Lymph nodes with Hodgkin disease (HD) harbor few neoplastic cells in a marked leukocytic infiltrate. Since chemokines are likely to be involved in the recruitment of these leukocytes, the expression of potentially relevant chemokines and chemokine receptors were studied in lymph nodes from 24 patients with HD and in 5 control lymph nodes. The expression of regulated on activation, normal T cell expressed and secreted (RANTES), monocyte chemotactic protein (MCP)–1, macrophage inflammatory protein (MIP)–1α, and MIP-1β was analyzed by in situ hybridization and that of CCR3 and CCR5 by immunohistochemistry and flow cytometry. It was found that, overall, the expression of all 4 chemokines was markedly enhanced, but the cellular source was different. RANTES was expressed almost exclusively by T cells whereas the expression of MCP-1, MIP-1α, and MIP-1β was confined largely to macrophages. In control lymph nodes, chemokine expression was low, with the exception of MIP-1α in macrophages. CCR3 and CCR5 were highly expressed in T cells of HD involved but not of control lymph nodes. CCR3 was equally distributed in CD4+ and CD8+ cells, but CCR5 was associated largely with CD4+ cells. In HD lymph nodes, CCR3 and CCR5 were also expressed in B cells, which normally do not express these receptors. All these chemokines and receptors studied, by contrast, were absent in the neoplastic cells. It was concluded that chemokines are involved in the formation of the HD nonneoplastic leukocytic infiltrate. Expression of CCR3 and CCR5 appears to be characteristic of HD, but the roles of these receptors' up-regulation for the disease process remain unclear.
Introduction
Hodgkin disease (HD) is characterized by the presence of few neoplastic mononuclear Hodgkin cells and polynuclear Reed-Sternberg (H-RS) cells surrounded by a dense nonneoplastic leukocytic infiltrate consisting of lymphocytes, plasma cells, granulocytes, and macrophages.1 In the nodular sclerosis (NS) and mixed cellularity (MC) subtypes, CD4+ T cells are particularly frequent in this infiltrate. They almost exclusively have the CD45RO+ and CD45RB+ phenotype and are in a state of activation that, based on their cytokine expression, appears to be related to a T-helper 2 (TH2)–mediated immune response.2-4 H-RS cells express various cell surface molecules, including CD58 (intercellular adhesion molecule 1), CD40, CD30, and CD80 (B7-1) as well as major histocompatibility complex class II molecules,5,4 and produce numerous cytokines, such as interleukin (IL)–1, IL-5, IL-6, IL-9, macrophage–colony stimulating factor, tumor necrosis factor (TNF)–α,6 and IL-13,7 that may have multiple effects on the surrounding leukocytes.
Few studies have addressed the role of chemokines in HD. For instance, the IL-8 gene expressed by reactive cells in lymph nodes involved by HD is related to the presence of neutrophilic granulocytes.8Studies based mainly on reverse-transcription polymerase chain reaction technology show that HD tissues also express higher levels of inducible protein 10 (IP-10), monokine induced by interferon γ (Mig), regulated on activation, normal T cell expressed and secreted (RANTES), macrophage inflammatory protein (MIP)–1α, and, in particular, eotaxin, when compared with control lymph nodes,9,10 but do not provide information on the cellular sources of these chemokines or on the expression of their receptors. It has also been recently demonstrated that the T-cell–directed chemokine thymus- and activation-regulated chemokine (TARC) is expressed in H-RS cells.11
It is likely that lymphocytes and other leukocytes in the background of HD do not solely represent residual nonfunctioning tissues but may be attracted by and also interact with neoplastic cells and potentially affect the tumor progression. Such an “inflammatory” infiltrate could therefore represent a potential therapeutic mechanism to control the spread of the disease or to reduce tumor growth and/or volume. Thus, to better understand the mechanisms involved in the recruitment or trapping of nonneoplastic leukocytic infiltrates in HD, we studied the expression patterns of the T-cell–attracting CC chemokines RANTES, monocyte chemotactic protein (MCP)–1, MIP-1α, and MIP-1β and their receptors CCR5 and CCR3 on tissues involved by this malignant lymphoma.
Materials and methods
Cell culture
The L428 and KMH2 Hodgkin-derived cell lines were kindly provided by M. G. Battelli, Bologna, Italy, and A. Ho, Amgen Institute, Mississauga, ON, Canada, respectively. Both cell lines were grown at 37°C in RPMI 1640 medium supplemented with penicillin, streptomycin, and 10% fetal calf serum. Cells were stimulated with Concanavalin (ConA) at 1 or 2 μg/mL or with lipopolysaccharide (LPS) at 1 μg/mL up to 10 days for studies on chemokine receptor expression.
Tissue samples
Formaldehyde-fixed, paraffin-embedded tissue samples with classical HD were obtained from 24 patients (14 men, 10 women; mean age, 39 years). Of these patients, 19 had HD that belonged to the NS, and 5 had HD belonging to the MC subtype. The least common variant of classical HD, the lymphocyte-depletion subtype, was excluded from the study. In 7 cases, frozen tissue specimens were available for immunohistochemical studies of chemokine receptor expression, and in 5 cases, fresh material was available for preparation of cell suspensions. In 20 patients, HD was diagnosed for the first time whereas 4 cases represented relapses. In addition, we studied 2 cases of large-cell peripheral T-cell lymphoma and 1 case of Hodgkin-like anaplastic large-cell lymphoma of T-lineage (ALCL). Control tissues consisted of 5 lymph nodes with follicular and diffuse hyperplasia.
Preparation of cell suspensions from fresh tissues
Control and HD lymph nodes were cut into small pieces and gently pressed with a glass homogenizer. Dissociated cells were collected, and the remaining tissue pieces were digested for 2 × 30 minutes at 37°C with collagenase V (1 mg/mL, Sigma Chemical, St Louis, MO) in 10% Hanks balanced salt solution (HBSS) supplemented with 5% horse serum (HS), 0.5 mM MgCl2, 0.6 mM MgSO4, and 1.3 mM CaCl2, and with Hepes added to 10 mM (pH 7.4). Cells were pooled and strained through a 40-μm–wide mesh, washed twice, and resuspended in HBSS and 5% HS at a final concentration of 107/mL.
35S-labeled riboprobes
Probes for in situ hybridization were prepared as previously described.12 Briefly, the following complementary DNA (cDNA) fragments were used: a 410–base pair (bp)EcoRI - ApaI fragment (position 1-411 of the coding sequence) of the human RANTES cDNA (Genentech, San Francisco, CA), subcloned into a pBluescript KS+ expression vector (Stratagene, La Jolla, CA); a 650-bp NotI - HindIII fragment of the human MCP-1 cDNA (provided by T. Yoshimura, Frederick, MD), subcloned into a pBluescript SK−; a 351-bp SmaIfragment (position 81-371 of the coding sequence) of the human MIP-1α cDNA, subcloned into a pGEM-7 (Invitrogen, Groningen, Netherlands); a 531-bp EcoRI fragment (position 1-531 of the coding sequence) of the human MIP-1β cDNA (provided by R. Gillitzer, Würzburg, Germany), subcloned into a pBluescript SK+; and a 1.1-kilobase BamHI - SalI fragment of human CCR5 cDNA (provided by C. Combadière, Paris, France), subcloned into a pBluescript II SK+. After linearization with the appropriate restriction enzymes, sense and antisense probes were generated by means of SP6, T3, or T7 RNA polymerases (Roche Diagnostics, Basel, Switzerland) and 35S-CTP (Amersham, Arlington Heights, IL). The labeled probes were size-reduced by alkaline hydrolysis to an average length of 100 to 200 bases before precipitation.
In situ hybridization
In situ hybridization of sections from formaldehyde-fixed and paraffin-embedded tissues was performed with minor modifications as previously described.12 Tissue sections were dewaxed and rehydrated in graded ethanol. After treatment with 100 μg/mL proteinase K (Roche Diagnostics) in 100 mM Tris-HCl, pH 8.0, and 50 mM EDTA at 37°C for 30 minutes, tissues were hybridized with the indicated labeled sense or antisense probes overnight at 50°C in a moist chamber. With the exception of proteinase K digestion at 1 μg/mL, sorted cells on slides were treated identically. Nonhybridized probe was removed by treatment with 20 μg/mL RNAse A and 1 U/mL RNAse T1 (Sigma Chemical). Slides were dipped into NTB-2 emulsion (Eastman-Kodak, New Haven, CT) diluted 1:2 in 800 mM ammonium acetate, pH 7.5. After exposure in the dark at 4°C for 4 weeks, slides were developed in Kodak PL-12 solution and counterstained with Gill's hematoxylin.
Cells were considered to be positive for messenger RNA (mRNA) expression when they had at least 3 times as many silver grains as the highest background obtained with the corresponding sense probe. In tissue sections, we evaluated 5 randomly selected fields (0.16 mm2) and calculated the mean value of positive cells, expressed as percentage of total nucleated cells. For evaluation of sorted cells, at least 500 cells per population were counted. The cells labeled with the sense probe never exceeded 5% of the cells labeled with the antisense probe.
Immunohistochemistry
All paraffin-embedded tissue samples were routinely studied by immunohistochemistry with commercially available antibodies directed against leukocyte common antigen, CD20, CD79a, CD3, CD30, CD15, EMA, Epstein-Barr virus (EBV)–latent membrane protein, J-chain as well as kappa and lambda light chains. Tissue from 7 HD and 4 control lymph nodes were snap-frozen and cut into 5-μm–thick sections. After fixation in acetone and rehydration, tissue sections were incubated for 60 minutes at room temperature with 1:100 dilution of anti-CCR5 (clone 2D7, PharMingen, San Diego, CA) or anti-CCR3 (clone 7B11, Leukosite, Cambridge, MA) as well as anti-CXCR3 (clone LS11). Isotype-matched mouse immunoglobulin (Ig)–G (Dako, Glostrup, Denmark) was used as control. After washing with Tris-buffered saline, slides were incubated for 45 minutes with a biotin-conjugated goat antimouse IgG (Dako). Following washing, slides were incubated for 45 minutes with an alkaline phosphatase– or horseradish peroxidase–conjugated avidin-biotin complex (Dako). The color reaction was developed with New Fuchsin (Sigma Chemical) or diaminobenzidine (Merck, Gibbstown, NJ). Sections were counterstained with Gill's hematoxylin.
Fluorescence-activated cell sorting and analysis
Cells isolated from 2 cases of NS and 2 control lymph nodes were labeled with anti-CD3, anti-CD4, anti-CD8, anti-CD14, anti-CD19, or anti-CD20 monoclonal antibodies (mAbs) (all from Pharmingen), diluted according to the manufacturer's instructions, sorted on polylysine-coated glass slides (Menzel Gläser, Braunschweig, Germany) using a FACS Vantage (Becton Dickinson Immunocytometry Systems, Basel, Switzerland), and subsequently hybridized with sense and antisense probes for RANTES, MCP-1, MIP-1α, MIP-1β, and CCR5.
For double immunostainings, cell suspensions were prepared from 5 fresh HD tissues and 5 control lymph nodes. Cells were first incubated with mouse Abs against CCR5 (clone 45523.111, R&D Systems, Minneapolis, MN), CCR3, or CXCR3 (see above) followed by phycoerythrin (PE)– or fluorescein isothiocyanate (FITC)–conjugated antimouse IgG F(ab′)2 fragments (Southern Biotechnologies, Birmingham, AL). Second antigen staining was performed with PE- or FITC-conjugated mouse Abs against CD3, CD4, CD8, and CD19 (all from PharMingen). Isotype-matched mouse IgG was used as a control.
Additionally, suspensions of L428 and KMH2 cell lines, cultured with or without ConA or LPS, were stained with mAbs against CCR5, CCR3, or CXCR3 (see above) and analyzed for receptor expression on FACScan.
Chemotaxis assay
Cell suspensions from lymph nodes with HD were prepared as described above and kept overnight in RPMI at 37°C. Cell migration was measured in 48-well chemotaxis chambers (Neuro Probe, Cabin John, MD). Phytohemagglutinin (PHA)–activated peripheral blood lymphocytes were used as a positive control in the migration assay. In brief, chemokines in Hepes-buffered RPMI 1640 supplemented with pasteurized plasma protein (Swiss Red Cross Laboratory, Bern, Switzerland) were added to the lower wells and 100 000 cells resuspended in the same medium to the upper wells. Polyvinylpyrrolidone-free polycarbonate membranes (Poretics, Livermore, CA) with 3-μm pores coated with type IV collagen were used. After incubation for 2 hours, the membrane was removed, washed on the upper side with phosphate-buffered saline, fixed, and stained. Migrated cells were counted at 1000 × magnification in 5 randomly selected fields per well. The assay was performed in triplicate.
Results
Expression of CC chemokine genes in lymph nodes with HD
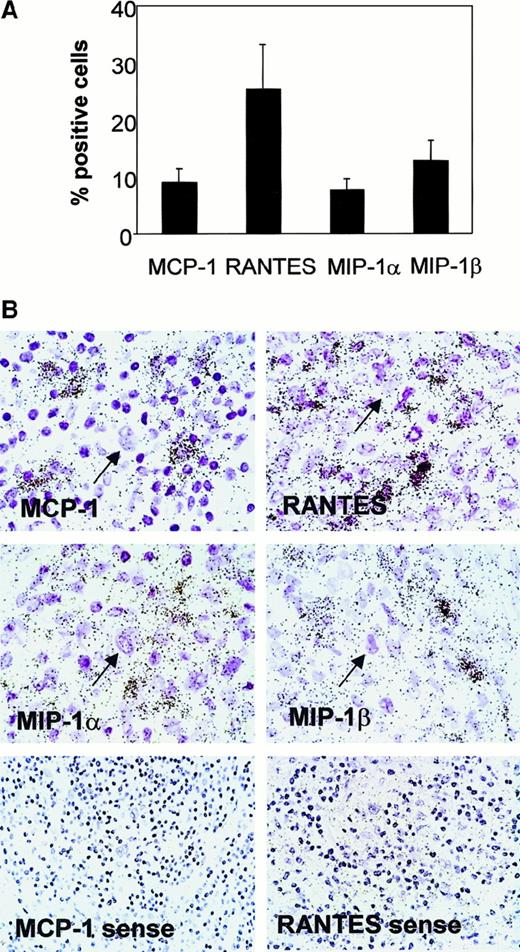
Cells expressing RANTES mRNA were the most numerous, were evenly distributed, and constituted 25% (SE, 9%) of all nucleated cells of the nonneoplastic leukocytic infiltrate of HD lymph nodes. The average percentage (SE) of cells expressing MCP-1, MIP-1α, and MIP-1β was 9.0% (2.5%), 7.5% (2.3%), and 12.5% (3.8%), respectively (Figure1). As observed for RANTES, positive cells were found largely in the background infiltrate. MCP-1 and, in particular, MIP-1α transcripts were occasionally found in connective-tissue fibroblasts, especially in the NS subtype. H-RS cells, recognized by their morphology, consistently lacked hybridization signals for all the chemokines analyzed (Figure 1). The chemokine gene expression pattern was similar, in both qualitative and quantitative terms, at the time of first diagnosis and in relapses. There was no dependence on the EBV status or on the histological subtype. In control lymph nodes with follicular or diffuse lymphatic hyperplasia, chemokine-expressing cells never exceeded 1% of the entire cell populations, and the positive cells were scattered throughout the lymphoid tissue without relation to any particular anatomical structure of the lymph node.
Chemokine gene expression in HD lymph nodes.
(A) Percentage of cells expressing RANTES, MCP-1, MIP-1α, and MIP-1β. Results represent the mean (± SE) from 19 cases hybridized with specific antisense probes and evaluated quantitatively. (B) Representative in situ gene expression left: All the chemokines are expressed in the nonneoplastic leukocytic infiltrates of HD but not in H-RS cells (arrows). The sense probe controls for MCP-1 and RANTES are shown. The sense probe controls for MIP-1α and MIP-1β were comparable (data not shown).
Chemokine gene expression in HD lymph nodes.
(A) Percentage of cells expressing RANTES, MCP-1, MIP-1α, and MIP-1β. Results represent the mean (± SE) from 19 cases hybridized with specific antisense probes and evaluated quantitatively. (B) Representative in situ gene expression left: All the chemokines are expressed in the nonneoplastic leukocytic infiltrates of HD but not in H-RS cells (arrows). The sense probe controls for MCP-1 and RANTES are shown. The sense probe controls for MIP-1α and MIP-1β were comparable (data not shown).
We also analyzed 3 T-cell lymphomas. Scattered signals for all 4 chemokines were observed throughout the involved lymph nodes. Interestingly, in a single case of ALCL, expression for RANTES and MCP-1 was restricted to neoplastic cells, with only rare transcripts in the reactive infiltrate (data not shown).
Chemokine expression pattern in T lymphocytes and macrophages
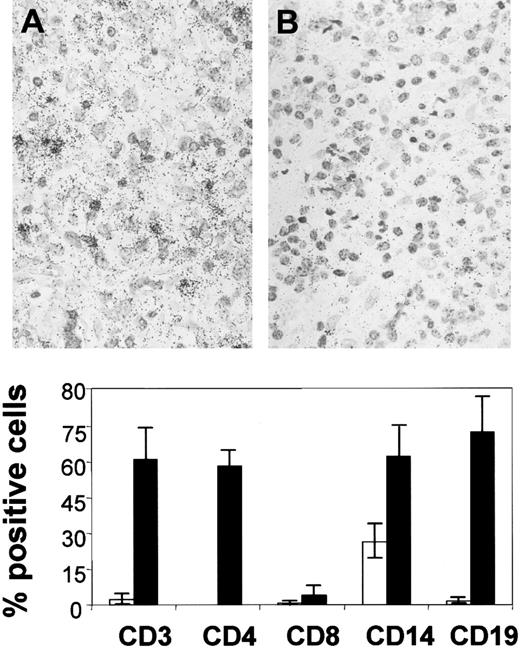
The markedly increased expression of RANTES, MCP-1, MIP-1α, and MIP-1β in HD as compared with control lymph nodes prompted us to assess the nature of the chemokine-producing cells. Single-cell suspensions were obtained from lymph nodes of 2 patients with NS subtype HD and negative EBV status and from 2 control lymph nodes. The cells were sorted for CD3, CD4, CD8, CD14, and CD19 and were analyzed on slides for chemokine expression by in situ hybridization. As shown in Table 1, in lymph nodes with HD, RANTES expression was markedly up-regulated in CD4+ and CD8+ T cells, which represented 46% (mean of 2 experiments) and 13.3% of the sorted cells, respectively. By contrast, the expression of MCP-1, MIP-1α, and MIP-1β was up-regulated in CD14+ macrophages, which constituted 9.0% of the cells sorted from the nonneoplastic leukocytic infiltrate. In control lymph nodes, chemokine expression was low or moderate, except for MIP-1α in macrophages.
Percentage of cells isolated from Hodgkin disease and control lymph nodes expressing the chemokine genes within a given cell subset
| . | RANTES . | MCP-1 . | MIP-1α . | MIP-1β . | ||||
|---|---|---|---|---|---|---|---|---|
| HD . | Controls . | HD . | Controls . | HD . | Controls . | HD . | Controls . | |
| CD3 | 76.4 | — | 2.5 | 3.0 | 1.4 | — | 3.2 | — |
| CD4 | 74.6 | — | 1.1 | 2.5 | 3.8 | — | 3.0 | — |
| CD8 | 86.4 | — | 4.6 | 5.4 | — | — | 2.8 | — |
| CD14 | 10.6 | 11.0 | 62.0 | — | 34.2 | 20.0 | 31.2 | — |
| CD19 | — | — | — | — | — | — | — | — |
| . | RANTES . | MCP-1 . | MIP-1α . | MIP-1β . | ||||
|---|---|---|---|---|---|---|---|---|
| HD . | Controls . | HD . | Controls . | HD . | Controls . | HD . | Controls . | |
| CD3 | 76.4 | — | 2.5 | 3.0 | 1.4 | — | 3.2 | — |
| CD4 | 74.6 | — | 1.1 | 2.5 | 3.8 | — | 3.0 | — |
| CD8 | 86.4 | — | 4.6 | 5.4 | — | — | 2.8 | — |
| CD14 | 10.6 | 11.0 | 62.0 | — | 34.2 | 20.0 | 31.2 | — |
| CD19 | — | — | — | — | — | — | — | — |
Results represent the mean percentage of 2 experiments. Controls indicate control lymph nodes. A dash indicates not detected.
RANTES indicates regulated on activation, normal T cell expressed and secreted; MCP, monocyte chemotactic protein; MIP, macrophage inflammatory protein; HD, Hodgkin disease.
Expression of CCR3 and CCR5
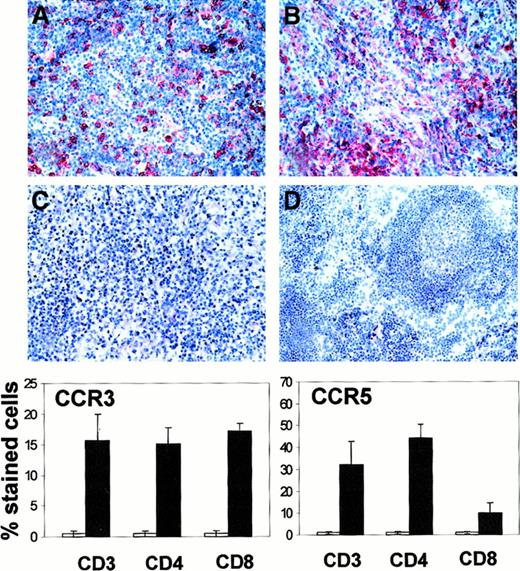
We studied the expression of 2 receptors that appear particularly relevant for the action of the detected chemokines, in particular CCR5 and CCR3 for RANTES and CCR5 for MIP-1α and MIP-1β. Cells expressing CCR3 and CCR5 were very rare in control lymph nodes. In contrast, both receptors were strongly expressed in about half the nonneoplastic leukocytes of lymph nodes involved by HD (Figure2). Immunohistochemical stainings suggested that both chemokine receptors were absent in H-RS cells. We thus analyzed 2 HD-derived cell lines, L428 and KMH2, by flow cytometry. Both were negative for CCR3 and CCR5 even after the cells were cultured for 10 days in the presence of ConA or LPS (data not shown). We also studied the expression of these receptors in T cells freshly isolated from control and HD lymph nodes. Compared with control lymph nodes, a marked up-regulation of CCR5 (expressed as mean percentage [SE]) was found in CD4+ T cells (1.5% [0.4%] and 44.2% [6.3%], respectively) but not in CD8+ T cells (1.3% [0.5%] and 10.0% [4.4%], respectively), whereas CCR3 expression was moderately up-regulated in both subsets (15.2% [2.5%] and 17.2% [1.1%] for CD4+ and CD8+ T cells, respectively), as shown in Figure 2. We then compared the expression of CCR3 and CCR5 in T cells from HD with the expression of CXCR3, which is the receptor for IP-10 and Mig, ie, chemokines involved in T-cell chemotaxis and known to be highly expressed in HD.9 Immunohistochemically, CXCR3 did not appear to be up-regulated in HD lymph nodes. It was less commonly expressed than CCR3 and CCR5, since it was detected only in about one fifth of the nonneoplastic leukocytes of both HD and control lymph nodes (data not shown). Flow cytometry studies on lymphocytes freshly isolated from HD and control lymph nodes, however, showed a moderate up-regulation of CXCR3 expression in CD4+T cells (51.7% [6.3%] and 28.2% [2.6%], respectively) but not in other lymphocytes subsets, ie, CD8+ cells or B cells.
CCR3 and CCR5 expression in HD and control lymph nodes.
Immunohistochemical staining of lymph nodes involved by HD with mAb directed against CCR5 (A), CCR3 (B), and isotype-matched control antibody (C). No CCR5 expression is detectable in control lymph nodes with follicular hyperplasia (D). Graphs show percentage of CCR5- and CCR3-expressing cells in T-cell subsets sorted from control (■) and HD lymph nodes (▪). The graphs represent the mean percentage (SE) of stained cells of 5 distinct experiments.
CCR3 and CCR5 expression in HD and control lymph nodes.
Immunohistochemical staining of lymph nodes involved by HD with mAb directed against CCR5 (A), CCR3 (B), and isotype-matched control antibody (C). No CCR5 expression is detectable in control lymph nodes with follicular hyperplasia (D). Graphs show percentage of CCR5- and CCR3-expressing cells in T-cell subsets sorted from control (■) and HD lymph nodes (▪). The graphs represent the mean percentage (SE) of stained cells of 5 distinct experiments.
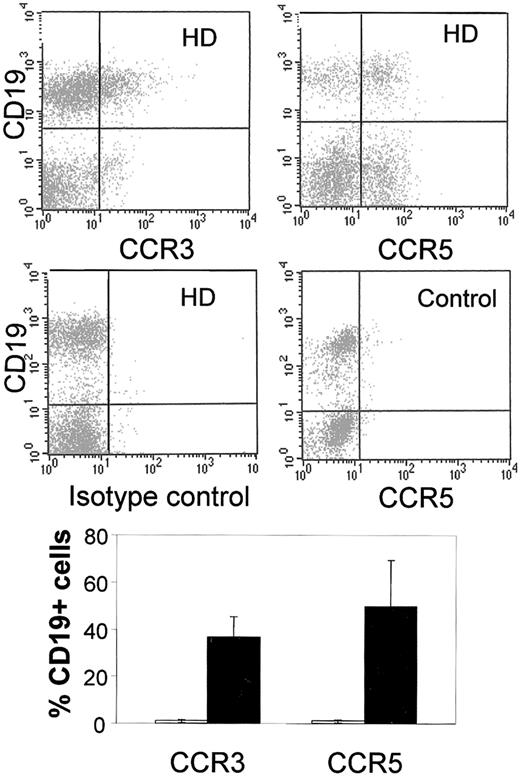
Of the nonneoplastic leukocytic infiltrate sorted from HD lymph nodes, 38% (SE, 3.2%) consisted of CD19+ B cells. Unexpectedly, 36.6% (8.8%) and 49.6% (19.9%) of this lymphocyte subset showed CCR3 and CCR5 expression, respectively. Similar results were obtained by sorting B cells with anti-CD20 antibodies. No expression of these receptors was found in B cells of control lymph nodes (Figure3). To assess whether CCR5 protein expression in HD lymph nodes is due to de novo synthesis or to relocation of pre-existing receptor to the cell surface, we performed in situ hybridization studies on formaldehyde-fixed and paraffin-embedded tissue samples. CCR5 gene expression could be detected in approximately 10% of the nonneoplastic cells of HD lymph nodes (Figure 4) whereas it was not detected in control lymph nodes. The studies on freshly isolated cells from HD lymph nodes showed CCR5 gene expression in the same cell subset identified by flow cytometry, namely, in CD4+ T cells and CD19+ B cells and, in addition, also in CD14+macrophages (Figure 4).
CCR3 and CCR5 expression in B lymphocytes.
Top: Double-staining of CD19+ B lymphocytes, freshly isolated from lymph nodes involved by HD with antibodies directed against CCR3 and CCR5 (upper diagrams), as well as isotype-matched controls (lower left diagram). No double-positive CD19/CCR5 cells were detected in control lymph nodes (lower right diagram). Results of 1 representative experiment of 5 are shown. Bottom: Percentage of CD19+ cells expressing CCR5 and CCR3 in HD (▪) and control lymph nodes (■). The figure represents the mean percentage (SE) of stained cells of 5 distinct experiments.
CCR3 and CCR5 expression in B lymphocytes.
Top: Double-staining of CD19+ B lymphocytes, freshly isolated from lymph nodes involved by HD with antibodies directed against CCR3 and CCR5 (upper diagrams), as well as isotype-matched controls (lower left diagram). No double-positive CD19/CCR5 cells were detected in control lymph nodes (lower right diagram). Results of 1 representative experiment of 5 are shown. Bottom: Percentage of CD19+ cells expressing CCR5 and CCR3 in HD (▪) and control lymph nodes (■). The figure represents the mean percentage (SE) of stained cells of 5 distinct experiments.
CCR5 gene expression.
Top: In situ mRNA expression of CCR5 in a lymph node involved by HD. Hybridizations with antisense (A) and sense (B) probes are shown. Bottom: CCR5 expression in isolated cells sorted from control (■) and HD lymph nodes (▪). The figure represents the mean percentage (SE) of 3 distinct experiments.
CCR5 gene expression.
Top: In situ mRNA expression of CCR5 in a lymph node involved by HD. Hybridizations with antisense (A) and sense (B) probes are shown. Bottom: CCR5 expression in isolated cells sorted from control (■) and HD lymph nodes (▪). The figure represents the mean percentage (SE) of 3 distinct experiments.
Migration assays with nonneoplastic cells of HD
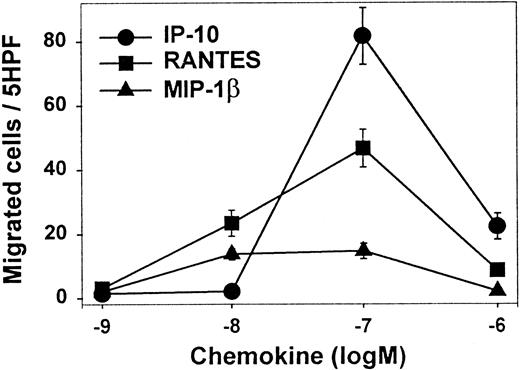
To further characterize the biological properties of the CCR5, CCR3, and CXCR3 receptors, we tested the migratory capacity of freshly isolated cells from HD lymph node. IP-10 (ligand of CXCR3) and RANTES (ligand of CCR3 and CCR5) elicted a migration response with typical bimodal concentration dependence (Figure5). The highest numbers of migrated cells were obtained with IP-10 and RANTES. Maximum migration activity was reached at 100 nM for both chemokines. Only a weak but significant migration was observed with MIP-1β, whereas no response to MCP-1 was observed (data not shown).
Migration assays in vitro.
Chemokine-induced in vitro migration of leukocytes from lymph nodes with HD. Shown are the mean numbers (SE) of migrated cells per 5 high-power fields (triplicate wells) (5HPF) in the presence of increasing molar (M) concentrations of IP-10, RANTES, and MIP-1β. PHA-activated peripheral blood lymphocytes were used as a positive control to confirm the functionality of the migration assay.
Migration assays in vitro.
Chemokine-induced in vitro migration of leukocytes from lymph nodes with HD. Shown are the mean numbers (SE) of migrated cells per 5 high-power fields (triplicate wells) (5HPF) in the presence of increasing molar (M) concentrations of IP-10, RANTES, and MIP-1β. PHA-activated peripheral blood lymphocytes were used as a positive control to confirm the functionality of the migration assay.
Discussion
We demonstrate a prominent expression of the T-cell–attracting chemokines RANTES, MCP-1, MIP-1β, and MIP-1α and of the receptors CCR5 and CCR3 in HD lymph nodes. In the nonneoplastic leukocytic infiltrates characteristic of HD, CD4+ T cells markedly up-regulate RANTES gene expression, whereas macrophages preferentially express MIP-1α, MIP-1β, or MCP-1. These findings are consistent with a recent report that also demonstrates RANTES and MIP-1α up-regulation in HD tissues.9 Further, the findings highlight the fact that distinct cellular subsets can be involved in the production of functionally similar chemokines. Indeed, RANTES attracts monocytes and CD4+/CD45R0+ memory T cells.13,14 MCP-1, MIP1α, and MIP-1β are also very potent in mediating T-cell migration.15-17 Together with CD28 ligand and antibodies to CD3, these chemokines enhance proliferation and IL-2 production in T cells.18 An excessive chemokine production and an IL-2–mediated stimulation of the expression of the corresponding receptors may thus represent an important activation loop responsible for the attraction of a considerable portion of T cells and macrophages into tissues involved by HD. In our study, we did not localize any of the chemokine genes analyzed (including eotaxin, data not shown) to the malignant H-RS cells. In contrast, previous reports found that the T-cell–directed chemokine TARC is expressed in H-RS cells. Our findings, however, are compatible with the fact that H-RS cells seem to originate from late germinal center B cells,19 for which no particular chemokine expression pattern has been reported.
Whether the marked up-regulation of all the 4 analyzed chemokines in HD represent a disease-specific phenomenon is arguable. However, we never observed a comparable high expression of RANTES in chronically inflamed tissues.20 In addition, in the 3 lymph nodes with T-cell lymphoma, including one Hodgkin-like anaplastic large-cell lymphoma, only few chemokine-expressing cells were detected by in situ hybridization in the nonneoplastic leukocytic infiltrates. Therefore, the chemokine profile observed in the present work can reflect an unusual state of cellular activation occurring specifically in HD.3,4 The marked up-regulation of CCR3 and CCR5 in HD as assessed by immunohistochemistry and by flow cytometry may also be related to similar mechanisms. CCR3 binds RANTES, MCP-2/3/4, eotaxin, and eotaxin-221-26 and is strongly expressed on eosinophils as well as on some T subsets.27 CCR5 recognizes MIP-1α and RANTES, but is the only receptor that binds MIP-1β. It is expressed on T cells and monocytes. CCR3 and CCR5 up-regulation in HD is therefore consistent with the accumulation of eosinophils, monocytes, and T lymphocytes. This assumption is also sustained by the findings that RANTES and MIP-1β can induce migration of freshly isolated cells from HD lymph nodes. The presence of CCR5 transcripts in tissue sections and in sorted cells from lymph nodes with HD indicates that CCR5 up-regulation is due at least in part to de novo protein synthesis. Alternatively, relocation to the cell surface of pre-existing intracellular receptor, as has been demonstrated for the CXCR4 receptor,28 29 cannot be excluded. This expression and/or relocation may have taken place in autochthonous cells of the lymph node, in cells recruited from other body compartments, or in both sites. The difference in the percentages of T lymphocytes expressing the CCR5 gene versus CCR5 proteins we found in this study may also suggest that mRNA expression and protein detection of CCR5 are not necessarily linked. However, the sensitivity of the different methodological approaches used in the present work should also be taken into account before drawing conclusions on this topic.
It is generally assumed that T lymphocytes in the nonneoplastic leukocytic infiltrates of HD are associated with a TH2-mediated immune response.2-4 Our findings of a prominent eotaxin gene expression in HD (data not shown) confirm previous studies9 and support the notion that T cells in the nonneoplastic infiltrate of HD may represent functionally differentiated TH2 cells. An increasing body of evidence, however, suggests that CCR5 is characteristic of the TH1 helper phenotype.30-33 Thus, the finding of CCR5 up-regulation in HD may indicate that the characteristics of T lymphocytes surrounding H-RS cells are consistent not only with a TH2 status, but also with a TH1 profile. A possible TH1 mediation of the local immune response is further sustained by our finding of a moderate CXCR3 up-regulation in CD4+ T cells from HD lymph nodes and by the migration activity of freshly isolated nonneoplastic cells from HD lymph nodes after exposure to the natural ligand of CXCR3, IP-10. It is possible that a deregulated cytokine production in HD leads to an excessive chemokine receptor up-regulation in T cells. However, cytokines known to be highly expressed in HD tissues, such as IL-13,7TNF-α, and interferon-γ,4,3 do not effectively stimulate chemokine receptors in cultured peripheral blood CD45R0+ T cells.34
A novel and unexpected finding of this study is the expression of CCR3 and CCR5 on B cells isolated from lymph nodes with HD. We did not detect CCR3 and CCR5 on B lymphocytes isolated from control lymph nodes. To our knowledge, studies on chemokine receptors have been carried out on peripheral blood lymphocytes, which, compared with lymphocytes directly isolated from diseased tissues, possibly have a different chemokine receptor expression. We do not know whether CCR3 and CCR5 expression on B cells reflects a HD-specific disregulation of the cytokine/chemokine network or if it represents a more general phenomenon. So far, we were unable to demostrate similar expression patterns on B lymphocytes isolated from inflamed nonneoplastic lymph nodes, a panel of non-Hodgkin lymphomas and B lymphocytes isolated from samples with colonic cancers (data not shown). The biological significance of this finding in the context of HD is unknown. Functional assays are clearly limited by the small numbers of available fresh tissues. The expression of CCR3 and CCR5 on B cells, however, is not necessarily linked to migration but may also mediate increased IgE and IgG4 production.35
In conclusion, our data indicate that CC chemokines attracting T cells play an important role in the formal pathogenesis of HD. The recruitment of nonneoplastic leukocytes in HD is most likely sustained by an autocrine loop of activation and may not be ascribed solely to the presence of H-RS cells. Up-regulation of CCR3 and CCR5 appears to be characteristic of the nonneoplastic leukocytic infiltrates of nodal HD. Additional studies are clearly needed to further elucidate the potential role of CC chemokine receptors in B-cell chemotaxis and/or activation in tissues.
We thank M. Lis for the excellent technical work, C. Vallan for cell-sorting expertise, and M. Baggiolini for useful discussion and critical reading of the manuscript.
Supported by Swiss National Science Foundation grants 31-52427.97 (L.M.) and 31-53961.98 (C.M.) and the Foundation San Salvatore, Lugano, Switzerland (L.M.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Luca Mazzucchelli, Institute of Pathology, University of Bern, Murtenstrasse 31, 3010 Bern, Switzerland; e-mail: mazzucch@patho.unibe.ch.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal