Abstract
Stromal cell–derived factor (SDF)-1α and its receptor, CXCR4, play an important role in cell migration, embryonic development, and human immunodeficiency virus infection. However, the cellular signaling pathways that mediate these processes are not fully elucidated. We and others have shown that the binding of SDF-1α to CXCR4 activates phosphatidylinositol-3 kinase (PI-3 kinase), p44/42 mitogen-associated protein kinase, and the transcription factor nuclear factor–κB, and it also enhances the tyrosine phosphorylation and association of proteins involved in the formation of focal adhesions. In this study, we examined the role of phosphatases in CXCR4-mediated signaling pathways. We observed significant inhibition of SDF-1α–induced migration by phosphatase inhibitors in CXCR4-transfected pre-B lymphoma L1.2 cells, Jurkat T cells, and peripheral blood lymphocytes. Further studies revealed that SDF-1α stimulation induced robust tyrosine phosphorylation in the SH2-containing phosphatase SHP2. SHP2 associated with the CXCR4 receptor and the signaling molecules SHIP, cbl, and fyn. Overexpression of wild-type SHP2 increased SDF-1α–induced chemotaxis. Enhanced activation of fyn and lyn kinases and the tyrosine phosphorylation of cbl were also observed. In addition, SDF-1α stimulation enhanced the association of cbl with PI-3 kinase, Crk-L, and 14-3-3β proteins. Our results suggest that CXCR4-mediated signaling is regulated by SHP2 and cbl, which collectively participate in the formation of a multimeric signaling complex.
Introduction
Chemokines and their receptors play important roles in immune and inflammatory responses through the regulation of cell migration and growth. In addition, chemokines participate in the pathogenesis of several diseases.1-5 Chemokine receptors act as coreceptors for the human immunodeficiency virus (HIV), and the expression of both chemokines and chemokine receptor homologues by Kaposi's sarcoma herpes virus (KSHV/HHV-8) has been implicated in the development of Kaposi's sarcoma.6 7
The α-chemokine, stromal cell–derived factor (SDF), is a widely expressed ligand of the CXCR4 receptor.8-11 It is a potent chemotactic factor for mature leukocytes such as monocytes and T lymphocytes as well as for CD34+progenitors.12-14 CXCR4 is a 7-transmembrane G protein–coupled receptor that is expressed by a variety of cells, including peripheral blood lymphocytes (PBLs), monocytes, thymocytes, pre-B cells, dendritic cells, and endothelial cells.15-17CXCR4 serves as a coreceptor for T-cell tropic HIV-1 strains and plays an important role in HIV pathogenesis.1,17,18 In addition, CXCR4 and SDF-1α are critical for embryonic development. Targeted disruption of either protein leads to severe defects in mice embryos that are lethal.19-22 Although CXCR4 and/or SDF-1α play important roles in both physiologic and pathologic processes, the cellular signaling pathways that mediate these effects are not fully elucidated.
Previously, we and others have shown that the association of SDF-1α with CXCR4 activates multiple signaling pathways.23-25 The tyrosine phosphorylation and association of proteins such as RAFTK, Crk, and paxillin, which are involved in the formation of focal adhesions, were enhanced upon SDF-1α stimulation.23,24Phosphatidylinositol-3 kinase (PI-3 kinase), which is an essential component of signaling pathways that induce chemotaxis, was also activated.23,26 In addition, selective activation of the downstream signaling molecule, p44/42 mitogen-activated protein kinase (MAPK), but not p38 MAPK or Jun N-terminal kinase, was observed as well as the activation of the transcription factor, nuclear factor (NF)-κB.23
In the present study, we have characterized the role of the tyrosine phosphatase SHP2 and adaptor molecule cbl in CXCR4-mediated signaling pathways. Protein tyrosine phosphatases (PTPs) play an important role in the regulation of signals generated by various stimuli.27-30 However, the role of individual PTPs in signaling is not uniform. SHP1, a PTP expressed predominantly in hematopoietic cells, and SHIP (SH2-containing inositol phosphatase) act as negative regulators of signaling.29-31 In contrast, the ubiquitously expressed PTP, SHP2, appears to play a positive role in growth factor–induced signaling pathways.32-35 In addition to their tyrosine phosphatase activity, SHP1 and SHP2 can function as adaptor molecules. Through their SH2 domains, SHP1 and SHP2 can bind to several proteins and then transduce signals.36-39
Both SHP1 and SHP2 play important roles in immune regulation and development.40-42 However, their roles in chemokine-mediated signaling are not fully understood. SHP1 was recently shown to mediate SDF-1α–induced chemotaxis. Alterations in chemotactic response to SDF-1α were observed in hematopoietic cells derived from mice lacking SHP1 (moth-eaten or viable).43
Cbl is a 120-kd protein present in lymphocytes that can function as an adaptor molecule in tyrosine phosphorylation–dependent signaling. It is rapidly phosphorylated by Src-like kinases upon stimulation of cell surface receptors and binds to several other signaling molecules, including ZAP-70, Syk, PI-3 kinase, Crk-L, and Vav.44-48Cbl also contains a ubiquitin-associated domain. It binds to and stimulates the ubiquitin-mediated degradation of active platelet-derived growth factor, epidermal growth factor, and colony-stimulating factor-1 receptors.49-51 In this way, cbl can act as a negative regulator of receptor and nonreceptor tyrosine kinases.
In this study, we demonstrate that SDF-1α treatment of CXCR4-positive cells resulted in the tyrosine phosphorylation of SHP2 and cbl and the activation of fyn kinase. Furthermore, we show that SHP2 is constitutively associated with the CXCR4 receptor and is recruited into the protein tyrosine kinase machinery by forming a complex with SHIP, cbl, and fyn. Cbl was also shown to form an activation-induced multimeric complex with PI-3 kinase, 14-3-3β, and Crk-L. Moreover, pretreatment of cells with the phosphatase inhibitors phenylarsine oxide (PAO) and sodium orthovanadate reduced the SDF-1α–induced migration of T and B cells. These results suggest that SHP2 and cbl are key mediators of SDF-1α–induced functional responses.
Materials and methods
Reagents and materials
Antibodies to SHP2, cbl, fyn, lyn, 14-3-3β, and Crk-L were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Monoclonal antiphosphotyrosine antibody (4G10) was a generous gift from Dr Brian Druker (Oregon Health Sciences University, Portland, OR). Antibody to PI-3 kinase was from Upstate Biotechnology (Lake Placid, NY). The GST-SH2 domain of SHP2 was obtained from Santa Cruz Biotechnology. Electrophoresis reagents and the nitrocellulose membrane were obtained from Bio-Rad Laboratories (Hercules, CA). The phosphatase inhibitors PAO and sodium orthovanadate and the protease inhibitors leupeptin and α 1-antitrypsin and all other reagents were acquired from Sigma Chemical Co (St Louis, MO).
Construction of stable CXCR4 transfectants
CXCR4 complementary DNA, tagged at the amino-terminus with a Flag epitope (Asp-Tyr-Lys-Asp-Asp-Asp-Asp-Lys), was subcloned into the pcDNAIII expression vector and then stably transfected into cells from the murine pre-B lymphoma cell line, L1.2, as described.52-54 Geneticin (G418)–containing medium was used to select for transfectants. Cell-surface expression of CXCR4 on the L1.2 transfectants was confirmed by flow cytometry.
Isolation of PBLs
PBLs were generated from peripheral blood mononuclear cells as described.55 56 Briefly, peripheral blood mononuclear cells were separated by Ficoll-Hypaque gradient centrifugation. After 2 rounds of adherence to plastic in RPMI 1640 with 10% fetal calf serum, 2 mM glutamine, 50 μg/mL penicillin, and 50 μg/mL streptomycin, the cells were grown in medium containing 5 μg/mL phytohemagglutinin for 3 days. The PBLs were then washed and grown in RPMI 1640 containing 15% fetal calf serum and 5% interleukin-2 (Advanced Biotechnologies, Columbia, MD). After 3 weeks, the activated T cells were found to be 50% to 60% positive for CXCR4 by flow cytometry and were then used for further studies.
Cell culture
The CXCR4 L1.2 cells were grown in RPMI 1640 with 10% fetal bovine serum, 2 mM glutamine, 1 mM sodium pyruvate, 50 μg/mL penicillin, 50 μg/mL streptomycin, 55 μM 2-mercaptoethanol, and 0.8 mg/mL G418 (Gibco BRL, Grand Island, NY). Jurkat T cells were grown in RPMI 1640 with 10% fetal calf serum, 2 mM glutamine, 50 μg/mL penicillin, and 50 μg/mL streptomycin.
Flow cytometry
Cells were washed twice with phosphate-buffered saline (PBS), resuspended in 500 μL of wash buffer (PBS containing 5% fetal calf serum) and 12G5 anti-CXCR4 antibody, and then incubated at 4°C for 30 minutes. The cells were next washed 3 times with wash buffer, resuspended in 500 μL of wash buffer and fluorescein isothiocyanate–coupled secondary antibody, and then incubated at 4°C for 30 minutes. Thereafter, the cells were washed 3 times, resuspended in 500 μL PBS, and analyzed by flow cytometry.
Stimulation of cells
Cells were washed twice with RPMI 1640 and serum-starved for 2 hours at 37°C in 1 × Hank's buffered salt solution at a concentration of 10 × 106 cells/mL. The cells were then treated with 100 ng/mL SDF-1α at 37°C. After different time periods, cells were lysed in modified radioimmunoprecipitation assay (RIPA) buffer (50 mM Tris-HCl, pH 7.4; 1% Nonidet P-40; 150 mM NaCl; 1 mM phenylmethylsulfonyl fluoride; 10 μg/mL each of aprotinin, leupeptin, and pepstatin; and 10 mM each of sodium vanadate, sodium fluoride, and sodium pyrophosphate). Total cell lysates were clarified by centrifugation at 10 000g for 10 minutes. Protein concentrations were determined by protein assay (Bio-Rad Laboratories).
Immunoprecipitation and immunoblotting
Equal amounts of protein from each sample were clarified by incubation with protein A–Sepharose CL-4B (Amersham Pharmacia Biotech, Piscataway, NJ) for 1 hour at 4°C. After the removal of protein A–Sepharose by brief centrifugation, the samples were incubated with a primary antibody for 2 hours or overnight at 4°C. The antibody-antigen complexes were immunoprecipitated by incubation with 50 μL protein A–Sepharose for 3 hours or overnight at 4°C. Nonspecific bound proteins were removed by washing the protein A–Sepharose beads 3 times with modified RIPA buffer and one time with PBS. Bound immunocomplexes were solubilized in 30 μL 2 × Laemmli buffer, separated on 8% or 12% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE), and transferred to nitrocellulose membranes. The membranes were blocked in 5% nonfat milk protein for 1 hour and probed with primary antibody for 2 hours at room temperature or overnight at 4°C. Immunoreactive bands were visualized using horseradish peroxidase–conjugated secondary antibody and the enhanced chemiluminescent system (Amersham Pharmacia Biotech). The immunoprecipitation and Western blotting data shown in “Results” are representative of findings from 3 independent experiments.
Glutathione-S-transferase–fusion protein binding studies
SH-PTP2 glutathione-S-transferase (GST)–fusion proteins were purchased from Santa Cruz Biotechnology. Fifty micrograms of GST-fusion protein were added to 1 mg cell lysate and incubated for 1 hour at 4°C. GST protein (Santa Cruz Biotechnology) was used as a control. The complexes were then preabsorbed with 50 μL Glutathione-Sepharose 4B beads (Amersham Pharmacia Biotech) and incubated for 3 hours or overnight at 4°C. The beads were centrifuged and washed 3 times with modified RIPA buffer and once with 1 × PBS. Bound proteins were eluted by boiling in Laemmli sample buffer and separated on 8% SDS-PAGE.
Migration assays
Cells were resuspended at 6.6 × 106/mL in RPMI 1640 medium containing 2.5% fetal calf serum. Twenty-four–well plates containing 5 μm porosity inserts (CoStar Corp, Kennebunk, ME) were used for the assays. A total of 25 ng/mL SDF-1α in 600 μL of medium was added to the bottom wells, and 150 μL of cells, untreated or treated with the phosphatase inhibitor PAO or sodium orthovanadate, was placed in the inserts. Controls were treated with appropriate solvents and SDF-1α under similar conditions. After 3 hours, cells that migrated to the bottom wells were collected and counted on a hemacytometer.
Fyn, lyn, and p44/42 MAPK assays
Cell lysates were immunoprecipitated with fyn, lyn, or Erk1 and Erk2 antibodies. For fyn and lyn kinase assays, the immune complexes were washed twice with RIPA buffer and twice with kinase buffer (20 mM HEPES, 50 mM NaCl, 10 μM Na3VO4, 5 mM MgCl2, and 5 mM MnCl2). The complexes were then incubated in kinase buffer containing 0.18 MBq (5 μCi) γ-32P-adenosine triphosphate (ATP) for 20 minutes at 30°C. The reaction was terminated by adding 4 × Laemmli sample buffer and boiling the samples for 5 minutes. Proteins were then separated on 8% SDS-PAGE and viewed by autoradiography. For p44/42 MAPK assays, the immune complexes were washed twice with RIPA buffer and twice with kinase buffer (50 mM HEPES, pH 7.4; 10 mM MgCl2; and 20 μM ATP). The complexes were then incubated in kinase buffer containing 7 μg of myelin basic protein (Upstate Biotechnology) and 0.18 MBq (5 μCi) of γ-32P-ATP for 30 minutes. Proteins were separated on 15% SDS-PAGE and viewed by autoradiography.
Transient transfection
Wild-type SHP2 (SHP2 WT) construct and control vector (kindly provided by Dr Benjamin G. Neel, Beth Israel Deaconess Medical Center) were transiently transfected into the JMC.T5 Jurkat cell line (kindly provided by Dr Hamid Band, Harvard Medical School) by the electroporation method as described.55 Briefly, 10 μg of plasmid carrying the control vector or SHP2 WT34 35 was added to the cell suspension (20 × 106 cells/mL) in a Gene Pulser cuvette and then incubated on ice for 10 minutes. Electric pulse was given by using the Gene Pulser II (Bio-Rad) set at 250 V, 950 μF. The cells were transferred to RPMI 1640 medium containing 10% fetal bovine serum and then grown for 72 hours. The expression of SHP2 was examined by immunoprecipitation as described above.
Results
Phosphatase inhibitors reduce SDF-1α– induced migration of CXCR4 L1.2, Jurkat T cells, and PBLs
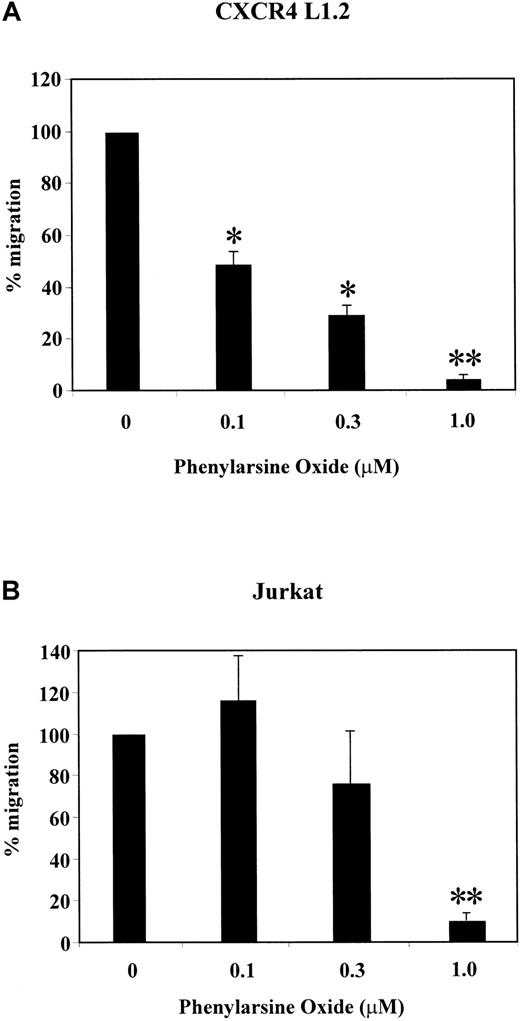
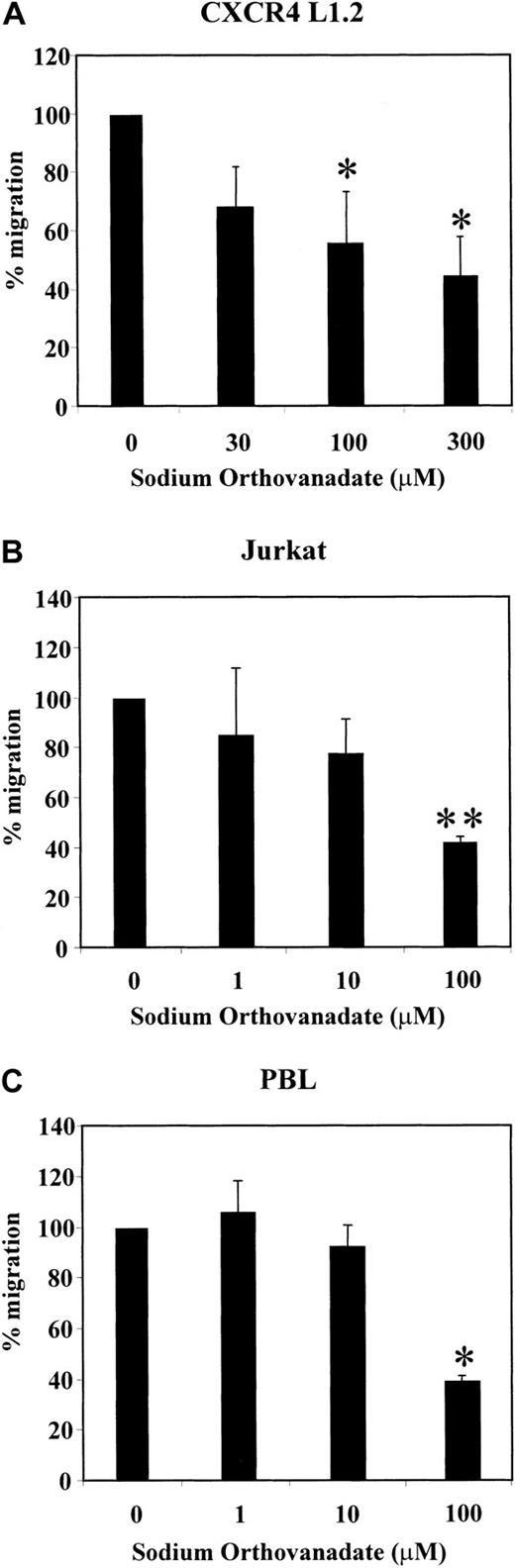
SDF-1α has been shown to act as a potent chemoattractant for various cell types.8,9,12 We and others have recently shown that SDF-1α–induced chemotaxis is regulated by PI-3 kinase.23,26 Because several signaling components are required for mediating chemotaxis, we analyzed the effect of the phosphatase inhibitors PAO and sodium orthovanadate on SDF-1α–induced migration of CXCR4 transfectants, Jurkat T cells, and PBLs. PAO and sodium orthovanadate inhibit the catalytic activity of PTPs, which modulate growth factor–mediated chemotaxis.57As shown in Figure 1A,B, PAO led to a dose-dependent decrease in SDF-1α–induced chemotaxis in CXCR4 L1.2 and Jurkat cells as compared with cells treated with solvent controls. Similarly, sodium orthovanadate treatment reduced the migration of CXCR4 L1.2, Jurkat T cells, and PBLs in a dose-dependent manner (Figure2A-C). At the highest concentration (1.0 μM), PAO reduced the migration of the CXCR4 transfectants to less than 5% (P ≤ .001) and the Jurkat cells to less than 10% (P ≤ .002) (Figure 1A,B). At 100 μM of sodium orthovanadate, the migration of the CXCR4 transfectants was reduced to 58% (P ≤ .034), the Jurkat T cells to 42% (P ≤ .002), and the PBLs to 39% (P ≤ .009) (Figure 2A-C). Under similar conditions, the inhibitors had no effect on the viability of the cells (data not shown). In addition, the phosphatase inhibitors had no effect on SDF-1α–induced p44/42 MAPK activation at 1 μM (PAO) and 100 μM (sodium orthovanadate), indicating that the observed inhibition of migration was not due to toxicity (data not shown).
Effect of PAO on SDF-1α–induced chemotaxis of CXCR4 L1.2 and Jurkat cells.
CXCR4 L1.2 (A) or Jurkat (B) cells were pretreated with 0, 0.1, 0.3, or 1.0 μM concentration of PAO for 45 minutes. Migration toward 25 ng/mL SDF-1α was measured after 3 hours. Chemotaxis of cells in the absence of inhibitor is considered 100% migration. *P < .05; **P < .005.
Effect of PAO on SDF-1α–induced chemotaxis of CXCR4 L1.2 and Jurkat cells.
CXCR4 L1.2 (A) or Jurkat (B) cells were pretreated with 0, 0.1, 0.3, or 1.0 μM concentration of PAO for 45 minutes. Migration toward 25 ng/mL SDF-1α was measured after 3 hours. Chemotaxis of cells in the absence of inhibitor is considered 100% migration. *P < .05; **P < .005.
Effect of sodium orthovanadate on SDF-1α–induced chemotaxis of CXCR4 L1.2 cells, Jurkat cells, and PBLs.
CXCR4 L1.2 (A), Jurkat (B), or PBL (C) cells were untreated (0) or pretreated with different concentrations of sodium orthovanadate for 45 minutes. Migration toward 25 ng/mL SDF-1α was measured after 3 hours. Chemotaxis of cells in the absence of inhibitor is considered 100% migration. *P < .05; **P < .005.
Effect of sodium orthovanadate on SDF-1α–induced chemotaxis of CXCR4 L1.2 cells, Jurkat cells, and PBLs.
CXCR4 L1.2 (A), Jurkat (B), or PBL (C) cells were untreated (0) or pretreated with different concentrations of sodium orthovanadate for 45 minutes. Migration toward 25 ng/mL SDF-1α was measured after 3 hours. Chemotaxis of cells in the absence of inhibitor is considered 100% migration. *P < .05; **P < .005.
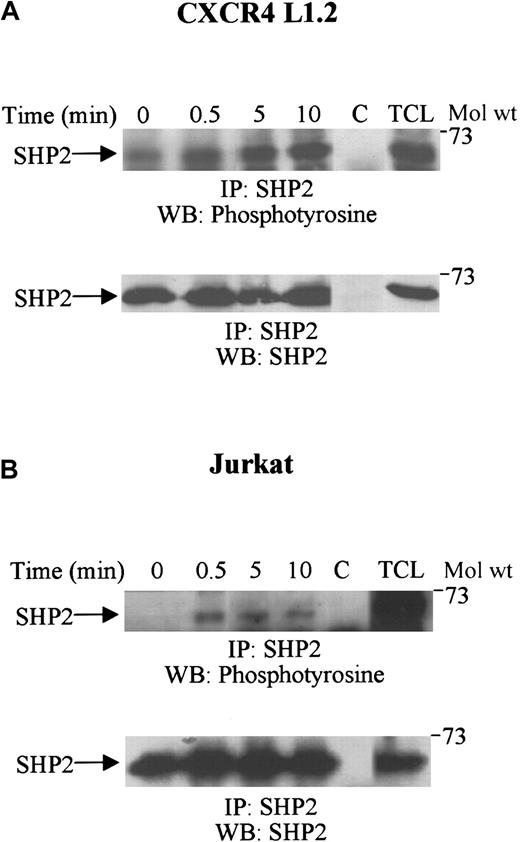
SHP2 is tyrosine-phosphorylated upon SDF-1α treatment of CXCR4 L1.2 and Jurkat T cells
Recently, the involvement of the tyrosine phosphatase, SHP1, in SDF-1α–induced chemotaxis has been shown in cells derived from SHP1-deficient moth-eaten mice.43 SHP2 has also been shown to regulate migration induced by various growth factors and cytokines.57 58 To characterize the role of SHP2 in CXCR4-mediated signaling pathways, CXCR4 transfectants and Jurkat T cells were treated with SDF-1α, and cell lysates were then analyzed for SHP2 tyrosine phosphorylation. Figure3A-B (top panels) shows that stimulation with SDF-1α resulted in a marked increase in the tyrosine phosphorylation of SHP2. This phosphorylation of SHP2 was rapid, and maximum phosphorylation was obtained after 5 to 10 minutes. Equal amounts of SHP2 protein were present in each lane (Figure 3A-B, bottom panels).
Tyrosine phosphorylation of SHP2 upon SDF-1α stimulation.
CXCR4 L1.2 (A) or Jurkat (B) cells were either unstimulated (0) or stimulated with 100 ng/mL SDF-1α for varying time periods. Cells were then lysed and immunoprecipitated with anti-SHP2 antibody. The immune complexes were run on SDS-PAGE and immunoblotted with antiphosphotyrosine antibody (top panels), followed by anti-SHP2 antibody (bottom panels). C represents the antibody control, and TCL is 50 μL of the total cell lysate. IP indicates immunoprecipitation; WB, Western blot.
Tyrosine phosphorylation of SHP2 upon SDF-1α stimulation.
CXCR4 L1.2 (A) or Jurkat (B) cells were either unstimulated (0) or stimulated with 100 ng/mL SDF-1α for varying time periods. Cells were then lysed and immunoprecipitated with anti-SHP2 antibody. The immune complexes were run on SDS-PAGE and immunoblotted with antiphosphotyrosine antibody (top panels), followed by anti-SHP2 antibody (bottom panels). C represents the antibody control, and TCL is 50 μL of the total cell lysate. IP indicates immunoprecipitation; WB, Western blot.
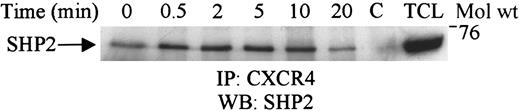
SHP2 associates with the CXCR4 receptor
SHP1 was recently shown to associate with CXCR4 upon SDF-1α stimulation.25 In the present study, we determined the association of SHP2 with CXCR4 upon SDF-1α stimulation of Jurkat cells. As shown in Figure 4, SHP2 constitutively associated with CXCR4, and this association was enhanced upon SDF-1α stimulation.
CXCR4 associates with SHP2 upon SDF-1α stimulation.
Lysates obtained from Jurkat cells unstimulated (0) or stimulated for varying time periods with 100 ng/mL SDF-1α were immunoprecipitated with CXCR4. The immune complexes were subjected to SDS-PAGE and immunoblotted with anti-SHP2 antibody. C represents the antibody control, and TCL is 50 μL of the total cell lysate.
CXCR4 associates with SHP2 upon SDF-1α stimulation.
Lysates obtained from Jurkat cells unstimulated (0) or stimulated for varying time periods with 100 ng/mL SDF-1α were immunoprecipitated with CXCR4. The immune complexes were subjected to SDS-PAGE and immunoblotted with anti-SHP2 antibody. C represents the antibody control, and TCL is 50 μL of the total cell lysate.
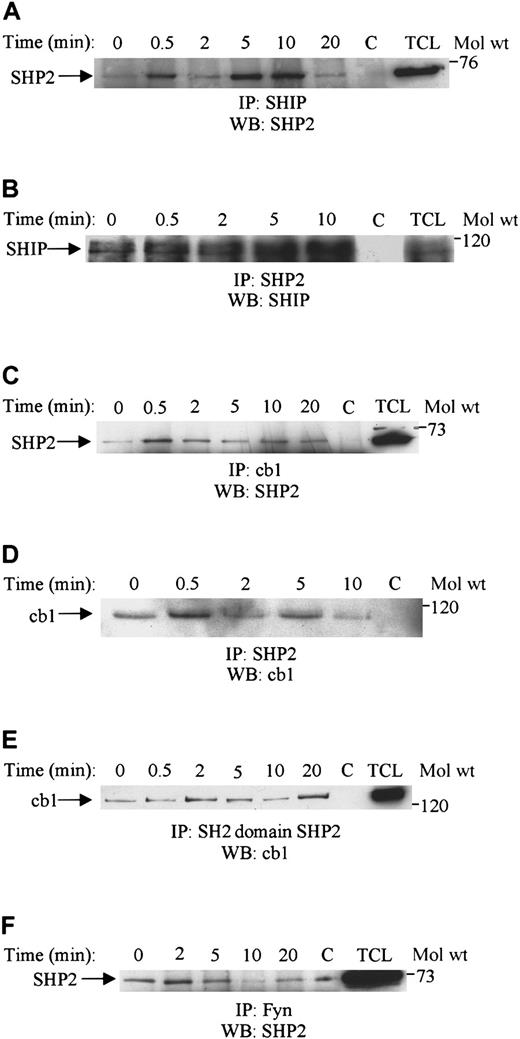
SHP2 associates with SHIP, fyn, and cbl upon SDF-1α stimulation of Jurkat T cells
SHP2 has been shown to associate with several signaling components upon growth factor or cytokine stimulation.36-39 Here, we examined its association with other signaling molecules upon SDF-1α stimulation. As shown in Figure 5A-F, SHP2 associated with the phosphatase SHIP, the adaptor molecule cbl, and fyn kinase upon SDF-1α stimulation of Jurkat T cells. Association with SHIP was rapid, reached a maximum level at 5 to 10 minutes, and was reduced thereafter (Figure 5A). This association was further confirmed by the reverse immunoprecipitation of blotting SHP2 immunoprecipitates with SHIP (Figure 5B). Association of SHP2 with cbl was rapid and sustained for up to 20 minutes of stimulation with SDF-1α (Figure 5C). The cbl association was also reconfirmed by blotting SHP2 immunoprecipitates with cbl (Figure 5D). Furthermore, cbl was shown to associate with the amino-terminal SH2 domain of SHP2 when using GST-fusion protein for the immunoprecipitation (Figure 5E). Fyn constitutively associated with SHP2, was slightly enhanced after 2 to 5 minutes of stimulation with SDF-1α, and was reduced thereafter (Figure 5F).
SHP2 associates with SHIP, fyn, and cbl upon SDF-1α stimulation.
Lysates obtained from Jurkat cells unstimulated (0) or stimulated with 100 ng/mL SDF-1α for various time periods were immunoprecipitated with either anti-SHIP (A), anti-SHP2 (B), anti-cbl (C), anti-SHP2 (D), the GST SH2-domain of SHP2 (E), or anti-Fyn (F). The immune complexes were then run on SDS-PAGE and immunoblotted with anti-SHP2 antibody (A,C,F) or anti-SHIP (B) or anti-cbl antibody (D,E). C represents the antibody control, and TCL is 50 μL of total cell lysate.
SHP2 associates with SHIP, fyn, and cbl upon SDF-1α stimulation.
Lysates obtained from Jurkat cells unstimulated (0) or stimulated with 100 ng/mL SDF-1α for various time periods were immunoprecipitated with either anti-SHIP (A), anti-SHP2 (B), anti-cbl (C), anti-SHP2 (D), the GST SH2-domain of SHP2 (E), or anti-Fyn (F). The immune complexes were then run on SDS-PAGE and immunoblotted with anti-SHP2 antibody (A,C,F) or anti-SHIP (B) or anti-cbl antibody (D,E). C represents the antibody control, and TCL is 50 μL of total cell lysate.
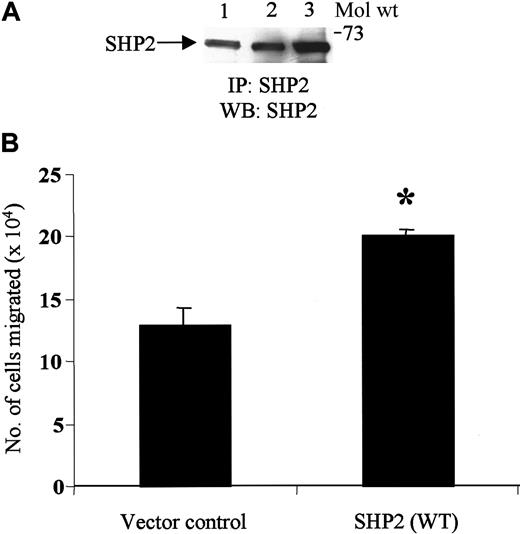
SHP2 overexpression enhances SDF-1α–induced migration
To address the functional role of SHP2 in SDF-1α–induced chemotaxis, we transiently transfected Jurkat cells with control vector or SHP2 WT. As shown in Figure 6A, a higher amount of SHP2 was expressed in SHP2 WT–transfected cells (lane 3) than in untransfected or control vector–transfected cells (lanes 1 and 2, respectively). Chemotaxis studies carried out with these transfectants revealed that overexpression of SHP2 WT protein enhanced SDF-1α–induced chemotaxis by 50% to 60% as compared with cells overexpressing the control vector (Figure 6B). Similar results were obtained in 3 different transfection experiments.
Overexpression of SHP2 in transfected JMC.T5 Jurkat cells enhances SDF-1α–induced migration.
(A) JMC.T5 Jurkat cells were untransfected or transfected with either control vector or SHP2 WT and then lysed with lysis buffer. Lysates (500 μg protein) from untransfected (lane 1), control vector–transfected (lane 2), or SHP2 WT–transfected (lane 3) cells were immunoprecipitated with SHP2 antibody. The immune complexes were then resolved on SDS-PAGE and blotted with SHP2 antibody. (B) The cells transfected with the control vector or SHP2 WT were subjected to migration assay with SDF-1α (50 ng/mL). The cells migrating to the bottom chamber were counted. *P < .005.
Overexpression of SHP2 in transfected JMC.T5 Jurkat cells enhances SDF-1α–induced migration.
(A) JMC.T5 Jurkat cells were untransfected or transfected with either control vector or SHP2 WT and then lysed with lysis buffer. Lysates (500 μg protein) from untransfected (lane 1), control vector–transfected (lane 2), or SHP2 WT–transfected (lane 3) cells were immunoprecipitated with SHP2 antibody. The immune complexes were then resolved on SDS-PAGE and blotted with SHP2 antibody. (B) The cells transfected with the control vector or SHP2 WT were subjected to migration assay with SDF-1α (50 ng/mL). The cells migrating to the bottom chamber were counted. *P < .005.
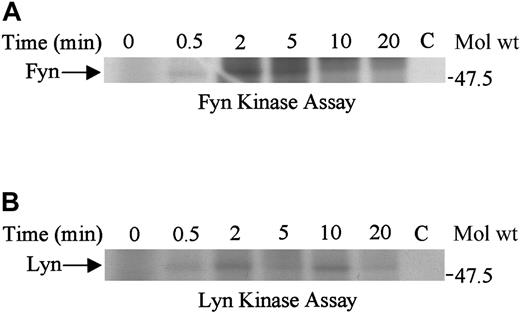
Fyn and lyn kinases are activated upon SDF-1α stimulation of Jurkat T cells
Because fyn associated with SHP2, we examined fyn and lyn kinase activation in SDF-1α–stimulated cells. We found that both fyn and lyn kinases were activated after 2 minutes of SDF-1α treatment, although the activation of fyn was greater than that of lyn (Figure7A-B). The activation of both fyn and lyn kinases was reduced after 5 to 10 minutes of stimulation.
Fyn and Lyn are activated upon SDF-1α stimulation.
Lysates obtained from Jurkat cells unstimulated (0) or stimulated with 100 ng/mL SDF-1α for various time periods were immunoprecipitated with anti-fyn (A) or anti-lyn (B) antibody. The immune complexes were subjected to in vitro kinase reactions. C represents the control antibody.
Fyn and Lyn are activated upon SDF-1α stimulation.
Lysates obtained from Jurkat cells unstimulated (0) or stimulated with 100 ng/mL SDF-1α for various time periods were immunoprecipitated with anti-fyn (A) or anti-lyn (B) antibody. The immune complexes were subjected to in vitro kinase reactions. C represents the control antibody.
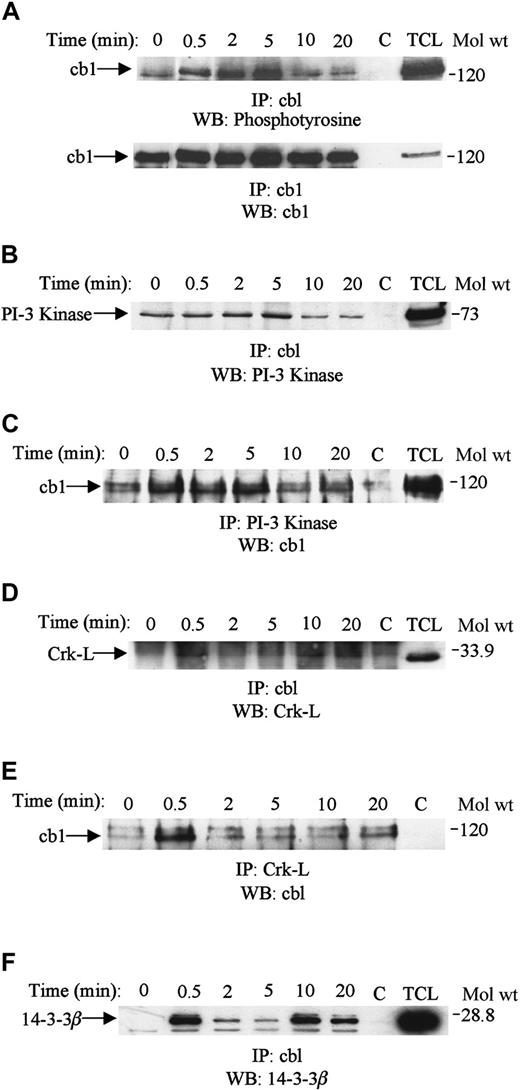
Cbl is phosphorylated upon SDF-1α stimulation of Jurkat T cells and associates with PI-3 kinase, 14-3-3β, and Crk-L
Cbl acts as an adaptor protein in tyrosine-dependent signaling and has been shown to associate with several signaling molecules in response to a variety of stimuli.44-48 Because cbl associated with SHP2, we explored its phosphorylation and association with other signaling molecules in response to SDF-1α treatment. Stimulation with SDF-1α increased the tyrosine phosphorylation of cbl in Jurkat cells (Figure 8A, upper panel). Maximum phosphorylation was obtained after 2 to 5 minutes of stimulation and declined thereafter. Equal amounts of cbl protein were present in each lane (Figure 8A, bottom panel). Furthermore, cbl associated with PI-3 kinase and the adaptor proteins Crk-L and 14-3-3β (Figure 8B-F). Cbl associated constitutively with PI-3 kinase, and the association was only slightly increased after 5 minutes of treatment with SDF-1α (Figure 8B). Similar results were obtained when PI-3 kinase immunoprecipitates were immunoblotted with cbl (Figure8C). The association of cbl with Crk-L was also enhanced upon SDF-1α stimulation (Figure 8D). This association was reconfirmed by immunoblotting Crk-L immunoprecipitates with cbl (Figure 8E). Cbl also associated with 14-3-3β upon SDF-1α stimulation (Figure8F).
Cbl is tyrosine-phosphorylated and associates with PI-3 kinase, Crk-L, and 14-3-3β upon SDF-1α stimulation.
Lysates obtained from Jurkat cells were unstimulated (0) or stimulated with 100 ng/mL SDF-1α for various time periods and then lysed. The cell lysates were immunoprecipitated with anti-cbl antibody run on SDS-PAGE and immunoblotted with antiphosphotyrosine antibody (A, top panel), followed by anti-cbl antibody (A, bottom panel). Equivalent amounts of cell lysate (500 μg) were immunoprecipitated with anti-cbl (B,D,F), anti–PI-3 kinase (C), or anti-Crk-L (E) and then run on SDS-PAGE and immunoblotted with anti–PI-3 kinase (B), anti-cbl (C,E), anti–Crk-L (D), or anti–14-3-3β (F). C represents the antibody control, and TCL is 50 μL of total cell lysate.
Cbl is tyrosine-phosphorylated and associates with PI-3 kinase, Crk-L, and 14-3-3β upon SDF-1α stimulation.
Lysates obtained from Jurkat cells were unstimulated (0) or stimulated with 100 ng/mL SDF-1α for various time periods and then lysed. The cell lysates were immunoprecipitated with anti-cbl antibody run on SDS-PAGE and immunoblotted with antiphosphotyrosine antibody (A, top panel), followed by anti-cbl antibody (A, bottom panel). Equivalent amounts of cell lysate (500 μg) were immunoprecipitated with anti-cbl (B,D,F), anti–PI-3 kinase (C), or anti-Crk-L (E) and then run on SDS-PAGE and immunoblotted with anti–PI-3 kinase (B), anti-cbl (C,E), anti–Crk-L (D), or anti–14-3-3β (F). C represents the antibody control, and TCL is 50 μL of total cell lysate.
Discussion
The α-chemokine, SDF-1α, and its cognate receptor, CXCR4, play a critical role in embryonic development, neuronal patterning, and HIV pathogenesis.1,17,19-22 The association of SDF-1α with CXCR4 also plays an important role in the immune system through the regulation of myeloid progenitor proliferation and the trafficking of leukocytes.12-14 However, the signaling pathways behind these normal and disease processes are only beginning to be understood. SDF-1α–induced activation of CXCR4 is coupled to the JAK/STAT, PI-3 kinase, p44/p42 MAPK, and NF-κB pathways.23-26 Recently, SDF-1α was shown to induce phosphorylation of the CXCR4 receptor at serine and tyrosine residues and association of the CXCR4 receptor with the tyrosine phosphatase, SHP1.25,59 Furthermore, the altered chemotactic response to SDF-1α observed in hematopoietic cells derived from SHP1-deficient mice indicates that SHP1 mediates SDF-1α–induced chemotaxis.43
In this study, we have further assessed the role of PTPs in SDF-1α–initiated signaling events. PTPs can regulate various cellular functions by initiating and terminating signals. We observed that the SDF-1α–induced chemotaxis of CXCR4+ cells was significantly reduced by the tyrosine phosphatase inhibitors PAO and sodium orthovanadate. However, PAO and sodium orthovanadate did not affect SDF-1α–induced p44/42 MAPK activation. The strong inhibition of migration by tyrosine phosphatase inhibitors suggests that tyrosine phosphatases are important mediators of SDF-1α–induced chemotaxis. PAO and sodium orthovanadate have been reported to suppress platelet-derived growth factor–induced migration and cell spreading of lung carcinoma cells by inhibiting SHP2 activity.51,60 We found that SDF-1α stimulation of T cells enhanced the tyrosine phosphorylation of SHP2, which has been shown to be tyrosine-phosphorylated in response to stimulation by growth factors and cytokines.32-35 Furthermore, we observed that overexpression of SHP2 WT enhanced SDF-1α–induced chemotaxis. In other studies, SHP2 has been shown to regulate chemotaxis induced by integrins. Its role in cell migration has also been confirmed in SHP2−/− knock-out mice; fibroblasts derived from these mice have slower spreading and motility as compared with cells from WT animals.35 SHP2 has been shown to regulate cell adhesion, spreading, and migration by modulating tyrosine phosphorylation of focal adhesion proteins.35,58,61 We and others have recently shown that SDF-1α induces the tyrosine phosphorylation of components of focal adhesion complexes.23 62
SHP2 was shown to associate with the CXCR4 receptor and the signaling molecules SHIP and PI-3 kinase. Tyrosine-phosphorylated SHP2 can act as an adaptor molecule and has been shown to bind to several signaling molecules, including growth factor receptors and the adhesion molecule PECAM-1.32-35,38 Recently, another PTP, SHP1, was shown to bind to the CXCR4 receptor.25 SHIP, which is a 145-kd inositol-5-phosphatase that selectively hydrolyzes the 5′ phosphate from both inositol 1,3,4,5 tetraphosphate (IP4) and phosphatidylinositol 3,4,5, triphosphate (PIP3), has previously been shown to bind to SHP2 in various cell types.31,63,64 SHIP seems to play an important role in chemokine-mediated signaling: Hematopoietic cells derived from SHIP−/− knock-out mice showed enhanced chemotactic responses to SDF-1α as compared with the cells from WT mice.65 SHP2 also binds to PI-3 kinase, which recently was shown to regulate SDF-1α–induced chemotaxis.23,26 Furthermore, SHIP may down-regulate PI-3 kinase–initiated signaling events through inhibition of protein kinase B activation by decreasing the levels of PIP3.66
Tyrosine phosphorylation of the adaptor protein cbl was enhanced upon SDF-1α stimulation. Therefore, cbl may play a role in SDF-1α–induced chemotaxis in T cells. Cbl is a prominent component of signaling events downstream of a variety of cell surface receptors, including T cells, B cells, epidermal growth factor, integrins, and cytokines.44-48 It is involved in ubiquitination and the endocytic degradation of various receptors.49-51
We observed that SDF-1α enhanced the association of cbl with SHP2, PI-3 kinase, Crk-L, and 14-3-3β. The reduction of cbl tyrosine phosphorylation and its association with membrane-localized PI-3 kinase activity have been shown in Src-family kinase mutants, which were defective in their ability to spread on fibronectin-coated surfaces. Furthermore, cbl antisense nucleotides and PI-3 kinase inhibitors also blocked the spreading of WT cells.47 Association of cbl with Crk-L was recently shown to be involved in Ba/F3 cell chemotactic events.67 Cbl also associates with 14-3-3β proteins upon SDF-1α stimulation. In addition, T-cell activation mediated by anti-CD3 has been shown to lead to the enhanced association of cbl with 14-3-3β proteins.68,69 These proteins bind to a wide variety of regulatory proteins and thereby participate in signaling events leading to cell differentiation and proliferation.70-72
The Src-related tyrosine kinases fyn and lyn were also activated upon SDF-1α stimulation, and fyn associated with SHP2. Fyn and lyn have been shown to associate with cbl and SHP2 and thereby regulate their functions.73-75 Both of these kinases play important roles in various growth factor and T- and B-cell receptor signaling by initiating changes in the phosphorylation of signaling intermediates that modulate further downstream events.76
Taken together, our studies suggest that the tyrosine phosphatase SHP2 and the adaptor cbl are key components of CXCR4-mediated signaling events induced by SDF-1α. SHP2 associates with CXCR4 and forms a multiprotein signaling complex with cbl, SHIP, and fyn. Cbl also forms an activation-induced complex with PI-3 kinase, Crk-L, and 14-3-3β proteins. The formation of these large multimeric signaling complexes may result in the activation of multiple downstream effectors, which mediate the various functional effects of the CXCR4 receptor.
Acknowledgments
We are thankful to Dr Jerome E. Groopman for his advice and support for our research project. We also thank Stephanie Brubaker for technical help, Janet Delahanty for editing, Daniel Kelley for preparation of the figures, and Simone Jadusingh for facilitating receipt of the reagents for the experiments.
Supported by National Institutes of Health grant CA76950 (R.K.G.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Ramesh K. Ganju, Division of Experimental Medicine, Harvard Institutes of Medicine-BIDMC, 4 Blackfan Circle, Room 343, Boston, MA 02115; e-mail: rganju@caregroup.harvard.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal