Posttransplant lymphoproliferative disease (PTLD) is a frequent and severe Epstein-Barr virus (EBV)–associated complication in transplantation recipients that is caused by iatrogenic suppression of T-cell function. The diagnostic value of weekly EBV DNA load monitoring was investigated in prospectively collected unfractionated whole blood and serum samples of lung transplantation (LTx) recipients with and without PTLD. In PTLD patients, 78% of tested whole blood samples were above the cut-off value of quantitative competitive polymerase chain reaction (Q-PCR) (greater than 2000 EBV DNA copies per mL blood), with the majority of patients having high viral loads before and at PTLD diagnosis. Especially in a primary EBV-infected patient and in patients with conversion of immunosuppressive treatment, rapid increases in peripheral blood EBV DNA load diagnosed and predicted PTLD. In non-PTLD transplantation recipients, only 3.4% of the whole blood samples was above the cut-off value (P < .0001) despite heavy immune suppression and cytomegalovirus (CMV)-related disease. These findings illustrate the clinical importance of frequent EBV DNA load monitoring in LTx recipients. The increased EBV DNA loads in PTLD patients were restricted to the cellular blood compartment, as parallel serum samples were all below cut-off value, which indicates absence of lytic viral replication. EBV+ cells in PTLD patients have a very short doubling time, which can be as low as 56 hours, thereby creating the need for high screening frequency in high-risk patients. Furthermore, it is shown that EBV and CMV can reactivate independently in LTx recipients and that EBV DNA load monitoring may be useful in discriminating PTLD from rejection.
Introduction
Posttransplant lymphoproliferative disease (PTLD) is a severe and frequent complication in allograft recipients, with mortality rates of 50% to 80%.1 Main risk factors for PTLD are primary Epstein-Barr virus (EBV) infection2,3 and intensity and type of immune suppression.4 The incidence of PTLD varies from 0.8% to 20% and is low for renal transplantation recipients and high for lung transplantation (LTx) recipients, which reflects the more intensive use of immunosuppressive drugs in the latter, possibly in combination with the different EBV load in the transplanted organ.1,5,6 PTLD is most likely caused by iatrogenical suppression of T-cell activity in transplantation recipients, which leads to inadequate immune surveillance against EBV-induced proliferation of infected B cells.7
PTLD initially presents as a reversible polymorphic polyclonal lymphoproliferation that may result in monoclonal malignant lymphoma if left untreated.6,8 PTLD is considered to present EBV latency pattern III phenotype. However, at the single cell level the lesions show a heterogenous EBV protein expression pattern with the presence of different latency types in individual tumor cells.9 10
For treatment strategy (ie, decrease, increase, or switch of immune suppression) it is important to demarcate PTLD from acute cellular rejection. In some cases, however, symptomatology does not discriminate between the 2 complications, and a specific diagnosis is required. Diagnosis based on assessment of biopsy material can be difficult because PTLD and rejection may have similar histologic features, including the presence of activated lymphocytes, blasts, and plasmacytoid lymphocytes,11,12 13 and thus PTLD requires the demonstration of EBV involvement.
Early identification of patients at risk for developing PTLD could reduce PTLD-related morbidity and mortality, thereby improving overall patient management. It was hypothesized in previous studies that EBV DNA load monitoring in peripheral blood could have diagnostic relevance for the development of PTLD, as EBV DNA load may reflect the immunopathologic changes preceding or underlying the genesis of PTLD. Rooney et al14 found a strong statistically significant correlation between the presence of biopsy-proven PTLD and EBV burden in peripheral blood of allogeneic bone marrow transplantation recipients. Riddler et al,15 Savoie et al,16Green et al,17 and Kenagy et al18 showed the same trend in solid-organ transplantation recipients. However, no systematic study has directly addressed the fluctuation of EBV DNA load in weekly samples obtained from a high-risk population.
The aim of our study was to investigate the dynamics and prognostic value of EBV DNA load in prospectively collected peripheral whole blood and serum samples of LTx recipients in order to predict PTLD. Unlike most other studies that used isolated peripheral blood mononuclear cells (PBMNCs) as input for polymerase chain reaction (PCR) assays, we determined EBV DNA load in unfractionated whole blood, which reflects the absolute viral burden in a defined unit of the circulation better than artificially enriched cell fractions. We show that EBV DNA load in whole blood can change rapidly, creating the need for frequent (weekly) monitoring in high-risk patients. The increase in EBV DNA load is shown to be restricted to the cellular fraction of the blood because parallel serum samples were all below cut-off value, thus reflecting direct proliferation of EBV+ cells.
Patients, materials, and methods
Patients and treatment
Approximately 1700 follow-up whole blood samples of 103 LTx recipients were collected prospectively from January 1997 through September 1999 at the Department of Pulmonary Diseases at University Hospital Groningen, Groningen, The Netherlands. Samples were obtained weekly during admissions and at all outpatient visits. From this cohort 6 patients with PTLD and a complete follow-up were included in this study. As controls, the first 8 LTx recipients who received transplantations after the start of the study and remained PTLD free were analyzed. Patient characteristics are summarized in Table1. Approval was obtained from the institutional review board for these studies. Informed consent was provided according to the Declaration of Helsinki.
Characteristics of patients who received LTx
| Patient no. . | Age at LTx, y/sex . | Pre-LTx EBV serostatus . | Localization of PTLD . | Time from LTx to PTLD, mo . | Conversion of IS prior to PTLD* . | CMV serostatus, recipient/donor . | Treatment for CMV . |
|---|---|---|---|---|---|---|---|
| 1 | 22/M | − | lung | 2 | — | −/+ | gancyclovir |
| 2 | 55/F | + | stomach | 19 | CsA to FK506 | −/+ | gancyclovir |
| 3 | 49/F | + | intestine | 32 | — | +/− | |
| 4 | 39/M | + | nasopharynx | 55 | — | −/− | |
| 5 | 43/M | + | liver | 67 | — | +/− | |
| 6 | 53/F | + | mouth | 78 | CsA to FK506 | −/+ | gancyclovir |
| 7 | 34/F | + | — | — | — | +/+ | |
| 8 | 54/F | + | — | — | — | +/− | gancyclovir |
| 9 | 35/M | − | — | — | — | +/− | |
| 10 | 55/M | + | — | — | — | −/− | |
| 11 | 49/F | + | — | — | — | −/− | |
| 12 | 25/F | + | — | — | — | +/− | |
| 13 | 50/M | + | — | — | — | +/+ | gancyclovir/foscavir/ immunoglobulines |
| 14 | 33/M | + | — | — | — | −/− |
| Patient no. . | Age at LTx, y/sex . | Pre-LTx EBV serostatus . | Localization of PTLD . | Time from LTx to PTLD, mo . | Conversion of IS prior to PTLD* . | CMV serostatus, recipient/donor . | Treatment for CMV . |
|---|---|---|---|---|---|---|---|
| 1 | 22/M | − | lung | 2 | — | −/+ | gancyclovir |
| 2 | 55/F | + | stomach | 19 | CsA to FK506 | −/+ | gancyclovir |
| 3 | 49/F | + | intestine | 32 | — | +/− | |
| 4 | 39/M | + | nasopharynx | 55 | — | −/− | |
| 5 | 43/M | + | liver | 67 | — | +/− | |
| 6 | 53/F | + | mouth | 78 | CsA to FK506 | −/+ | gancyclovir |
| 7 | 34/F | + | — | — | — | +/+ | |
| 8 | 54/F | + | — | — | — | +/− | gancyclovir |
| 9 | 35/M | − | — | — | — | +/− | |
| 10 | 55/M | + | — | — | — | −/− | |
| 11 | 49/F | + | — | — | — | −/− | |
| 12 | 25/F | + | — | — | — | +/− | |
| 13 | 50/M | + | — | — | — | +/+ | gancyclovir/foscavir/ immunoglobulines |
| 14 | 33/M | + | — | — | — | −/− |
IS indicates immune suppression; M, male; F, female; +, seropositive; −, seronegative; and CsA, cyclosporine A.
Indicates conversion from CsA to FK506 before presentation of PTLD.
Of the 14 patients entered in this study, 2 patients were EBV− before receiving transplantations. Patient no. 1 had a primary EBV infection as determined by EBV-specific immunoglobulin G (IgG) and IgM serology and subsequently underwent 3 PTLD episodes. The proliferating cells in the PTLD lesion were of recipient origin, as determined by HLA typing of tumor cells grown in vitro from the PLD biopsy specimen. The other EBV− patient did not seroconvert for more than one year after LTx and probably received a transplantation from an EBV− donor. Twelve of the 14 patients were EBV+ prior to transplantation. Seven of these 12 patients remained PTLD free, and the other 5 patients developed late PTLD (diagnosis more than one year after transplantation). Of these 5 patients, 2 developed PTLD within one year after conversion of immune suppression (ie, replacement of cyclosporine A by FK506).
Diagnosis of PTLD was made by immunohistochemical staining and Epstein-Barr virus–encoded RNA–1/2 RNA in situ hybridization (EBER-RISH) of biopsy specimens as described by Verschuuren et al. All patients received standard immunosuppressive treatment with 3 mg/kg rabbit antithymocyte globulin (Thymoglobulin; Pasteur-Merieux, Lyon, France) 2-5 times after transplantation, 1.5-3 mg/kg/d azathioprine, cyclosporine A (dose adjusted to whole blood trough levels of 400 μg/L, tapering to levels of 150 μg/L within 3 weeks), prednisolone (3 times 125 mg the first day, 0.2 mg/kg/d from day 2 to third month, and 0.1 mg/kg/d thereafter), 960 mg Co-trimaxozole on alternate days for Pneumocystis carinii prophylaxis, and 200 mg acyclovir four times a day for 6 months. Acute rejection was treated with pulse therapy of 500-1000 mg/d IV methylprednisolone for 3 days. Recurrent rejection was treated by replacement of cyclosporine by FK506 (Prograft; Fujisawa) and subsequently from azathioprine to mycophenolate mofetil (Cellcept; Roche). CMV-related disease was diagnosed by increased pp65-antigenemia levels and treated with IV gancyclovir (Cymevene, Roche) or foscarnet (Foscavir, Astra Pharmaceuticals, Wilmington, DE) until pp65-antigenemia levels dropped below limit of detection.19 PTLD episodes were treated by tapering cyclosporine A to 50% of standard levels (75-100 μg/L) combined with high-dose acyclovir administration (800 mg/d for 5 days) or 1000 mg valacyclovir three times a day.
Nontransplantation controls
Whole blood samples from healthy individuals (n = 50) were obtained from blood bank Den Bosch, 'S-Hertogenbosch, The Netherlands. Follow-up whole blood samples (n = 27) were obtained from healthy volunteers (n = 7) from our institute. Follow-up time ranged from 2-21 months, and 2-7 samples were obtained from each donor.
DNA isolation
We lysed 1 mL fresh unfractionated whole blood in 9 volumes of lysis buffer comprising 5 M guanidine isothiocyanate, 1.2% Triton X-100, 20 mM ethylenediamine tetraacetic acid (EDTA), and 0.1 M (tris[hydroxymethyl] aminomethane–hydrochloride (Tris-HCl) (pH 6.4) (Organon Teknika BV, Boxtel, The Netherlands) and stored samples at −80°C until use. DNA was isolated from 1 mL lysate by silica-based extraction as described previously.20 One negative control (water) was included for each run of 10 whole blood samples.
DNA was isolated from 100 μL serum using the High Pure PCR Template Purification method (Boehringer Mannheim, Mannheim, Germany) according to the manufacturer's instructions. As positive control, 100 μL serum from a healthy donor was spiked with DNA from 100 EBV+ JY cell lines prior to DNA isolation. Serum from a healthy EBV− donor and water served as negative controls.
Quantitative EBV DNA PCR
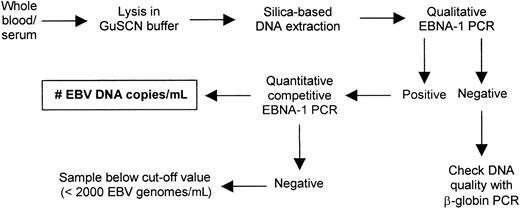
The experimental approach for EBV DNA load determination in clinical specimens is depicted in Figure1. First, the DNA equivalent of 5 μL whole blood or serum was amplified in qualitative EBNA-1 PCR.21 This allowed the detection of samples with loads exceeding 2000 genome copies per mL blood, which is equivalent to the presence of 200-400 EBV+ B cells, a level that is above EBV DNA detected in peripheral blood of the large majority of healthy EBV carriers.22-24 EBV DNA load in PCR+samples was subsequently determined by quantitative competitive EBNA-1 PCR exactly as described recently.21 The cut-off value used in Q-PCR was 2000 copies per mL blood, which is the detection limit of this assay. DNA quality of whole blood samples was checked by β-globin PCR according to de Roda Husman et al.25
Experimental approach for EBV DNA load determination in whole blood and serum.
Fresh whole blood or serum was lysed in guanidine isothiocyanate (GuSCN) buffer, and DNA was isolated by silica-based extraction. EBV status was assessed by qualitative EBNA-1 PCR, and EBV DNA in positive samples was quantified by quantitative competitive PCR. DNA quality was checked with β-globin PCR.
Experimental approach for EBV DNA load determination in whole blood and serum.
Fresh whole blood or serum was lysed in guanidine isothiocyanate (GuSCN) buffer, and DNA was isolated by silica-based extraction. EBV status was assessed by qualitative EBNA-1 PCR, and EBV DNA in positive samples was quantified by quantitative competitive PCR. DNA quality was checked with β-globin PCR.
To ensure the validity of the results, several precautions were taken to avoid contamination of PCR, as described previously.26As control for accuracy and reproducibility of quantification, a fixed amount of WT plasmid DNA was quantified in each experiment in duplicate. In addition, all samples were screened blindly, and appropriate negative and positive controls for DNA isolation, preparation of PCR master mix, and enzyme immunoassay detection were included (one negative control for each 10 tested samples and one positive control per experiment).
EBER RNA in situ hybridization and CD20 double-staining
PBMNCs were isolated from fresh whole blood by cell-separation tube centrifugation. Cytospins of isolated PBMNCs were made using standard procedures, and glass slides containing approximately 50 000 cells were vacuum-sealed and stored at −80°C in vacuum. After thawing, the vacuum seal was opened, and slides were fixed in 4% paraformaldehyde in phosphate-buffered saline. EBER-RISH was performed as described previously.27 EBV+ JY cells were used as positive control. As negative control, a sense riboprobe inverse complementary to the specific antisense probe was hybridized. After EBER-RISH as described above, PBMNC cytospins were stained for CD20 (L26; Dako, Glostrup, Denmark) for one hour at room temperature (diluted 1:100) employing a 3-step method using biotinylated rabbit antimouse monoclonal antibody (mAb) and streptavidin-biotin horseradish peroxidase (HRP) complex (Dako). HRP was visualized with di-amino benzidine, and slides were counterstained with hematoxylin.
EBV serology
EBV-specific IgM and IgG antibodies were determined by a semiquantitative synthetic peptide-based enzyme-linked immunosorbent assay (ELISA) (Organon Teknika), and immunoblot analysis as described by Verschuuren et al. Active EBV infection was diagnosed by the presence of IgM antibodies to VCA and EA and/or a significant rise in VCA-IgG antibody level, which was occasionally accompanied by the temporal presence of EA-IgG.
Results
EBV Q-PCR positivity in LTx recipients with and without PTLD
A total of 14 LTx recipients were included in the follow-up study. Six of these 14 patients developed PTLD, and the total patient follow-up time for the PTLD patients was 90 months. In total 141 whole blood samples from the PTLD group were analyzed, and 96 (68%) samples were above cut-off value in Q-PCR (ie, more than 2000 EBV DNA copies per mL blood) versus only 4 (3.4%) of 117 samples in the non-PTLD group (P < .0001) (Table2). Concerning samples before PTLD diagnosis, 50 (78%) of 64 samples were above cut-off value, which was also significantly higher than in the non-PTLD LTx recipients, indicating that increased EBV DNA loads may identify patients at risk for developing PTLD. In addition, the number of samples above cut-off value before PTLD diagnosis was higher than the number of samples above cut-off value after diagnosis (P = .02) (Table 2), indicating that EBV DNA loads decrease after PTLD diagnosis as a consequence of therapy.
Detection of EBV DNA in peripheral blood of LTx recipients with and without PTLD by Q-PCR
| Patients . | Total samples tested, no. . | Samples above cut-off value in Q-PCR, no. (%) . | Samples tested before PTLD, no. . | Samples above cut-off value in Q-PCR before PTLD, no. (%) . | Samples tested after PTLD, no. . | Samples above cut-off value in Q-PCR after PTLD, no. (%) . |
|---|---|---|---|---|---|---|
| PTLD LTx (n = 6) | 141 | 96 (68) | 64 | 50 (78) | 77 | 46 (60) |
| Non-PTLD LTx (n = 8) | 117 | 4 (3.4) | NA | NA | NA | NA |
| Total | 258 | 100 (39) | — | — | — | — |
| Patients . | Total samples tested, no. . | Samples above cut-off value in Q-PCR, no. (%) . | Samples tested before PTLD, no. . | Samples above cut-off value in Q-PCR before PTLD, no. (%) . | Samples tested after PTLD, no. . | Samples above cut-off value in Q-PCR after PTLD, no. (%) . |
|---|---|---|---|---|---|---|
| PTLD LTx (n = 6) | 141 | 96 (68) | 64 | 50 (78) | 77 | 46 (60) |
| Non-PTLD LTx (n = 8) | 117 | 4 (3.4) | NA | NA | NA | NA |
| Total | 258 | 100 (39) | — | — | — | — |
Percentage of the total samples above cut-off value in Q-PCR is based on more than 2000 EBV DNA copies per mL blood. The followingP values were determined: The total number of samples above cut-off value in PTLD patients versus non-PTLD patients:P < .0001; number of samples above cut-off value before diagnosis of PTLD versus number of samples above cut-off value in non-PTLD patients: P < .0001; and number of samples above cut-off value before diagnosis of PTLD versus number of samples above cut-off value after diagnosis of PTLD patients: P< .02. NA indicates not applicable.
Overall EBV DNA load in PTLD patients ranged from 2000 to 308 000 EBV DNA copies per mL blood, with EBV DNA loads (copies per mL blood) at PTLD diagnosis of 66 600, 95 400, and 24 800 (patient no. 1); 15 000 (patient no. 2); less than 2000 (patient no. 3); 29 800 (patient no. 4, sample obtained 3 weeks before PTLD, as indicated below); 4500 (patient no. 5); and 7000 (patient no. 6) (Table3). We followed 8 PTLD-free LTx recipients for a total of 91 months. Only 4 (3.4%) of 117 tested samples were above cut-off value, with a maximum EBV DNA load of 6600 EBV DNA copies per mL blood.
EBV DNA loads in peripheral blood of 6 PTLD patients and 8 non-PTLD LTx recipients
| Patient no. . | PTLD, mo . | First EBV+ sample, mo/d after LTx . | Follow-up period, mo . | Total samples . | EBV DNA load range, copies per mL blood . | Samples before PTLD . | EBV DNA load at PTLD diagnosis, copies per mL blood . | ||
|---|---|---|---|---|---|---|---|---|---|
| No. tested . | Above Q-PCR cut-off value, no. (%) . | No. tested . | Above Q-PCR cut-off value, no. (%) . | ||||||
| 1 | 2 | 1 mo | 13 | 29 | 23 (79) | 3400-308 000 | 7 | 4 (57) | 66 600, 95 400, 24 800 |
| 2 | 19 | 2 mo | 19 | 38 | 34 (89) | 2400-98 000 | 31 | 27 (87) | 15 000 |
| 3 | 32 | — | 15 | 15 | 1 (6) | 5500 | 6 | 0 (0) | <2000 |
| 4 | 55 | 483-150mo | 17 | 23 | 19 (83) | 2700-108 800 | 6 | 6 (100) | 29 8003-151 |
| 5 | 67 | 613-150 mo | 13 | 19 | 5 (26) | 2400-11 900 | 4 | 4 (100) | 4500 |
| 6 | 78 | 73-150mo | 13 | 17 | 14 (82) | 2000-13 600 | 9 | 9 (100) | 7000 |
| 7 | — | 11-25 d | 15 | 16 | 3 (19) | 4500-6600 | — | — | — |
| 8 | — | 3 mo | 14 | 13 | 1 (8) | 4000 | — | — | — |
| 9 | — | — | 26 | 36 | 0 (0) | — | — | — | — |
| 10 | — | — | 6 | 17 | 0 (0) | — | — | — | — |
| 11 | — | — | 8 | 9 | 0 (0) | — | — | — | — |
| 12 | — | — | 8 | 8 | 0 (0) | — | — | — | — |
| 13 | — | — | 8 | 8 | 0 (0) | — | — | — | — |
| 14 | — | — | 6 | 10 | 0 (0) | — | — | — | — |
| Patient no. . | PTLD, mo . | First EBV+ sample, mo/d after LTx . | Follow-up period, mo . | Total samples . | EBV DNA load range, copies per mL blood . | Samples before PTLD . | EBV DNA load at PTLD diagnosis, copies per mL blood . | ||
|---|---|---|---|---|---|---|---|---|---|
| No. tested . | Above Q-PCR cut-off value, no. (%) . | No. tested . | Above Q-PCR cut-off value, no. (%) . | ||||||
| 1 | 2 | 1 mo | 13 | 29 | 23 (79) | 3400-308 000 | 7 | 4 (57) | 66 600, 95 400, 24 800 |
| 2 | 19 | 2 mo | 19 | 38 | 34 (89) | 2400-98 000 | 31 | 27 (87) | 15 000 |
| 3 | 32 | — | 15 | 15 | 1 (6) | 5500 | 6 | 0 (0) | <2000 |
| 4 | 55 | 483-150mo | 17 | 23 | 19 (83) | 2700-108 800 | 6 | 6 (100) | 29 8003-151 |
| 5 | 67 | 613-150 mo | 13 | 19 | 5 (26) | 2400-11 900 | 4 | 4 (100) | 4500 |
| 6 | 78 | 73-150mo | 13 | 17 | 14 (82) | 2000-13 600 | 9 | 9 (100) | 7000 |
| 7 | — | 11-25 d | 15 | 16 | 3 (19) | 4500-6600 | — | — | — |
| 8 | — | 3 mo | 14 | 13 | 1 (8) | 4000 | — | — | — |
| 9 | — | — | 26 | 36 | 0 (0) | — | — | — | — |
| 10 | — | — | 6 | 17 | 0 (0) | — | — | — | — |
| 11 | — | — | 8 | 9 | 0 (0) | — | — | — | — |
| 12 | — | — | 8 | 8 | 0 (0) | — | — | — | — |
| 13 | — | — | 8 | 8 | 0 (0) | — | — | — | — |
| 14 | — | — | 6 | 10 | 0 (0) | — | — | — | — |
PTLD is given in months after LTx. The patient follow-up period started at LTx.
Follow-up started at first collected positive sample.
Indicates the value of the last sample obtained before PTLD (3 weeks prior to diagnosis) because PTLD was diagnosed elsewhere.
EBV DNA load dynamics in peripheral blood of PTLD patients
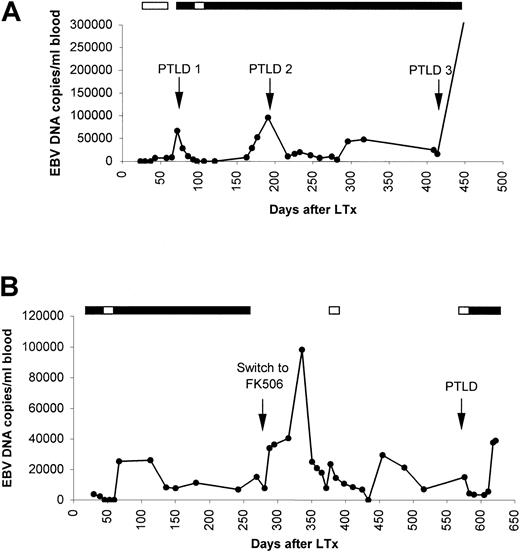
One EBV− recipient who developed PTLD shortly after primary EBV infection was included in this study (patient no. 1) (Figure 2). This patient developed PTLD within 3 months after LTx, shortly following recovery from a symptomatic CMV infection that was acquired at 3-6 weeks after LTx and was successfully treated with gancyclovir. The PTLD episode coincided with strongly increased levels of EBV DNA load in the peripheral blood. Increasing EBV DNA loads could be detected approximately one month prior to PTLD, preceding the serologic response with about 1.5 months. PTLD was diagnosed at the same day of a peak in EBV DNA load. The EBV DNA levels in the circulation rapidly dropped upon reduction of immune suppression and administration of acyclovir. High-dose acyclovir was maintained for several months. Following a period of decreasing lung function, which was treated by repeated methylprednisolone because of suspected rejection, a recurrent PTLD was diagnosed by EBER-RISH of a transbroncheal biopsy at 6 months after LTx. This PTLD episode was again preceded by increasing EBV DNA loads one month prior to diagnosis and coincided with a peak in EBV DNA load at diagnosis.
EBV DNA load dynamics in unfractionated whole blood of PTLD patient nos. 1 and 2.
(A) Patient no. 1 developed 3 episodes of PTLD after primary EBV infection, all preceded by increased EBV DNA loads. (B) Patient no. 2 was converted from cyclosporine A to FK506 as therapy for chronic rejection, which was followed by dramatically increasing EBV DNA loads that fluctuated strongly. EBV+ plasmacytoma in the mouth was diagnosed approximately 300 days later. Black boxes indicate acyclovir treatment and white boxes indicate gancyclovir treatment.
EBV DNA load dynamics in unfractionated whole blood of PTLD patient nos. 1 and 2.
(A) Patient no. 1 developed 3 episodes of PTLD after primary EBV infection, all preceded by increased EBV DNA loads. (B) Patient no. 2 was converted from cyclosporine A to FK506 as therapy for chronic rejection, which was followed by dramatically increasing EBV DNA loads that fluctuated strongly. EBV+ plasmacytoma in the mouth was diagnosed approximately 300 days later. Black boxes indicate acyclovir treatment and white boxes indicate gancyclovir treatment.
Subsequently, upon adjustment of the immunosuppressive treatment, EBV DNA loads decreased, but they persisted at levels of 10 000 to 20 000 EBV copies per mL blood. Ten months after LTx, when the patient was monitored prospectively, EBV DNA loads increased again to 40 000 copies per mL blood. A lung biopsy confirmed the third suspected PTLD. Subsequent liver and renal dysfunction in this patient, causing initially unrecognized highly elevated cyclosporine A blood levels, was associated with a rapid and extreme increase of EBV DNA load of more than 300 000 copies per mL blood.
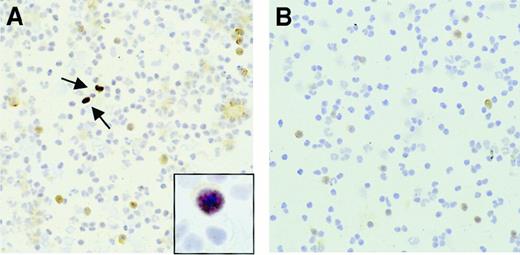
To confirm the presence of circulating EBV+ (tumor) cells, EBER-RISH was performed on cytospins of blood leukocytes obtained from patient no. 1 at the time of the third PTLD diagnosis, which showed numerous EBV+ B cells. Double-staining with CD20 indicated that about 6.5% of the B cells in peripheral blood were EBV+. A simultaneously obtained control slide from a healthy EBV+ donor did not show any signal in EBER-RISH (Figure 3).
EBER-RISH and CD20 staining of PBMNCs obtained from PTLD patient no. 1 and a healthy EBV+ donor.
(A) EBER-RISH shows numerous EBV+ B cells (indicated by arrows) in the peripheral blood of PTLD patient no. 1 at the period of the third PTLD diagnosis. Insert shows closeup of EBER and CD20+ cell. (B) In the healthy donor, there was no EBER signal detected, thereby confirming the low EBV loads in peripheral blood of healthy individuals.
EBER-RISH and CD20 staining of PBMNCs obtained from PTLD patient no. 1 and a healthy EBV+ donor.
(A) EBER-RISH shows numerous EBV+ B cells (indicated by arrows) in the peripheral blood of PTLD patient no. 1 at the period of the third PTLD diagnosis. Insert shows closeup of EBER and CD20+ cell. (B) In the healthy donor, there was no EBER signal detected, thereby confirming the low EBV loads in peripheral blood of healthy individuals.
Preliminary studies using the RNA equivalent of 5 μL whole blood as input indicate that besides EBER RNA and a very weak signal for LMP-1 RNA, there was no coding EBV RNA (eg, LMP-2 and EBNA-1) detectable despite the high abundance of EBV DNA in the samples, which confirms the findings by Babcock et al.28
Five patients in this study developed late PTLD (diagnosis more than 1 year after transplantation). In patient no. 2 (Figure 2) EBV DNA became detectable 2 months after LTx, and 27 of 31 samples before PTLD diagnosis were above cut-off value of Q-PCR. At the time of diagnosed PTLD, 15 000 EBV DNA copies per mL blood were found. This patient had been converted to FK506 before PTLD because of chronic rejection. Interestingly, in retrospect we detected strong increases in EBV DNA load shortly after the switch from cyclosporine A to FK506, which diminished upon stabilized FK506 blood levels (Figure 2).
All samples of patient no. 3 obtained before PTLD diagnosis were below 2000 EBV DNA copies per mL blood. Only one sample, taken 6 months after PTLD and after discontinuation of valacyclovir, was positive (5500 EBV DNA copies per mL blood). This patient presented with an acute abdomen due to a perforation in a Meckel diverticulum. It was resected, and PTLD was demonstrated. PTLD was totally resected, and no other localizations were found. The patient is now free of PTLD more than 18 months later.
Patient no. 4 was diagnosed elsewhere with an EBV+plasmacytoma of the nasopharynx 55 months after LTx. Treatment was started elsewhere as well, so there was no sample available at the day of diagnosis. A sample taken 3 weeks before PTLD contained an EBV DNA load of 29 800 copies per mL blood. The other samples taken before PTLD were between 12 000 and 31 800 EBV DNA copies per mL blood. The plasmacytoma was resected, and high-dose acyclovir was started. After initiation of therapy, EBV levels dropped to 2800 copies per mL blood. Later, the plasmacytoma disseminated, and very high EBV DNA loads, up to 108 800 copies per mL blood, were detected. Currently, this patient is being treated with chemotherapy.
Patient no. 5 presented with abdominal discomfort, which proved to be PTLD located in the liver. All blood samples tested during the 6 months before diagnosis were above cut-off value of Q-PCR, with 2400 to 11 900 EBV DNA copies per mL blood. EBV levels became undetectable after initiation of chemotherapy.
Patient no. 6 presented with oral ulcerations, which proved to be PTLD. All samples obtained during the 8 months prior to diagnosis were EBV+, with levels ranging from 4200 to 11 800 copies per mL blood. This patient had been converted from cyclosporine A to FK506 6 months before PTLD diagnosis because of ongoing rejection.
EBV DNA load dynamics in peripheral blood of LTx recipients without PTLD
The first 8 LTx recipients in this series who remained PTLD free were included as control in this study. All control patients received standard immunosuppression and antirejection treatments, and patient nos. 8 and 13 also were treated for active CMV infection (Table 1). Patient no. 7 showed low EBV DNA loads of 4500, 5300, and 6000 EBV DNA copies per mL blood in the first 3 samples obtained after LTx (11-27 days after LTx), which coincided with 2 occasions of methylprednisolone administration. At subsequent methylprednisolone administrations, the EBV DNA load in this patient remained undetectable. Patient no. 8 had one sample with detectable EBV DNA load (4000 EBV copies per mL blood) 3 months after LTx, while in all other samples, EBV DNA was undetectable, despite 7 rejection episodes treated with increased immunosuppression. The patient has remained EBV− DNA ever since. Patient no. 9 was EBV− before LTx and probably received an EBV− allograft. Donor serum was not available, but EBV DNA was never detected in this patient, and 18 months after transplantation, he had not seroconverted. Patient nos. 10-14 were tested starting at transplantation, and despite all patients being EBV+, EBV DNA was never detected in peripheral blood.
Five of the PTLD-free LTx recipients were CMV+ before transplantation. All 5 patients showed reactivating CMV infection during the follow-up period that was controlled by reduction of azathioprine in 3 patients and additional administration of gancyclovir in 2 patients. CMV reactivation in these patients was not accompanied by any detectable EBV reactivation. Three patients were CMV− and had received a transplantation from a seronegative donor (Table 1).
Healthy blood donors
To determine the peripheral blood EBV DNA load in immunocompetent individuals, 50 whole blood samples were obtained from a local blood bank. One sample was excluded from the study due to negative β-globin PCR after silica-based DNA extraction. All remaining 49 samples had less than 2000 EBV DNA copies per mL blood as determined by EBNA-1 Q-PCR. Follow-up of healthy seropositive lab volunteers (n = 7) indicated that the low level of EBV DNA remained stable during a 2-21 month period.
Comparison of EBV DNA load in whole blood and serum
Fifteen serum samples of patient no. 1, with extreme high EBV DNA loads of up to 308 000 EBV DNA copies per mL in the corresponding, simultaneously obtained whole blood samples, were all below cut-off value in Q-PCR, which indicates the absence of cell-free EBV DNA in the circulation. Six serum samples of patient no. 2, with corresponding whole blood levels of 15 000 to 98 200 EBV DNA copies per mL, were below cut-off value, as were 3 serum samples of patient no. 4 and 3 serum samples of patient no. 6, all obtained at high EBV DNA loads in whole blood. To check whether the negative results of the serum eluates were caused by inhibition, a second PCR was performed in which eluates were spiked with 100 copies of plasmid containing the EBNA-1 target. All samples were positive in subsequent PCR, indicating the absence of PCR inhibitors. Furthermore, all serum samples were positive in β-globin PCR, indicating the presence of cellular DNA.
Discussion
This study aimed to investigate the dynamics of EBV DNA load fluctuations in weekly whole blood samples obtained from LTx recipients. The results underline the importance and diagnostic value of EBV DNA load monitoring in peripheral blood for early identification of PTLD. The cut-off value of 2000 EBV genome equivalents per mL blood, initially based on the low EBV DNA levels in the circulation of healthy carriers,21 was shown in this study to be clinically relevant because nearly all samples of control patients were below this value. Four of 5 PTLD patients were above the cut-off value at PTLD diagnosis with 50/64 (78%) of all samples tested before diagnosis being above this value. Only 4 (3.4%) of 117 samples originating from 2 of the 8 PTLD-free LTx patients were positive at isolated time-points (P < .0001). EBV DNA in PTLD patients could be detected a long time before diagnosis, indicating a long subclinical period of active EBV infection ultimately resulting in PTLD. During this subclinical infection period, viral loads fluctuated with immune suppression.
The clinical consequence of our findings is obvious: There is good opportunity for therapeutic intervention, for example, by temporal reduction of immune suppression, possibly combined with the administration of antiviral drugs or pre-emptive use of rituximab. Moreover, our results confirm a recent study showing that EBV reactivation occurs early after transplantation at highest immune suppression and that patients at risk for PTLD can be identified during these first months after transplantation.29 A similar situation may apply to periods following a switch in immune suppressive medication, eg, a switch from cyclosporine A to FK506.
By weekly measurement of EBV DNA load using a reproducible quantitative competitive PCR assay, it was shown for the first time that the EBV+ cells in PTLD patients have a very short doubling time in vivo because EBV DNA loads can change from undetectable to extremely elevated numbers in a very limited period of time. In patient no. 1, EBV DNA loads increased from 8600 to 66 600 copies per mL blood between days 65 and 72 after LTx, with a doubling time of only 56 hours. In the period of the third PTLD episode, the increase was more than 14 000 copies per mL blood per day due to extreme high cyclosporine A blood levels. In patient no. 2, EBV DNA load increased from undetectable to 25 000 copies per mL blood in a week's time (553-560 days after LTx). Apparently EBV+ B cells both in tissue and peripheral blood have an enormous growth potential when CTL activity is decreased. On the contrary, decreasing immune suppression quickly reduces peripheral blood EBV DNA load, which may correspond with improved anti-EBV CTL responses and clinical regression of PTLD.30
In contrast to the unfractionated whole blood samples, all tested serum samples of PTLD patients were below the cut-off level despite highly elevated EBV DNA levels in simultaneously obtained unfractionated whole blood samples. This indicates that the elevated EBV DNA loads are associated with the cellular blood compartment, ie, the EBV-infected B cell originating either from the PTLD itself or from aberrant expansion of the infected B-cell pool in the circulation in absence of lytic viral replication. Possibly the use of the antiviral drugs acyclovir and gancyclovir in the LTx population prevents lytic replication despite strong immune suppression.
There were no elevated EBV DNA loads observed in transplantation recipients without PTLD, even if the recipients were treated for rejection episodes with high levels of methylprednisolone. This is in agreement with Green et al,17 who found a temporal relationship between peripheral blood EBV DNA load and onset of rejection, with only very low EBV DNA loads at diagnosed rejection in all cases. Clearly this shows an important feature contained within EBV DNA load monitoring, as it may discriminate PTLD from acute rejection.
Several of the 8 non-PTLD control patients suffered from episodes of CMV reactivation and rejection. Despite this we found no simultaneous EBV reactivation. On the other hand several PTLD patients had no CMV-related problems. These findings are in contrast to data published by Hornef et al,31 who found coincidence of symptomatic CMV infection, rejection episodes, and serologic signs of EBV reactivation in renal transplantation recipients. In patient no. 1, who developed early PTLD after primary EBV infection, we observed delayed EBV-specific IgM and IgG response compared to EBV DNA detection, indicating serology to be an insufficient late marker for EBV infection and unsuitable for PTLD diagnosis (E.A.M.V. et al, unpublished data, September 2000). We conclude that different mechanisms (eg, differences in virus-specific CTLs, different responses to antiviral drugs, and differences in cell tropism) are involved in EBV and CMV reactivation and the 2 herpesviruses react differently in response to fluctuations in immune status of the immunocompromised individual.
The finding that EBV DNA load can increase rapidly in short periods of time before PTLD diagnosis has profound consequences for patient management. We propose that measurement of EBV DNA load in peripheral blood at least once a week is essential in high-risk patients for accurate monitoring of the dynamic balance between EBV-induced B-cell proliferation and CTL-mediated clearance. The proposed frequent monitoring can probably be limited to high-risk periods such as active primary EBV infection and following switch in immune suppression (eg, cyclosporine A replacement with FK506).
The limited fluctuations of EBV DNA in peripheral blood of EBV+ control LTx recipients without PTLD indicate that these patients still manage to limit B-cell proliferations. On the other hand, in patients prone to develop PTLD, a different EBV expression program may be switched on in premalignant EBV-infected cells. This is in part indicated by our findings of a rather heterogeneous EBV gene expression at the single cell level in PTLD tissue.9,10 Preliminary results show that a limited EBV gene expression profile exists in the circulation, confirming studies by Babcock et al.28 Further analysis of the EBV RNA expression patterns in circulating B cells or B-cell subpopulations of healthy donors and PTLD patients may increase our knowledge of the different forms of EBV burden that are transmitted to or exist in transplantation recipients.
We are grateful to Antoinette Brink for assistance with RNA in situ hybridization and immunohistochemistry, to Danny Dukers for help with generating Figure 3, and to Conny de Boer for technical assistance. We thank the laboratory of Transplant Immunology of the University Hospital Groningen, Groningen, The Netherlands, for collection of the whole blood samples.
Supported by grant 1C-18-CT96-0132 from the European Community, Brussels, Belgium.
S.J.C.S. and E.A.M.V. contributed equally to this article.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Servi J. C. Stevens, Department of Pathology, University Hospital Vrije Universiteit, De Boelelaan 1117, 1081 HV, Amsterdam, The Netherlands; e-mail: s.stevens@azvu.nl.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal