Abstract
Flt-1, also known as vascular endothelial growth factor receptor 1 (VEGFR-1), is a high-affinity tyrosine kinase receptor for VEGF and is expressed almost exclusively on vascular endothelial cells. As an exception, Flt-1 transcript was recently found to be expressed in human peripheral blood monocytes. However, the protein of the Flt-1 receptor on the cell surface of monocytes is yet to be identified, and whether the Flt-1 protein is expressed during the differentiation of monocyte-macrophage lineage cells has not been examined. Using monoclonal antibodies against 2 different antigenic epitopes on the Flt-1 extracellular domain, this study found that the major population of the monocyte-marker CD97+ cells in human peripheral blood express Flt-1 as a cell surface molecule. VEGFR-2 (KDR/Flk-1) was not expressed at detectable levels in these cells. An Flt-1 neutralizing monoclonal antibody significantly suppressed VEGF-induced migration of the monocytes, suggesting an important role for Flt-1 in the biologic function of monocytes. Furthermore, CD34+cells in human cord blood, originally negative for the Flt-1 expression, differentiated into Flt-1+ cells in association with the appearance of monocyte-macrophage markers after a 2-week culture in the presence of hematopoietic cytokines. In addition, the Flt-1+CD11b+ cell fraction from CD34+ cells was found to efficiently differentiate into multinuclear osteoclasts in the presence of macrophage colony-stimulating factor and osteoclast differentiation factor. These results strongly suggest that Flt-1 is a novel cell surface marker as well as a biologically functional molecule for monocyte-macrophage lineages in humans.
Introduction
Vascular endothelial growth factor (VEGF) and its receptors Flt-1 (VEGFR-1) and KDR/Flk-1 (VEGFR-2) are well established as the essential regulatory system for blood vessel formation in embryogenesis.1-4 Other VEGF family members such as VEGF-C and VEGF-D and their specific receptor Flt-4 (VEGFR-3) are also important for angiogenesis as well as lymphoangiogenesis in the embryo.5 The specificity of VEGF and its receptor system for vascular endothelial cells appears to be based on at least 2 factors, the endothelial cell–specific expression of Flt-1 and KDR/Flk-16,7 and a unique signaling pathway from the receptors. We have recently shown that VEGF-dependent DNA synthesis in primary endothelial cells is mainly mediated via the phospholipase Cγ-protein kinase C-mitogen-activated protein (MAP) kinase pathway, different from the signal transduction of the representative receptor tyrosine kinases such as EGF receptor.8 9
Most of the signals for VEGF-induced cell proliferation and vascular permeability are considered to be mediated by KDR/Flk-1, which has strong tyrosine kinase activity.10-12 On the other hand, the Flt-1 bears an affinity for VEGF that is about 10-fold higher than KDR, but contains much weaker tyrosine kinase activity, suggesting a regulatory function in the VEGF signaling in endothelial cells.13-16
Recently, in addition to vascular endothelial cells, human peripheral blood monocytes were reported to express flt-1 gene messenger RNA (mRNA).17,18 A population of these cells showed a VEGF-mediated migration even in the presence of KDR-neutralizing polyclonal antibody, suggesting that this cell migration signal is mediated by Flt-1.18 We have recently demonstrated that Flt-1 tyrosine kinase domain–deficient mice lost the capacity for VEGF-dependent cell migration of peritoneal macrophages, indicating that the Flt-1 tyrosine kinase activity is important for the VEGF-induced cell migration of macrophages in mice.16Although these results strongly suggest an intimate relationship between Flt-1 and the monocyte-macrophage lineages, it is still not clear which population of monocyte-macrophages expresses the Flt-1 molecule and carries the Flt-1–dependent signaling pathway, because monoclonal antibodies (mAbs) against the extracellular domain of Flt-1 have not been established yet.
Monocyte-macrophages are known to differentiate into a variety of cell types such as osteoclasts in bone, Kupffer cells in liver, dendritic cells in the immune system, and mature macrophages in a number of tissues. Furthermore, they are involved in a variety of major diseases such as inflammation, metabolic diseases, and cancer. Therefore, it appears important to clarify in which population of monocyte-macrophages Flt-1 is expressed on the cell surface and is biologically functional. In addition, because Flt-1 is a transmembrane receptor-type molecule, it seems worth examining whether it is useful as a cell surface marker for the differentiation of monocyte-macrophage lineages in FACS analysis.
To address these issues, we have established a series of mAbs against Flt-1 in humans. Here we report that Flt-1 but not KDR/Flk-1 is a novel cell surface marker for monocyte-macrophage lineages in adults and is gradually up-regulated during the differentiation and maturation of macrophages originating from the CD34+ hematopoietic progenitor cells in cord blood.
Materials and methods
Cells
The establishment of NIH 3T3 cells overexpressing either Flt-1 (VEGFR-1) or KDR/Flk-1 (VEGFR-2) was described previously.14 19 These cells were cultured in 10% calf serum containing Dulbecco modified Eagle medium (DMEM; Nissui, Tokyo, Japan) with 200 μg/mL of G-418. Human umbilical vein endothelial cells (HUVECs) were purchased from Clonetics Corp (San Diego, CA) and cultured in HuMedia-EG2 (Kurabo, Osaka, Japan). Human peripheral blood and cord blood from placenta were obtained from a healthy donor with informed consent.
Preparation of soluble VEGF receptors
Soluble VEGF receptor protein, Flt-1 7N, carrying the N-terminal first to seventh and Flt-1 3N carrying first to third Ig-like domains of Flt-1, were obtained by the method described previously.20 KDR 7N carrying the N-terminal first to seventh Ig-like domains and KDR 5Δ1N carrying second to fifth Ig-like domains of KDR fused with the Fc portion of an IgG (KDR 7N-Fc and KDR 5Δ1N-Fc, respectively) were obtained by the method described previously.21
Anti-VEGF receptors mAbs
The production of antihuman Flt-1 mAbs including KM1730 (mouse IgG1) and KM1732 (mouse IgG1) and antihuman KDR mAb including KM1992 (mouse IgG1) and KM1995 (mouse IgG2b) was carried out as follows. B3C3F1 mice (Charles River Japan, Kanagawa, Japan) at 6 to 8 weeks old were immunized by intraperitoneal injection of Flt-1 7N or KDR 5Δ1N-Fc. Spleen cells were fused with mouse myeloma cells and hybridoma cells producing the mAbs reactive with Flt-1 3N or KDR 7N-Fc were selected. Antihuman Flt-1 mAbs KM1730 and KM1732 react with the second Ig-like domain of Flt-1 and antihuman KDR mAbs KM1992 and KM1995 react with the fourth Ig-like domain of KDR. Mouse IgG1 mAb KM1135 or KM231 was used as a control mAb.
VEGF binding assay for soluble VEGF receptors
Fifty μL/well of 1.6 μg/mL Flt-1 7N or 4 μg/mL KDR 7N-Fc in phosphate-buffered saline (PBS, 137 mM NaCl, 2.7 mM KCl, 9.7 mM sodium phosphate buffer, pH 7.4) was applied to the surface of methanol-treated 96-well immobilon-P filtration plates (Millipore, Bedford, MA) overnight at 4°C, and nonspecific binding sites were blocked with 1% bovine serum albumin (BSA) in PBS. After 3 washes with PBS, 50 μL purified mAbs as competitor and 50 μL of 3 ng/mL [125]I-VEGF (Amersham-Pharmacia Biotech, Buckinghamshire, England) were added to each well and allowed to react for 1.5 hours at room temperature. After 3 washes with 0.05% Tween 20 in PBS, 30 μL microscinti-o (Packard, Meriden, CT) was added to each well and the radioactivity was measured in Topcount (Packard).
Flow cytometric analysis
Biotinylation of mAb was performed as described by Shitara and colleagues.22 For indirect immunofluorescence, the NIH 3T3–Flt-1 cells, NIH 3T3–KDR cells, NIH 3T3–Neo cells, or HUVECs (1 × 106) were incubated with 10 μg/mL mAbs or biotinylated mAbs for 30 minutes at 4°C. After the reaction with 30-fold diluted fluorescein isothiocyanate (FITC)-conjugated rabbit F(ab)′2 antimouse IgG (H+L) (Wako, Osaka, Japan) or 5 μg/mL streptavidin-phycoerythrin (Gibco, Gaithersburg, MD), the reactivity was analyzed in an EPICS Elite flow cytometer (Beckman-Coulter, Fullerton, CA).
Reaction of anti–Flt-1 and other antibodies with human peripheral monocytes
The mononuclear cells were isolated from human adult peripheral blood by the Ficoll/Paque density gradient method. The mononuclear cells were washed once with PBS, then suspended in 0.1% BSA-containing PBS. An aliquot of the mononuclear cells (3 × 106) was incubated with FITC-labeled anti-CD97 antibody (Pharmingen, San Diego, CA), phycoerythrin (PE)-labeled anti-CD11c antibody (Beckman-Coulter), and biotin-labeled anti-CD11b (Beckman-Coulter), respectively, at room temperature for 15 minutes. The samples were washed by centrifugation, reacted with streptavidin-APC (Becton Dickinson, Franklin Lakes, NJ), then once washed, and analyzed with an EPICS Elite flow cytometer (Beckman-Coulter).
For double labeling with anti–Flt-1 mAb KM1730 and anti-CD34 antibody, 3 × 106 mononuclear cells in 0.3 mL 0.1% BSA-PBS were incubated with anti–Flt-1 mAb KM1730 at room temperature for 15 minutes. After a wash, the cells were resuspended in 300 μL 0.1% BSA-PBS and reacted with PE-labeled antimouse-IgG goat antibody (Becton Dickinson). The cells were washed, then reacted with PC5-labeled anti-CD34 (Beckman-Coulter) at room temperature for 15 minutes. The cells were washed again and analyzed by flow cytometry.
For the double labeling of monocytes with anti–Flt-1 and anti-CD97 mAbs,23 the mononuclear cells were first reacted with anti–Flt-1 mAb KM1730, then with PE-labeled antimouse-IgG goat antibody as described above. Next, the cells were reacted with FITC-labeled anti-CD97 antibody (Pharmingen). After washing the samples were analyzed by flow cytometry.
VEGF-dependent monocyte migration
About 2 × 107 mononuclear cells obtained from healthy adult human peripheral blood were suspended in 10 mL 10% fetal calf serum (FCS; JRH Biosciences, Lenexa, KS) and kanamycin (40 μg/mL)-containing RPMI 1640 medium. The cells were plated on a culture dish that was preincubated with 10% FCS-RPMI 1640 overnight, and incubated at 37°C for 2 hours to attach the monocytes. Nonattached cells were removed from the plate and the attached monocytes were washed twice with PBS. The monocytes were recovered by incubating these plates with 5 mL 0.2% EDTA-containing 5% FCS-PBS for 20 minutes. The monocytes were washed 3 times with 10% FCS-RPMI 1640, and suspended at 1 × 106/mL in 0.5% FCS-RPMI 1640.
For the cell migration assay, a Neuroprobe 48-well microchemotaxis chamber (Neuroprobe, Bethesda, MD) was used. In the lower chamber, 25 μL 0.5% FCS-RPMI 1640 medium was added with or without purified recombinant VEGF. In some wells of the lower chamber, a neutralizing mAb against Flt-1 (KM1732) was added at 1 μg/mL. As a positive control, fMLP (N-formyl-methyonyl-leucyl-phenylalanine; Sigma, St Louis, MO) was added at a concentration of 5 nM. A filter (5-μm-pore-size PVP(−) polycarbonate filter, Neuroprobe) was overlaid; then, in the upper chamber 50 μL of the human monocytes (1 × 106/mL) was placed and incubated at 37°C for 2 hours in a 5% CO2 incubator. The filter was recovered, and the cells that migrated from the upper to lower layer were stained with Diff-Quik (Kokusai-Shiyaku, Kobe, Japan). The migrated monocytes were counted in 10 areas under a microscope to obtain an average value of cell migration.
In addition, macrophagelike cells were obtained from cord blood cells after a 12- to 14-day culture (see below) and used for cell migration assay as indicated for peripheral blood monocytes.
In vitro culture of cord blood CD34+cells for the proliferation and differentiation of macrophagelike cells
A mononuclear cell fraction containing monocytes and lymphocytes was isolated from human cord blood of placenta by Ficoll/Paque density gradient centrifugation. These cells were washed once with PBS, then suspended in 0.1% BSA-PBS. Mononuclear cells (3 × 106) in 0.3 mL 0.1% BSA-PBS were added with anti–Flt-1 mAb KM1730 and incubated at room temperature for 15 minutes. After being washed, the cells were reacted with PE-labeled antimouse-IgG goat antibody at room temperature for 15 minutes.
The cells were washed and reacted with PC5-labeled anti-CD34 antibody (Beckman-Coulter) and FITC-labeled anti-CD38 antibody (Beckman-Coulter) for 15 minutes. After washing, the cells were analyzed by flow cytometry.
The CD34+CD38+ cell fraction was recovered by cell sorting, and the cells were incubated at 37°C for 12 to 14 days under 5% CO2 in 30% FCS containing α-MEM supplemented with a cytokine mixture of interleukin (IL)-3 (200 U/mL, Kirin, Tokyo, Japan), IL-6 (200 U/mL, Ajinomoto, Tokyo, Japan), macrophage colony-stimulating factor (M-CSF; 100 ng/mL, Morinaga Milk Industry, Tokyo, Japan), granulocyte-macrophage colony-stimulating factor (GM-CSF; 20 ng/mL, Kirin), and Flt-3 ligand (100 ng/mL, R&D Systems, Minneapolis, MN).24 In the experiments for osteoclast differentiation, stem cell factor (SCF; 100 ng/mL, Amgen) was used instead of GM-CSF and Flt-3 ligand to increase monocyte-macrophage lineage cells. After incubation, the cells were harvested in 0.1% BSA-PBS, and then reacted with anti–Flt-1, anti-CD34, or anti-CD97 mAb as described above. These labeled cells were analyzed by flow cytometry.
Differentiation of macrophagelike cells to osteoclasts in vitro
Macrophagelike cells obtained from CD34+ cells after a 2-week incubation were washed and recultured with M-CSF and ODF (osteoclast differentiation factor)/OPGL (osteoproteregin ligand)/sRANKL (soluble RANK ligand)25,26 in fibronectin-coated dishes for 10 days. After the culture, cells were examined for phagocytic activity and for vitronectin receptor expression. For phagocytosis assay, cultured osteoclasts and macrophages were incubated with diluted (1 × 107/mL) yellow-green fluorescent beads, whose diameters were around 2 μm (Polysciences, Warrington, PA) for 2 hours at 37°C in a humidified atmosphere flushed with 5% CO2 in air. Then, adherent cells were washed 3 times with PBS and fixed with 5% formaldehyde for 15 minutes at room temperature. In addition, osteoclasts were specifically stained with antivitronectin receptor mAb (a mixture of anti-CD51 and anti-CD61 mAbs or clone 23C6 mAb) and detected with alkaline phosphatase antialkaline phosphatase (APAAP).27
Results
The mAbs against the extracellular domain of Flt-1 recognize the cell surface–expressed Flt-1 but not KDR/Flk-1
To obtain mAbs against Flt-1, several constructs of the extracellular domain of human Flt-1 were expressed in the Baculovirus-insect cell system, and the purified peptides were used as antigens to immunize mice. In parallel, mAbs against human KDR were prepared in a similar procedure (see “Materials and methods”).
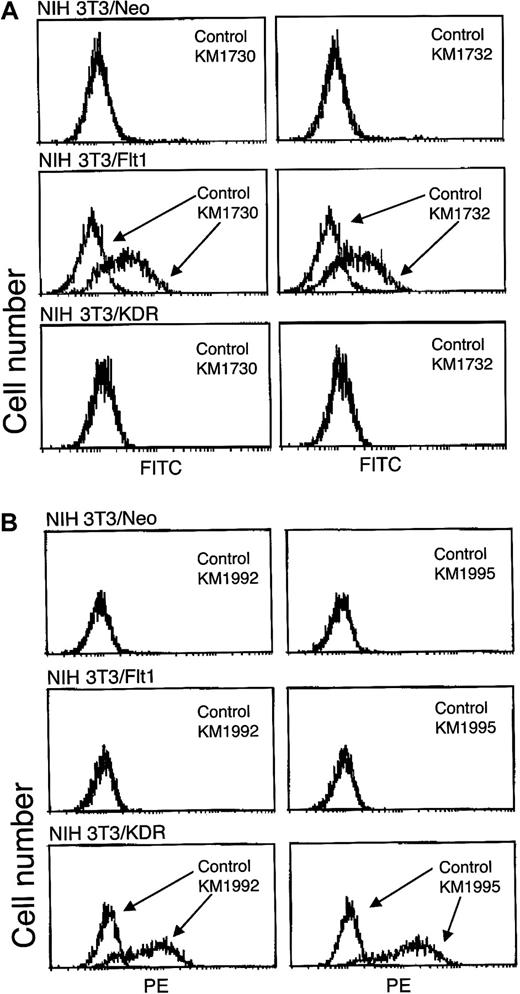
Figures 1 and2 indicate the specificity of the anti–Flt-1 mAbs. The KM1730 and KM1732 anti–Flt-1 mAbs recognized the Flt-1 expressed on the surface of NIH 3T3 cells, but not the KDR expressed on NIH 3T3 cells (Figure 1A). In addition, KM1732, which recognizes the second Ig domain of Flt-1, blocked the binding of VEGF to Flt-1 but not to KDR, indicating that KM1732 is a neutralizing mAb (Figure 2A). The second Ig region of Flt-1 and its surrounding structures were previously shown to be the direct binding site for VEGF.20,28 29 These anti–Flt-1 mAbs also recognized Flt-1 endogenously expressed on HUVECs (Figure 2B). On the other hand, anti-KDR mAbs, KM1992 and KM1995, recognized only the KDR molecule expressed on HUVECs and NIH 3T3 cells (Figures 1B,2B).
Specificity of mAbs KM1730 and KM1732 against Flt-1.
(A) Flow cytometric analysis of the cell binding of antihuman Flt-1 mAbs, KM1730 and KM1732. NIH 3T3/Neo, NIH 3T3/Flt-1, and NIH 3T3/KDR cells were stained with KM1730, KM1732, or control mouse IgG1 mAb and analyzed by flow cytometry. (B) Flow cytometric analysis of the cell binding of antihuman KDR mAbs, KM1992 and KM1995.
Specificity of mAbs KM1730 and KM1732 against Flt-1.
(A) Flow cytometric analysis of the cell binding of antihuman Flt-1 mAbs, KM1730 and KM1732. NIH 3T3/Neo, NIH 3T3/Flt-1, and NIH 3T3/KDR cells were stained with KM1730, KM1732, or control mouse IgG1 mAb and analyzed by flow cytometry. (B) Flow cytometric analysis of the cell binding of antihuman KDR mAbs, KM1992 and KM1995.
Inhibition of VEGF binding to Flt-1 by mAb KM1732 and flow cytometric analysis of HUVEC with mAbs.
(A) Inhibition of specific 125I-VEGF binding to Flt-1 7N or KDR 7N-Fc by anti–Flt-1 mAb, KM1732. Experimental details are given in “Materials and methods.” The profiles of KM1732 (○) and control KM231 (▴) are shown. Arrowhead (dashed line), inhibitor 0 μg/mL. (B) Flow cytometric analysis of HUVEC, binding with antihuman Flt-1 mAbs, KM1730 and KM1732, or antihuman KDR mAb, KM1992. HUVECs were stained with KM1730, KM1732, KM1992, or control mouse IgG1 mAb and analyzed by flow cytometry.
Inhibition of VEGF binding to Flt-1 by mAb KM1732 and flow cytometric analysis of HUVEC with mAbs.
(A) Inhibition of specific 125I-VEGF binding to Flt-1 7N or KDR 7N-Fc by anti–Flt-1 mAb, KM1732. Experimental details are given in “Materials and methods.” The profiles of KM1732 (○) and control KM231 (▴) are shown. Arrowhead (dashed line), inhibitor 0 μg/mL. (B) Flow cytometric analysis of HUVEC, binding with antihuman Flt-1 mAbs, KM1730 and KM1732, or antihuman KDR mAb, KM1992. HUVECs were stained with KM1730, KM1732, KM1992, or control mouse IgG1 mAb and analyzed by flow cytometry.
The major population of human peripheral blood monocytes expresses Flt-1 as a cell surface molecule
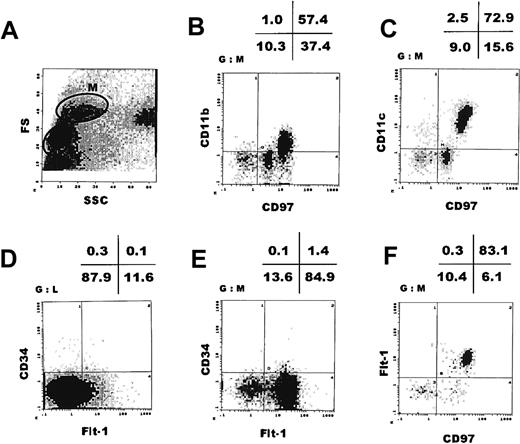
To examine whether Flt-1 is present on the cell surface of adult human monocytes, we carried out a cell fractionation study of normal monocytes by cell sorting of human peripheral blood mononuclear cells. With the cell size and the cell complexity as markers, we obtained a monocyte fraction (Figure 3A, M-fraction). We have previously shown that this M-fraction basically contains peripheral blood monocytes. In this fraction, cells were positive for CD97 (about 90%) as well as for CD11b and CD11c (58% and 75%, respectively), confirming that they are of monocyte-macrophage lineage30 (Figure 3B,C). CD97 in humans is known to be structurally closely related to a macrophage marker F4/80 in mice.
The major population of human peripheral blood monocytes expresses Flt-1.
Flow cytometric analysis of peripheral blood mononuclear cells obtained by Ficoll/Paque density gradient centrifugation. The M-fraction (monocyte fraction) in panel A was further analyzed by double staining with mAbs indicated in panels B, C, E, and F. (D) The L-fraction (lymphocyte fraction) in panel A was analyzed by double staining with anti-CD34 and anti–Flt-1 mAbs. FS indicates forward scatter; SSC, side scatter.
The major population of human peripheral blood monocytes expresses Flt-1.
Flow cytometric analysis of peripheral blood mononuclear cells obtained by Ficoll/Paque density gradient centrifugation. The M-fraction (monocyte fraction) in panel A was further analyzed by double staining with mAbs indicated in panels B, C, E, and F. (D) The L-fraction (lymphocyte fraction) in panel A was analyzed by double staining with anti-CD34 and anti–Flt-1 mAbs. FS indicates forward scatter; SSC, side scatter.
When these cells were sorted with anti–Flt-1 mAb KM1730 labeled with PE, at least 2 cellular populations were obtained. About 83% of the M-fraction clearly expressed human Flt-1 on the cell surface, whereas about 17% of these cells were only very weakly Flt-1+ or Flt-1− (Figure 3E,F). The lymphocyte fraction (Figure 3A, L-fraction) did not express Flt-1 (Figure 3D). In addition, anti–Flt-1 mAb KM1732, which recognizes an epitope different from the one KM1730 mAb recognizes, showed essentially the same pattern, that is, about 83% of the peripheral monocytes were Flt-1+ (data not shown). On average, M-fractions taken from 5 individuals showed 70.7% ± 17.0% Flt-1+ (Table1).
KM1730-staining–positive (Flt-1+) monocytes in human peripheral blood
| Case no. . | CD11b+ . | CD11c+ . | CD97+ . | KM1730+ . |
|---|---|---|---|---|
| 1 | 94.0 | 87.6 | 94.2 | 83.4 |
| 2 | 92.0 | 92.2 | 86.6 | 86.3 |
| 3 | 94.1 | 98.2 | 95.3 | 44.6 |
| 4 | 94.3 | 98.5 | 92.4 | 75.6 |
| 5 | 58.4 | 75.4 | 94.8 | 63.5 |
| Mean | 86.6 | 90.4 | 92.7 | 70.7 |
| SD | 15.8 | 9.5 | 3.6 | 17.0 |
| Case no. . | CD11b+ . | CD11c+ . | CD97+ . | KM1730+ . |
|---|---|---|---|---|
| 1 | 94.0 | 87.6 | 94.2 | 83.4 |
| 2 | 92.0 | 92.2 | 86.6 | 86.3 |
| 3 | 94.1 | 98.2 | 95.3 | 44.6 |
| 4 | 94.3 | 98.5 | 92.4 | 75.6 |
| 5 | 58.4 | 75.4 | 94.8 | 63.5 |
| Mean | 86.6 | 90.4 | 92.7 | 70.7 |
| SD | 15.8 | 9.5 | 3.6 | 17.0 |
Mononuclear cells were obtained from peripheral blood and monocyte fraction was further analyzed with anti-CD11b, CD11c, CD97, or Flt-1 (KM1730) antibody (see “Materials and methods”).
Therefore, we conclude that human peripheral blood monocytes can be separated into 2 populations in terms of the expression level of Flt-1, and that the major population of monocytes are clearly Flt-1+.
The Flt-1 receptor is involved in monocyte migration
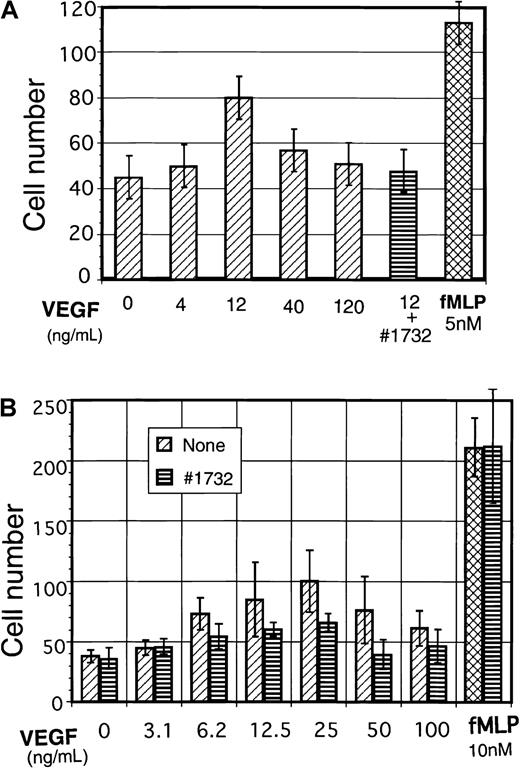
Next we examined the biologic significance of the Flt-1 expressed in peripheral cells of monocyte-macrophage lineage. Monocytes were selected from peripheral mononuclear cells by attachment to culture dishes and examined for VEGF-dependent cell migration using a Neuroprobe microchemotaxis chamber. As shown in Figure4A, VEGF stimulated cell migration in a dose-dependent manner, but at doses higher than 40 ng/mL, it was rather suppressive. Suppression of cell migration at higher doses of VEGF was also observed by other investigators.17 18 A neutralizing mAb KM1732 against Flt-1, which binds the second Ig region of Flt-1 and blocks VEGF binding to Flt-1, efficiently suppressed this VEGF-induced monocyte migration.
VEGF induces cell migration of human peripheral monocytes and macrophagelike cells differentiated from cord blood cells.
(A) Human peripheral blood monocytes were analyzed for VEGF-dependent cell migration. Experimental procedures are outlined in detail in “Materials and methods.” In some wells, anti–Flt-1 neutralizing mAb KM1732 (1 μg/mL) was used for the blocking of Flt-1. (B) Human macrophagelike cells (adherent cells) were obtained from CD34+ cord blood cells after 12- to 14-day cultures (see “Materials and methods”), and 1 × 106/mL of cells in RPMI 1640 medium (FCS-free) were used for VEGF-dependent cell migration assay as indicated in panel A.
VEGF induces cell migration of human peripheral monocytes and macrophagelike cells differentiated from cord blood cells.
(A) Human peripheral blood monocytes were analyzed for VEGF-dependent cell migration. Experimental procedures are outlined in detail in “Materials and methods.” In some wells, anti–Flt-1 neutralizing mAb KM1732 (1 μg/mL) was used for the blocking of Flt-1. (B) Human macrophagelike cells (adherent cells) were obtained from CD34+ cord blood cells after 12- to 14-day cultures (see “Materials and methods”), and 1 × 106/mL of cells in RPMI 1640 medium (FCS-free) were used for VEGF-dependent cell migration assay as indicated in panel A.
To further confirm VEGF-dependent monocyte-macrophage cell migration and its suppression by anti–Flt-1–neutralizing mAb, CD34+cord blood cells were differentiated into macrophage lineage (see below and “Materials and methods”) and the macrophagelike cells were used for cell migration assay. We obtained essentially the same results as monocytes in peripheral blood (Figure 4B). On the other hand, KM1732 mAb did not alter the cell migration in negative control (minus VEGF) nor that in positive control with fMLP. These results suggest that the Flt-1 expressed on the cell surface is important to the functions of monocyte-macrophages such as ligand-dependent cell migration.
Generation of macrophagelike cells from CD34+hematopoietic progenitor cells is associated with the expression of Flt-1 on the cell surface
To see whether we could induce the expression of Flt-1 on the cell surface in vitro under conditions of commitment and differentiation into cells of macrophage lineage, we took advantage of cord blood of placenta, which contains a relatively high percentage of pluripotent progenitor cells compared with peripheral blood mononuclear cells in adult stages. The CD34+ cells, shown to be mainly hematopoietic progenitor cells in the embryo,30 were isolated by a sorting procedure. About 90% of these cells were CD97−, and so not of the monocyte-macrophage lineage (Figure 5A,B). More than 95% of these CD34+ cells were also Flt-1−, as determined with anti–Flt-1 mAb KM1730 (Figure 5C).
CD34+Flt-1− cells in cord blood differentiate to CD97+Flt-1+macrophagelike cells after long-term culture.
(A) Flow cytometric analysis of human cord blood mononuclear cells. The L-fraction was further analyzed by double staining with CD34 and CD97 (B), with CD34 and Flt-1 mAbs (C), or with CD34 and CD38 mAbs (D). After a 2-week culture with a cytokine mixture (see “Materials and methods”), CD34+CD38+ cells were again analyzed by flow cytometry (E). The M-fraction in panel E was further analyzed by double staining with CD34 and CD97 (F), or with CD34 and Flt-1 mAbs (G). FS indicates forward scatter; SSC, side scatter.
CD34+Flt-1− cells in cord blood differentiate to CD97+Flt-1+macrophagelike cells after long-term culture.
(A) Flow cytometric analysis of human cord blood mononuclear cells. The L-fraction was further analyzed by double staining with CD34 and CD97 (B), with CD34 and Flt-1 mAbs (C), or with CD34 and CD38 mAbs (D). After a 2-week culture with a cytokine mixture (see “Materials and methods”), CD34+CD38+ cells were again analyzed by flow cytometry (E). The M-fraction in panel E was further analyzed by double staining with CD34 and CD97 (F), or with CD34 and Flt-1 mAbs (G). FS indicates forward scatter; SSC, side scatter.
The fraction of CD34+CD38+ cells was isolated by cell sorting and cultured for 12 to 14 days in the presence of a cytokine mixture (IL-3, IL-6, Flt-3 ligand, GM-CSF, and M-CSF). The CD34+CD38+ cell fraction covers about 95% of total CD34+ cell population (Figure 5D) and is known to efficiently respond to cytokines for colony formation. After this long-term culture, a large population of macrophagelike cells was found to be generated based on the cell size and complexity (Figure 5E). This macrophagelike cell fraction was isolated and further examined for the expression of monocyte-macrophage marker CD97 and Flt-1. As indicated in Figure 5F, about 98% of these cells expressed CD97, indicating proliferation and differentiation of the progenitor cells into macrophage-lineage cells. Interestingly, these cells separated into at least 2 major subpopulations, Flt-1 negative to weakly positive and Flt-1 strongly positive (Figure 5G, 6A). In an average of 6 experiments, Flt-1 weakly positive cells were 44.7% ± 13.2% and Flt-1 strongly positive cells were 20.1% ± 4.7% (Table 2). Therefore, these results clearly indicate that under culture conditions for differentiation into macrophage-lineage cells, Flt-1 is expressed on the cell surface. The different levels of Flt-1 in macrophagelike cells suggest that the commitment and differentiation into a mature macrophage is a stepwise process, and that Flt-1 is a useful marker for distinguishing these subpopulations.
Precursor cells for multinuclear osteoclasts exist in the Flt-1+CD11b+ cell fraction obtained from CD34+Flt-1− cord blood mononuclear cells.
Flt-1+CD11b+ and Flt-1++CD11b++ cell fractions obtained from CD34+Flt-1− cord blood mononuclear cells after a 2-week incubation (A) were further cultured with M-CSF and ODF/sRANKL cytokines (see “Materials and methods”). Flt-1+CD11b+ cells but not Flt-1++CD11b++ cells (B) efficiently differentiated into multinuclear osteoclasts, which are negative for phagocytic activity but positive for expression of VNR. Scale bar indicates 100 μm in length. The numbers of the multinuclear (▪) and mononuclear (░) osteoclasts started from 3 × 104Flt-1+CD11b+ or Flt-1++CD11b++ cells are indicated in panel C.
Precursor cells for multinuclear osteoclasts exist in the Flt-1+CD11b+ cell fraction obtained from CD34+Flt-1− cord blood mononuclear cells.
Flt-1+CD11b+ and Flt-1++CD11b++ cell fractions obtained from CD34+Flt-1− cord blood mononuclear cells after a 2-week incubation (A) were further cultured with M-CSF and ODF/sRANKL cytokines (see “Materials and methods”). Flt-1+CD11b+ cells but not Flt-1++CD11b++ cells (B) efficiently differentiated into multinuclear osteoclasts, which are negative for phagocytic activity but positive for expression of VNR. Scale bar indicates 100 μm in length. The numbers of the multinuclear (▪) and mononuclear (░) osteoclasts started from 3 × 104Flt-1+CD11b+ or Flt-1++CD11b++ cells are indicated in panel C.
KM1730-staining–positive (Flt-1+) macrophagelike cells differentiated from CD34+ cord blood cells
| Case no. . | CD11b+ . | CD97+ . | KM1730 . | |
|---|---|---|---|---|
| (+) . | (++) . | |||
| 1 | 57.8 | 98.1 | 52.1 | 26.0 |
| 2 | 54.9 | 96.4 | 56.9 | 19.3 |
| 3 | 66.8 | 99.4 | 23.3 | 18.9 |
| 4 | 33.0 | 99.5 | 46.1 | 15.7 |
| 5 | 50.5 | 98.8 | 55.3 | 25.6 |
| 6 | 49.7 | 97.8 | 34.7 | 15.1 |
| Mean | 52.1 | 98.3 | 44.7 | 20.1 |
| SD | 11.2 | 1.2 | 13.2 | 4.7 |
| Case no. . | CD11b+ . | CD97+ . | KM1730 . | |
|---|---|---|---|---|
| (+) . | (++) . | |||
| 1 | 57.8 | 98.1 | 52.1 | 26.0 |
| 2 | 54.9 | 96.4 | 56.9 | 19.3 |
| 3 | 66.8 | 99.4 | 23.3 | 18.9 |
| 4 | 33.0 | 99.5 | 46.1 | 15.7 |
| 5 | 50.5 | 98.8 | 55.3 | 25.6 |
| 6 | 49.7 | 97.8 | 34.7 | 15.1 |
| Mean | 52.1 | 98.3 | 44.7 | 20.1 |
| SD | 11.2 | 1.2 | 13.2 | 4.7 |
CD34+ but Flt-1− cells were obtained from human cord blood and differentiated into monocyte-macrophage lineages. Macrophagelike cell fraction was further analyzed with anti-CD11b, CD97, or Flt-1 (KM1730) antibody (see “Materials and methods”).
Differentiation of Flt-1+CD11b+ cells into multinuclear osteoclasts in vitro
Recently, VEGF has been shown to be closely involved in bone remodeling in vivo.31 32 To examine the characteristics of the Flt-1+ cells obtained from the CD34+population in vitro, particularly in terms of the potentiality to differentiate into osteoclasts, we collected the Flt-1+CD11b+ cells and Flt-1++CD11b++ cells, separately (Figure 6A), and further cultured them with M-CSF and ODF for 10 days.
Vitronectin receptors (VNR) are known to be a specific cell marker for osteoclasts. VNR+ osteoclasts were obtained both from Flt-1+CD11b+ cells and Flt-1++CD11b++ cells (Figure 6B). However, the number of multinuclear osteoclasts in the Flt-1+CD11b+ cell fraction was about 4-fold that in the Flt-1++CD11b++ fraction (Figure6B,C). Further, the numbers of macrophages surrounding the osteoclasts markedly increased in Flt-1+CD11b+ cells during the culture, whereas the macrophagelike cells in the Flt-1++CD11b++ fraction did not proliferate even in the presence of these cytokines (Figure 6). These results suggest that although the Flt-1++CD11b++ cells still contain osteoclast precursor cells as well as macrophages, they are highly differentiated and have lost the potential to proliferate. Physiologic osteoclasts are essentially multinuclear giant cells morphologically very similar to those in Figure 6B; thus, we conclude that the Flt-1+CD11b+ cell fraction bears the normal osteoclast precursor cells and retains cell proliferative activity.
Discussion
Flt-1 tyrosine kinase was originally isolated as a new type of receptor tyrosine kinase specifically expressed on vascular endothelial cells as well as in placental tissue.33-36 In this study, we have shown that, in addition to vascular endothelial cells and placental trophoblasts, Flt-1 is a useful cell surface marker for monocyte-macrophages in humans. Recently, VEGF has been shown to participate the differentiation and function of chondroclasts and osteoclasts in vivo.31 32 Therefore, our results seem consistent with those of other reports.
We previously reported that the cell type–specific flt-1gene expression is regulated by cooperation between the Ets family and CRE/ATF family transcription factors.37 Furthermore, we demonstrated that at least a part of the cellular signaling from Flt-1 is mediated via the PLCγ and PKC pathway.38 It seems worth elucidating whether the expression and function of Flt-1 in monocyte-macrophages is dependent on these transcription factors and signaling molecules.
Acknowledgments
We thank Drs Tatsuo Suda and Naoyuki Takahashi (Showa University Medical School, Tokyo) for supplying ODF.
Supported by Grants-in-Aid for Special Project Research on Cancer- Bioscience 04253204 from the Ministry of Education in Japan and for the program “Research for the Future” of the Japan Society for Promotion of Science.
A.S. and S.I. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Masabumi Shibuya, Department of Genetics, Institute of Medical Science, University of Tokyo, 4-6-1 Shirokane-dai, Minato-ku, Tokyo 108-8639, Japan; e-mail: shibuya@ims.u-tokyo.ac.jp.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal