Abstract
The study identified 10 patients from 6 families with prolonged bleeding time, decreased von Willebrand factor (vWF) ristocetin cofactor activity (RCoF) to vWF:Ag (antigen) ratio, and reduced ristocetin-induced platelet agglutination as well as ristocetin- or botrocetin-induced binding of plasma vWF to platelet glycoprotein Ib (GpIb). In addition, all patients showed a decrease of intermediate-molecular-weight (intermediate-MW) and high-molecular-weight (HMW) multimers of vWF. In the heterozygous state, a cysteine-to-threonine (C → T) transversion was detected at nucleotide 4193 of the VWF gene of all patients and lead to the arginine (R)522C substitution in the A1 loop of vWF mature subunit (R1315C in the preprovWF). By in vitro mutagenesis of full-length complementary DNA (cDNA) of vWF and transient expression in COS-7 cells, the mutated C552 recombinant vWF (C552rvWF) was found to exhibit decreased expression, abnormal folding, and lack of intermediate-MW and HMW multimers. In addition, direct binding of botrocetin to C552rvWF, as well as ristocetin- and botrocetin-induced binding of C552rvWF to GpIb, was markedly decreased. Although being localized in an area of the A1 loop of vWF where most of the type 2B mutations that induce a gain-of-function have been identified, the R552C mutation induces a 2A-like phenotype with a decrease of intermediate-MW and HMW multimers as well as a loss-of-function of vWF in the presence of either ristocetin or botrocetin.
Introduction
von Willebrand factor (vWF) is a large multimeric plasma glycoprotein (Gp) with molecular weight (MW) ranging from 0.5 to 15 million. It plays an essential role in hemostasis as a mediator of platelet adhesion to the subendothelium of injured vessels and as a carrier of factor VIII (VIII).1 The vWF binds to VIII, to components of the subendothelium such as collagen, and to the platelet receptors GpIb and GpIIb/IIIa.1 In plasma vWF does not spontaneously interact with platelet GpIb. In vitro this interaction can be induced by the antibiotic ristocetin or by the snake venom protein botrocetin. The mechanism of binding induced by ristocetin appears distinct from that induced by botrocetin: Ristocetin can bind to both vWF and platelets, whereas botrocetin binds to vWF but not to GpIb, and both modulators act at different sites of vWF.2-4
The binding domain of vWF to platelet GpIb has been localized between amino acid (aa) 449 and 728 of the mature vWF subunit.5This region overlaps the A1 domain that is characterized by the presence of a large disulfide bond loop between cysteine (C)509 and C695. The complex role of the conformation of the vWF binding domain to GpIb has been investigated by deletions or by aa substitutions in this domain.4,6-10 Crucial sequences of the A1 loop are involved in the modulation of vWF binding to GpIb (inhibitor sequences: aa 497-511, 540-578, and 687-698; activator sequences aa 520-534 and 626) or directly interact with GpIb (aa 596-616, 629-632, and 642-645).9 Recently 2 groups showed that the lysine (K)599 residue is strictly required for the binding of vWF to GpIb.11,12 The crystal structure of the A1 domain of vWF13 14 is also helpful for better understanding the molecular switches that activate vWF and the mechanism of vWF binding to GpIb.
Mutations found in 2 well-identified molecular variants of von Willebrand disease (vWD), types 2B and 2M, are localized in the A1 domain. The mutations of type 2B vWD, which is characterized by an increased affinity of vWF for platelet GpIb, are mostly clustered within the aa 540-578 inhibitory sequence of the GpIb-binding domain.15,16 These 2B mutations are believed to modify the conformation of the A1 loop that becomes activated for vWF binding to GpIb 9. Thus, type 2B mutations induce a gain-of-function of vWF. Several point mutations—glycine-561-alanine (G561A), G561S (serine), glutamic acid (E)596K, phenylalanine-606-isoleucine (F606I), and I662F—and 2 deletions—deletion arginine-629-glutamine639 (delR629-Q639) and delK642—have also been identified within the A1 loop in type 2M vWD, which is characterized by a decreased platelet-dependent function not caused by the absence of high-MW (HMW) multimers.17-19 Studies using mutated recombinant vWFs (rvWFs) have shown that type 2M mutations are responsible for a loss-of-function of vWF by maintaining the A1 loop in an inactive conformation.17 19
Type 2A vWD refers to variants with decreased platelet-dependent function that is associated with the absence of HMW multimers. The large majority of mutations found in type 2A have been localized within the A2 domain of vWF between aa 742 and 909.15,16,18 The identification of mutations in the A1 loop inducing a 2A phenotype of vWD is still a matter of debate. Indeed, 5 mutations—C509R, C509G, R545C, valine (V)551F, and V553M (methionine)—have been identified within the A1 disulfide loop in patients with an apparent 2A phenotype.18,20-22 However, the corresponding mutated rvWF exhibited an increased affinity for platelet GpIb, as observed in patients with type 2B vWD.21,23-25 Moreover, the study of platelet vWF in a patient with the V553M mutation showing an increased affinity for GpIb is also in favor of type 2B vWD.22
In the present paper we report the identification of an arginine-to-cysteine mutation at position 552 of the mature vWF subunit (R1315C in the preprovWF) in 10 patients from 6 families showing a 2A-like phenotype. Using site-directed mutagenesis and transient expression in COS-7 cells, we demonstrate for the first time that a mutation located in the A1 loop of vWF induces a defect of expression, multimerization, folding, and GpIb binding capacity of vWF. Therefore, our data show that a type 2A-like mutation may occur in the A1 loop of vWF where all the vWD type 2B and type 2M mutations are also localized.
Patients, materials, and methods
Phenotypic and genotypic characterization of patients
The study involved 10 patients from 6 French families with vWD. All patients were aware of the investigative nature of the analysis and gave their consent. Response to DDAVP was evaluated in one patient and measured before and one hour after intravenous infusion of 0.3 μg/kg MinirinB (Ferring Laboratory, Gentilly, France). As a control, plasma from 2 patients fulfilling the criteria of type 2A and type 2M vWD was also used in the present study. F751C and I662F mutations were found in the heterozygous state in the gene of the type 2A and type 2M patients, respectively.
Study of bleeding time and vWF
Measurements of Ivy bleeding time using simplate (BT), VIII coagulant activity (VIII:c), vWF antigen level (vWF:Ag), vWF ristocetin cofactor activity (RCoF), and vWF collagen binding activity (vWF:CBA) were performed as reported.26 27 One hundred units of VIII:c, vWF:Ag, RCoF, and vWF:CBA are defined as the amount present in a 1-dL pool of 20 normal plasmas calibrated with the international plasma standard for VIII and vWF. Ristocetin-induced platelet agglutination (RIPA) was performed on platelet-rich plasma (PRP) by measuring the extent of agglutination using various concentrations of ristocetin.
Multimer analysis
The multimeric structure of patient plasma vWF (1 mU vWF:Ag per deposit) was analyzed by 0.1% sodium dodecyl sulfate (SDS)–1% agarose gel electrophoresis. The multimers were visualized using rabbit anti-vWF polyclonal antibodies (pAbs) labeled with125I (iodine 125).28 The relative percentage of low-MW (≤ 5 mers), intermediate-MW (5 < mers ≤ 10), and HMW multimers (> 10mers) was determined after densitometric scanning. The satellite bands in plasma vWF multimers were visualized after 0.1% SDS–2.5% agarose gel electrophoresis using rabbit anti-vWF pAbs conjugated to alkaline phosphatase, as previously described.29
vWF-GpIb binding assays
Platelet binding assays of patient plasma vWF were performed in the presence of various concentrations of agonist (ristocetin or botrocetin) using an anti-vWF monoclonal antibody (mAb) that does not inhibit the vWF-GpIb interaction as a marker. Ristocetin-induced platelet binding assays of plasma vWF of patients from family A were performed in the presence of various concentrations of ristocetin (0-1 mg/mL) as described by Hilbert et al.30 These researchers used anti-vWF 125I-mAb 32B12, which has an epitope between aa 51 and 60 within the N-terminal part of vWF subunit and recognizes all multimeric forms of vWF.31 Ristocetin- and botrocetin-dependent platelet-binding assays of plasma vWF of patients from families B to F were performed in the presence of various concentrations of ristocetin (0-1.5 mg/mL) or botrocetin (0-1 μg/mL), as described by Siguret et al,25 who used125I-mAb anti-vWF 505, which has an epitope localized in the A3 domain between aa 911 and 1114 of vWF subunit.32The binding was expressed as the percentage of bound radioactivity to platelets.
Identification of the molecular abnormality in theVWF gene
The 5′ part of exon 28 of the VWF gene from members of family A was amplified by the polymerase chain reaction (PCR) and sequenced using Thermo Sequenase Kit (Amersham, Les Ulis, France) as previously described by Hilbert et al.30 Exon 28 of theVWF gene from members of families B to F was amplified by PCR and analyzed by denaturing gradient gel electrophoresis (DGGE) as previously described.33 PCR fragments with altered electrophoretic mobility were excised from the gel and reamplified, and both strands were sequenced using dRhodamine Terminator Cycle Sequencing Ready Reaction kit (PerkinElmer, Foster City, CA).
Study of the intron 40 polymorphism of theVWF gene
The VWF gene polymorphism related to a variable number of tandem repeats (VNTRs) in intron 40 was used as a genetic marker to study the segregation of the VWF gene alleles in the different patients (except in patient D1, where a DNA sample was no longer available). This investigation was performed according to Mercier et al34 with the modifications described by Gaucher et al.35 Each intron 40 allele is represented by an arbitrary letter code and is identified by the length (expressed in base pair [bp]) of the 3 AluI fragments of the VNTR region. For example, allele A is characterized by 3 bands of 240, 276, and 340 bp, whereas allele E is identified by bands of 248, 292, and 324 bp.
Expression and characterization of C552rvWF
Expression vector construction.
Plasmid pSVvWFA containing the normal human full-length complementary DNA (cDNA) of vWF was obtained as previously described.29A vWF cDNA fragment of 1.2 kb was derived from digestion of pSVvWFA with enzymes AflII (nucleotide [nt] 4137 = aa 533) and NheI (nt 5358 = aa 941). Plasmid pSL-AN was obtained by subcloning the 1.2-kb fragment into the pSL1180 Superlinker Phagemid (Pharmacia Biotech, Saclay, France) at the polylinker site.
Site-directed mutagenesis was performed onto pSL-AN with the QuickChange Kit (Stratagene, La Jolla, CA) using 2 phosphorylated and complementary mutagenesis primers (Oligo Express, Paris, France): strand 552: 5′-CAGAAGTGGGTCTGCGTGGCCGTGG-3′; antistrand 552: 5′-CCACGGCCACGCAGACCCACTTCTG-3′ (the mutated nt is underlined). After verifying by sequencing that no undesired nucleotide substitution was present, the mutated AflII-NheI fragment was subcloned into pSVvWFA digested in parallel with the same enzymes to obtain the mutated full-length cDNA plasmid pSVvWFR552C.
Cell culture and transfection.
COS-7 cells were cultured with Dulbecco modified Eagle medium (DMEM) containing 2 mM L-glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin (all from Gibco-BRL, Cergy Pontoise, France) and 10% (vol/vol) fetal calf serum (FCS) (Boehringer Ingelheim Bioproducts, Gagny, France). Transfections were performed using the diethylaminoethyl (DEAE) dextran method as previously described.30 The recombinant proteins produced by plasmids pSVvWFA and pSVvWFR552C are called WTrvWF and C552rvWF, respectively. The hybrid rvWF resulting from 1:1 cotransfection experiments, performed to mimic the heterozygous vWF assumed to be present in patient plasma, is called R/C552rvWF.
Radio-labeling of proteins
Immunoglobulin G (IgG) from antibodies and purified botrocetin was labeled with 125I (Amersham) using the Iodo-Gen (Pierce Chemical, Rockford, IL) method.36 Specific radioactivity was 0.037-0.111 MBq/μg (1-3 μCi/μg) for anti-vWF mAbs and 0.37 MBq/μg (10 μCi/μg) for pAbs and botrocetin.
vWF:Ag determination
The amount of vWF:Ag present in the conditioned medium after transfection was determined by 2-site enzyme-linked immunosorbent assay (ELISA) used for coating and detecting a pool of mAbs with epitopes distributed along the mature subunit of vWF.25
Multimers and subunit analysis
The multimeric composition of rvWFs was analyzed by 0.1% SDS–1.5% agarose gel electrophoresis and visualized using rabbit anti-vWF pAbs labeled with alkaline phosphatase as previously described.29 We performed rvWF subunit analysis by electrophoresis on 0.1% SDS–5% polyacrylamide gels under reducing conditions, then transferred the gels onto nitrocellulose membranes and stained them with 125I-pAbs to vWF. MW markers used were commercial low-MW and HMW markers (Pharmacia).
Reactivity of anti-vWF mAbs toward the mutated C552rvWF
The mAbs 418 and 522 are directed against the N-terminal part of vWF mature subunit (aa 2-53 and 35-81, respectively)32 and inhibit the binding of vWF to VIII. The mAbs 328 and 724 have a conformation-dependent epitope against the A1 disulfide loop and inhibit the ristocetin-induced binding to GpIb2 and the binding of vWF to botrocetin.37 The mAb 505, directed against the A3 domain, inhibits the binding of vWF to collagen.
The reactivity of radio-labeled mAb 505 with rvWFs was analyzed by 2-site immunoradiometric assay (IRMA) using the pool of mAbs for coating (same pool of mAbs used for vWF:Ag determination). The reactivity of mAbs 418, 522, 328, and 724 with rvWFs was analyzed by IRMA using each mAb for coating and radio-labeled mAb 505 for detection.
vWF-botrocetin binding assays
Botrocetin was purified from Bothrops jararaca venom as previously described.38 A pool of mAbs (anti-Nter [N-terminal] mAbs) was constituted with anti-vWF mAbs having their epitopes evenly distributed along the N-terminal part of vWF subunit (aa 1-272) and no inhibitory effect upon vWF binding to botrocetin. Wells of 96-well microtiter plates were coated for 16 hours at 4°C with 100 μL anti-Nter mAbs at 10 μg/mL with 50 mM sodium carbonate (Na2CO3) at pH 9.6. After washing the wells were saturated with a solution of 3% bovine serum albumin (BSA) and incubated with 100 μL dilution rvWF (0-10 U/dL vWF:Ag) overnight at 37°C. Then 100 μL radio-labeled purified botrocetin solution (100 000 cpm per well) was added for 5 hours at 37°C. After drying, each well was cut out, and bound radioactivity was estimated. The amount of immobilized rvWFs was determined by IRMA using anti-Nter mAbs for coating and the 125I-mAb 505 as second antibody. Supernatant from mock-transfected cells was used as negative control.
vWF-GpIb binding assays
Ristocetin- and botrocetin-dependent platelet-binding assays of rvWFs were performed as described above for plasma vWF.25
Results
Phenotypic characterization of the 10 vWD patients
This study was performed on 10 patients from 6 French families. Family A was studied in Lille, France, while the 5 others were investigated in Bicêtre, France. All patients had a moderate-to-severe hemorrhagic syndrome with bruising, epistaxis, and gingivorrhagia, and the women had menorrhagia and post-partum bleeding.
All patients (Table 1) showed a normal platelet count; markedly prolonged BT; and profound deficiency of vWF levels, with a significantly decreased mean ratio of RCoF to vWF:Ag (0.38). When tested, levels of vWF:CBA paralleled those of RCoF. The response to DDAVP was tested in patient E1: BT was shortened to 12 minutes but was not normalized, no thrombocytopenia occurred, and levels of vWF:Ag and RCoF doubled 1 hour after infusion. In all patients RIPA was nil or markedly decreased at 1.3 mg/mL ristocetin and nil at 0.5 mg/mL agonist (Table 1).
Phenotypic data of the 10 patients with von Willebrand disease
| Patient . | Sex . | Age, y . | BT, min . | vWF:Ag, U/dL . | RCoF, U/dL . | RCoF/ vWF:Ag . | vWF:CBA, U/dL . | VIII:c, U/dL . | RIPA, 1.3 mg/mL . | RIPA, 0.5 mg/mL . | Platelet count, 109cells per L . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| A1 propositus | M | 44 | >20 | 17 | 6 | 0.35 | 8 | 25 | — | — | 412 |
| A2 sister | F | 41 | — | 18 | 9 | 0.50 | 12 | 29 | nil* | nil | 294 |
| A3 son | M | 12 | 17 | 20 | 6 | 0.30 | 7.5 | 38 | — | — | 445 |
| A4 nephew | M | 10 | >20 | 21 | 8 | 0.38 | 14 | 42 | nil | nil | 296 |
| B1 propositus | F | 31 | >20 | 19 | 5 | 0.26 | — | 24 | decreased | nil | 221 |
| B2 uncle | M | 47 | >20 | 25 | 12 | 0.48 | — | 57 | nil | nil | 218 |
| C1 propositus | F | 44 | 15.5 | 18 | 5 | 0.28 | — | 48 | nil | nil | 284 |
| D1 propositus | F | 33 | 20 | 20 | 4 | 0.20 | — | 27 | nil | nil | 227 |
| E1 propositus | F | 26 | >20 | 30 | 18 | 0.60 | — | 58 | decreased | nil | 307 |
| F1 propositus | M | 54 | >20 | 18 | 8 | 0.44 | 9 | 50 | decreased | nil | 254 |
| normal range | 4-8 | 50-150 | 50-150 | ≥0.7 | 50-150 | 50-150 | 150-400 |
| Patient . | Sex . | Age, y . | BT, min . | vWF:Ag, U/dL . | RCoF, U/dL . | RCoF/ vWF:Ag . | vWF:CBA, U/dL . | VIII:c, U/dL . | RIPA, 1.3 mg/mL . | RIPA, 0.5 mg/mL . | Platelet count, 109cells per L . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| A1 propositus | M | 44 | >20 | 17 | 6 | 0.35 | 8 | 25 | — | — | 412 |
| A2 sister | F | 41 | — | 18 | 9 | 0.50 | 12 | 29 | nil* | nil | 294 |
| A3 son | M | 12 | 17 | 20 | 6 | 0.30 | 7.5 | 38 | — | — | 445 |
| A4 nephew | M | 10 | >20 | 21 | 8 | 0.38 | 14 | 42 | nil | nil | 296 |
| B1 propositus | F | 31 | >20 | 19 | 5 | 0.26 | — | 24 | decreased | nil | 221 |
| B2 uncle | M | 47 | >20 | 25 | 12 | 0.48 | — | 57 | nil | nil | 218 |
| C1 propositus | F | 44 | 15.5 | 18 | 5 | 0.28 | — | 48 | nil | nil | 284 |
| D1 propositus | F | 33 | 20 | 20 | 4 | 0.20 | — | 27 | nil | nil | 227 |
| E1 propositus | F | 26 | >20 | 30 | 18 | 0.60 | — | 58 | decreased | nil | 307 |
| F1 propositus | M | 54 | >20 | 18 | 8 | 0.44 | 9 | 50 | decreased | nil | 254 |
| normal range | 4-8 | 50-150 | 50-150 | ≥0.7 | 50-150 | 50-150 | 150-400 |
BT indicates bleeding time; vWF, von Willebrand factor; Ag, antigen; RCoF, ristocetin cofactor activity; CBA, collagen binding activity; VIII:c, factor VIII coagulant activity; RIPA, ristocetin-induced platelet agglutination.
Undetectable RIPA at the ristocetin concentration tested.
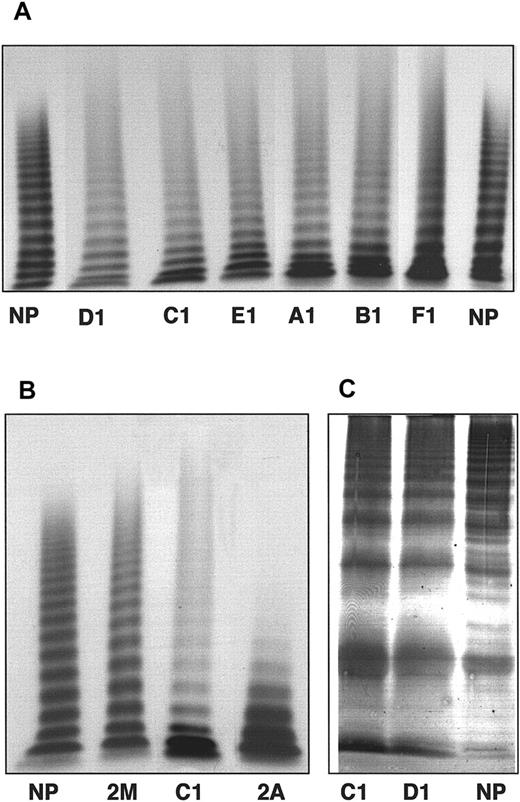
The 9 patients tested for multimeric analysis in low-resolution gels showed a marked decrease of the intermediate-MW and HMW multimers (> 5 mers) (Table 2), an increase in the lowest multimers (≤ 5 mers), and a smear in the upper part of the gels (Figure 1A) for the multimeric profile of the different propositi. Such a multimeric pattern appeared distinct from those observed when testing patients with typical type 2A and 2M vWD (Figure 1B). After electrophoresis in high-resolution gels, the marked increase of the vWF low-MW multimers already observed in the low-resolution gels was confirmed. In addition, a significant decrease in the intensity of satellite bands could be noted in patient plasma compared to the pool of normal plasma, as exemplified in Figure 1B for patients C1 and D1.
Multimeric distribution of patient plasma von Willebrand factor after 0.1% SDS–1% agarose gel electrophoresis
| Multimers . | Patients . | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| NP . | A1 . | A2 . | A3 . | A4 . | B1 . | C1 . | D1 . | E1 . | F1 . | |
| Low MW (≤ 5 mers), % | 43 | 68 | 67 | 72 | 65 | 57 | 65 | 52 | 62 | 57 |
| Intermediate MW (5 < mers ≤ 10), % | 37 | 18 | 21 | 19 | 23 | 27 | 21 | 28 | 23 | 26 |
| HMW (> 10 mers), % | 20 | 14 | 12 | 9 | 12 | 16 | 14 | 20 | 15 | 17 |
| Multimers . | Patients . | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| NP . | A1 . | A2 . | A3 . | A4 . | B1 . | C1 . | D1 . | E1 . | F1 . | |
| Low MW (≤ 5 mers), % | 43 | 68 | 67 | 72 | 65 | 57 | 65 | 52 | 62 | 57 |
| Intermediate MW (5 < mers ≤ 10), % | 37 | 18 | 21 | 19 | 23 | 27 | 21 | 28 | 23 | 26 |
| HMW (> 10 mers), % | 20 | 14 | 12 | 9 | 12 | 16 | 14 | 20 | 15 | 17 |
Results are expressed as the relative percentage of each group of multimers.
MW indicates molecular weight; HMW, high MW.
Eight patients tested for multimeric analysis.
(A) Multimer analysis of plasma vWF from the propositus of each family. (B) Comparison of sample from the propositus C1 with type 2A (F751C) and type 2M (I662F) plasma vWF. The plasma samples were electrophoresed in 1% agarose gels and visualized using 125I-labeled anti-vWF pAbs. (C) Analysis of triplet structure of plasma vWF multimers. The samples from propositi C1 and D1 were electrophoresed in 2.5% agarose gels and visualized using anti-vWF pAbs labeled with alkaline phosphatase. NP indicates normal plasma.
Eight patients tested for multimeric analysis.
(A) Multimer analysis of plasma vWF from the propositus of each family. (B) Comparison of sample from the propositus C1 with type 2A (F751C) and type 2M (I662F) plasma vWF. The plasma samples were electrophoresed in 1% agarose gels and visualized using 125I-labeled anti-vWF pAbs. (C) Analysis of triplet structure of plasma vWF multimers. The samples from propositi C1 and D1 were electrophoresed in 2.5% agarose gels and visualized using anti-vWF pAbs labeled with alkaline phosphatase. NP indicates normal plasma.
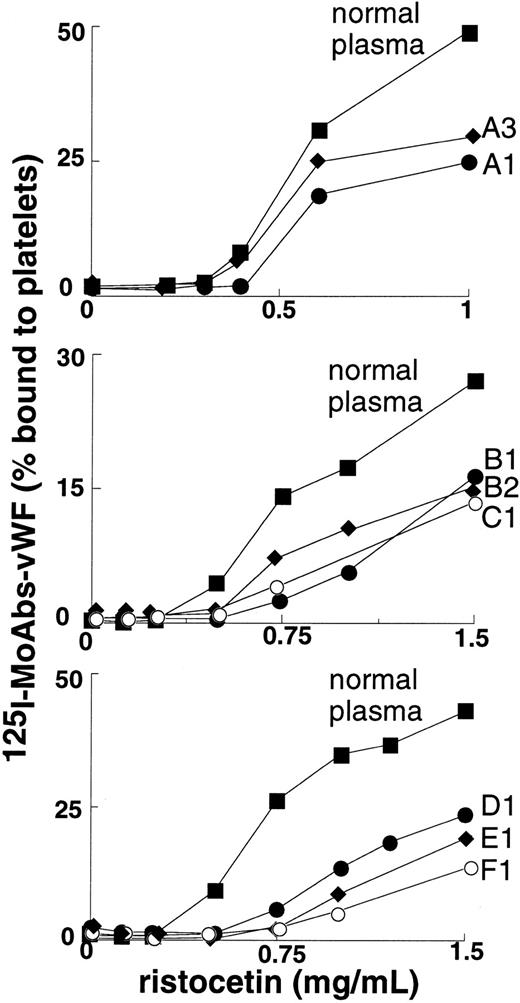
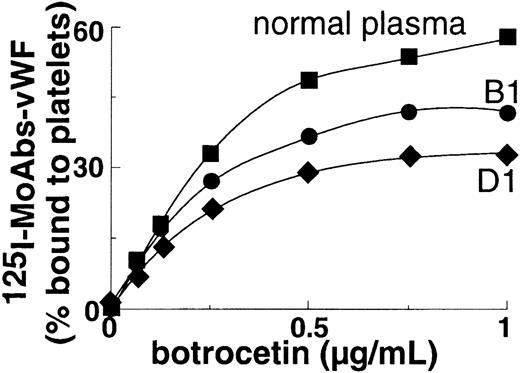
Binding of plasma vWF to platelet GpIb was moderately to severely decreased in all patients at concentrations of ristocetin ranging from 0.5-1.5 mg/mL (Figure 2); binding was nil at concentrations of ristocetin less than or equal to 0.5 mg/mL. In the patients who were tested (B1 and D1), binding of plasma vWF to GpIb was also significantly decreased at concentrations of botrocetin of at least 0.25 μg/mL (Figure 3).
Ristocetin-induced binding to platelets of plasma vWF from 8 patients.
Normal plasma or patient plasma vWF was indirectly labeled with 125I-labeled anti-vWF mAb and then incubated with fixed platelets in the presence of ristocetin. The binding was expressed as the percentage of platelet-bound radioactivity to total radioactivity. Results represent the mean of 2 experiments.
Ristocetin-induced binding to platelets of plasma vWF from 8 patients.
Normal plasma or patient plasma vWF was indirectly labeled with 125I-labeled anti-vWF mAb and then incubated with fixed platelets in the presence of ristocetin. The binding was expressed as the percentage of platelet-bound radioactivity to total radioactivity. Results represent the mean of 2 experiments.
Botrocetin-induced binding to platelets of plasma vWF from propositi B1 and D1.
Normal plasma or patient plasma vWF was indirectly labeled with125I-labeled anti-vWF mAb and then incubated with fixed platelets in the presence of botrocetin. The binding was expressed as the percentage of the platelet-bound radioactivity to total radioactivity. Results represent the mean of 2 experiments.
Botrocetin-induced binding to platelets of plasma vWF from propositi B1 and D1.
Normal plasma or patient plasma vWF was indirectly labeled with125I-labeled anti-vWF mAb and then incubated with fixed platelets in the presence of botrocetin. The binding was expressed as the percentage of the platelet-bound radioactivity to total radioactivity. Results represent the mean of 2 experiments.
Identification of a missense mutation in the vWF A1 disulfide loop
The 5′ part of exon 28 of the VWF gene from the propositus in family A (patient A1) was amplified by PCR and sequenced. A single C → T transversion was found in the heterozygous state at nt 4193, resulting in the substitution of the R552 by a cysteine in the mature subunit of vWF (R1315C in the preprovWF). This molecular abnormality has also been found in the heterozygous state in theVWF gene of the affected relatives of patient A1 who were available for the study (patients A2, A3, and A4).
Exon 28 of the VWF gene from 2 family B patients (patients B1 and B2) and other patients (patients C1, D1, E1, and F1) was amplified by PCR, analyzed by DGGE, and sequenced. The same C → T substitution at nt 4193 was observed in the heterozygous state. So far, between 73% and 88% of the VWF gene of patients from families B, C, D, and E have been analyzed by DGGE, and no other molecular abnormality has been found.
Study of the intron 40 polymorphism of theVWF gene
The R552C substitution was found to segregate with alleleA in family A. The propositus (patient A1) and her sister (patient A2) have both inherited the same paternal and maternal alleles and have transmitted this allele A, which carries the mutation, to their 2 children, who have been analyzed in this study. As patient F1 also displayed allele A, this suggests that he may be related to family A members, although these 2 families were apparently unaware of this relationship. In family B the R552C mutation segregated with allele E. Patients C1 and E1 displayed AluI bands that could not be related to an allele already identified in the other patients (data not shown).
Expression and characterization of C552rvWF
To determine the effect of the missense mutation R552C on vWF structure and function, the nucleotide substitution was introduced by site-directed mutagenesis. The corresponding mutated full-length cDNA was transiently expressed in COS-7 cells, and cotransfections, with equal amounts of WT and mutated cDNAs, were performed to mimic the heterozygous protein present in patient plasma. Whereas the amount of WTrvWF released into the cell culture medium was between 7 and 11 U/dL/72 h (n = 10 transfections), it was only between 2 and 3.5 U/dL/72 h for C552rvWF (n = 10 transfections) and 5 and 7 U/dL/72 h for the hybrid rvWF (n = 4 cotransfections). For further analysis, conditioned media were concentrated to 40 to 100 U/dL of vWF:Ag.
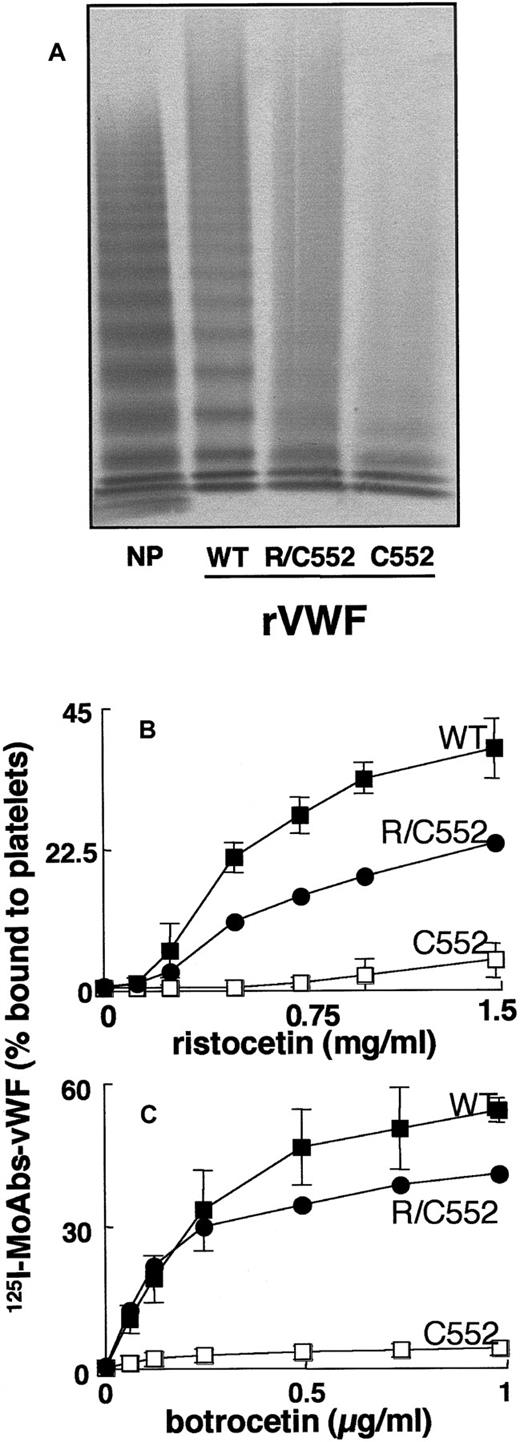
Analysis by 0.1% SDS–1.5% agarose gel electrophoresis showed that WTrvWF contained the full range of multimers (Figure4A). In contrast, the mutated C552rvWF showed a lack of intermediate-MW and HMW forms and exhibited only the lowest MW multimers (Figure 4A). The hybrid R/C552rvWF displayed an intermediate-MW multimeric pattern between those of WTrvWF and C552rvWF (Figure 4A). The number of multimers present in the hybrid and mutated proteins was difficult to determine due to the smear always observed in the upper part of the electrophoresis gels. When analyzed by SDS-PAGE (polyacrylamide gel electrophoresis) under reducing conditions, WTrvWF and C552rvWF showed a similar pattern, with a major band corresponding to the mature subunit of vWF (MW, 275 kd).
Structural and functional characterization of rvWFs.
(A) Multimeranalysis. The samples were electrophoresed in 1.5% agarose gels. vWF was visualized using anti-vWF pAbs labeled with alkaline phosphatase. Binding of rvWFs to platelets in the presence of (B) ristocetin or (C) botrocetin. The binding was expressed as the percentage of platelet-bound radioactivity to total radioactivity. Non-specific binding estimated with conditioned media from mock-transfected cells was less than 2%. Results represent the mean ± SD of 3 experiments performed in duplicate. In the figure, ■ indicates WTrvWF; ●, R/C552rvWF; and □, C552rvWF.
Structural and functional characterization of rvWFs.
(A) Multimeranalysis. The samples were electrophoresed in 1.5% agarose gels. vWF was visualized using anti-vWF pAbs labeled with alkaline phosphatase. Binding of rvWFs to platelets in the presence of (B) ristocetin or (C) botrocetin. The binding was expressed as the percentage of platelet-bound radioactivity to total radioactivity. Non-specific binding estimated with conditioned media from mock-transfected cells was less than 2%. Results represent the mean ± SD of 3 experiments performed in duplicate. In the figure, ■ indicates WTrvWF; ●, R/C552rvWF; and □, C552rvWF.
The reactivity of 5 anti-vWF mAbs was tested toward the C552rvWF by IRMA. The mutated protein was not recognized by mAbs 328 and 724 directed against the A1 domain, but it was normally recognized by mAb 505 directed against the A3 domain and by mAbs 418 and 522, of which the epitopes are in the N-terminal part of the vWF subunit (data not shown).
In the absence of modulator, WTrvWF and C552rvWF did not bind to platelets. In the presence of ristocetin or botrocetin, WTrvWF bound to platelets in a dose-dependent manner, whereas C552rvWF did not bind significantly to platelets (< 5%) whatever the concentration of inducer was (Figure 4B,C). The hybrid rvWF showed intermediate binding values between those obtained for WTrvWF and C552rvWF in the presence of ristocetin (Figure 4B), whereas this binding was slightly reduced at concentrations of at least 0.25 μg/mL botrocetin (Figure 4C).
To determine the effect of the R552C mutation upon the interaction of vWF with botrocetin, we analyzed the binding of a constant amount of125I-botrocetin to immobilized mutated rvWF using serial dilutions of rvWFs. In parallel, the amount of immobilized rvWF was determined by IRMA using the 125I-mAb 505 for revelation. The results showed that C552rvWF did not bind to purified botrocetin (data not shown).
Discussion
In the present study, we report the identification of a single C → T substitution at nt 4193 of the VWF gene, thereby changing the R552 to cysteine of the mature vWF subunit in 10 affected patients belonging to 6 families from different areas of France. Patients showed a moderate-to-severe hemorrhagic syndrome with prolonged BT, diminished levels of vWF and VIII, and marked decrease of RIPA. The multimeric profile of their plasma vWF was abnormal, with a markedly decreased proportion of intermediate-MW and HMW multimers and the presence of a smear. This structural abnormality is associated with a loss-of–function of vWF as determined by testing the GpIb-binding capacity of patient plasma vWF. In all cases, both ristocetin- and botrocetin-induced binding to platelets was significantly reduced. Our 10 patients thus have characteristics of type 2A vWD.39 The R552C substitution has also been identified by Casana et al40 in a single family with apparent type 2M vWD. The 2 patients were reported to display low levels of vWF and strongly decreased RIPA, but normal multimeric structure of plasma vWF. A discrepancy for classification between the latter patients and our 10 patients thus resides in the interpretation of vWF multimeric structure by SDS–agarose gel electrophoresis. Casana et al40 used 1.4% agarose, whereas we tested a lower agarose concentration (1%), which appears more adequate to detect a decrease or a lack of HMW multimers, and we confirmed our observations by densitometric analysis.
Thus, according to the classification of vWD,39 the decreased binding of patient plasma vWF to platelet GpIb and that of intermediate-MW and HMW multimers led us to conclude that the phenotype of our patients is closer to type 2A than type 2M vWD. However, we also used high agarose gels to study the characteristics of the satellite bands of the vWF multimers, and we observed that their intensity was markedly reduced. The presence of satellite bands reflects the proteolysis of vWF in plasma,41 which is enhanced in patients with typical type 2A vWD, which is classified as group 2 by Lyons et al.42In contrast, our data suggest that the R552C substitution decreases the sensitivity of vWF to physiologic degradation. Thus the phenotype of our patients appears somewhat distinct from classical type 2A vWD.
It is interesting to note that the R552C substitution is localized between V551 and V553, which have been found mutated in patients with either 2B or 2A phenotype18,21,22,43,44 but 2B genotype. The latter discrepancy may be explained by the spontaneous binding to platelets of plasma intermediate-MW and HMW multimers, which are cleared in vivo from the circulation. The remaining low-MW multimers are unable to function normally, as observed in type 2A vWD. Thus, the position of R552C mutation within the A1 loop and the uncertain classification of the corresponding phenotype prompted us to express rvWF containing the R552C mutation, by transient transfection in COS-7 cells, and to assess the effect of this substitution on the structure and function of vWF. The mutated protein C552rvWF showed a marked expression defect and an abnormal multimerization with the presence of low-MW multimers but a lack of intermediate-MW and HMW multimers and the existence of a smear. The hybrid rvWF resulting from cotransfections between normal and mutated vWF cDNA was better secreted and multimerized than the mutant, but it remained abnormal when compared with the WTrvWF. The presence of an additional cysteine residue, which may disturb the normal pairing of cysteine residues and lead to the formation of unnatural disulfide bonds within the multimers, is possibly responsible for the smear observed in the multimeric profiles of both plasma vWF and mutated rvWFs. Such a smear has been already reported for the rvWF harboring the R611C mutation.29
The study of the multimeric structure of mutated and hybrid rvWFs thus confirms that the abnormal multimerization of our patient plasma vWF is due to the presence of the R552C mutation. It also demonstrates that the mutation induces an additional defect leading to decreased vWF secretion, which is similar to that originally described by Lyons et al42 for vWF mutated in the A2 domain and classified as type 2A vWD group 1. However, the functional study of our mutant protein also underlines differences with this latter subtype.
We studied the capacity of C552rvWF and hybrid R/C552rvWF to bind to GpIb in the presence of inducers such as ristocetin and botrocetin. As expected from the multimeric structure, the C552rvWF did not bind to GpIb in the presence of either agonist. In addition, we observed that it was unable to bind to purified botrocetin. The hybrid R/C552rvWF showed a binding to GpIb that was significantly decreased in the presence of ristocetin and slightly reduced in the presence of botrocetin. These results confirm the data obtained with the plasma vWF of the patients described in this study. These data also indicate that besides the defect of secretion and of proteolysis, the R552C substitution induces the impairment of the vWF binding site for botrocetin. This can occur via either a direct modification of the binding site itself or a striking change of the conformation of the A1 domain leading to a major alteration of both the function and the maturation of vWF. Taken together, our results using conformational mAbs and those reported by several groups dealing with rvWF mutated at position 552 strongly suggest that this latter hypothesis is the most likely.
mAbs 724 and 328 have conformational epitopes within the A1 domain. mAb 724 inhibits binding of vWF to botrocetin, and mAb 328 inhibits ristocetin-induced binding of vWF to GpIb. We observed that neither mAb recognizes the mutated C552rvWF. Matsushita and Sadler9 showed that the doubly mutated rvWF549-552A was normally secreted and multimerized, but demonstrated a markedly reduced ristocetin- or botrocetin-induced binding to GpIb and direct binding to botrocetin. Kroner and Frey10 showed that the single mutant A552rvWF was normally secreted, but it had an incomplete multimeric structure; failed to interact with platelets in the presence of ristocetin; and had a moderately decreased binding to platelets in the presence of high concentrations of botrocetin, which suggests a decreased binding of botrocetin itself to their mutant. The discrepancies observed in secretion level and multimerization profile between R552C and R552A mutants thus appear to reside in the nature of the mutation. Finally Masushita et al11 produced and studied mutant R552A. Its expression and multimeric structure were found to be similar to those of WTrvWF, but when using conformational mAbs and binding assays, the substitution R552A was shown to cause significant misfolding of the A1 domain, with a decreased binding to conformation-dependent mAbs, botrocetin, and GpIb. Our results are thus in agreement with these reports and confirm the major role of residue 552 to maintain the normal folding and function of vWF.
The crystal structure of the A1 domain of vWF has been elucidated and is extremely useful for understanding the relationship between vWF structure and function.13,14 The A1 domain of vWF displays an α/β fold, with a central β-sheet formed by 5 parallel and one antiparallel β-strands flanked by 3 α-helices on each side. The R552 is localized in the βB-strand at the amino edge of the β-sheet and forms a salt bridge with the buried aspartic acid (D)514 localized at the bottom of the βA-strand.13 This contributes to the stabilization of the salt-bridge network elaborated between some of the charged amino acids of the A1 domain. As suggested by these authors, some of these salt bridges are important elements of folding, and therefore the mutation of R552 involved in a salt bridge may induce the abnormal folding of the A1 loop.
In conclusion, we have demonstrated that although being localized in an area of the A1 loop where most of the type 2B mutations inducing a gain-of-function of vWF have been identified, the R552C mutation induces a loss-of-function of vWF, thereby resulting in a type 2A-like phenotype that we observed in 10 patients from 6 families. This mutation strongly alters the secretion and multimerization of vWF as well as the folding of the A1 loop and, consequently, the biological activity of vWF. As ristocetin- and botrocetin-induced binding of vWF to platelets are known to be dependent upon the multimer size in plasma,38 the absence of binding of patient plasma vWF to GpIb is probably the result of a combined effect of the R552C mutation on the folding of the A1 loop and on the maturation of vWF leading to the decrease of HMW multimers. However, the respective contribution of each phenomenon in the binding to GpIb remains to be clarified.
Acknowledgments
The authors thank Sabine Belmont, Ghislaine Cherel, Denis Hoguet, Anne Houllier, and Bernadette Obert for their expert technical assistance.
A.-S.R. and L.H. contributed equally to this work.
Submitted March 2, 2000; accepted October 16, 2000.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
A.-S. Ribba, INSERM U.143, Hôpital de Bicêtre, 84 rue du Général Leclerc, 94276 le Kremlin-Bicêtre Cedex, France; e-mail: ribba@infobiogen.fr.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal