoxic through the Fas-mediated pathway may be limited, but the pore-forming CTLs can be used for the treatment of drug-resistant myeloma.
In conclusion, our results demonstrate that by using DCs as APCs, autologous Id-specific CTLs can be generated from myeloma patients. These T cells are efficient in killing Id-pulsed autologous DCs and primary myeloma cells from patients. Thus, our study provides strong and direct evidence to support the application of Id-based immunotherapies in MM.
Supported in part by a grant from the Leukemia and Lymphoma Society. Q.Y. is a recipient of the Leukemia and Lymphoma Society Translational Research Award.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Qing Yi, Myeloma and Transplantation Research Center, University of Arkansas for Medical Sciences, 4301 W Markham St, Slot 776, Little Rock, AR 72205; e-mail: yiqing@exchange.uams.edu.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal