Abstract
A case of a novel mutation in the F7gene that results in factor VII coagulant activity (VII:c) of less than 1% and VII antigen (VII:Ag) levels of 10% is presented. DNA analysis revealed a homozygous 15–base pair (bp) in-frame insertion-type mutation at nucleotide 10554. This insertion consisted of a duplication of residues leucine (L)213 to aspartic acid (D)217 (leucine, serine, glutamic acid, histidine, and aspartic acid), probably arising by slipped mispairing between 2 copies of a direct repeat (GCGAGCACGAC) separated by 4 bp. Molecular graphic analyses showed that the insertion is located at the surface of the catalytic domain in an exposed loop stabilized by extensive salt-bridge and hydrogen bond formation at which the calcium binding site is located. The mutation probably interferes with protein folding during VII biosynthesis and/or diminishes functional activity through the loss of calcium binding. In vitro expression studies demonstrated that the levels of VII:Ag in lysates of cells transfected with wild type VII (VIIWT) were equivalent to those with mutant type VII (VIIMT), but the level of secreted VIIMT was 5% to 10% that of VIIWT. Pulse chase studies demonstrated that VIIMT did not accumulate intracellularly, and studies with inhibitors of protein degradation showed that recombinant VIIMT was partially degraded in the pre-Golgi compartment. Accordingly, only small amounts of VIIMT with undetectable procoagulant activity were secreted into conditioned media. These results demonstrate that a combination of secretion and functional defects is the mechanism whereby this insertion causes VII deficiency.
Introduction
Factor VII (VII) has a central role in the initiation of blood coagulation. This glycoprotein circulates in blood as a single-chain zymogen composed of 406 amino acid residues1 with a molecular weight of 50 kd. After the formation of a one-to-one stoichiometric complex with its cell surface receptor and cofactor tissue factor (TF) and in the presence of calcium ions, VII is rapidly cleaved to its active form, VIIa. This activation occurs through proteolytic cleavage at a single site (arginine (R)152-isoleucine (I)153).2,3 VIIa converts zymogen factors X and IX to the corresponding active enzymes.2VIIa is composed of an N-terminal light chain (152 amino acids) and a C-terminal heavy chain (254 amino acids) linked by a disulfide bond. The light chain contains an amino terminal γ-carboxy-glutamic acid–rich domain followed by 2 epidermal growth factor (EGF)–like modules. The heavy chain consists of the catalytic domain. TheF7 gene is 12.8 kb in length and is located 2.8 kb away from the F10 gene on the long arm of chromosome 13.4The mature protein is encoded by exons 2 to 8.
VII deficiency is a rare recessive bleeding disorder with a relatively poor correlation between VII coagulant activity and hemorrhagic symptoms.5 Several missense mutations and a few small deletions and insertions in the human F7 gene have been reported,6,7 but no insertion-type mutation or mutations affecting the VII calcium site in the catalytic domain have yet been documented (see the VII mutation database athttp://europium.csc.mrc.ac.uk). Here, using expression studies, we describe and characterize the first case of VII deficiency caused by a homozygous insertion-type mutation consisting of a duplication of a 15-bp segment within exon 8 (leucine [L]213-aspartic acid [D]217) in the catalytic domain {73-77} (Figure1). (Chymotrypsinogen numbering is denoted throughout by curved brackets.) Crystal and solution scattering structures are available for VIIa and its complex with TF.8-11 We show, using molecular graphic analysis, that the insertion is proximate to the calcium binding site of the catalytic domain and propose that this is responsible for the complete loss of VIIa activity. In vitro transient and stable expression studies demonstrated reduced levels of VII:Ag (antigen) in conditioned media, supporting the hypothesis of a secretion defect caused by the insertion mutation. This mutation probably does not interfere with VII synthesis, but it is associated with various defects including abnormal folding, intracellular degradation, secretion failure, and lack of coagulant activity. To our knowledge this is the first instance of a VII deficiency caused by a perturbation at its calcium binding site in the catalytic domain.
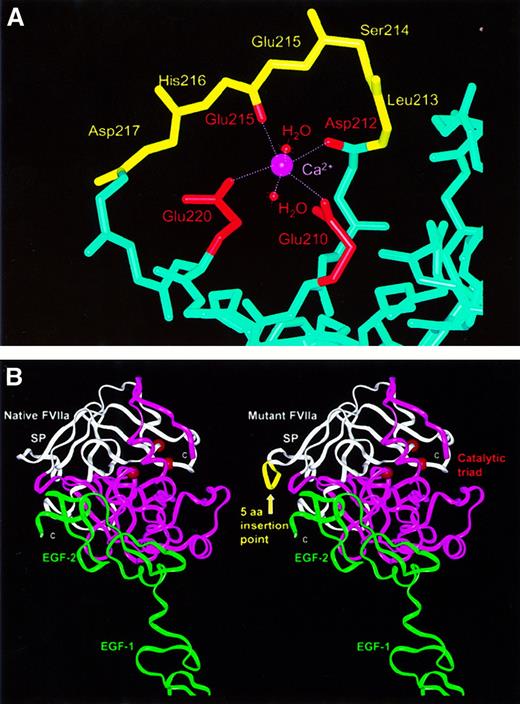
Molecular graphic views of the 15-bp insertion mutation (L213-D217ins) in the VII catalytic domain.
(A) In native VIIa (PDB code, 1cvw), the polypeptide main-chain backbone is shown in light blue, with the exception of residues L213-D217, which are shown in yellow. The calcium ion is shown in magenta, with its 6 ligands being the side-chain carboxyl oxygen atoms of E210 and E220, the main-chain carbonyl oxygen atoms of D212 and E215, and 2 water molecules. With the exceptions of E210 and E220, the side chains are not shown. (B) Structural comparison of native VIIa (left) with a model of VIIa containing the 15-bp insertion mutation (right) (PDB code, 1dan). In native VIIa, the sequence of the β-strands G and H (underlined) and the loop between them isLIAVLGEHDLSEHDGDEQSRRVAQVIIP. In the mutant this sequence becomesLIAVLGEHDLSEHDLSEHDGDEQSRRVAQVIIP.7The 2 structures are shown in ribbon views, where the N-terminal and C-terminal subdomains of the catalytic domain are shown in white and magenta, respectively. The EGF-1 and EGF-2 domains are shown in green and are proximate to the 2 TF domains at the bottom (not shown). The insertion mutation is shown as a yellow ribbon, while the catalytic triad is represented by 3 red spheres on the opposite side of the domain to that of the 15-bp insertion.
Molecular graphic views of the 15-bp insertion mutation (L213-D217ins) in the VII catalytic domain.
(A) In native VIIa (PDB code, 1cvw), the polypeptide main-chain backbone is shown in light blue, with the exception of residues L213-D217, which are shown in yellow. The calcium ion is shown in magenta, with its 6 ligands being the side-chain carboxyl oxygen atoms of E210 and E220, the main-chain carbonyl oxygen atoms of D212 and E215, and 2 water molecules. With the exceptions of E210 and E220, the side chains are not shown. (B) Structural comparison of native VIIa (left) with a model of VIIa containing the 15-bp insertion mutation (right) (PDB code, 1dan). In native VIIa, the sequence of the β-strands G and H (underlined) and the loop between them isLIAVLGEHDLSEHDGDEQSRRVAQVIIP. In the mutant this sequence becomesLIAVLGEHDLSEHDLSEHDGDEQSRRVAQVIIP.7The 2 structures are shown in ribbon views, where the N-terminal and C-terminal subdomains of the catalytic domain are shown in white and magenta, respectively. The EGF-1 and EGF-2 domains are shown in green and are proximate to the 2 TF domains at the bottom (not shown). The insertion mutation is shown as a yellow ribbon, while the catalytic triad is represented by 3 red spheres on the opposite side of the domain to that of the 15-bp insertion.
Patient, materials, and methods
Patient
The patient, born from a consanguineous marriage, is a 5-year-old female from Oman. The first bleeding episode was noted after female circumcision. Hematuria occurred in her first year of life, and several episodes of epistaxis and hematuria occurred at age 3. These bleeding episodes were severe enough to require transfusions of fresh frozen plasma and red cells. Informed consent to perform the research studies was obtained from the patient's family.
Collection and processing of blood samples
Blood was collected by atraumatic venipuncture into plastic tubes containing 1/10th volume 0.129 M buffered trisodium citrate. Plasma was obtained by centrifugation at 2500g for 15 minutes at 4°C, transferred into plastic tubes, and stored along with leukocytes at −80°C until use.
VII assays
Plasma factor VII coagulant activity (VII:c) was measured by a one-stage prothrombin time-based assay using rhTF (RecombiPlastin; Ortho Diagnostic Systems, Raritan, NJ) and VII-deficient plasma (Dade International, Miami, FL). Plasma VII:Ag was measured with an enzyme-linked immunoabsorbent assay (ELISA) with murine monoclonal antibodies (mAbs) against VII.12 A normal plasma pool was constructed by mixing equal volumes of plasma from 40 healthy subjects, 20 men and 20 women (not pregnant and not taking oral contraceptives). In the expression studies, levels of recombinant VII:c and VII:Ag were determined using the functional assay described above and ELISA (American Bioproducts Co, Parsippany, NJ) using a polyclonal (pAb) rabbit antiserum against human VII, respectively.
DNA isolation and in vitro amplification using polymerase chain reaction
Genomic DNA was isolated from peripheral blood leukocytes by established techniques.13 The coding regions, intron/exon boundaries, and 490 bp of 5′ flanking region of the F7 gene were amplified by polymerase chain reaction (PCR) and analyzed for mutations by single-strand conformational polymorphism (SSCP).14 PCR amplifications were carried out in 100-μL volumes comprising 100-500 ng DNA, 100 pmoles of each oligonucleotide primer, 200 mM of each dNTP (deoxynucleoside 5′-triphosphate), 1.5 mM MgCl2, 50 mM potassium (KCl), 10 mM Tris-HCl (tris[hydroxymethyl] aminomethane–hydrochloride) (pH 8.3), and 2 units Taq polymerase (Perkin-Elmer Cetus, Norwalk, CT). Reactions were denatured at 94°C for 5 minutes, then 40 cycles of amplification were performed, with a 10-minute extension time in the last cycle. For SSCP analysis, 1 μL of each final PCR product was mixed with 5 μL denaturation buffer (95% formamide, 0.05% xylene cyanol, and 0.05% bromphenol blue), which was denatured for 10 minutes at 94°C and then placed on ice for 2 minutes. We loaded 3-4 μL of each denatured sample onto a 10% polyacrylamide gel (acrylamide:bisacrylamide, 99:1) in Tris-borate ethylenediamine tetraacetic acid (EDTA) buffer (0.09 M Tris-borate and 2 mM EDTA) supplemented with 7.5% urea. Following electrophoresis DNA fragments were visualized by silver staining (Promega Corp, Southampton, England). PCR fragments showing abnormal SSCP migration were purified by Bioline PCRapid Purification kit (Bioline, London, England) and directly sequenced using an ABI 377 automated DNA sequencer (Perkin-Elmer Applied Biosciences, Warrington, England).
Antibodies
The production and purification of mAb1476, a murine mAb that recognizes an epitope in the amino terminal region of VII, has been described.15 This antibody was used to detect VII in pulse chase experiments.
Structural analysis of the mutant protein
The crystal coordinates of the TF-VIIa complex and unbound VIIa at resolutions of 0.2-0.28 nm (2.0-2.8 Å)9-11were obtained from the Protein Databank (PDB codes, 1dan, 1qfx, and1cvw). Protein structures were visualized using INSIGHT II software (MSI, San Diego, CA) on Silicon Graphics INDY Workstations (Silicon Graphics, Mountain View, CA) in conjunction with stereo glasses. The VIIa secondary structures and side-chain solvent accessibilities are as reported previously.7 The rigid body fragment assembly method in the HOMOLOGY and DISCOVER modules (MSI) was used to model the insertion. For reason of their positions in the exposed surface loop, histidine (H)216 {76} and D219 {79} were fixed and used to define the insertion point, and D217 {77} and glycine (G)218 {78} were deleted. The 7-residue fragment DLSEHDG was inserted by standard homology modeling methods.16 A precalculated Cα distance matrix identified known loops in the Protein Databank that best fitted the corresponding Cα distance matrix based on the positions of H216 {76} and D219 {79} and other flanking residues in VIIa. Side-chain atoms were automatically generated for the inserted region using template structures and general rules for residue exchanges. The final inserted loop structure showed no steric overlap with the remaining VIIa structure and was refined using 300 steps of steepest descent energy minimization.
Cloning of the insertion mutation
The 15-bp insertion mutation identified at the very beginning of exon 8 was inserted into the VIIWT (wild type) complementary DNA (cDNA) by overlapping PCR. For the first PCR reaction, the VIIWT cDNA was used as a template. The upstream primer extended from positions 8906 to 8937 in exon 6, including the following underlined XbaI restriction site (5′-CCATGTGGAAAAATACCTATTCTAGAAAAAAG-3′). The downstream primer began at position 9726, the junction of exons 7 and 8 (5′-CCAGCACCG CGATCAAATTTCTCCAGTTC-3′), and included silent mutations (underlined) in codons 202 to 204, which introduced anApoI restriction site (AGGAACCTG to AGAAATTTG). For the second PCR reaction, the template was the PCR product of exon 8 from the patient's genomic DNA. The upstream primer for this reaction included a 5′ extension of 29 bases of exon 7 sequence (positions 9698 to 9726) containing the silent mutations as well as the first 30 bases of exon 8 sequence (positions 10543 to 10572) and 15 bp of insertion (lower case): (5′-GAACTGGAGAAATTTGATCGCGGTGCTGGGCGAGCACGACCTCAGCGAGCACGACCTCAGCGAGCACGACGGGG-3′). The downstream primer extended from position 10927 to 10905 within exon 8 (5′-GGTTGCGCAGCCCTGGCCCCAGC-3′). For the third PCR reaction, the products of the first and second reactions and the upstream primer from reaction 1 and downstream primer from reaction 2 were used to generate the overlapping PCR fragment containing the insertion. The presence of the mutation was confirmed byApoI digestion.
The construction of plasmid pT7SalIVIIWTEcoRIwas described previously.17 The product of the third PCR reaction was purified, digested with XbaI andKpnI, and then ligated into this vector, which had been digested with the same enzymes, to make the clone pT7SalIVIIMTEcoRI. Cloned inserts were sequenced, which confirmed the correct sequence including the insertion mutation.
Construction of expression vectors
SalIVIIWTEcoRIandSalIVIIMTEcoRIfragments were prepared from the corresponding pT7 vectors and ligated into the expression plasmid pED-mtxr, which was provided by Dr Randal J. Kaufman. The resulting plasmids, pEDVIIWT and pEDVIIMT, are dicistronic messenger RNA (mRNA) mammalian expression vectors carrying the WT or MT VII cDNAs at the 5′ open reading frame and the dihydrofolate reductase (DHFR) cDNA at the 3′ open reading frame.17
Cell culture and transfection assays
For transient transfection experiments, African green monkey COS-1 cells (ATCC CRL1650) were maintained in Dulbecco modified Eagle medium (DMEM) supplemented with 10% (vol/vol) fetal bovine serum (FBS), 2 mM L-glutamine, 10 mM HEPES (4-(2-Hydroxyethyl)-1-piperazineethanesulfonic acid) (pH 7.2), 100 U/mL penicillin G, 100 μg/mL streptomycin, and 5 μg/mL vitamin K1 (Phytonadione; Abbott Laboratories, North Chicago, IL) in a 5% carbon dioxide (CO2) atmosphere at 37°C. Twenty hours prior to transfection, COS-1 cells were plated on 100-mm culture dishes at a density of 3 × 106 cells per dish. We transfected 15 μg of the pEDVIIWT or pEDVIIMT constructs into cells by lipofectamine (Gibco-BRL, Gaithersburg, MD) according to the manufacturer's instructions. After 16 hours, medium was changed, and 36 hours later, supernatants and cell lysates were harvested and assayed for VII:c and VII:Ag.
To obtain stable cell lines expressing recombinant VIIWT and pEDVIIMT, DHFR-deficient CHO cells (CHO-DUKX-B11)18(gift of Dr Barbara C. Furie, Boston, MA) were transfected with the pED expression vectors. These cells were grown in alpha modified essential medium (AMEM) supplemented with 10% FBS, 2 mM L-glutamine, 10 mM HEPES (pH 7.2), 100 U/mL penicillin G, 100 μg/mL streptomycin, 5 μg/mL vitamin K1, 10 μg/mL adenosine, 10 μg/mL deoxyadenosine, and 10 μg/mL thymidine. CHO-DUKX-B11 cells were plated on 100-mm culture dishes at a density of 3 × 106cells per dish. Transfections were performed as described above with 15 μg of either pEDVIIWT or pEDVIIMT plasmid and 45 μL lipofectamine. Two days after transfection, cells were divided at a 1:8 ratio and selected for DHFR expression using medium deficient in ribonucleosides and deoxyribonucleosides. Twelve days after transfection, 14 colonies were picked at random and cultured in 12-well (24-mm) plates. At day 20, when the cells achieved confluence, each well was split into 2 35-mm dishes. At 90% confluence, cell lysates were prepared and assayed for VII:Ag by ELISA.
Metabolic labeling studies
Nearly confluent 100-mm dishes of transiently transfected COS-1 cells or stably transfected CHO cells expressing recombinant VII were used for pulse chase experiments. Fresh media with FBS was added 4 hours before cells were deprived of methionine for 30 minutes and labeled for 15 minutes with 2 mL methionine-free AMEM (Gibco) containing 1.48 MBq (400 μCi) Expre35S (sulfur 35) Protein Labeling Mix (approximately 73% L–35S-methionine and approximately 22% L–35S-cysteine) (DuPont NEN Research Products, Billerica, MA) in a 5% CO2 atmosphere at 37°C. A chase was then performed in 2 mL medium containing an excess of unlabeled L-methionine (Gibco) for various time periods. At each time-point, medium was harvested and PMSF added to a final concentration of 1 mM. Cell extracts were prepared in 600 μL ice-cold NP-40 lysis buffer—50 mM sodium chloride (NaCl), 50 mM Tris (pH 8.0), and 1% (wt/vol) NP-40—supplemented with 1 mM PMSF. The cell lysates were precleared overnight at 4°C with 100 μL 20% (vol/vol) fixedStaphylococcus aureus Cowan I (SAC) coupled with a rabbit antimouse immunoglobulin G (IgG) (Sigma Chemical Co, St Louis, MO) in NP-40 lysis buffer.
Immunoprecipitation of VII was accomplished by incubating precleared cell lysates and conditioned media with 25 μg mAb MC1476 for 4 hours at 4°C. The resulting immune complexes were adsorbed with 70 μL 20% (vol/vol) Protein A Sepharose (Sigma) coupled 5:1 (vol/vol) with rabbit antimouse IgG antiserum in NP-40 lysis buffer. Pellets were washed 4 times in NP-40 lysis buffer, resuspended either in buffer for further enzymatic digestion (see below) or in polyacrylamide gel electrophoresis (PAGE) sample buffer with or without reducing agents, and denatured by heating to 95°C for 5 minutes. The immunoprecipitated proteins were resolved by sodium dodecyl sulfate (SDS)-PAGE in 8% (wt/vol) gels, and the radioactivity incorporated into VII bands was analyzed using a Bio-Rad model 501 PhosphorImager (Bio-Rad Laboratories) and radiography on X-OMAT-AR film (Eastman-Kodak Co, Rochester, NY).
In vitro transcription/translation of VIIWT and VIIMT
We linearized 1 μg pT7-VIIWT and pT7-VIIMT by EcoRI digestion and incubated the plasmids in rabbit reticulocyte-coupled transcription/translation reactions (Promega Company, Madison, WI) for 90 minutes at 30°C. We included 0.74 MBq (20 μCi) of translation-grade methionine (DuPont-New England Nuclear, Billerica, MA) in the reactions to label the synthesized VII protein, and a control reaction to which no plasmid DNA had been added was run in parallel. Aliquots of the reactions were electrophoresed on a 12% polyacrylamide gel under denaturing conditions.
Effect of protein degradation inhibitors on VIIWT and VIIMT levels
To study the effect of protein degradation inhibitors on VII biosynthesis, confluent stably transfected CHO cells grown in 100-mm dishes were incubated with media containing 50 mM ammonium chloride, 100 μM leupeptin, 50 μg/mL N-acetyl-leucine (L)-L-Norleucinal, or 10 μg/mL Brefeldin A (Sigma) dissolved according to the manufacturer's recommendations and used at previously published concentrations.19-23 After 4 hours, cell lysates were harvested and assayed for VII:Ag.
Results
Coagulation assays and genomic studies
A 5-year-old female patient from Oman exhibited moderately severe bleeding episodes. Coagulation assays revealed a severe VII deficiency with less than 1% VII:c and 10% VII:Ag. The asymptomatic parents had VII:c levels of 50% (mother) and 60% (father). To determine the molecular abnormality causing the VII deficiency in the patient, the entire coding region and 490 bp of promoter region of the F7gene were amplified and screened by SSCP and heteroduplex analysis. An abnormally migrating fragment was detected in exon 8 by SSCP analysis. Sequence analysis of this fragment demonstrated a homozygous insertion-type mutation located at nucleotide 10554. This insertion was an in-frame 15-bp (5-codon) duplication from L213 {73} to D217 {77} inclusive (CTC AGC GAG CAC GAC) and was located between codons 217 and 218.
Structural analysis of the mutant VIIa
The importance of the 5-residue insertion for VIIa function was revealed by the superimposition and viewing of the crystal structure for the VIIa-TF complex and 2 structures for unbound VIIa (PDB codes,1dan, 1qfx, and 1cvw). All 3 structures showed that the L213-D217 {73-77} sequence had identical conformations at a surface loop in the first subdomain of the catalytic serine protease domain. They occur within a sequence 210-EHDLSEHDGDEQSRR-224, in which 10 residues possess charged side chains, and half are buried from solvent. A network of buried hydrogen bonds and salt bridges occur within this loop, which is highly exposed to solvent and is adjacent to the active site cleft between the 2 subdomains of the catalytic domain. The loop is followed by the long β-strand G with many buried side chains that pass over the top of the domain surface and end at A242 {102} of the H-D-S (serine) catalytic triad.7 The insertion of 5 residues at this location is therefore expected to disrupt the correct formation of the loop and the insertion of β-strand G into the protein surface, and the insertion may lead to misfolded protein that may be degraded or not secreted.
In addition to the possibility of misfolding, the insertion coincides with a calcium binding site formed by direct metal contacts with the side chains of G210 {70} and G220 {80} and with the main-chain oxygen atoms of D212 {72} and G215 {75}. Two water molecules fill the 2 remaining calcium coordination sites (Figure2A). All 6 oxygen-calcium bond lengths range between 0.21-0.24 nm (2.1-2.4 Å) (PDB code, 1cvw). The insertion will directly affect the G215-calcium interaction, as Figure 2A shows that the correct main-chain conformation on the loop from D212 {72} to G215 {75} is critical for this calcium binding site. It is also likely that the 15-bp insertion will disrupt the G220 interaction with calcium. To visualize the possible effects of the L213-D217 insertion on VIIa, its sequence was added to the crystal structure of VIIa by homology modeling methods. This was most simply achieved by the deletion of H216 {76} and D217 {77} and reorienting these 2 residues outwards in order to permit the subsequent addition of the 5 additional amino acid residues. Figure 2B shows that the loop could be readily incorporated into the VIIa structure without steric problems. This indicated that mutant VIIa might form a folded protein, although it was not possible to comment on the probability of this correct folding taking place.
In vitro transcription/translation of VIIWT and VIIMT.
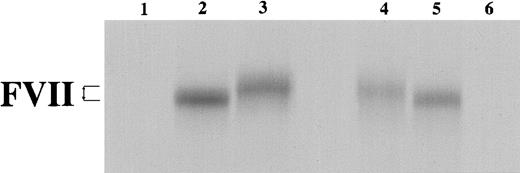
In vitro transcription/translation in a rabbit reticulocyte lysate system was completed with either no added DNA template (lanes 1 and 6) or with closed circular pT7VII plasmid (WT, lane 2; MT, lane 3), or with linearized pT7VII plasmid (WT, lane 5; MT, lane 4). Samples were analyzed on a 12% SDS-PAGE under reducing conditions. The difference in electrophoretic mobility is shown by arrows.
In vitro transcription/translation of VIIWT and VIIMT.
In vitro transcription/translation in a rabbit reticulocyte lysate system was completed with either no added DNA template (lanes 1 and 6) or with closed circular pT7VII plasmid (WT, lane 2; MT, lane 3), or with linearized pT7VII plasmid (WT, lane 5; MT, lane 4). Samples were analyzed on a 12% SDS-PAGE under reducing conditions. The difference in electrophoretic mobility is shown by arrows.
Expression studies
To investigate the influence of the insertion mutation, the pEDVIIWT and pEDVIIMT vectors were expressed in COS-1 cells by transient transfection. ELISA assays of the cell lysates demonstrated that VII:Ag levels of WT (80 ng/mL in 3 × 106 cells plated in 36 hours) and MT (75 ng/mL in 3 × 106 cells plated in 36 hours) were similar. However, the VII:Ag levels in the conditioned media of MT were significantly reduced to 5% (7 ng/mL) of WT (140 ng/mL) (Figure3). No VII procoagulant activity was detectable in the conditioned media of cells transfected with VIIMT (VII:c < 1%), which is consistent with the result obtained by assay of the patient's plasma. Recombinant VIIWT, in contrast, had normal procoagulant activity that was similar to the level of VII:Ag.
Pulse-chase of cell lysate and conditioned media of the cells stably transfected with pEDVIIWT or pEDVIIMT.
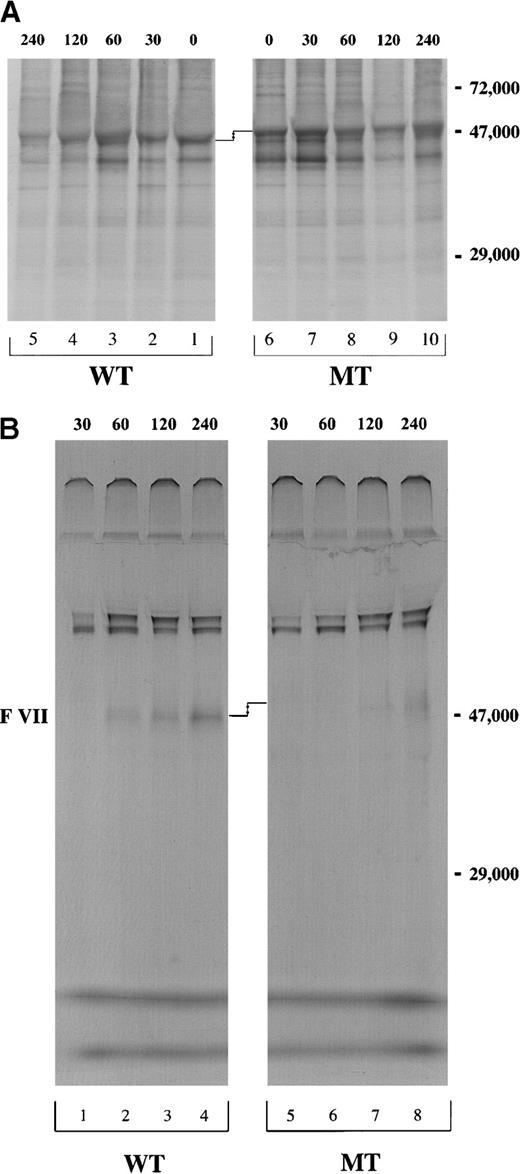
After 15 minutes of pulse with 35S-methionine, cells were chased for 30, 60, 120, and 240 minutes. Equivalent amounts of (A) cell lysate and (B) conditioned media for both WT and MT constructs were immunoprecipitated using mAb1476 against VII and analyzed by 8% SDS-PAGE under nonreducing conditions. The molecular weight marker is localized at the right side of the figures. The difference in electrophoretic mobility is shown by arrows.
Pulse-chase of cell lysate and conditioned media of the cells stably transfected with pEDVIIWT or pEDVIIMT.
After 15 minutes of pulse with 35S-methionine, cells were chased for 30, 60, 120, and 240 minutes. Equivalent amounts of (A) cell lysate and (B) conditioned media for both WT and MT constructs were immunoprecipitated using mAb1476 against VII and analyzed by 8% SDS-PAGE under nonreducing conditions. The molecular weight marker is localized at the right side of the figures. The difference in electrophoretic mobility is shown by arrows.
To compare the synthesis of VIIWT and VIIMT proteins, in vitro transcription/translation assays were performed. This experiment showed that when both the mRNA and the nascent protein were protected from degradation, equivalent amounts of VIIWT and VIIMT proteins were produced. It was noted that the VIIMT protein, which contained the additional 5 amino acids, migrated more slowly than the VIIWT protein under denaturing conditions (Figure 2).
We then investigated VII biosynthesis and secretion in CHO cells stably transfected with pEDVIIWT or pEDVIIMT cDNA. Following a 15-minute pulse with 35S-methionine, a chase was performed at 0, 30, 60, 120 and 240 minutes. At each time-point, the recombinant VIIWT in cell lysates was immunoprecipitated with mAb1476. Detectable levels of VIIWT were maximal at 60-120 minutes and decreased after this time as a consequence of protein secretion. Approximately similar amounts of intracellular protein appeared to be synthesized for VIIWT and VIIMT (Figure 3A, lanes 1-5 and 6-10). No intracellular accumulation was observed for VIIMT (Figure 3A, lanes 1-10). However, in the conditioned media, VIIMT was barely detectable at 120 minutes (Figure 3B, lane 7), and there was only a very small amount (5% to 10% VIIWT) after 240 minutes (Figure 3B, lane 8). The same difference in the electrophoretic mobility between VII WT and VIIMT in the transcription/translation experiment was also present in the immunoprecipitation analysis (Figure3A,B).
Effects of protein degradation inhibitors on VII biosynthesis
Because metabolic labeling studies indicated that the VIIMT protein was neither accumulating within the cell nor being secreted, we hypothesized that it might undergo intracellular degradation. We analyzed by ELISA the effects of various inhibitors of protein degradation on the intracellular VII levels in stably transfected CHO cells (Table 1). NH4Cl, a general inhibitor of lysosomal proteolysis, modestly increased WT VII:Ag levels (P = .001) but not MT VII:Ag levels (P = .74). Also, treatment with leupeptin, which inhibits cathepsins B, D, H, and L, did not change VII:Ag levels significantly, demonstrating that VIIMT is not likely to be degraded in the lysosome. We also investigated the effect of Brefeldin A on intracellular levels of VII:Ag. Brefeldin A is a compound that blocks protein transport from the endoplasmic reticulum to the Golgi complex and causes retrograde translocation of Golgi components back to the endoplasmic reticulum. Intracellular levels of VIIWT were increased by 55% after treatment with Brefeldin A (P < .0001), but there was no significant difference for levels of VIIMT (P = .12). This result suggests that partial degradation of the mutant protein occurs in a preGolgi compartment.
Effect of inhibitors of protein degradation and Brefeldin A on intracellular levels of VIIWT and VIIMT
| . | VIIWT, % . | VIIMT, % . |
|---|---|---|
| Media alone | 95 (±5) | 116 (±4) |
| NH4Cl (50 mM) | 121 (±4) | 115 (±3) |
| Leupeptin (100 μM) | 87 (±18) | 99 (±16) |
| Brefeldin A (10 μg/mL) | 150 (±3) | 88 (±24) |
| . | VIIWT, % . | VIIMT, % . |
|---|---|---|
| Media alone | 95 (±5) | 116 (±4) |
| NH4Cl (50 mM) | 121 (±4) | 115 (±3) |
| Leupeptin (100 μM) | 87 (±18) | 99 (±16) |
| Brefeldin A (10 μg/mL) | 150 (±3) | 88 (±24) |
Confluent CHO cells expressing recombinant VIIWT and VIIMT were incubated for 4 hours in fresh media containing 10% FBS in the presence or absence of agents. The results report the levels of VII:Ag in cell lysates, expressed as the percentage of VII:Ag (mean ± SE) from 3 independent experiments. The P values (see text) were calculated by the t test.
Discussion
The vast majority of mutations in the F7 gene are missense mutations, but a few nonsense mutations and small deletions have also been reported.6,7 We report a novel homozygous insertion-type mutation that causes a severe deficiency of VII. The insertion was localized between codons 217 and 218, leading to a duplication of codons 213 to 217. This mutation appears to be a classic case of slipped mispairing between 2 copies of a direct repeat (GCG AGC ACG AC) separated by 4 bp, in which the wild type evolved by internal duplication, thereby setting up the possibility of a mutation caused by slipped mispairing. Molecular graphic analyses of the 5–amino acid insertion in the VIIa crystal structure revealed that the insertion occurs at the calcium binding site in the catalytic domain. The 6 calcium-oxygen ligands are the same as those reported for bovine trypsin.24 As the intracellular concentration of calcium is 1.5 mM and its plasma concentration is 2.5 mM, VII is expected to interact with calcium as soon as it is synthesized. This report describes experiments that characterize the first mutation directly affecting this VII calcium binding site, and the lack of detectable procoagulant activity in VIIMT stresses the importance of this site for the normal function of VII.
Similar intracellular levels of VII:Ag measured by ELISA or detected by immunoprecipitation confirmed the normal synthesis of VIIMT. In contrast, the reduced levels of VII:Ag observed in conditioned media supported our hypothesis of a secretion defect caused by the insertion mutation. After 240 minutes of chase, very small amounts of labeled VIIMT were detected in the conditioned media. However no intracellular VII accumulation was seen, which suggests the additional possibility of intracellular degradation. Thus, we chose to block various pathways of protein biosynthesis and transportation using NH4Cl (an inhibitor of lysosomal degradation) and leupeptin (an inhibitor of cathepsins B, D, H, and L). These inhibitors did not markedly change intracellular VII:Ag levels. However, treatment with Brefeldin A, an agent that usually blocks protein transport from the endoplasmic reticulum to the Golgi complex and causes translocation of Golgi components back to the endoplasmic reticulum, caused the intracellular level of VIIWT to increase by 55%, whereas no significant difference was seen for VIIMT.
The small amount of VIIMT protein secreted by transfected cells showed no coagulant activity in a one-stage coagulation assay, and this confirmed the phenotype of the patient. The lack of activity in secreted VIIMT is well-explained in terms of the direct perturbation of 2 calcium binding sites at the main-chain carbonyl atoms of D212 and G215, which can affect either the activation of VIIa and/or the substrate or TF binding activity of VIIa. The insertion of 5 residues implies that the loop main-chain structure will be modified and that many of the side-chain interactions between buried residue pairs involving H216, D217, D219, G220, G221, and arginine (R)223 will no longer be possible. Thus, the insertion is expected to disrupt the side-chain positions of the 2 other calcium binding ligands at G210 and G220. In agreement with the modeling, alanine-scanning mutagenesis has shown that mutations in the side chains of G210, D212, L213, and G220 lead to marked decreases in proteolytic function without affecting TF binding.25 Mutants involving G220 reduced the coagulant activity of VII to 0.1%.26 Even though the homology modeling shows that it was possible to add a surface loop to the surface of VII, the correct formation of folded mutant VIIa may be difficult to achieve for reason of the extensive side-chain interactions in this loop region. If so, this would lead to the misfolding of the catalytic domain of VIIMT. Accordingly VIIMT may either be secreted at lower levels or be abnormally degraded. This explanation would account for the observed reduction in the VIIMT:Ag level when this was measured using 2 mAbs specific for the VIIa light chain.9 12
In conclusion, we report the first insertion mutation in theF7 gene and the first naturally occurring mutation to be associated with the VII calcium binding site in the catalytic domain. This mutation is associated with various defects including abnormal folding, intracellular degradation, secretion failure, and lack of detectable procoagulant activity.
Acknowledgments
We thank Drs P. V. Jenkins and J. Hinshelwood for assistance with the molecular graphics modeling.
Supported by the Katharine Dormandy Trust for Haemophilia and Allied Disorders and the Medical Research Service of the Department of Veterans Affairs.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Flora Peyvandi, Angelo Bianchi Bonomi Hemophilia and Thrombosis Center, Via Pace, 9–20122, Milan, Italy; e-mail:flora.peyvandi@unimi.it.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal