SB-251353 is an N-terminal truncated form of the human CXC chemokine GROβ. Recombinant SB-251353 was profiled in murine and rhesus monkey peripheral blood stem cell mobilization and transplantation models. SB-251353 rapidly and transiently mobilized hematopoietic stem cells and neutrophils into the peripheral blood after a single subcutaneous injection. Transplantation of equivalent numbers of hematopoietic stem cells mobilized by SB-251353 into lethally irradiated mice resulted in faster neutrophil and platelet recovery than stem cells mobilized by granulocyte colony-stimulating factor (G-CSF). A single injection of SB-251353 in combination with 4 days of G-CSF administration resulted in augmented stem and progenitor cell mobilization 5-fold greater than G-CSF alone. Augmented stem cell mobilization could also be demonstrated in mice when a single injection of SB-251353 was administered with only one-day treatment with G-CSF. In addition, SB-251353, when used as a single agent or in combination with G-CSF, mobilized long-term repopulating stem cells capable of hematopoietic reconstitution of lethally irradiated mice. In rhesus monkeys, a single injection of SB-251353 induced rapid increases in peripheral blood hematopoietic progenitor cells at a 50-fold lower dose than in mice, which indicates a shift in potency. These studies provide evidence that the use of SB-251353 alone or in combination with G-CSF mobilizes hematopoietic stem cells with long-term repopulating ability. In addition, this treatment may (1) reduce the number of apheresis sessions and/or amount of G-CSF required to collect adequate numbers of hematopoietic stem cells for successful peripheral blood cell transplantation and (2) improve hematopoietic recovery after transplantation.
Introduction
Peripheral blood stem cell (PBC) transplantation has gained widespread acceptance as a therapy to reconstitute hematopoiesis following myeloablative chemotherapy in patients with a variety of tumor types.1-3 Mobilization regimens used to collect hematopoietic stem cells via apheresis include administration of chemotherapy, hematopoietic cytokines such as granulocyte-macrophage colony-stimulating factor (GM-CSF) and G-CSF, or the combination of chemotherapy and cytokines.3 4
SB-251353 is a recombinant N-terminal 4–amino acid truncated form of the human chemokine GROβ, identified as a hematopoietic bioactivity in marrow stromal cell cultures.5 GROβ is a member of the CXC chemokine family, which includes the related ligands GROα, GROγ, ENA78, NAP-2, GCP-2, IP10, and interleukin-8 (IL-8), and it has biological activities related to specific binding to the CXCR2 receptor.6,7 SB-251353 specifically binds only to CXCR2 and with greater potency than full-length GROβ5(A.G.K., unpublished observations, November 1999). The CXC chemokine family is involved in chemotaxis and activation of neutrophils.6 In addition to chemo-attractant activity, selected chemokines have diverse biological and hematopoietic activities including antiviral activity,8,9 activation of myeloid cell populations,10 and inhibition or stimulation of early hematopoietic progenitor cell proliferation.11,12After a single injection in mice and rabbits, in vivo administration of IL-8 induces a rapid neutrophilia13 and mobilization of hematopoietic stem and progenitor cells into the peripheral blood of mice and monkeys.14-16 In mice, IL-8–mobilized stem cells demonstrate long-term hematopoietic repopulating ability alone or in combination with stem cell factor (SCF).17 The mechanism of IL-8–induced stem cell mobilization appears to involve increased matrix metalloproteinase-9 (MMP-9) activity that is detected immediately before the appearance of progenitors in the blood of mice and monkeys.18
In the present study we report the rapid mobilization of long-term repopulating hematopoietic stem cells (LTRCs) and multilineage as well as lineage restricted progenitor cells after single administration of SB-251353 and in combination with G-CSF therapy. In a murine peripheral blood transplantation model, LTRCs mobilized by SB-251353 alone or in combination with G-CSF demonstrated enhanced neutrophil and platelet engraftment compared with LTRCs mobilized by G-CSF alone. The hematopoietic cell mobilization potential of SB-251353 was confirmed in rhesus monkeys, with greater potency (approximately 50-fold) than in murine studies, which indicates some species preference. In addition, SB-251353–induced progenitor cell mobilization was dependent on MMP-9 activity in mice.
Materials and methods
Animals
The mice (C57Bl/6 × DBA/2)F1 (BDF1) (Harlan Sprague-Dawley, Indianapolis, IN) used were 8-12 weeks of age. All mice were provided with acidified water and sterilized rodent chow and housed along with sentinel mice that were routinely screened and shown to be pathogen-free. The SmithKline Beecham Animal Care and Use Committee, Collegeville, PA, approved all protocols.
Male rhesus monkeys, Macaca mulatta, with a mean weight of 3.6 ± 0.2 kg, were housed in individual cages in conventional holding rooms at the Veterinary Resources Department at the University of Maryland Greenebaum Cancer Center, Baltimore, MD, which is accredited by the American Association for Accreditation of Laboratory Animal Care, Rockville, MD. Monkeys were provided 10 air changes per hour of 100% fresh air, conditioned to 22.2 ± 2°C (72 ± 2°F) with relative humidity of 50% ± 20% and maintained on a 12-hour light, 12-hour dark full-spectrum light cycle. Monkeys were provided with commercial primate chow supplemented with fresh fruit and tap water ad libitum. Research was conducted according to the principles enunciated in the “Guide for the Care and Use of Laboratory Animals” prepared by the Institute of Laboratory Resources, National Research Council, Washington, DC.
Cytokines and antibodies
Recombinant SB-251353 was produced and purified as previously described.5 N-terminal amino acid sequencing and/or MALDI-MS analysis confirmed production of pure protein (greater than 99%). Preparations of SB-251353 were endotoxin-free (less than 0.05 EU/mg). We used recombinant human (rH)G-CSF (Neupogen; Hanna's Pharmaceuticals, Wilmington, DE) and neutralizing anti–MMP-9 (clone 6-6B) and isotype control antibody (Oncogene Research Products, Cambridge, MA).
Hematologic evaluation of mouse blood
Mice were injected intravenously (IV) or subcutaneously (SQ) with SB-251353 in 200 μL phosphate-buffered saline (PBS). In all experiments, the number of mice per group was at least 3. In some experiments mice were injected SQ with 50 μg/kg G-CSF twice daily (bid) for 1-4 days and bled on day 5. In combination regimens, SB-251353 was administered as a single injection the day following bid administration of G-CSF for the indicated number of days. Peripheral blood was obtained by cardiac puncture following carbon dioxide (CO2) asphyxiation using a heparinized syringe. Blood was transferred to tubes containing ethyelenediamine tetraacetic acid (EDTA) for complete blood cell (CBC) analysis. CBC analysis was performed on a Technicon H1 hematology analyzer equipped with veterinary software (Bayer, Tarrytown, NY).
Murine clonogenic assays
Low-density PBCs (LD-PBCs) were prepared by centrifugation over Lympholyte M (Cedarlane Laboratories Ltd, Hunby, Ontario, Canada) at 800g for 30-40 minutes at room temperature; washed; resuspended in McCoy 5A modified media with L-glutamine; and supplemented with 100 U/mL penicillin, 100 μg/mL streptomycin, 0.6 × MEM (modified essential medium) vitamin solution, 1 mM sodium pyruvate, 0.8 × MEM essential amino acids, 0.6 × MEM nonessential amino acids, 0.05% sodium bicarbonate (all from Gibco-BRL Life Technologies, Grand Island, NY), and 15% heat-inactivated fetal bovine serum (Hyclone Sterile Systems, Logan, UT). Cells (8 × 103 to 4 × 105) were mixed with 0.3% agar (Difco Laboratories, Detroit, MI) in McCoy 5A medium with 10 ng/mL recombinant murine (rm)IL-1α (R&D Systems, Minneapolis, MN), 10 ng/mL rmGM-CSF (R&D), and 50 ng/mL rmSCF (stem cell factor) (gift of Dr Karl Nocka, UCB Research, Cambridge, MA). This mixture was added to 12-well tissue culture plates at 0.5 mL per well with 3 wells per sample.
In some experiments LD-PBCs were cultured in MethoCult GF M3434 (Stem Cell Technologies, Vancouver, BC, Canada) containing a cocktail of cytokines to enumerate colony-forming unit granulocyte-macrophage (CFU-GM), CFU-GEMM (granulocyte-erythrocyte-monocyte-megakaryocyte), CFU-E (erythrocyte), and burst-forming unit erythrocyte (BFU-E). Cultures were incubated for 14 days at 37°C with 6% CO2and 7% O2 in a fully humidified incubator. CFU-G was enumerated by culturing cells with 100 ng/mL G-CSF in Methocult GF M3234 for 7 days under the above conditions. These incubation conditions were found to be optimal for enumerating the high-proliferative progenitor cells mobilized into peripheral blood. CFU-Meg (megakaryocyte) was quantitated using the MegaCult-C reagents following manufacturer's instructions. Total colonies per mL blood were determined by multiplying the CFU frequencies by the number of low-density cells per mL blood.
PBC transplantation in mice
In transplantation experiments, recipient BDF1 mice were irradiated with 1250 cGy (100% lethal dose) with a Gammacel-40 irradiator (Nordion International, Kanata, ON, Canada) in 2 sessions approximately 5-6 hours apart. Recipient mice were injected via the tail vein with LD-PBCs in a volume of 0.2 ml PBS.
Hematologic evaluation of rhesus monkey blood
Peripheral blood was obtained from the saphenous vein of primates anesthetized with 10 mg/kg intramuscular Ketaset (Fort Dodge Laboratories, Fort Dodge, IA) in order to obtain CBC and clonogenic assays. EDTA-anticoagulated blood was used to assay CBC (Sysmex K-4500; Sysmex, Long Grove, IL), and white blood cell differential counts were obtained using Wright-Geimsa stain (Ames Automated Slide Stainer, Elkhart, IN). Clonogenic cells were assayed using heparinized blood. Red blood cells were removed by ammonium chloride lysis, and nucleated cells were cultured in medium containing 0.9% methylcellulose (Methocult H4230) in Iscove modified Dulbecco medium (IMDM) with 10 000 U each 10% penicillin/streptomycin, 1% fungizone, and L-glutamine. In addition, 5 ng/mL rHG-CSF, 50 ng/mL SCF, 2 U/mL erythropoietin (EPO), 20 ng/mL megakaryocyte growth and development factor (Amgen, Thousand Oaks, CA), 20 ng/mL IL-3, 5 ng/mL GM-CSF, and 40 ng/mL IL-6 (Sandoz Pharmaceuticals, East Hanover, NJ) were added to each culture dish. Cells were cultured at 0.5-1 × 105 cells per mL for 10 days at 37°C with 5% CO2 in air in a fully humidified incubator. CFU-GM–, CFU-Meg–, and BFU-E–derived colonies (greater than 50 cells) were expressed as an absolute number per mL. CFU-Meg (10-50 cells per colony) was distinguished from smaller colonies.
Y chromosome polymerase chain reaction analysis
LD-PBCs containing mobilized stem cells were obtained from male BDF1 mice mobilized with a single SQ injection of 2.5 mg/kg SB-251353 as described above and 106 cells injected into lethally irradiated female BDF1 mice. After 100 days bone marrow was harvested from individual recipient mice, mixed with Methocult M3434 plus cytokines, and added to 96-well plates in limiting dilutions to obtain single colonies per well. After 14 days colonies were picked and diluted in PBS to 1000 cells per 10 μL. We added 50 μL 50 mM Tris-HCl (tris[hydroxymethyl] aminomethane–hydrochloride) (pH 8.5), 1 mM EDTA, 0.5% Tween 20, and 200 μg/mL proteinase K, and the mixture was incubated at 56°C for one hour followed by boiling for 10 minutes. Primers specific for a 400–base pair (bp) sequence of the sex-determining region of the Y chromosome were used to detect donor cell colonies.19 Primers detecting a 700-bp sequence of the PDGF receptor were used as a positive polymerase chain reaction (PCR) control.20 PCR amplification was performed for 35 cycles at an annealing temperature of 61°C. PCR products were analyzed using 10% acrylamide TBE gels (Novex, San Diego, CA) and stained with ethidium bromide. For each gel, colonies from healthy male and female mice were included as controls.
Antibody neutralization in vivo
Mice were injected IV via the tail vein with either isotype-matched control or 3 mg/kg neutralizing anti–MMP-9 antibody 2 hours before drug treatment. Fifteen minutes after a single SQ dose of 2.5 mg/kg SB-251353, mice were bled to evaluate progenitor cell mobilization, and 45 minutes after a single SQ dose of 0.1 mg/kg SB-251353, mice were bled to evaluate neutrophil count changes.
Statistical analysis
Differences were evaluated using the Student t test.P < .05 was considered statistically significant. Survival analysis was performed using a Fisher exact test. Comparisons of cell recovery rates were performed using nonlinear regression analysis with 4 parameter-weighted fits.
Results
Rapid mobilization of CFU-GM by SB-251353 in mice
SB-251353 was tested as a single SQ agent at a dose of 2.5 mg/kg, and the kinetics of peripheral blood CFU-GM mobilization was monitored for a period of 60 minutes. In representative data from 13 experiments, CFU-GM mobilization peaked (9-fold increase compared with baseline) approximately 15 minutes after SQ injection and persisted for 60 minutes, although declining levels were noted by 30 minutes (Table1).
Kinetics of hematopoietic progenitor cell mobilization in mice injected with a single subcutaneous dose of SB-251353
| . | Minutes after SB-251353 administration . | ||||
|---|---|---|---|---|---|
| 0 . | 15 . | 30 . | 45 . | 60 . | |
| CFU-GM/mL blood | 48 ± 4 | 486 ± 38 | 269 ± 44 | 134 ± 16 | 117 ± 15 |
| . | Minutes after SB-251353 administration . | ||||
|---|---|---|---|---|---|
| 0 . | 15 . | 30 . | 45 . | 60 . | |
| CFU-GM/mL blood | 48 ± 4 | 486 ± 38 | 269 ± 44 | 134 ± 16 | 117 ± 15 |
BDF1 mice (n = 3 per time point per experiment) were injected SQ with 2.5 mg/kg SB-251353 and bled at the indicated times. Day 14 CFU-GM (more than 50 cells) stimulated by 10 ng/mL rmIL-1α, 10 ng/mL rmGM-CSF, and 50 ng/mL rmSCF was enumerated in triplicate plates per experiment. Data are presented as the mean ± SEM of 13 experiments.
CFU-GM indicates colony-forming unit granulocyte-macrophage; SQ, subcutaneous; rmGM-CSF, recombinant murine granulocyte-macrophage colony-stimulating factor; SCF, stem cell factor.
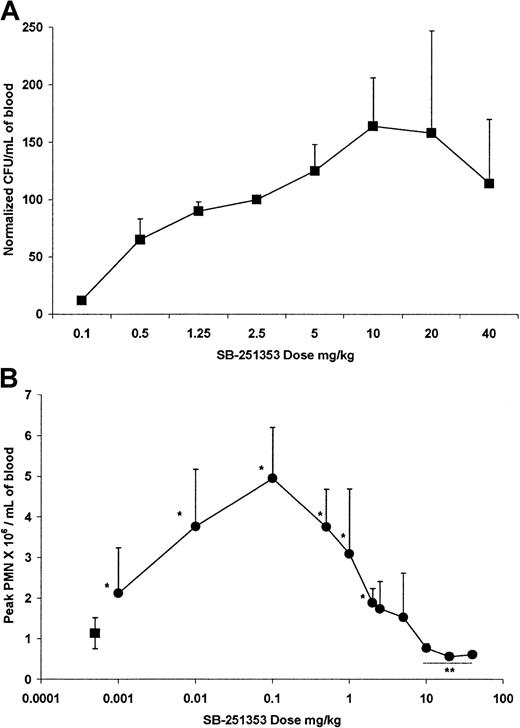
Dose-related effects of SB-251353 on hematopoietic progenitor cell and polymorphonuclear neutrophil mobilization in mice
Dose response analysis of the effects of SB-251353 on CFU-GM mobilization was performed during a series of experiments (Figure1A). Due to variations in the number of CFU-GM mobilized by a single SQ injection of 2.5 mg/kg SB-251353 (range, 400-2076 CFU-GM/mL) between experiments, CFU-GM data were normalized by designating the number of CFU-GM mobilized by the SQ dose of 2.5 mg/kg SB-251353 (included in each experiment) as 100%, then comparing CFU-GM mobilized at other doses. Maximal dose-dependent CFU-GM mobilization was obtained after stimulation by an SQ dose of 2.5 mg/kg SB-251353, and the mobilization remained elevated at doses up to 40 mg/kg SB-251353. Compared with the 2.5 mg/kg SB-251353, P= .43 for a 5 mg/kg dose, and P = .18 for a 10 mg/kg dose. (Compared with the 2.5 mg/kg dose, P = .43 for the 5 mg/kg dose, and P = .18 for the 10 mg/kg dose.) Minimal CFU-GM mobilization was noted after IV bolus administration of SB-251353 (data not shown).
Dose-related effect of single SQ injection of SB-25153 on blood CFU-GM neutrophil mobilization in mice.
(A) Normalized CFU-GM data are presented as the number of CFU-GM mobilized per mL blood at the optimal time (15 minutes after dosing) in comparison to 2.5 mg/kg SB-251353 (100%) in the same experiment. Data are presented as the mean ± SEM of 8 experiments. All doses above 0.1 mg/kg increased CFU-GM mobilization compared with PBS controls (P < .01). Values at the 5-40 mg/kg doses were not statistically different from the 2.5 mg/kg dose (P > .05). (B) Dose-related effect of single SQ injection of SB-251353 (●) on absolute neutrophil counts. ANCs represent the peak values observed within 60 minutes after injection. Data are presented as the mean ± SEM of 3-37 experiments. *P < .01 increase in ANCs compared with PBS controls (▪). **P < .05 decrease in ANCs compared with PBS controls.
Dose-related effect of single SQ injection of SB-25153 on blood CFU-GM neutrophil mobilization in mice.
(A) Normalized CFU-GM data are presented as the number of CFU-GM mobilized per mL blood at the optimal time (15 minutes after dosing) in comparison to 2.5 mg/kg SB-251353 (100%) in the same experiment. Data are presented as the mean ± SEM of 8 experiments. All doses above 0.1 mg/kg increased CFU-GM mobilization compared with PBS controls (P < .01). Values at the 5-40 mg/kg doses were not statistically different from the 2.5 mg/kg dose (P > .05). (B) Dose-related effect of single SQ injection of SB-251353 (●) on absolute neutrophil counts. ANCs represent the peak values observed within 60 minutes after injection. Data are presented as the mean ± SEM of 3-37 experiments. *P < .01 increase in ANCs compared with PBS controls (▪). **P < .05 decrease in ANCs compared with PBS controls.
SB-251353 also mobilized neutrophils to peripheral blood (Figure 1B); however, the dose response for neutrophilia in the mouse was distinct from the CFU-GM mobilization dose response curve (Figure 1A). The neutrophil mobilizing dose response was bell-shaped, and increased neutrophil counts occurred after either SQ (Figure 1B) or IV (data not shown) administration. Peak neutrophil counts occurred after administration of 0.1 mg/kg SB-251353; doses greater than 10 mg/kg significantly decreased neutrophil counts for at least 60 minutes. In all dose groups, neutrophil counts normalized within 24 hours.
Administration of SB-251353 in combination with G-CSF in mice
The effects of a single SQ dose of 2.5 mg/kg SB-251353 following 4-day mobilization by 50 μg/kg G-CSF SQ bid for 4 days were evaluated to identify additive or synergistic potential. The data in Table2 represent peak CFU-GM/mL blood at the optimal time: (1) 15 minutes after 2.5 mg/kg SB-251353, (2) following 4 days of G-CSF (16 hours after last dose) as a single agent, or (3) 15 minutes after administration of 2.5 mg/kg SB-251353 to mice pretreated with 50 μg/kg G-CSF SQ bid for 4 days (16 hours after the last dose) for every experiment performed. The 25% and 75% quartile values measure overall experimental variation. Mice receiving single administration of SB-251353 or multiday administration of G-CSF were directly compared with control mice given PBS. SB-251353 and G-CSF treatment resulted in 11-fold and 30-fold mean increases in CFU-GM mobilized compared with controls, respectively. In experiments directly comparing administration of SB-251353 plus G-CSF, the combination resulted in significantly greater (5-fold) mobilization of CFU-GM compared with G-CSF alone (P < .01). Mobilization of CFU-GM induced by SB-251353 alone was not significantly different (P = .15) than mobilization induced by G-CSF.
Summary of mouse peripheral blood progenitor cell mobilization
| Mobilization method . | Experiments, no. . | Peak CFU-GM/mL blood . | |
|---|---|---|---|
| Mean . | Quartile, 25% to 75% . | ||
| Vehicle alone | 89 | 47 | 24-63 |
| SB-251353 alone | 107 | 525* | 314-602 |
| G-CSF alone | 31 | 1432* | 452-1911 |
| SB-251353 + G-CSF | 14 | 4483*,† | 1808-3377 |
| Mobilization method . | Experiments, no. . | Peak CFU-GM/mL blood . | |
|---|---|---|---|
| Mean . | Quartile, 25% to 75% . | ||
| Vehicle alone | 89 | 47 | 24-63 |
| SB-251353 alone | 107 | 525* | 314-602 |
| G-CSF alone | 31 | 1432* | 452-1911 |
| SB-251353 + G-CSF | 14 | 4483*,† | 1808-3377 |
BDF1 mice were injected with either PBS, a single SQ dose of 2.5 mg/kg SB-251353, 50 μg/kg G-CSF SQ bid for 4 days, or the combination of G-CSF followed by a single SB-251353 treatment 16 hours after the last dose of G-CSF. Peak CFU-GM mobilization for each experiment is presented as a pooled value from a minimum of 3 mice per group per experiment. The 25% to 75% quartile range is presented as an indication of variability.
CFU-GM indicates colony-forming unit granulocyte-macrophage; G-CSF, granulocyte colony-stimulating factor.
P < .001 compared with PBS.
P = .0019 compared with G-CSF alone.
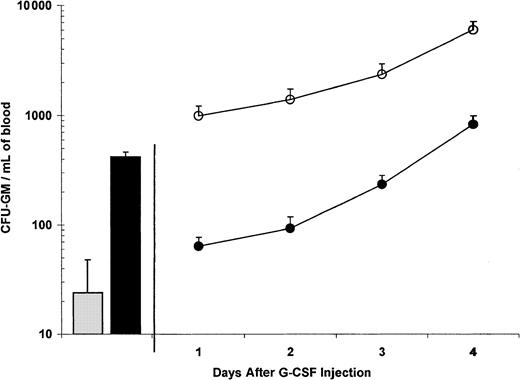
G-CSF induces significant CFU-GM mobilization in mice after 4 days (Figure 2), with minimal activity after 1 or 2 days. A single injection of SB-251353 administered 16 hours after one-day administration of G-CSF (ie, 50 μg/kg G-CSF for 2 doses) resulted in peak CFU-GM mobilization equal to that seen after administration of G-CSF alone for 4 days (ie, 50 μg/kg G-CSF for 8 doses). The combination of a single dose of SB-251353 administered after 3 or 4 days of G-CSF administration also resulted in augmented CFU-GM mobilization in peripheral blood, which was 7-fold to 10-fold (2344 vs 234 CFU/mL on day 3 and 6005 vs 825 CFU/mL on day 4, respectively) more than CFU-GM mobilization seen with G-CSF alone (P < .05).
Combination of a single SQ injection of SB-251353 after 0, 1, 2, 3, and 4 days of G-CSF treatment.
Blood CFU-GM was collected 15 minutes after a single SQ injection of 2.5 mg/kg PBS (░) or SB-251353 (▪). Blood was collected 16 hours after the last dose of G-CSF in the combination groups. Data are presented as the mean ± SEM CFU-GM per mL blood from a representative of 3 experiments with similar results. All combination SB-251353 and G-CSF values were significantly higher than G-CSF alone (P < .05). ●, G-CSF plus PBS; ○, G-CSF plus SB-251353.
Combination of a single SQ injection of SB-251353 after 0, 1, 2, 3, and 4 days of G-CSF treatment.
Blood CFU-GM was collected 15 minutes after a single SQ injection of 2.5 mg/kg PBS (░) or SB-251353 (▪). Blood was collected 16 hours after the last dose of G-CSF in the combination groups. Data are presented as the mean ± SEM CFU-GM per mL blood from a representative of 3 experiments with similar results. All combination SB-251353 and G-CSF values were significantly higher than G-CSF alone (P < .05). ●, G-CSF plus PBS; ○, G-CSF plus SB-251353.
Characterization of mobilized hematopoietic progenitor cell subtypes
Fifteen minutes after a single SQ injection of 2.5 mg/kg SB-251353, there were statistically significant (P < .05) increases in CFU-GEMM, BFU-E, CFU-Meg, CFU-G, and CFU-GM (Table3). Administration of 50 μg/kg G-CSF SQ bid for 4 days resulted in a similar profile of progenitor cell mobilization with a trend toward higher numbers of CFU-G and CFU-GM compared with SB-251353 alone (P > .2). The combination of 4-day G-CSF treatment plus a single injection of SB-251353 resulted in statistically significant increases in both CFU-G and CFU-Meg (P < .05) compared with G-CSF alone. The greatest degree of synergistic mobilization was noted for CFU-Meg, where combination treatment mobilized an average of more than 4-fold CFU-Meg compared with G-CSF or SB-251353 alone.
Mobilization of hematopoietic progenitor subsets after treatment with SB-251353, granulocyte colony-stimulating factor, and the combination of SB-251353 and granulocyte colony-stimulating factor
| Progenitor subset . | Mobilization method . | |||
|---|---|---|---|---|
| PBS alone . | SB-251353 alone . | G-CSF alone . | SB-251353 + G-CSF . | |
| CFU-G | 43 ± 14 | 179 ± 46 | 662 ± 291 | 1842 ± 6453-150 |
| CFU-GM | 136 ± 28 | 513 ± 130 | 1807 ± 611 | 3075 ± 479 |
| BFU-E | 18 ± 11 | 102 ± 49 | 188 ± 86 | 368 ± 151 |
| CFU-GEMM | 14 ± 10 | 58 ± 23 | 68 ± 49 | 292 ± 192 |
| CFU-Meg | 35 ± 12 | 147 ± 48 | 378 ± 161 | 1469 ± 6333-150 |
| Progenitor subset . | Mobilization method . | |||
|---|---|---|---|---|
| PBS alone . | SB-251353 alone . | G-CSF alone . | SB-251353 + G-CSF . | |
| CFU-G | 43 ± 14 | 179 ± 46 | 662 ± 291 | 1842 ± 6453-150 |
| CFU-GM | 136 ± 28 | 513 ± 130 | 1807 ± 611 | 3075 ± 479 |
| BFU-E | 18 ± 11 | 102 ± 49 | 188 ± 86 | 368 ± 151 |
| CFU-GEMM | 14 ± 10 | 58 ± 23 | 68 ± 49 | 292 ± 192 |
| CFU-Meg | 35 ± 12 | 147 ± 48 | 378 ± 161 | 1469 ± 6333-150 |
BDF1 mice were injected with either PBS, 2.5 mg/kg SB-251353 SQ, 50 μg/kg G-CSF SQ bid for 4 days, or the combination of G-CSF followed by a single administration of SB-251353 16 hours after the last dose of G-CSF. Blood samples were collected 15 minutes after injection of PBS or SB-251353. Progenitor subtypes were determined as defined in “Materials and methods.” Data are presented as the mean ± SEM per mL blood of 3-4 experiments. P < .05 compared with PBS for all other mobilization methods.
PBS indicates phosphate-buffered saline; G-CSF, granulocyte colony-stimulating factor; CFU, colony-forming unit; G, granulocyte; GM, granulocyte-macrophage; BFU-E, burst forming unit-erythrocyte; GEMM, granulocyte-erythrocyte-monocyte-megakaryocyte; Meg, megakaryocyte.
P < .05 compared with G-CSF alone.
Kinetics of neutrophil and platelet recovery after transplantation
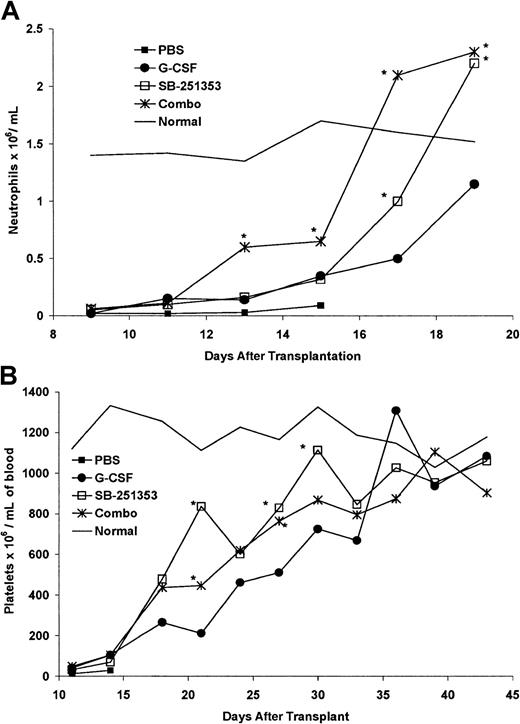
We evaluated the recovery kinetics for mature blood cells in lethally irradiated mice that received transplantations with equivalent numbers of LD-PBCs containing mobilized stem cells derived from cohorts of mice administered PBS (control), SB-251353 alone, G-CSF alone, or the combination of SB-251353 and G-CSF (Figure3A). Mice that received a transplantation from PBS-treated donors failed to recover blood cells and died. Healthy nonirradiated mice were bled daily for comparison. SB-251353–mobilized LD-PBCs restored recipient absolute neutrophil counts (ANCs) faster than G-CSF–mobilized cells (P < .05). LD-PBCs mobilized by the combination of SB-251353 and G-CSF restored ANCs faster than either SB-251353– or G-CSF–mobilized stem cells (P < .05).
Neutrophil and platelet recovery in lethally irradiated mice that received transplantations with 2 × 106mobilized LD-PBCs from treated donor mice.
(A) Neutrophil recovery in lethally irradiated mice that received transplantations with 2 × 106 mobilized LD-PBCs from donor mice treated with PBS, SB-251353, G-CSF, and SB-251353 plus G-CSF. Each time point represents the mean ANCs of 3 mice per time point. *P < .05 increase compared with G-CSF alone. (B) Platelet recovery in lethally irradiated mice that received transplantations with 2 × 106 mobilized LD-PBCs from donor mice treated with PBS, SB-251353, G-CSF, or SB-251353 plus G-CSF. Each time point represents the mean platelet count of 3 mice per time point. *P < .05 increase compared with G-CSF alone on the indicated day. The rates of cell recovery for SB-251353 alone and in combination with G-CSF were significantly higher than cell recovery using G-CSF alone, as determined by nonlinear regression analysis based upon 4-parameter weighted fits (P < .01).
Neutrophil and platelet recovery in lethally irradiated mice that received transplantations with 2 × 106mobilized LD-PBCs from treated donor mice.
(A) Neutrophil recovery in lethally irradiated mice that received transplantations with 2 × 106 mobilized LD-PBCs from donor mice treated with PBS, SB-251353, G-CSF, and SB-251353 plus G-CSF. Each time point represents the mean ANCs of 3 mice per time point. *P < .05 increase compared with G-CSF alone. (B) Platelet recovery in lethally irradiated mice that received transplantations with 2 × 106 mobilized LD-PBCs from donor mice treated with PBS, SB-251353, G-CSF, or SB-251353 plus G-CSF. Each time point represents the mean platelet count of 3 mice per time point. *P < .05 increase compared with G-CSF alone on the indicated day. The rates of cell recovery for SB-251353 alone and in combination with G-CSF were significantly higher than cell recovery using G-CSF alone, as determined by nonlinear regression analysis based upon 4-parameter weighted fits (P < .01).
In additional experiments platelet recovery was monitored from days 11-44 after transplantation (Figure 3B). Platelet count recovery in mice that received transplantations with SB-251353–mobilized blood cells was faster than in mice that received transplantations with G-CSF–mobilized blood cells (P < .05 for individual days by Student t test; P < .01 for entire time period by nonlinear regression analysis). Mobilized LD-PBCs from G-CSF– plus SB-251353–treated mice accelerated platelet recovery that was slightly slower than treatment with SB-251353 alone, but faster than treatment with G-CSF alone. These data indicate that SB-25135–mobilized LD-PBCs engraft in recipient mice and with more rapid neutrophil and platelet recovery rates than observed with G-CSF–mobilized LD-PBCs.
Mobilization of long-term repopulating stem cells in mice
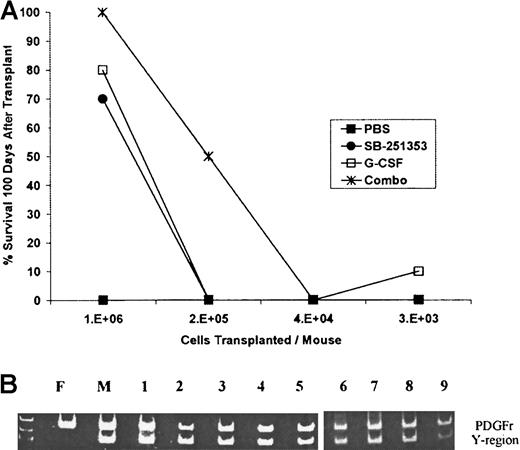
Stem cells capable of long-term hematopoietic reconstitution (LTRCs) were evaluated in lethally irradiated mice that received transplantations with LD-PBCs (8 × 103 to 1 × 106 cells per mouse) from SB-251353– or G-CSF–mobilized syngeneic donors. The ability of mobilized LD-PBCs collected from mice treated with either PBS, SB-251353 and G-CSF, or 4-day G-CSF dosing followed by a single injection of SB-251353 to rescue lethally irradiated mice was compared (Figure4A). One million LD-PBCs collected from PBS-treated mice did not contain sufficient quantities of LTRCs, and LD-PBCs protected less than 5% of the mice 100 days after transplantation. Stem cells (1 × 106 cells per transplantation) mobilized by a single injection of SB-251353 or a multiday G-CSF regimen reconstituted 14 (70%) of 20 recipient mice and 16 (80%) of 20 recipient mice, respectively. This difference in survival rates between SB-251353 and G-CSF was not statistically different (P = .76). Stem cells mobilized by the combination of G-CSF plus a single injection of SB-251353 protected 20 (100%) of 20 recipient mice at 1 × 106 cells and 5 (50%) of 10 mice at 2 × 105 cells per transplantation, whereas 2 × 105 cells mobilized by either agent alone was not protective. A bone marrow transplantation group was included as a positive control, and 100% of mice receiving 1 × 106bone marrow cells were alive at day 100 (data not shown). These data demonstrate that a single administration of SB-251353 and a 4-day multidose G-CSF regimen mobilized comparable numbers of LTRCs. The combination of G-CSF and SB-251353 mobilized significantly greater numbers of LTRCs than either G-CSF or SB-251353 alone (P = .0004).
Comparison of the ability of mobilized LD-PBCs collected from treated mice followed by a single injection of SB-251353 to rescue lethally irradiated mice.
(A) Long-term (more than 100 days) survival after transplantation of SB-251353– and/or G-CSF–mobilized LD-PBCs. Analysis of mobilized long-term repopulating stem cells by SB-251353 alone, G-CSF alone, or the combination of SB-251353 plus G-CSF was determined by limiting dilution analysis. SB-251353 was administered as a single SQ injection (2.5 mg/kg), and 50 μg/kg G-CSF was administered SQ bid for 4 days. Irradiated recipients were injected with mobilized LD-PBCs by tail vein injection. Each time point represents n = 20-30 mice per time point. Survival of at least 50% was statistically increased compared with PBS by a nonparametric Fischer exact test (P < .001). (B) PCR analysis of donor origin of long-term hematopoietic progenitor cells from mice that received transplantations with SB-251353–mobilized stem cells. Bone marrow from 7 of 10 surviving mice more than 100 days after transplantation was grown in a CFU-GM assay. Representative analysis of the Y chromosome region in 9 CFU-GMs isolated from marrow cultures of a single female mouse at more than 100 days after transplantation with SB-251353–mobilized LD-PBCs from syngeneic male donor mice. Similar analysis of 42 CFU-GMs from 6 additional mice that received transplantations (4-9 CFU-GMs per mouse) demonstrated that 98% of colonies were positive for the Y region. All colonies were analyzed for RNA for both PDGF receptor (700-bp band) and the Y chromosome (400-bp band) by PCR. PCR analysis on CFU-GM from normal male (M) and female (F) mice was used as control.
Comparison of the ability of mobilized LD-PBCs collected from treated mice followed by a single injection of SB-251353 to rescue lethally irradiated mice.
(A) Long-term (more than 100 days) survival after transplantation of SB-251353– and/or G-CSF–mobilized LD-PBCs. Analysis of mobilized long-term repopulating stem cells by SB-251353 alone, G-CSF alone, or the combination of SB-251353 plus G-CSF was determined by limiting dilution analysis. SB-251353 was administered as a single SQ injection (2.5 mg/kg), and 50 μg/kg G-CSF was administered SQ bid for 4 days. Irradiated recipients were injected with mobilized LD-PBCs by tail vein injection. Each time point represents n = 20-30 mice per time point. Survival of at least 50% was statistically increased compared with PBS by a nonparametric Fischer exact test (P < .001). (B) PCR analysis of donor origin of long-term hematopoietic progenitor cells from mice that received transplantations with SB-251353–mobilized stem cells. Bone marrow from 7 of 10 surviving mice more than 100 days after transplantation was grown in a CFU-GM assay. Representative analysis of the Y chromosome region in 9 CFU-GMs isolated from marrow cultures of a single female mouse at more than 100 days after transplantation with SB-251353–mobilized LD-PBCs from syngeneic male donor mice. Similar analysis of 42 CFU-GMs from 6 additional mice that received transplantations (4-9 CFU-GMs per mouse) demonstrated that 98% of colonies were positive for the Y region. All colonies were analyzed for RNA for both PDGF receptor (700-bp band) and the Y chromosome (400-bp band) by PCR. PCR analysis on CFU-GM from normal male (M) and female (F) mice was used as control.
To confirm that donor LTRC engraftment was responsible for survival, PCR analysis was performed in recipient female mice that received transplantations with 1 × 106 SB-251353–mobilized PBCs per mouse from male donor mice (Figure 4B). Of 10 female mice that received transplantations with SB-251353–mobilized blood cells, 7 mice survived, as expected. PCR analysis was conducted on single CFU-GM colonies picked from methylcellulose cultures of bone marrow from the 7 long-term surviving (more than 100 days) mice. CFU-GM colonies picked from untreated male and female mice were used as positive controls. All colonies were PCR+ for the PDGF receptor (to verify the presence of cellular DNA). Of 51 CFU-GM colonies picked from marrow cultures from the 7 mice that received transplantations with SB-251353–mobilized stem cells, 98% of the colonies were positive for the Y chromosome, thereby verifying complete donor stem cell engraftment.
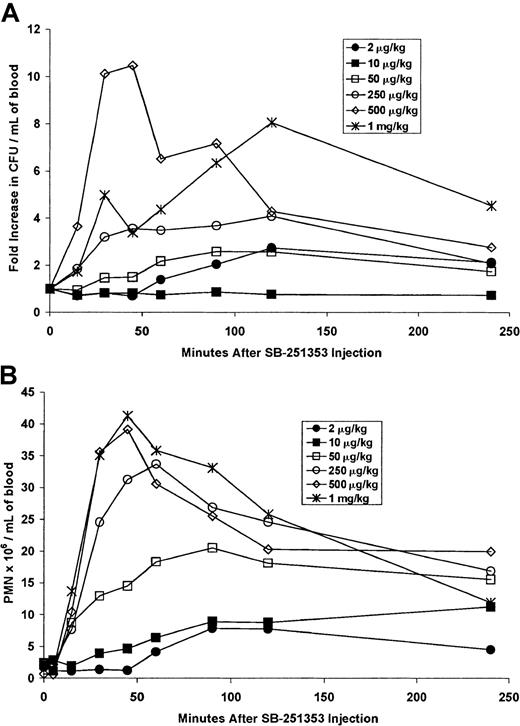
Rapid effects of SB-251353 on CFU-GM and neutrophil mobilization in rhesus monkeys
Dose response analysis was performed with a single SQ injection of SB-251353 in rhesus monkeys (Figure 5A). These studies were designed to evaluate species-related changes in potency rather than optimization of the SB-251353 dose. Hematopoietic progenitor cell mobilization occurred at doses of at least 50 μg/kg. Increases in CFU-GM of 3.5-fold to 10.5-fold compared with baseline were observed at SB-251353 doses of at least 250 μg/kg. Increased CFU-GM mobilization was sustained for 2-4 hours in rhesus monkeys, in contrast to mice, where progenitor cell mobilization was more transient. In addition to CFU-GM mobilization, the numbers of CFU-Meg were consistently increased following SB-251353 administration. Baseline numbers of CFU-Meg ranged from 0-20 CFU-Meg/mL blood, and peak values in animals administered SB-251353 (at doses greater than 50 μg/kg) ranged from 45-150 CFU-Meg/mL blood (data not shown). Evaluation of other progenitor types was not performed.
Dose response analysis was performed with a single SQ injection of SB-251353 in rhesus monkeys.
(A) Hematopoietic progenitor cell mobilization in rhesus monkeys after a single SQ injection of SB-251353. Blood collected after a single injection of SB-251353 at the doses indicated was analyzed for the presence of hematopoietic progenitors. Data are presented as the fold increase compared with baseline values (23, 159, 171, 77, 17, and 41 CFU-GM/mL blood for 2, 10, 50, 250, 500, and 1000 μg/kg, respectively). (B) Neutrophil count increases in rhesus monkeys after a single SQ injection of SB-251353. Blood cell count differentials were performed on samples collected after injection. For panels A and B, data are shown as absolute counts for a single monkey dosed at each concentration of 2, 10, 50, 100, 500, and 1000 μg/kg and 3 monkeys dosed at 250 μg/kg. PMN indicates polymorphonucleated cell.
Dose response analysis was performed with a single SQ injection of SB-251353 in rhesus monkeys.
(A) Hematopoietic progenitor cell mobilization in rhesus monkeys after a single SQ injection of SB-251353. Blood collected after a single injection of SB-251353 at the doses indicated was analyzed for the presence of hematopoietic progenitors. Data are presented as the fold increase compared with baseline values (23, 159, 171, 77, 17, and 41 CFU-GM/mL blood for 2, 10, 50, 250, 500, and 1000 μg/kg, respectively). (B) Neutrophil count increases in rhesus monkeys after a single SQ injection of SB-251353. Blood cell count differentials were performed on samples collected after injection. For panels A and B, data are shown as absolute counts for a single monkey dosed at each concentration of 2, 10, 50, 100, 500, and 1000 μg/kg and 3 monkeys dosed at 250 μg/kg. PMN indicates polymorphonucleated cell.
In addition to rapid CFU-GM mobilization, neutrophils were increased after SB-251353 administration in rhesus monkeys (Figure 5B). Neutrophil count decreased transiently within 5 minutes of injection with the majority of doses tested. This decrease was rapidly followed by neutrophilia, which peaked after 50-60 minutes and was sustained for more than 4 hours, reaching levels of 40-fold compared with baseline (greater than 40 × 106 cells per mL). In comparison, administration of 10 μg/kg/d G-CSF SQ for 3-4 days resulted in ANCs between 10-25 × 106 cells per mL (data not shown). A single IV bolus injection of SB-251353 resulted in dose-related increases in both CFU-GM and ANCs (data not shown). However, although similar in maximal response, the duration of increased CFU-GM and ANCs was lower after IV bolus administration of SB-251353 compared with SQ administration.
Combination effects of SB-251353 and G-CSF on CFU-GM and neutrophil mobilization in rhesus monkeys
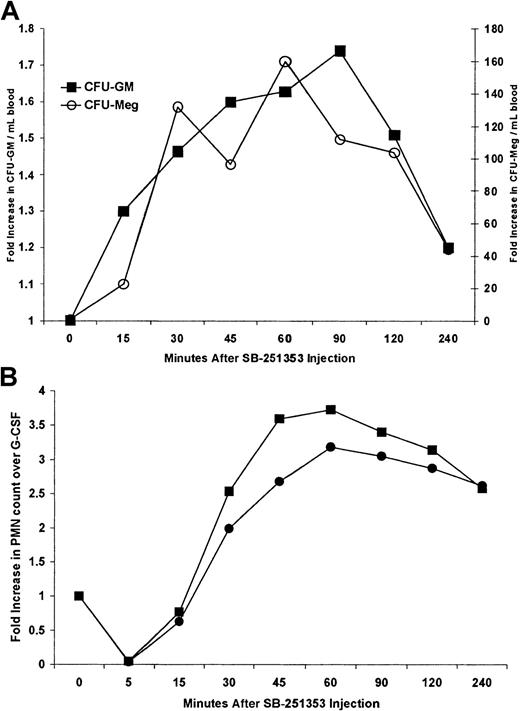
G-CSF induced an 11.4 ± 6.2–fold increase in CFU-GM mobilization compared with baseline values (n = 8 monkeys). Administration of G-CSF with a single injection of 250 μg/kg SB-251353 on day 5 was performed on 2 monkeys to test the concept of enhanced CFU-GM mobilization in combination with G-CSF, as observed in mice. However, we did not perform dose optimization. In 2 monkeys G-CSF administration resulted in 17-fold and 24-fold increases in CFU/mL blood compared with baseline values after 4 days (162-2736 and 68-1651 CFU/mL, respectively). The addition of a single dose of SB-251353 24 hours after the last dose of G-CSF boosted these levels to 26.1-fold and 26.7-fold compared with baseline, representing a mean 2-fold increase in CFU-GM and a 200-fold increase in CFU-Meg compared with that observed using G-CSF alone (Figure6A). Mobilization of CFU-Meg colonies was not routinely observed following administration of G-CSF alone in this set of animals (data not shown). Fold increases in CFU-Meg assumed a baseline of one CFU-Meg/mL because none were detected at baseline or after G-CSF.
Combination of a single SQ dose of SB-251353 after G-CSF treatment in Rhesus monkeys.
(A) Effects of the combination of 10 μg/kg G-CSF SQ for 4 days and a single SQ injection of 250 μg/kg SB-251353 on CFU mobilization in rhesus monkeys. Data represent the kinetics of mobilized CFU-GM (▪) and CFU-Meg (○) in response to G-CSF in combination with SB-251353, expressed as mean fold increase compared with G-CSF alone for 2 identically treated rhesus monkeys. Baseline and G-CSF–induced blood CFU-GM were on average 117 and 2194 CFU-GM/mL, respectively. There were no CFU-Meg colonies detected at baseline or after G-CSF treatment. Fold increases in CFU-Meg assumed a baseline of 1 CFU/mL. (B) Neutrophil counts following a single SQ injection of 250 μg/kg SB-251353 in G-CSF–pretreated rhesus monkeys. Two G-CSF–pretreated monkeys (10 μg/kg/d SQ for 4 days) were injected with a single dose of SB-251353 24 hours after the last G-CSF dose. Neutrophil counts were obtained at baseline (before G-CSF therapy began, 1.68 and 1.34 × 106 cells per mL, respectively) and immediately before SB-251353 injection (after G-CSF pretreatment, 16.51 and 7.52 × 106 cells per mL, respectively). Data are presented as the fold increase in neutrophil counts compared with G-CSF alone at the indicated times after SB-251353 administration.
Combination of a single SQ dose of SB-251353 after G-CSF treatment in Rhesus monkeys.
(A) Effects of the combination of 10 μg/kg G-CSF SQ for 4 days and a single SQ injection of 250 μg/kg SB-251353 on CFU mobilization in rhesus monkeys. Data represent the kinetics of mobilized CFU-GM (▪) and CFU-Meg (○) in response to G-CSF in combination with SB-251353, expressed as mean fold increase compared with G-CSF alone for 2 identically treated rhesus monkeys. Baseline and G-CSF–induced blood CFU-GM were on average 117 and 2194 CFU-GM/mL, respectively. There were no CFU-Meg colonies detected at baseline or after G-CSF treatment. Fold increases in CFU-Meg assumed a baseline of 1 CFU/mL. (B) Neutrophil counts following a single SQ injection of 250 μg/kg SB-251353 in G-CSF–pretreated rhesus monkeys. Two G-CSF–pretreated monkeys (10 μg/kg/d SQ for 4 days) were injected with a single dose of SB-251353 24 hours after the last G-CSF dose. Neutrophil counts were obtained at baseline (before G-CSF therapy began, 1.68 and 1.34 × 106 cells per mL, respectively) and immediately before SB-251353 injection (after G-CSF pretreatment, 16.51 and 7.52 × 106 cells per mL, respectively). Data are presented as the fold increase in neutrophil counts compared with G-CSF alone at the indicated times after SB-251353 administration.
Administration of G-CSF resulted in 6-fold and 10-fold increases in ANCs in 2 monkeys (1.68-16.51 × 106 and 1.39-7.52 × 106 cells per mL) (Figure 6B). A single SQ injection of 250 μg/kg SB-251353 following the G-CSF regimen resulted in further increased ANCs in both monkeys, rising 3-fold and 4-fold more than levels already elevated by G-CSF (18-fold and 37-fold compared with basal levels). Sustained increases in ANCs were observed for at least 4 hours. Combination studies with IV bolus administration of 50 μg/kg SB-251353 and a SQ dose of 10 μg/kg/d G-CSF for 4 days were performed in 2 monkeys and resulted in similar increases in ANCs (not shown).
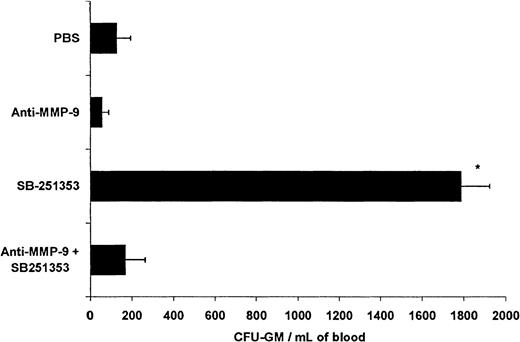
Effect of MMP-9 neutralizing monoclonal antibody on SB-251353–induced CFU-GM and neutrophil mobilization in mice
A neutralizing antibody against MMP-9 was used to determine if the mechanism of SB-251353–induced stem cell mobilization involved MMP-9 activity in vivo. Injection of SB-251353 resulted in a 14-fold increase in CFU-GM in the blood compared with PBS controls (Figure7); the increase was completely prevented by pre-administration of anti–MMP-9. In another experiment isotype control antibody pretreatment had no effect on SB-251353–induced CFU-GM mobilization: PBS, 94 CFU-GM/mL; SB-251353 alone, 798 CFU-GM/mL; and isotype control antibody pretreatment plus SB-251353, 920 CFU-GM/mL). Anti–MMP-9 (and an isotype control antibody) had no significant effect on basal circulating CFU-GM numbers.
Anti–MMP-9 neutralization of SB-251353–induced hematopoietic progenitor cell mobilization in the mouse.
We injected 3 mg/kg neutralizing antihuman MMP-9 antibody (clone 6-6B) IV 2 hours prior to SQ administration of 2.5 mg/kg SB-251353. Isotype control antibody does not inhibit SB-251353–induced CFU-GM mobilization (data not shown). Each time point represents data from 3-4 mice. *P < .01 increase in CFU-GM/mL blood compared with PBS control and antibody-pretreated mice.
Anti–MMP-9 neutralization of SB-251353–induced hematopoietic progenitor cell mobilization in the mouse.
We injected 3 mg/kg neutralizing antihuman MMP-9 antibody (clone 6-6B) IV 2 hours prior to SQ administration of 2.5 mg/kg SB-251353. Isotype control antibody does not inhibit SB-251353–induced CFU-GM mobilization (data not shown). Each time point represents data from 3-4 mice. *P < .01 increase in CFU-GM/mL blood compared with PBS control and antibody-pretreated mice.
Administration of 0.1 mg/kg SB-251353 SQ resulted in a rapid 5-fold increase in ANCs, but administration of a stem cell mobilization dose, ie, 2.5 mg/kg SB-251353 SQ, had no effect on ANCs (Figure 1B). Pretreatment of mice with 3 mg/kg IV neutralizing anti–MMP-9 antibody or isotype control failed to inhibit an SB-251353–induced increase in ANCs that reached more than 5-fold compared with baseline 45 minutes after injection (data not shown).
Discussion
In mice and nonhuman primates, hematopoietic stem cell mobilization has been demonstrated with a variety of agents used alone and in combination: G-CSF,21,22 G-CSF with SCF19,20,23 or flt-3,24-28 IL-3, GM-CSF with IL-3,29,30 myelopoietin,31IL-1,32 IL-7,33 and anti-VLA4 antibody.34,35 In addition, the CC chemokine MIP-1α36 and the CXC chemokines IL-814-16 and MIP-237 also induce stem cell mobilization in animal models. In this report we demonstrate that a specific truncated form of the CXC chemokine GROβ, SB-251353, mobilizes stem cells in both mice and rhesus monkeys. Unlike IL-8, an agonist for both CXCR1 and CXCR2, SB-251353 is a specific CXCR2 receptor agonist5 and is a more potent CXCR2 agonist than GROβ in receptor binding and in vitro cell-based assays.5 A single injection of SB-251353 results in significant mobilization of a broad spectrum of hematopoietic progenitor cells and long-term repopulating cells within 15 minutes following administration.
The combination of SB-251353 with a multiple-day regimen of G-CSF results in augmented stem cell mobilization compared with the use of G-CSF alone. The G-CSF response in mice mimics the range of stem and progenitor cell mobilization observed in clinical studies, as defined by CFU-GM and/or CD34+ cell increase.38,39 The addition of SB-251353 after only one day of G-CSF treatment results in stem and progenitor cell mobilization equal to 4 days of G-CSF treatment, suggesting that SB-251353 may decrease the frequency of administration and total amount of G-CSF required for harvesting sufficient blood stem cells for transplantation. The mechanism by which SB-251353 synergizes with G-CSF to augment stem cell mobilization is unclear. Recent studies in G-CSF receptor knockout mice have demonstrated that the G-CSF receptor may play a role in regulating cellular responses to chemokines.40 In fact, G-CSF receptor–deficient mice fail to mobilize hematopoietic progenitor cells in response to G-CSF or IL-8.24
The primary goal of our nonhuman primate studies was to evaluate potential species-related differences in potency before the start of human clinical trials. Recombinant human SB-251353 induced rapid hematopoietic progenitor cell mobilization in the rhesus monkey in the μg/kg dose range, suggesting that the mg/kg dose levels required in mice may be due to species selectivity. Murine MIP-2α mobilizes hematopoietic stem cells in the mouse in the μg/kg dose range, which is consistent with potential species preference of these CXC chemokines in vivo.37 Combination studies with G-CSF in monkeys demonstrated augmented CFU-GM and CFU-Meg mobilization after SB-251353 administration. The optimal doses of SB-251353 for mobilization when used alone or in combination with G-CSF was not determined due to the limited number of animals available. However, dose-related changes in stem cell mobilization and functional analysis of stem cells were performed in murine transplantation models. Stem cells mobilized by SB-251353 alone or in combination with G-CSF were capable of long-term hematopoietic reconstitution in mice (survival monitored for more than one year after transplantation). The LTRCs mobilized by a single injection of SB-251353 were similar in numbers to those obtained after multiple doses of G-CSF, but the combination of SB-251353 and G-CSF resulted in a 5-fold increase in LTRC frequency.
Of particular interest in these studies was the observation that transplantation of an equal number of SB-251353–mobilized LD-PBCs resulted in faster neutrophil and platelet recovery compared with G-CSF–mobilized LD-PBCs. The number of CFUs transplanted does not explain this phenomenon. In fact, animals that received transplantations with SB-251353–mobilized blood received less CFUs (66% to 83%) than the G-CSF group. These data suggest that blood stem cells mobilized by SB-251353 were able to engraft more efficiently in recipient mice than G-CSF–mobilized stem cells. Alternatively, the more efficacious engraftment rates observed might be due to mobilization into the blood of early hematopoietic stem cells with short-term repopulating capacity, which are not detected in standard colony assays.41
In addition to rapidly mobilizing hematopoietic stem cells, both SB-251353 and IL-8 rapidly alter neutrophil counts in mice and monkeys. Initially, chemokine administration is associated with a leukopenia within 5 minutes of injection followed by a period of neutrophilia by 30-45 minutes.16 In our studies ANCs increased 6-fold in mice 45 minutes after SQ injection of 0.1 mg/kg SB-251353 alone, which is a noneffective stem cell mobilizing dose. Interestingly, at stem cell mobilizing doses, ie, 2.5 mg/kg in mice, there was no observed increase in ANCs. In comparison, the dose response curves in rhesus monkeys for mobilization of neutrophils versus CFU-GM were similar. Rapid increases (3-fold to 4-fold compared with G-CSF alone) in neutrophil counts were also demonstrated by a single injection of SB-251353 16 hours after the last dose of a 4-day G-CSF regimen.
The mechanism of action of SB-251353–induced stem and progenitor cell mobilization appears similar to IL-8, which involves up-regulation of MMP-9 activity.18 IL-8 induces MMP-9 activity in the plasma of rhesus monkeys immediately before the appearance of CFU in the blood. Administration of neutralizing anti–MMP-9 antibodies in rhesus monkeys completely blocked IL-8–induced progenitor cell mobilization.18 We have confirmed up-regulation of plasma MMP-9 activity in rhesus monkeys immediately before appearance of CFU following administration of SB-251353, thereby suggesting a CXCR2-mediated effect (data not shown). Our studies in mice also demonstrated a relationship between increased MMP-9 activity (as determined by zymography) and CFU mobilization (data not shown).42 Pretreatment of mice with neutralizing anti–MMP-9, but not isotype control antibody, completely blocked SB-251353–induced stem cell mobilization,43 indicating that increased MMP-9 enzyme activity is a key mediator in CXC chemokine-induced stem cell mobilization.
Induction of stem cell mobilization and neutrophilia appear to occur by different mechanisms. In mice a difference in the dose response curves for SB-251353–induced progenitor cell mobilization and neutrophilia was observed. In rhesus monkeys the dose response curves were more similar, albeit in limited number of animals. Pretreatment of mice with anti–MMP-9 antibodies had no effect on SB-251353–induced neutrophilia, but the antibodies completely blocked SB-251353–induced CFU-GM mobilization. In rhesus monkeys IL-8–induced progenitor cell mobilization, but not neutrophilia, were completely blocked by pretreatment with neutralizing anti–MMP-9 antibodies.18These data suggest that SB-251353 and IL-8 have multiple modes of action in regulating cell migration and trafficking. The role of CXCR1 versus CXCR2 in IL-8–induced stem cell mobilization is unclear; however, robust stem cell mobilization induced by the CXCR2-specific agonist SB-251353 suggests that CXCR2 may be the dominant receptor for CXC chemokine-induced stem cell mobilization.
In summary, our results demonstrate that the human CXCR2 selective ligand SB-251353 induces rapid mobilization of hematopoietic stem and progenitor cells in mice and monkeys and synergizes with G-CSF. These results provide a rationale for the potential clinical utility of SB-251353 alone with G-CSF to mobilize hematopoietic stem cells for use in peripheral blood stem cell transplantation. Further studies are needed to delineate the molecular mechanisms involved in SB-251353–induced stem cell mobilization and the cellular nature of the increased engraftment properties of SB-251353–mobilized blood cells observed in murine transplantation models.
We thank Robert Gagnon for his input into statistical analysis and nonlinear regression analysis and Silas Nwagwu, Brett Claffee, and William Schneider for scale-up and production of SB-251353.
A.G.K., D.H., S.B.D. and R.L. are employed by SmithKline Beecham Pharmaceuticals.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Andrew G. King, SmithKline Beecham Pharmaceuticals, Department of Molecular Virology and Host Defense, Mail code UP 1455, 1250 S Collegeville Rd, Collegeville, PA 19426-0989; e-mail: andrew_g_king@sbphrd.com.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal