Genetic deficiency in CD18 leads to disease characterized by myeloid hyperplasia, including profound granulocytosis and splenomegaly. Myeloid hyperplasia could directly result from the disruption of CD18 functions essential to granulopoiesis or basal leukocyte trafficking. Alternatively, myeloid hyperplasia could be reactive in nature, due to disruption of essential roles of CD18 in leukocyte responses to microbial challenge. To distinguish between these mechanisms, the hematopoietic systems of lethally irradiated wild-type (WT) mice were reconstituted with either WT fetal liver cells or CD18-deficient fetal liver cells, or an equal mixture of both types of cells. Granulocytosis and splenomegaly developed in mice that received CD18-deficient fetal liver cells. Splenomegaly was prevented and granulocytosis was inhibited by more than 95% in mice that had received both CD18-deficient and WT fetal liver cells, suggesting that myeloid hyperplasia was largely reactive in nature. Consistent with this postulate, the circulating life spans in the blood and the fraction of neutrophils that incorporated BrdU in the bone marrow were not increased for CD18-deficient neutrophils compared with the WT. However, these animals did develop mild granulocytosis compared with mice reconstituted with WT cells alone, and a higher percentage of CD18-deficient leukocytes were neutrophils compared with the WT leukocytes. These observations suggest that the granulocytosis observed in the absence of CD18 occurs through at least 2 mechanisms: one that is dramatically improved by the presence of WT cells, likely reactive in nature, and a second that is independent of the WT hematopoietic cells, involving an alteration in the lineage distribution of blood leukocytes.
Introduction
Leukocyte adhesion deficiency (LAD) is characterized by frequent soft tissue infections and premature mortality.1-3 LAD type I is caused by a genetic deficiency of CD18, the β-chain of the leukocyte integrins, which are heterodimers of CD11 (CD11a, -b, -c, or -d) and CD18. This deficiency leads to a loss of all immunologically recognizable CD11 and CD18 and the absence of all CD11/CD18 function.1,2 Infected tissues from patients with LAD are generally devoid of extravascular neutrophils, suggesting that CD11/CD18 complexes are often essential for neutrophils to emigrate from blood vessels, and that this defect is responsible for the increased sensitivity to infection. Supporting this hypothesis is the observation that neutrophils from CD18-deficient patients exhibit defective adherence to endothelium.1,2Mice lacking CD18 as the result of gene targeting demonstrate similar symptoms to patients with LAD, including defective neutrophil emigration and frequent soft tissue infections.4-6
In addition to frequent infections, patients and animals lacking CD18 develop marked peripheral blood granulocytosis and myeloid hyperplasia in the spleen and marrow.5 However, the mechanisms that lead to the development of granulocytosis have not been clearly delineated. In this report, we have attempted to distinguish between a mechanism in which granulocytosis persists in the presence of wild-type (WT) hematopoietic cells, which we will refer to as an intrinsic mechanism, and a mechanism in which granulocytosis is resolved by the presence of WT hematopoietic cells, which we will refer to as a reactive mechanism. Intrinsic mechanisms of granulocytosis may include alterations in the production, emigration, or circulating life span of neutrophils, or their precursors. For instance, the absence of CD18 on hematopoietic precursors may lead to the excessive production of neutrophils. Another integrin, β1, has been reported to influence the frequency of hematopoietic precursors.7 Other possibilities include defective emigration of CD18-deficient neutrophils from the circulation,4,6,8-12 resulting in peripheral blood granulocytosis, whereas longer transit times within the sites of hematopoiesis, such as the bone marrow,13could lead to the accumulation of neutrophils in the bone marrow and spleen. Neutrophils lacking CD11b, one of the binding partners of CD18, exhibit reduced rates of spontaneous apoptosis,14 raising the possibility that decreased apoptosis of CD18-deficient neutrophils could play an etiologic role in the development of granulocytosis. If any of these mechanisms predominate, then granulocytosis should persist in the presence of WT hematopoietic cells. In contrast to an intrinsic mechanism, a reactive mechanism for the development of granulocytosis may involve excessive microbial overgrowth in the face of defective neutrophil function,1,2,5 15 leading to the chronic stimulation of the bone marrow and the production of large numbers of granulocytes. This reaction would be suppressed by the presence of sufficient numbers of WT neutrophils, because these cells should provide normal antimicrobial function.
To distinguish between an intrinsic and a reactive mechanism of granulocytosis, we endeavored to compare the properties of WT and CD18-deficient neutrophils in the presence of WT hematopoietic cells. To accomplish this, mice with mixtures of WT and CD18-deficient neutrophils in their blood were generated both stably, by reconstitution of the hematopoietic system of lethally irradiated WT mice with mixtures of WT and CD18-deficient fetal liver cells, and transiently, by adoptive transfer of a mixture of WT and CD18-deficient blood cells into nonirradiated WT hosts. Our results suggest that granulocytosis occurs through both reactive and intrinsic mechanisms.
Materials and methods
Hematopoietic reconstitutions
The hematopoietic systems of C57BL/6-CD45.1 host mice were reconstituted with fetal liver cells as described.6 16Briefly, CD18-deficient mice or WT mice of similar randomly mixed C57BL/6 X129/Sv backgrounds, which express the CD45.2 allele of leukocyte common antigen, were mated with the like genotype. Fetal livers were harvested on day 14 of gestation and single-cell suspensions prepared. 2 × 106 fetal liver cells of either genotype or a mixture containing 1 × 106 fetal liver cells of each genotype were injected intravenously into host C57BL/6-CD45.1 mice that had received radiation doses of 800 and 400 rads from a cesium-137 source separated by 3 hours. After transplantation, mice received trimethoprim-sulfamethoxazole in their drinking water for 1 month. Mice were maintained in full-barrier facilities.
Flow cytometry
For flow cytometric analysis of blood leukocytes, the following antibodies were used: fluorescein isothiocyanate (FITC)–conjugated CD11a (M17/4, Pharmingen, San Diego, CA), phycoerythrin (PE)–conjugated Gr-1 (RB6-8C5, Pharmingen), PE-conjugated B220 (Pharmingen), and biotinylated CD45.2 (104, Pharmingen). Biotin was revealed with either streptavidin-Red670 (Gibco/BRL, Gaithersburg, MD) or streptavidin-allophycocyanin (Pharmingen). Gr-1bright cells had forward- and side-scatter characteristics consistent with neutrophils. Gr-1 was used to identify neutrophils, and CD45.2 was used to identify donor cells. In all experiments, reconstitution with CD45.2+ donor cells was essentially 100%. The total absolute neutrophil count (ANC) was determined by multiplying the fraction of CD45.2+leukocytes that were Gr-1bright by the white blood cell (WBC) count. In mixed chimeras, the ANC for each genotype was determined by multiplying the fraction of Gr-1bright cells that were CD11a+ or CD11a− by the ANC. The WBC count was determined with a hemocytometer.
Analysis of colony-forming units in culture
The 1.5 × 104 bone marrow cells from hosts animals that had received 1:1 mixtures of WT and CD18-deficient fetal liver cells 6 months previously were plated in Methocult GF M3543 (Stemcell Technologies, Vancouver, BC, Canada). Seven to 10 days later, well-separated colonies were harvested and incubated overnight at 55°C in 0.2 M NaCl, 5 mM EDTA, 100 mm Tris-HCl pH 8.0, 0.4% sodium dodecyl sulfate, and 100 μg/mL proteinase K. The following day, DNA was extracted with phenol:chloroform. The 2 μL of DNA was used in a 25 μL 3 primer polymerase chain reaction (PCR) (94°C for 1 minute, 66°C for 2.5 minutes × 40 cycles) with the following primers: CD18-5′WT-5′-CTT CCT GGG ATC TGG TGA GTT CTG-3′; CD18-3′-WT-5′-ATT CCT GGG ACA CAG CTG GGG AGA C-3′; CD18-3′NEO-5′-GAG AAC CTG CGT GCA ATC CAT CTT G-3′. WT (smaller) and CD18-deficient (larger) PCR products could be distinguished by size with standard 2% agarose/Tris/borate/EDTA electrophoresis. Of 84 colonies analyzed, 74 gave unambiguous results.
Adoptive transfer
For adoptive transfer experiments, blood was collected from the inferior vena cava of donor mice into a heparinized syringe. Blood from WT and CD18-deficient animals was mixed and 250 μL immediately injected into unirradiated C57BL/6-CD45.1 hosts. Hosts were bled by retro-orbital puncture at the indicated times after injection. The percentage of donor neutrophils and the ratio of WT to CD18 neutrophils within the donor population were determined by flow cytometry.
BrdU labeling of bone marrow neutrophils
For determining BrdU-labeling index of bone marrow neutrophils, host animals were injected with 0.6 mg of BrdU intraperitoneally twice, separated by 12 hours.17 Twenty-four hours after the first injection, bone marrow cells were harvested and red blood cells were lysed. Cells were stained with PE-conjugated Gr-1 and biotinylated CD11a, and biotin was revealed with streptavidin-Red670. After washing, the cells were resuspended in 0.5 mL of cold 0.15 M NaCl and 1.2 mL of ice-cold 100% ethanol was added dropwise, followed by a 30-minute incubation on ice. Cells were washed with cold phosphate-buffered saline (PBS) and resuspended in 1% paraformaldehyde in PBS with 0.05% Tween-20 and incubated for 30 minutes at room temperature and then overnight at 4°C. The following day, cells were resuspended in 1 mL of a solution consisting of 0.15 M NaCl, 4.2 mM MgCl2, 10 μM HCl, and 100 μ/mL DNase (Sigma, St Louis, MO, DN-25), and incubated for 30 minutes at 25°C. Cells were washed with cold PBS and resuspended in 50 μL of FITC-conjugated anti-BrdU (Becton Dickinson, San Jose, CA), diluted 1:5 in PBS, and incubated at 25°C for 30 minutes. Cells were washed in PBS and then analyzed by flow cytometry.
Statistics
Data sets were compared by analysis of variance using Statistica software (Statsoft, Tulsa, OK). Because data collected from the 6-month animals failed Levene's test for homogeneity of variance, and the means and standard deviations were positively correlated, all data from 6-month animals were log transformed before statistical analyses. Individual groups were compared post hoc by using either the Tukey honest significant different test or Spjotvoll and Stoline's Tukey honest significant difference test for unequal sample sizes. Differences between groups were considered statistically significant when P < .05.
Results and discussion
Granulocytosis develops after hematopoietic reconstitution with CD18-deficient fetal liver cells
Expression of the β2-integrins is limited to hematopoietic tissue, and therefore phenotypic defects observed in animals genetically deficient in CD18 are also assumed to be limited to the hematopoietic system. To test this assumption, WT mice were lethally irradiated and injected with either WT or CD18-deficient fetal liver cells. Donor cells carried the CD45.2 allele of leukocyte common antigen, whereas host mice did not, such that donor cells could be definitively identified by expression of the CD45.2 allele. One or 6 months after transplantation, flow cytometry demonstrated that host animals were reconstituted with donor-derived hematopoietic cells and that WT donor neutrophils expressed CD11a, whereas CD18-deficient neutrophils did not. Animals reconstituted with CD18-deficient donor fetal liver cells had significantly higher circulating ANCs 1 month after transplantation (Figure 1A-B). After 6 months, both total WBC counts and ANCs were increased in the animals that received CD18-deficient cells (Figure 1A-B). Furthermore, animals reconstituted with CD18-deficient fetal liver cells demonstrated significant splenomegaly at 6 months (Figure 1C), and histologic analysis suggested that this was secondary to myeloid hyperplasia within the red pulp (data not shown). These findings are characteristic of CD18-deficient mice and demonstrate that the absence of CD18 in hematopoietic cells alone is sufficient to cause granulocytosis.
Myeloid hyperplasia is inhibited by the presence of WT hematopoietic cells.
(A) WBC counts of reconstituted animals 1 and 6 months after transplantation. WT refers to animals that received only WT fetal liver cells, CD18 to animals that received only CD18-deficient fetal liver cells, and Mix to animals that received 50:50 mixtures of WT and CD18-deficient fetal liver cells. (B) Absolute neutrophil counts of reconstituted animals 1 and 6 months after transplantation. (C) Splenic weights of reconstituted animals 6 months after transplantation. *, increase in Mix is significant (P < .05) compared with WT at same time point. **, increase in CD18 is significant (P < .05) compared with WT or Mix at same time point.
Myeloid hyperplasia is inhibited by the presence of WT hematopoietic cells.
(A) WBC counts of reconstituted animals 1 and 6 months after transplantation. WT refers to animals that received only WT fetal liver cells, CD18 to animals that received only CD18-deficient fetal liver cells, and Mix to animals that received 50:50 mixtures of WT and CD18-deficient fetal liver cells. (B) Absolute neutrophil counts of reconstituted animals 1 and 6 months after transplantation. (C) Splenic weights of reconstituted animals 6 months after transplantation. *, increase in Mix is significant (P < .05) compared with WT at same time point. **, increase in CD18 is significant (P < .05) compared with WT or Mix at same time point.
The presence of wild-type hematopoietic cells limits granulocytosis
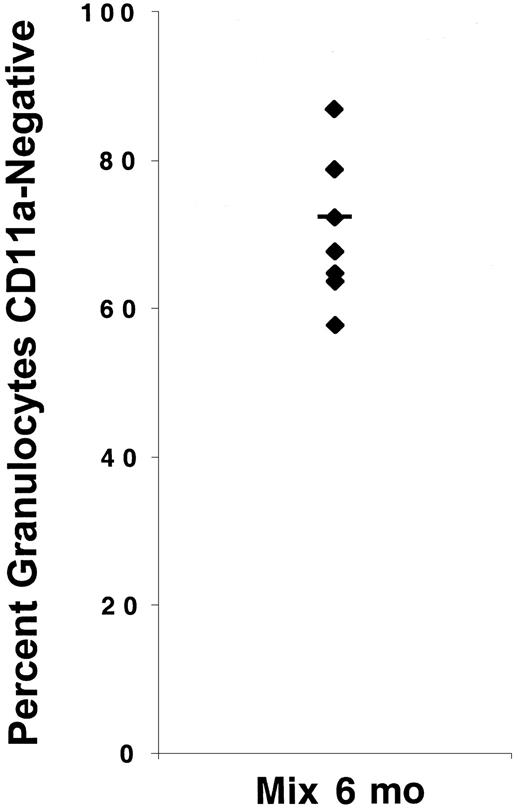
To determine whether granulocytosis would develop in animals reconstituted with mixtures of WT and CD18-deficient hematopoietic cells, lethally irradiated hosts were injected with 50:50 mixtures of WT and CD18-deficient fetal liver cells. Six months after transplantation, the presence of both WT and CD18-deficient neutrophils in the blood of host animals was verified by flow cytometry (Figure2). Although donor fetal liver cells were mixed at a 1:1 ratio, we found that the percentage of neutrophils that were CD18-deficient varied widely but in these experiments was greater than 50% for all animals analyzed (Figure 2). These data demonstrate that experimental animals were reconstituted by both WT and CD18-deficient fetal liver cells. One month after transplantation, there was a small but not statistically significant increase in the ANCs of these animals compared with control animals. By 6 months after adoptive transfer, ANCs were significantly increased compared with the animals transplanted with WT cells, but markedly lower than the ANCs in animals transplanted with CD18-deficient fetal liver cells alone (Figure 1B). Although the mean ANC increased 65-fold in mice reconstituted with CD18-deficient cells alone, the mean ANC increased only 2-fold in mice reconstituted with mixtures of WT and CD18-defcient cells. Six months after the transfer of mixtures of both WT and mutant cells, neither splenic weights nor total circulating WBC counts differed from the animals reconstituted with WT cells alone, whereas as demonstrated previously, both parameters were significantly increased in animals reconstituted with CD18-deficient cells alone (Figure 1A,C). There were only minimal signs of myeloid hyperplasia within the splenic red pulp of mice reconstituted with mixtures of WT and CD18-deficient cells (data not shown). These results demonstrate that the presence of WT hematopoietic cells largely, although not completely, suppresses the development of granulocytosis by cells lacking CD18. This suggests that granulocytosis in the absence of CD18 is caused predominantly by a reactive mechanism.
Host animals were reconstituted with both WT and CD18-deficient neutrophils.
The percentage of neutrophils that were CD18-deficient was determined by flow cytometry 6 months after injection of host animals with 50:50 mixtures of WT and CD18-deficient fetal liver cells.
Host animals were reconstituted with both WT and CD18-deficient neutrophils.
The percentage of neutrophils that were CD18-deficient was determined by flow cytometry 6 months after injection of host animals with 50:50 mixtures of WT and CD18-deficient fetal liver cells.
An important caveat to the interpretation of this mixing experiment is the possibility that CD18-deficient hematopoietic precursors compete poorly with WT hematopoietic precursors. This could lead to a situation in which granulocytosis in the presence of WT cells is suppressed because there are fewer CD18-deficient precursors, despite the ability of these precursors to produce higher numbers of neutrophils than WT precursors. To address this possibility, we compared the percentage of colony-forming units in culture (CFU-C) in the bone marrow that were CD18-deficient to the percentage of neutrophils in the blood that were CD18-deficient in 2 animals that were reconstituted with mixtures of WT and CD18-deficient fetal liver cells. To determine CFU-C genotypes, bone marrow cells were plated in methylcellulose with appropriate growth factors, and 7 to 10 days later individual CFUs were harvested and DNA prepared. The genotype of each CFU was determined by PCR. In the 2 animals examined, the percentage of CD18-deficient CFU-C was 80% and 92%, whereas the percentage of CD18 neutrophils in the blood was 85% and 77%, respectively. This limited experiment suggests that the lack of extensive granulocytosis in animals that received mixtures of WT and CD18-deficient fetal liver cells is not caused by the inability of CD18-deficient granulocyte precursors to compete with WT precursors in the bone marrow.
CD18-deficiency does not increase the time spent in blood or bone marrow
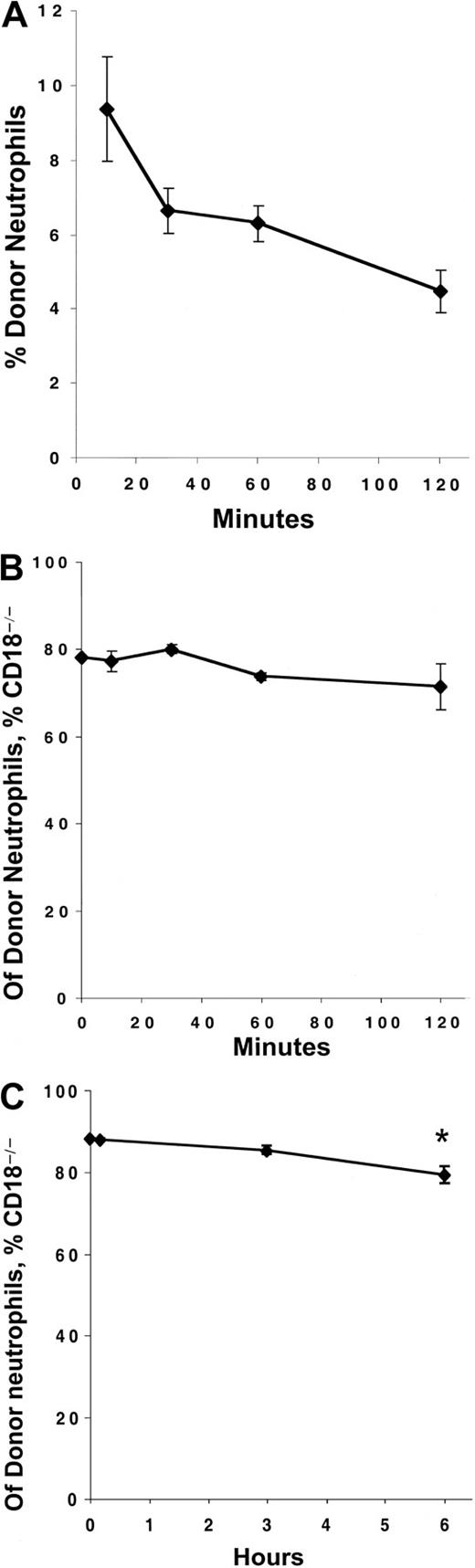
Although the vast majority of the peripheral blood granulocytosis was prevented by the presence of WT cells, the ANCs remained significantly elevated in mice that had received a mixture of WT and CD18-deficient fetal liver cells 6 months previously. Because CD11/CD18 facilitates neutrophil adhesion to endothelial cells lining the blood vessels, and because CD11/CD18 can expedite neutrophil apoptosis,14 it has been proposed that the loss of CD18 could directly contribute to peripheral blood granulocytosis by increasing the circulating life span of neutrophils. Indeed, examination of a CD18-deficient human indicated that this patient's neutrophils circulated longer than historic control values,18 although the disease state of this patient complicated the interpretation. To compare the circulating kinetics of WT and CD18 neutrophils in the same physiologic environment, we performed an adoptive transfer experiment. Blood was collected from WT and CD18-deficient mice, mixed, and immediately injected intravenously into 4 C57BL/6-CD45.1 hosts. Blood was sampled from host mice at various times after injection, and the percentage of donor (CD45.2+) neutrophils and the distribution of WT to CD18-deficient neutrophils within the donor population was determined simultaneously by flow cytometry. As expected, the number of donor neutrophils in the circulation decreased over time (Figure3A). However, the proportions of WT and CD18-deficient donor neutrophils remained virtually constant throughout the 2 hours sampling period (Figure 3B), and were comparable to the original mixture before injection.
The circulating life spans of CD18-deficient neutrophils is not increased compared with WT.
C57BL/6-CD45.1 host animals were injected intravenously with mixtures of blood collected from WT and CD18-deficient animals. (A) The numbers of blood neutrophils (Gr-1bright), which were of donor origin (CD45.2+), were determined by counting and flow cytometry at the indicated time points after injection. (B, C) The percentage of donor neutrophils (CD45.2+, Gr-1bright) that were CD18-deficient (CD11a−) was determined by flow cytometry at the indicated time points. The data point at time 0 refers to the percentage of CD18-deficient neutrophils in the original mixture before injection into the host animal. * indicates significant (P < .05) decrease in the percentage of CD18-deficient neutrophils compared with the earlier time points. Each point represents the mean ± SEM for 4 host mice.
The circulating life spans of CD18-deficient neutrophils is not increased compared with WT.
C57BL/6-CD45.1 host animals were injected intravenously with mixtures of blood collected from WT and CD18-deficient animals. (A) The numbers of blood neutrophils (Gr-1bright), which were of donor origin (CD45.2+), were determined by counting and flow cytometry at the indicated time points after injection. (B, C) The percentage of donor neutrophils (CD45.2+, Gr-1bright) that were CD18-deficient (CD11a−) was determined by flow cytometry at the indicated time points. The data point at time 0 refers to the percentage of CD18-deficient neutrophils in the original mixture before injection into the host animal. * indicates significant (P < .05) decrease in the percentage of CD18-deficient neutrophils compared with the earlier time points. Each point represents the mean ± SEM for 4 host mice.
To determine whether longer periods would reveal differences in circulating kinetics between WT and CD18-deficient neutrophils, a second set of 4 C57BL/6-CD45.1 host mice received intravenous injections of blood mixed from WT and CD18-deficient donor mice. Again, the percentage of donor neutrophils that were CD18-deficient was comparable to the original injection after 10 minutes, and it did not significantly differ after 3 hours (Figure 3C). However, by 6 hours, the percentage of circulating donor neutrophils that were CD18-deficient significantly decreased compared with either 10 minutes or 3 hours after injection (Figure 3C). These data suggest that, between 3 and 6 hours after injection, the CD18-deficient neutrophils disappeared from the circulation at a greater rate than the WT neutrophils. The mechanisms responsible for this accelerated loss of CD18-deficient neutrophils compared with the WT, and the physiologic significance of this alteration, remain uncertain. However, because this alteration would tend to decrease rather than increase the number of CD18-deficient neutrophils in the blood, these data suggest that the peripheral blood granulocytosis of CD18-deficient mice does not result from prolonged circulating life spans of CD18-deficient neutrophils.
To determine the transit time neutrophils spend in the bone marrow, newly formed neutrophils were labeled with the thymidine analog BrdU. BrdU labeling has been used previously to evaluate the transit time of postmitotic hematopoietic cells through the bone marrow. The Gr-1+ compartment within the bone marrow has been reported to be predominantly postmitotic19 and our preliminary experiments confirmed this (data not shown). A BrdU-labeling index was calculated as the percentage of Gr-1bright cells recovered from the bone marrow that were labeled with BrdU. If CD18-deficient neutrophils spend greater time in the bone marrow, they should have a decreased BrdU-labeling index. To compare the BrdU-labeling index, host animals reconstituted with equal mixtures of WT and CD18-deficient fetal liver cells 6 months previously were injected with BrdU twice, at times 0 and 12 hours. Twenty-four hours after the first injection, the animals were killed, and the bone marrow cells were stained simultaneously for CD11a, Gr-1, and BrdU. As shown in Table1, the labeling index of WT and CD18-deficient bone marrow neutrophils was virtually identical (36% ± 1.1% vs 37% ± 2.6%). The observation that approximately 30% of the Gr-1+ positive cells were labeled within a 24-hour period is consistent with previous estimates that used BrdU-labeling techniques in which the transit time of neutrophils through the postmitotic pool in the bone marrow was about 60 hours.20 This suggests that the bone marrow transit times of the WT and CD18-deficient neutrophils are similar.
Percentage of wild-type and CD18−/− bone marrow neutrophils that incorporated BrdU
| Neutrophil genotype . | Animal . | Average . | ||
|---|---|---|---|---|
| 1 . | 2 . | 3 . | ||
| Wild type | 37 | 35 | 36 | 36 ± 1.1 |
| CD18−/− | 35 | 39 | 38 | 37 ± 2.6 |
| Neutrophil genotype . | Animal . | Average . | ||
|---|---|---|---|---|
| 1 . | 2 . | 3 . | ||
| Wild type | 37 | 35 | 36 | 36 ± 1.1 |
| CD18−/− | 35 | 39 | 38 | 37 ± 2.6 |
Animals reconstituted with mixtures of wild-type and CD18−/− hematopoietic cells 6 months previously were labeled with BrdU for 24 hours. Expression of Gr-1 and incorporation of BrdU were determined by flow cytometry. The percentages of wild-type and CD18−/− neutrophils (Gr-1+) that incorporated BrdU are shown. The percentages of Gr-1+ cells that were CD18-deficient were 85%, 83%, and 79% in animals 1, 2, and 3, respectively. A separate experiment, again with 3 mice, confirmed no differences in BrdU-labeling indices between WT and CD18-deficient neutrophils in the marrow.
Altogether, these data demonstrate that, within the same physiologic environment, neither the circulating life span nor the bone marrow transit time of CD18-deficient neutrophils are increased compared with the WT. These results are consistent with the hypothesis that granulocytosis in the absence of CD18 is not caused by intrinsic defects in neutrophil trafficking.
Alteration in leukocyte distribution in the absence of CD18
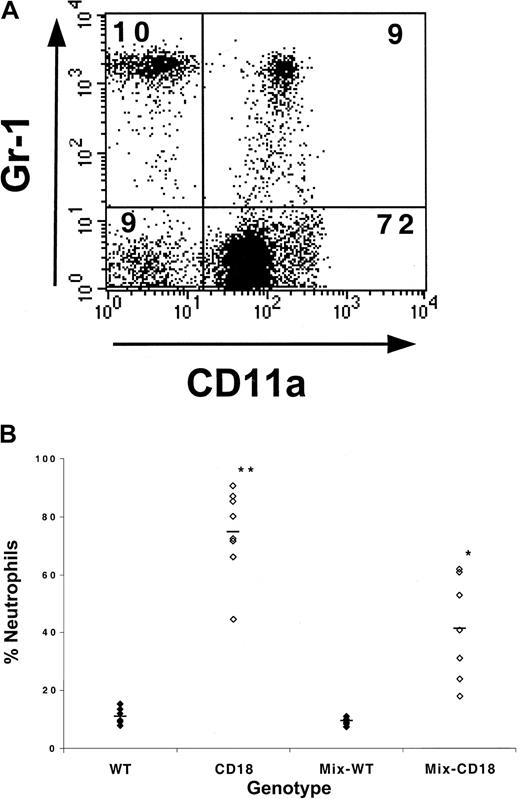
The data presented above strongly argue that granulocytosis in the absence of CD18 is predominantly a reactive process because it can be repressed by the presence of WT hematopoietic cells. Nonetheless, there have been reports that other integrin molecules can influence the development of hematopoietic precursors,7 and it has been well-documented that β2-integrins are expressed in certain hematopoietic precursor populations.21 22 To evaluate the lineage distribution of WT and CD18-deficient leukocytes, we compared the percentage of WT or CD18-deficient leukocytes that were neutrophils in the blood of animals that had received either WT fetal liver cells or CD18-deficient fetal liver cells, or mixtures of both WT and CD18-deficient fetal liver cells. To accomplish this, blood leukocytes were stained with CD11a, Gr-1, and CD45.2 and analyzed by flow cytometry. Donor leukocytes were identified by forward- and side-scatter characteristics and staining with CD45.2. Within this gate, the percentage of CD11a+ cells that were Gr-1bright was determined as a measure of the percentage of WT leukocytes that were neutrophils, and the percentage of CD11a− cells that were Gr-1bright indicated the percentage of CD18-deficient leukocytes that were neutrophils (Figure 4A). As expected, we observed an increase in the percentage of leukocytes that were neutrophils in animals that had received CD18-deficient fetal liver cells alone (Figure 4B). More surprisingly, in animals that had received mixtures of WT and CD18-deficient fetal liver cells, there was also an increase in the percentage of CD18-deficient leukocytes that were neutrophils compared with the percentage of WT leukocytes that were neutrophils (Figure 4B). This was accompanied by a reciprocal decrease in the percentage of CD18-deficient leukocytes that were presumably mononuclear cells (ie, not Gr-1bright). Independent staining for B cells with B220 reflected this change, as the percentage of CD18-deficient leukocytes that were B cells was decreased compared with the percentage of WT leukocytes that were B cells (data not shown).
WT WBCs only partially prevent the increase in CD18-deficient leukocytes that are neutrophils.
(A) To determine the percentage of WT or CD18-deficient leukocytes that were Gr-1+, blood leukocytes were stained and analyzed by flow cytometry as described in the text. The dot plot is an example of blood leukocytes derived from a CD45.1+ host that had received a 1:1 mixture of WT and CD18-deficient fetal liver cells 6 months previously. The plot shows the CD11a and Gr-1 staining profiles of CD45.2+ cells that fall within the forward- and side-scatter leukocyte gate. The percentage of cells that fall within each quadrant is shown. (B) The percentage of WT or CD18-deficient leukocytes that were neutrophils was determined by flow cytometry. WT and CD18 refer to WT or CD18-deficient leukocytes from host animals that had received either WT or CD18−/− fetal liver cells alone. Mix-WT and Mix-CD18 refers to WT and CD18−/−leukocyte populations, respectively, from animals that had received mixtures of both WT and CD18-deficient fetal liver cells. *, increase in the percentage of Mix-CD18 is significant (P < .05) compared with WT or Mix-WT. **, increase in CD18 is significant (P < .05) compared with WT, Mix-WT, and Mix-CD18.
WT WBCs only partially prevent the increase in CD18-deficient leukocytes that are neutrophils.
(A) To determine the percentage of WT or CD18-deficient leukocytes that were Gr-1+, blood leukocytes were stained and analyzed by flow cytometry as described in the text. The dot plot is an example of blood leukocytes derived from a CD45.1+ host that had received a 1:1 mixture of WT and CD18-deficient fetal liver cells 6 months previously. The plot shows the CD11a and Gr-1 staining profiles of CD45.2+ cells that fall within the forward- and side-scatter leukocyte gate. The percentage of cells that fall within each quadrant is shown. (B) The percentage of WT or CD18-deficient leukocytes that were neutrophils was determined by flow cytometry. WT and CD18 refer to WT or CD18-deficient leukocytes from host animals that had received either WT or CD18−/− fetal liver cells alone. Mix-WT and Mix-CD18 refers to WT and CD18−/−leukocyte populations, respectively, from animals that had received mixtures of both WT and CD18-deficient fetal liver cells. *, increase in the percentage of Mix-CD18 is significant (P < .05) compared with WT or Mix-WT. **, increase in CD18 is significant (P < .05) compared with WT, Mix-WT, and Mix-CD18.
In animals that received mixtures of WT and CD18-deficient fetal liver cells, the increase in ANCs and the selective expansion of CD18-deficient neutrophils relative to other blood cells suggest that CD18 plays a direct role in determining the lineage distribution of circulating leukocytes. This alteration in leukocyte distribution was a consistent finding in all mice that received mixtures of CD18-deficient and WT fetal liver cells, regardless of the ratio of CD18-deficient to WT leukocytes in the particular animal. Furthermore, this alteration was observed in animals that received lower ratios of CD18-deficient to WT fetal liver cells in the initial transplantation step (data not shown). Given our inability to identify defects in the trafficking of postmitotic neutrophils, we suggest that this alteration is caused by an intrinsic alteration of hematopoiesis in the absence of CD18. Further studies will be necessary to conclusively determine the roles of CD18 in establishing the lineage distribution of circulating leukocytes. The lineage distribution was further skewed in the mice reconstituted with CD18-deficient cells alone than in the CD18-deficient population of leukocytes in mice reconstituted with mixtures of WT and CD18-deficient cells, suggesting that a reactive component may exacerbate the skewing of lineage distributions intrinsic to CD18-deficient leukocytes.
Humans and mice with CD18-deficiency develop granulocytosis. There appears to be several mechanisms underlying this abnormality. More than 95% of the granulocytosis is suppressed when WT neutrophils are present. We suggest that the most likely explanation for this phenomenon is that CD18-deficient neutrophils exhibit defective antimicrobial functions, resulting in infection, subsequent stimulation of the bone marrow, and reactive granulocytosis. When WT neutrophils are present, they provide antimicrobial functions that limit granulocytosis, despite the presence of neutrophils lacking CD18. However, even in the presence of WT neutrophils, a mild but significant granulocytosis develops over time, and the percentage of leukocytes that are neutrophils is consistently higher for the CD18-deficient population of leukocytes than for the WT population. These data suggest that intrinsic mechanisms may be contributing to the granulocytosis as well. Alterations in circulating life span appear unlikely, based on the observation that CD18-deficient neutrophils are cleared from the circulation no slower than WT neutrophils. The observation that the BrdU labeling is similar suggests that turnover rate in the bone marrow is unchanged, although this does not exclude an intrinsic alteration in hematopoietic lineage commitment or an alteration in release from the bone marrow after a stressful stimulus. The data suggesting that the percentages of WT and CD18-deficient CFU-C in the marrow parallels the percentage of WT and CD18-deficient neutrophils in the blood argues that if there is an alteration in hematopoietic lineage commitment, it is occurring before development of CFU-C. It is possible that a defect in the ability of an early CD18-deficient hematopoietic precursor or stem cell to compete with WT cells could lead both to the suppression of granulocytosis and the alteration of lineage commitment that we have observed. In this scenario, a small number of CD18-deficient hematopoietic precursors could be producing an increased number of granulocytes per precursor. This could lead to equivalent numbers of WT and CD18-deficient neutrophils in the blood but a paucity of CD18-deficient mononuclear cells, leading to a skewing of the lineage distribution. Evaluating this possibility will require detailed analyses of early hematopoietic progenitors beyond the scope of this study. Thus, the exact mechanisms underlying the intrinsic component of the granulocytosis remain to be determined. The dysregulation of circulating neutrophils resulting from CD18 deficiency is clearly complex and multifactorial, likely resulting from the loss of CD18 functions essential to leukocyte lineage distribution combined with the loss of CD18 functions essential to host defense and homeostasis.
Supported by National Institutes of Health grant HL48160, a Clinical Scientist Award in Translational Research from the Burroughs Wellcome Fund, a research grant from the American Lung Association, and a Parker B. Francis Fellowship from the Francis Families Foundation.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Bruce H. Horwitz, LMRC-511, Immunology Research Division, Department of Pathology, Brigham and Women's Hospital, 221 Longwood Ave, Boston, MA 02115; email: bhorwitz@rics.bwh.harvard.edu.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal