Abstract
A preliminary study has linked raised blast glutathione levels with chemoresistance in acute myeloid and lymphoblastic leukemia in adults and children. In this study, therefore, the relationship between leukemic blast glutathione levels and prognosis in childhood acute lymphoblastic leukemia (ALL) was investigated. A total of 77 childhood ALL samples were analyzed, 62 at initial presentation and 15 at relapse. A 20-fold interindividual variation in glutathione levels at presentation (median, 6.54 nmol/mg protein; range, 1.37 to 27.9) was demonstrated. The median level in T-lineage ALL was 2.3-fold higher than in B-lineage ALL (Mann-Whitney test,P < .0001). There was a significant correlation between presenting white cell count (WBC) and glutathione level (Spearman rank correlation coefficient, ρ = 0.45, P = .001). A high DNA index correlated with low glutathione levels (Mann-Whitney test,P = .013). There was no significant relationship between glutathione levels and in vitro drug sensitivity. Patients with glutathione levels above the median had a significantly greater risk of relapse (log-rank test statistic, 5.55; P = .018), and the overall survival rate was significantly reduced (log-rank test statistic, 4.38; P = .04). Multivariate analysis demonstrated that glutathione concentration was of independent prognostic value when assessed in conjunction with age, gender, WBC, and immunophenotype. The association of elevated blast glutathione levels with an increased risk of relapse suggests that glutathione-depleting agents may be of therapeutic value in patients who present with a high WBC.
Introduction
With the use of regimes such as those developed in the United States, the United Kingdom, and Germany, overall survival rates for children with acute lymphoblastic leukemia (ALL) are currently over 70%.1-3 However, progress has been accompanied by the development of treatment-related toxicity. Periods of consolidation therapy are associated with hospitalization, acute morbidity, and an increased use of resources. A number of survivors suffer long-term side effects from their treatment, including intellectual impairment, disorders of growth, cardiac damage, and second malignancies.4-8 A reduction in both the mortality rate and the toxic consequences of treatment remains a challenge for the improvement of ALL therapy in the future.
Future progress in childhood ALL will be facilitated by the identification of accurate prognostic indicators and the introduction of new therapeutic agents. Accurate stratification will allow intensive treatment, ie, bone marrow transplantation, to be targeted to those patients who are unlikely to be cured by conventional therapy, while sparing those patients with a good prognosis the hazards of the associated toxic side effects. Currently available indicators of prognosis in childhood ALL include the age and sex of the patient, leukemic blast immunophenotype, karyotype, and the peripheral blood white cell count (WBC) at presentation. Together, they allow broad stratification of patients, enabling more intensive treatment to be targeted to those with a higher risk of relapse. Although these current parameters provide a guide to the relative risk of relapse, their predictive power in identifying resistant disease is limited.9 Investigation of factors that govern lymphoblast chemosensitivity may aid further differentiation of risk groups and possibly provide new targets for drug development. In vitro studies have supported a role for glutathione in cell protection, both in drug detoxification pathways10-12 and in the control of the apoptotic threshold.13-16 A number of in vivo studies on solid tumors report that elevated glutathione levels are associated with resistant disease.17 In a small preliminary study of childhood and adult leukemias, Maung et al18 reported that the concentration of glutathione in leukemic blasts predicted treatment response. Conversely, in a study of glutathione levels in adult leukemia, there was no relationship between blast glutathione levels and clinical outcome.19 There have been no previous studies reported that examine the relationship between blast glutathione levels, clinical outcome, and established indicators of prognosis in childhood ALL. Here we report that the concentration of glutathione in lymphoblasts at diagnosis is related to treatment outcome in childhood ALL. (Preliminary analysis of the results were presented at the third meeting of Drug Resistance in Leukemia and Lymphoma, Amsterdam, the Netherlands20.)
Patients, materials, and methods
Patients
Patients for this study were recruited from The Netherlands, Denmark, and Germany according to locally agreed-upon ethical guidelines. Samples of bone marrow or peripheral blood were sent to the Haematology, Oncology and Immunology Laboratory, Department of Paediatrics, Free University, Amsterdam, The Netherlands, where blasts were isolated by centrifugation over Ficoll prior to cryopreservation in liquid nitrogen. All samples contained more than 90% blasts as determined by analysis of cytospins.
A total of 77 samples from children with ALL were obtained, 62 at initial presentation and 15 on relapse. Of the presentation ALL cases, 8 samples were sent from Germany, 13 from Denmark, and 41 from Dutch medical centers. Patients were treated according to their respective national trials: the German Berlin-Frankfurt-Munster, the Danish Nordic Society of Paediatric Haematology and Oncology, and the Dutch Childhood Leukaemia Study Group (SNWLK) protocols. We studied 37 male and 25 female patients; 51 samples expressed B-lineage and 11 T-lineage markers. The median age at presentation was 68 months (range, 3-165 months). Patients were followed up for a maximum of 103 months.
We analyzed 15 samples from patients with ALL at relapse. All were diagnosed in Dutch medical centers and treated according to the SNWLK relapse protocols. Of these patients, 10 were males and 5 females. None were from patients who had also been examined at diagnosis. The mean age was 55 months (range, 0-131 months, including 2 infants). There were 10 B-lineage and 5 T-lineage ALLs.
In vitro drug sensitivity assay
In vitro drug sensitivity was tested by means of the the MTT (3-[4,5-dimethylthiazol-2,5-diphenyl] tetrazolium bromide) assay. In vitro drug sensitivity was tested on fresh samples within 24 hours of their arrival in the laboratory. The assay was performed according to the methodology previously published by Pieters et al.20The drugs and their maximum-well concentration were etoposide, 0.05 mg/mL (Bristol-Meyers, The Netherlands); cytarabine, 0.01 mg/mL (Upjohn, The Netherlands); 6-thioguanine, 0.05 mg/mL (Sigma, St Louis, MO); doxorubicin, 0.008 mg/mL (Farmitalia, The Netherlands); 6-mercaptopurine, 0.50 mg/mL (Sigma); dexamethasone, 0.006 mg/mL (Brocacef, The Netherlands); prednisolone, 0.250 mg/mL (Brocacef, The Netherlands); vincristine, 0.050 mg/mL (Lilly, The Netherlands); daunorubicin, 0.002 mg/mL (Rhone-Poulnec, The Netherlands); L-asparaginase, 10 U/mL (Bayer, The Netherlands); and ifosfamide, 0.1 mg/mL (Astra-Medica, The Netherlands).
The cells were cultured with or without drug in 96-well, round-bottomed microtiter plates (Greiner Labortechnik), in humidified air containing 5% carbon dioxide at 37°C. On day 4, we added 50μg of MTT (stock solution, 5 mg/mL) (Sigma) to each well and incubated these at 37°C for 6 hours. The MTT is reduced to formazen crystals by viable cells. The formazen crystals were dissolved in 100 μL of acidified isopropanol. The absorbance of the dissolved formazen crystals was measured spectrophotometrically at 562 nm in an EL-312 microtiter plate reader (Bio-Tek Instruments, Winooski, VT). The optical density is linearly related to the number of viable cells in each well.20 The drug concentration, which results in 50% leukemic cell survival (LC50), was calculated from x-y plots of drug concentration against the percentage cell kill.
Measurement of cellular glutathione levels
After thawing at 37°C, any contaminating red cells were lysed by resuspending the cell pellets in an ice-cold solution containing 0.155 mM NH4Cl, 0.1 mM Na2EDTA, and 10 mM KHCO3 for 4 minutes prior to washing in ice-cold phosphate-buffered saline. Cell viability was measured by means of the trypan blue exclusion technique; trypan blue cannot enter a cell if the cell membrane is intact. A cell suspension was mixed in equal volumes with the trypan blue solution. The cells were examined by light microscopy at 400 × magnification in a cell-counting chamber (Neubauer, The Netherlands). The percentage of cells excluding trypan blue represented the percentage of viable cells. Viable cells were counted after thawing and red cell lysis had been performed. The cells were suspended in phosphate-buffered saline at a concentration of 1 × 107 live cells per milliliter. The glutathione assay was performed on aliquots of 4 × 106 cells that were pelleted, lysed in 0.1% Triton-X 100 (vol/vol) in 10 mM HCl, and kept at 4°C prior to analysis.
A modification of the enzyme-recycling method of Tietze22 was used to measure total (ie, oxidized and reduced) intracellular glutathione. The assay is based on the continued enzymatic regeneration of reduced glutathione (GSH) from the reduction of oxidized glutathione (GSSG, glutathione disulfide) by the enzyme glutathione reductase and the GSH-mediated reduction of the sulfhydryl reagent 5,5′-dithiobis-2-nitrobenzoic acid to a chromogenic product. Details of the adaptations we have made to the assay for the measurement of glutathione in leukemic blasts have been previously published.23
The optimized Tietze reaction was performed in a 96-well microtiter plate (flat-bottomed cell-culture plates) (Costar, The Netherlands). A standard curve was prepared with the use of reduced glutathione (GSSG) (Sigma) and was measured in duplicate. This assay measured total GSH, including all GSH generated by reduction of GSSG. Each mole of GSSG is reduced to 2 moles of GSH; therefore, measured GSH levels were expressed as GSH equivalents.
Each sample was analyzed in 3 pairs of wells; 2 pairs contained known quantities of GSSG. The reaction was initiated by the addition of glutathione reductase (type IV from Baker's yeast) (Sigma). The final reaction concentrations per well were as follows: 0.345 mM 5,5′-dithiobis-2-nitrobenzoic acid (DTNB) (Sigma), 0.24 mM β–nicotinamide adenine dinucleotide phosphate (reduced form) (Sigma), and 0.34 IU/mL glutathione reductase. The absorbance at 405 nm was measured for 16 minutes at 4-minute intervals, and the data were analyzed at a time point at which the reaction was linear.
Standards, samples, and assay solutions were always kept on ice. The prepared plate was kept at 4°C prior to analysis. The plate was incubated for 2 minutes at room temperature and then placed in an EL-312 microtiter plate reader. The reaction was initiated by the addition of glutathione reductase and followed kinetically, with the absorbance measured at 405 nm at 4-minute intervals from time 0 to 16 minutes. The plate was shaken automatically within the plate reader prior to each reading. The results were analyzed by means of Prism computer software (GraphPad, CA). Each measurement was corrected for background absorbance at 0 μM GSH. The linearity of the rate of reaction over a period of 16 minutes was determined for each GSH concentration in the standard curve. The linearity at each time point for the range of concentrations used was also determined. The standard curve was generated by plotting the standard dilutions, in GSH equivalents, against absorbance at 405 nm recorded at a time point when the reaction rate was demonstrated to be linear.
A recovery experiment was performed for each patient sample by adding known amounts of GSSG to the samples (2 and 4 μM GSH equivalents). Curves were plotted from measurements with and without the addition of known amounts of GSH to assess the recovery of GSH for each sample. Known amounts of GSH to the samples were added to determine any errors due to the presence of glutathione reductase inhibitors.
The protein concentration in the lysates was measured by the method of Bradford by means of commercially available reagents (BioRad, Hemel Hempstead, United Kingdom). Glutathione results were expressed as nmol/mg cellular protein.
The sensitivity and reproducibility of this methodology to measure glutathione levels in cryopreserved lymphoblasts were evaluated. The intra-assay variability was below 5%. The interassay variability was 13% for the high GSH standard and 37% for the low GSH standard. However, the 95% confidence intervals (CIs) were narrow for both standards. The low GSH internal standard mean was 2.3 μM with 95% CI, 2.05-2.55 (46 picomoles per well; 95% CI, 41-51), and the high GSH internal standard mean was 11 μM with 95% CI, 10.6-11.4 (220 picomoles per well; 95% CI, 212-228).
Statistics
Nonparametric statistics were used, including Spearman rank correlation and the Mann-Whitney test. The Kaplan-Meier method was applied to generate the survival curves, which were compared by the log-rank test. Multivariate analysis was performed by means of Cox regression. Statistical data were tested for significance by 2-tailed analysis.
Results
Presentation ALL
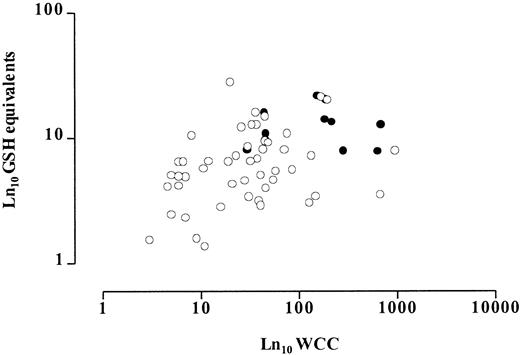
There was a 20-fold variation in lymphoblast glutathione levels. The median value was 6.54 nmol/mg protein (range, 1.37 to 27.9), with the median glutathione level in T-lineage ALL being 2.3-fold higher than in B-lineage ALL (Mann-Whitney test, P < .0001) (Figure 1). No significant relationship was found between lymphoblast glutathione level and patient gender (data not shown). The data were also analyzed in age-defined prognostic groups: younger than 12 months, 12 to 120 months, and older than 120 months. There was no significant difference between lymphoblast glutathione levels in the good prognosis group at 12 to 120 months and in the poor prognosis groups at younger than 12 months and older than 120 months (Mann-Whitney test, P = .66 and .61, respectively). Higher glutathione levels were significantly associated with a higher WBC (Spearman rank correlation coefficient, ρ = 0.45, P = .001) (Figure2). In general, a high WBC is seen in T-lineage ALL. It was therefore possible that this relationship was simply a reflection of the correlation observed between glutathione levels and immunophenotype. However, analysis of glutathione levels in B-lineage samples alone demonstrated that this relationship was independent of immunophenotype (Spearman rank correlation coefficient, ρ = 0.33, P = .02).
Comparison of glutathione levels in B- and T-lineage lymphoblasts obtained at diagnosis from children with ALL.
Horizontal lines represent the median values. The median value in T lymphoblasts was 2.3-fold higher than in B lymphoblasts. This difference is statistically significant (P < .0001, Mann-Whitney test).
Comparison of glutathione levels in B- and T-lineage lymphoblasts obtained at diagnosis from children with ALL.
Horizontal lines represent the median values. The median value in T lymphoblasts was 2.3-fold higher than in B lymphoblasts. This difference is statistically significant (P < .0001, Mann-Whitney test).
Relationship between presenting WBC and lymphoblast glutathione levels.
Open circles represent B-lineage and closed circles T-lineage cases. There is a statistically significant correlation (ρ = 0.45,P = .001), which persists if only B-lineage cases are analyzed (ρ = 0.33, P = .02).
Relationship between presenting WBC and lymphoblast glutathione levels.
Open circles represent B-lineage and closed circles T-lineage cases. There is a statistically significant correlation (ρ = 0.45,P = .001), which persists if only B-lineage cases are analyzed (ρ = 0.33, P = .02).
The DNA index was available on 47 of the 62 cryopreserved samples analyzed. Blast glutathione levels were 45% lower in patients with a DNA index exceeding 1.16, which is a recognized indicator of a good prognosis (Mann-Whitney test, P = .013). Lymphoblast glutathione levels were examined within different karyotype groups. Although the lowest median glutathione level was observed in the good-prognosis, hyperdiploid, group (more than 50 chromosomes), the highest median glutathione level was seen in the poor-prognosis, hypodiploid, group. The full lymphoblast chromosomal karyotype was available in only 37 samples; therefore, the prognostic groups were too small to perform valid statistical analyses.
Kaspers et al23 proposed in vitro drug sensitivity of lymphoblasts as a sensitive indicator of prognosis in childhood ALL. In this study, in vitro sensitivities to cytotoxic agents commonly used in the treatment of childhood ALL were measured by the MTT assay. The IC50 was defined as the lowest concentration of a drug required to kill 50% of cells in the MTT assay. A few samples demonstrated extreme resistance to prednisolone, dexamethasone, and 6-mercaptopurine. The IC50 recorded was therefore the maximum dose of the drug to which the cells were exposed, but a 50% cell kill was not achieved. For the purpose of statistical analysis, the rank positions of these cases were not affected. The relationship between the IC50 for each cytotoxic drug and the lymphoblast glutathione level was examined. No statistical relationships were found between blast glutathione levels and in vitro drug sensitivities for any of the drugs tested (Table1).
Statistical analysis of the relationship between lymphoblast glutathione levels and in vitro drug sensitivity in childhood acute lymphoblastic leukemia
| Drug . | No. . | ρ* . | P† . | MWU test‡P value . |
|---|---|---|---|---|
| 6-Mercaptopurine | 51 | −0.12 | .40 | .83 |
| Thioguanine | 55 | −0.08 | .56 | .92 |
| Dexamethasone | 42 | −0.18 | .27 | .29 |
| Prednisolone | 55 | −0.10 | .48 | .63 |
| Daunorubicin | 57 | 0.01 | .95 | .46 |
| Doxorubicin | 44 | −0.03 | .87 | .63 |
| Asparaginase | 58 | 0.17 | .21 | .31 |
| Vincristine | 56 | 0.25 | .06 | .041-153 |
| Ifosfamide | 17 | 0.46 | .07 | .031-153 |
| Cytarabine | 56 | −0.08 | .54 | .64 |
| Drug . | No. . | ρ* . | P† . | MWU test‡P value . |
|---|---|---|---|---|
| 6-Mercaptopurine | 51 | −0.12 | .40 | .83 |
| Thioguanine | 55 | −0.08 | .56 | .92 |
| Dexamethasone | 42 | −0.18 | .27 | .29 |
| Prednisolone | 55 | −0.10 | .48 | .63 |
| Daunorubicin | 57 | 0.01 | .95 | .46 |
| Doxorubicin | 44 | −0.03 | .87 | .63 |
| Asparaginase | 58 | 0.17 | .21 | .31 |
| Vincristine | 56 | 0.25 | .06 | .041-153 |
| Ifosfamide | 17 | 0.46 | .07 | .031-153 |
| Cytarabine | 56 | −0.08 | .54 | .64 |
The relationship between lymphoblast glutathione levels and the IC50 for each cytotoxic drug studied was tested by means of the Spearman rank correlation and the Mann-Whitney test (MWU test). The IC50 was defined as the lowest concentration of a drug required to kill 50% of cells in the MTT assay.
ρ = Spearman rank correlation coefficient.
P = Significance tested by 2-tailed test.
For the Mann-Whitney test, the samples were grouped as high or low glutathione levels. A high glutathione level was defined as greater than the median for the cohort of samples analyzed.
Significant P values.
For the purpose of analysis, samples were also grouped as having high or low lymphoblast glutathione levels. A high lymphoblast level of glutathione was defined as being greater than the median value for the cohort studied. The IC50 for lymphoblasts with a high glutathione level was compared with the IC50 for patients with low lymphoblast glutathione levels by means of the Mann-Whitney test. A significant correlation was demonstrated with in vitro drug resistance to ifosfamide and vincristine (P = .03 and .04, respectively), but no significant relationship was demonstrated between GSH levels and in vitro sensitivity to the other drugs tested (Table1).
Survival data were available on all except one patient. Kaplan-Meier analysis showed that the event-free 5-year survival in this cohort of patients was 66% and the overall survival at 5 years was 75%. Cox regression analysis was performed to assess the prognostic value of age, gender, immunophenotype, and WBC in this cohort of patients (Table 2). Only the presenting WBC was of independent prognostic significance (hazard ratio, 4.6; 95% CI, 1.29-16.04).
Cox regression analysis of event-free survival in childhood acute lymphoblastic leukemia analyzing the relative risk of established indicators of prognosis
| Variable* . | Hazards ratio . | 95% confidence intervals . | |
|---|---|---|---|
| Lower . | Upper . | ||
| Age | 0.70 | 0.15 | 3.39 |
| Immunophenotype | 0.30 | 0.49 | 1.81 |
| Gender | 2.19 | 0.71 | 6.72 |
| WBC | 4.56 | 1.29 | 16.04 |
| Variable* . | Hazards ratio . | 95% confidence intervals . | |
|---|---|---|---|
| Lower . | Upper . | ||
| Age | 0.70 | 0.15 | 3.39 |
| Immunophenotype | 0.30 | 0.49 | 1.81 |
| Gender | 2.19 | 0.71 | 6.72 |
| WBC | 4.56 | 1.29 | 16.04 |
WBC indicates white cell count.
Variables defined according to risk groups as follows: age, good risk 1 to 10 years old, poor risk < 1 and > 10 years old; immunophenotype, good-risk B lineage, poor-risk T lineage; gender, good risk girls, poor risk boys; WBC good risk < 50 × 109/L, poor risk > 50 × 109/L.
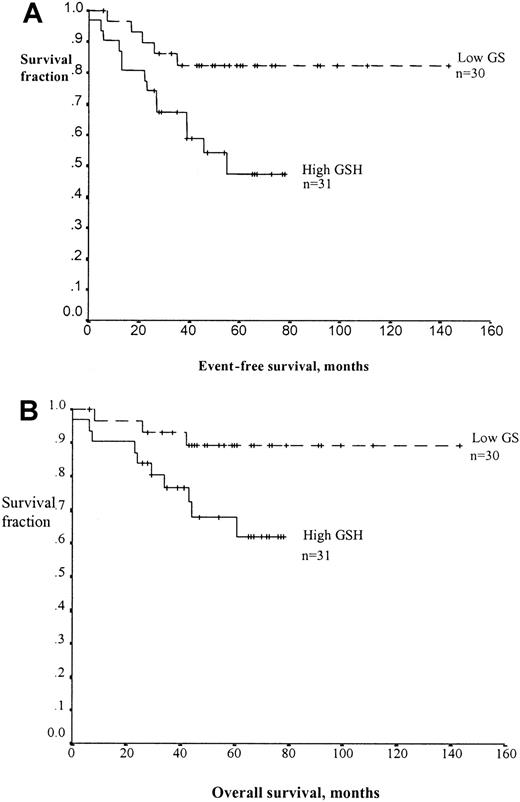
To examine the relationship between lymphoblast glutathione level and clinical outcome, we defined a high lymphoblast glutathione level as being greater than the median value for the cohort studied. Patients with high lymphoblast glutathione level had a significantly lower event-free survival (log-rank test statistic, 5.55;P = .018) (Figure 3A). The overall survival rate for patients with high glutathione levels was also significantly reduced (log-rank test statistic 4.38;P = .04, Figure 3B). Cox regression analysis was performed to assess the independence of glutathione as an indicator of risk of relapse. This confirmed that a high lymphoblast glutathione level was an independent indicator of poor prognosis in childhood ALL (Table 3). Children with a high lymphoblast glutathione level had a 4.5-fold increased risk of relapse (95% CI, 1.4-14.6). The only other independent prognostic indicator among those tested was the presenting WBC. Those with a count above 50 × 109/L had a 5.35-fold increased risk of relapse (95% CI, 1.35-21.12).
Kaplan-Meier curves demonstrating the effect of blast glutathione on survival in childhood ALL.
Event-free survival is shown in panel A and overall survival in panel B. The patients were stratified according to lymphoblast glutathione levels. A high level (solid line) is defined as being above the median blast glutathione concentration for this cohort of patients (6.54 nmol/mg cytosolic protein) and a low level (dashed line) as being below the median value. In both cases, the difference between the curves is statistically significant (P = .018 for event-free survival, P = .04 for overall survival by log-rank analysis).
Kaplan-Meier curves demonstrating the effect of blast glutathione on survival in childhood ALL.
Event-free survival is shown in panel A and overall survival in panel B. The patients were stratified according to lymphoblast glutathione levels. A high level (solid line) is defined as being above the median blast glutathione concentration for this cohort of patients (6.54 nmol/mg cytosolic protein) and a low level (dashed line) as being below the median value. In both cases, the difference between the curves is statistically significant (P = .018 for event-free survival, P = .04 for overall survival by log-rank analysis).
Cox regression analysis of event-free survival in childhood acute lymphoblastic leukemia analyzing the relative risk of prognostic indicators including glutathione
| Variable3-150 . | Hazards ratio . | 95% confidence intervals . | |
|---|---|---|---|
| Lower . | Upper . | ||
| Age | 0.64 | 0.13 | 3.07 |
| Immunophenotype | 0.12 | 0.02 | 0.93 |
| Gender | 2.46 | 0.74 | 8.18 |
| WBC | 5.35 | 1.35 | 21.12 |
| Glutathione | 4.52 | 1.40 | 14.61 |
| Variable3-150 . | Hazards ratio . | 95% confidence intervals . | |
|---|---|---|---|
| Lower . | Upper . | ||
| Age | 0.64 | 0.13 | 3.07 |
| Immunophenotype | 0.12 | 0.02 | 0.93 |
| Gender | 2.46 | 0.74 | 8.18 |
| WBC | 5.35 | 1.35 | 21.12 |
| Glutathione | 4.52 | 1.40 | 14.61 |
WBC indicates white cell count.
Table 2 footnote gives risk-group definitions for the variables.
Relapsed ALL
The median lymphoblast glutathione level in samples from patients at relapse was 6.85 nmol/mg protein (range, 1.49-16.5). This was not significantly different from the result obtained from samples at presentation (Mann-Whitney test, P = .92). The median level in T-lineage samples was 10.82 nmol/mg protein, 1.7-fold higher than the median level in B-lineage samples (6.4 nmol/mg protein). This difference was comparable to that in samples obtained at presentation, but a small number of patients were studied and the difference was not statistically significant (Mann-Whitney test,P = .82).
Discussion
Previous evidence for the role played by glutathione in determining prognosis in leukemia has been conflicting. Paydas et al19 did not find any relationship between treatment response or hematologic parameters and blast glutathione levels. However, their data included a heterogeneous group of adult leukemias, ie, acute and chronic lymphoid leukemia and acute and chronic myeloid leukemia. Conversely, Maung et al18 demonstrated a significant relationship between treatment response and blast glutathione levels in ALL. Only 16 childhood and 5 adult presenting samples were included in their study group. In this study, glutathione levels were measured by means of a high-performance liquid chromatography technique based on the detection of glutathione-monobromobiamine conjugates. This method required fresh blasts for analysis, which limited the number of patients, particularly children, who could be included in their study. We have previously reported that the enzyme-recycling method is a reliable technique for the analysis of glutathione levels in cryopreserved samples,23 thereby allowing us to undertake a larger retrospective study of children with ALL.
We demonstrated that the leukemic blast glutathione concentration varied 20-fold among individual patients and that there were significant differences among subgroups of leukemia. Blast glutathione levels were shown to be significantly related to clinical outcome in childhood ALL. The risk of relapse in ALL was 4.5-fold higher in patients with greater than the median blast glutathione level. It was apparent that glutathione levels in pretreatment blasts were higher in association with certain established markers of resistant disease. Blast glutathione levels were higher in association with the T-lineage immunophenotype, in patients with a high presenting WBC, and in those with a DNA index below 1.16. There was, however, no difference in blast glutathione levels in samples taken at relapse compared with those taken at initial presentation in ALL. Multivariate analysis demonstrated that the blast glutathione level was an independent predictor of relapse risk and not simply a marker for existing indicators of prognosis. The mechanism by which blast glutathione levels determine the risk of relapse in childhood ALL is unclear, and the correlation with clinical outcome does not prove any direct involvement.
Kaspers et al24 proposed in vitro drug sensitivity as a marker for prognosis in ALL. They identified the level of in vitro sensitivity to prednisolone, vincristine, and L-asparaginase as particularly important in the determination of clinical outcome. A relationship between blast glutathione levels and the IC50measured by the MTT assay could be expected if in vitro resistance was mediated by glutathione. However, no strong correlation between lymphoblast glutathione concentration and in vitro drug sensitivity could be demonstrated. A Mann-Whitney test comparing drug sensitivity in high and low glutathione groups suggested a significant relationship for vincristine and ifosfamide (P = .04 and 0.03, respectively); however, the correlations were weak, as reflected by the Spearman rank correlation coefficient, which was not statistically significant (0.06 and 0.07, respectively). The involvement of glutathione in determining prognosis may not therefore be as a direct mediator of resistance to a specific cytotoxic agent. It is interesting to note that in vitro drug sensitivity is not always related to other factors known to determine prognosis. For example, Kaspers et al25 reported that there was no significant relationship between in vitro drug sensitivity and presenting WBC in ALL.
In this study, glutathione levels were not measured in normal tissues in the same patients. It therefore remains to be investigated whether high blast glutathione concentrations reflect a constitutional increase or an elevation occurring exclusively in the leukemic cells. From a study of chronic lymphatic leukemia, there is some evidence for glutathione being higher in leukemic cells compared with lymphocytes.26 Elevated glutathione levels in tumor tissue compared with the equivalent normal tissue have been reported27; these findings would support the proposal that high glutathione levels are specific to the leukemic cells.
It could be argued that blast glutathione levels are a marker of a high presenting WBC and that the effect on risk of relapse is a reflection of the power of the WBC as a prognostic indicator. However, multivariate analysis clearly demonstrated that glutathione levels and WBC have independent effects on survival. Differences in blast glutathione levels may represent functional differences in leukemic cell biology that determine the in vivo response to treatment. The simplest explanation might be that high glutathione levels directly render the cells resistant to cytotoxic drugs. Glutathione has been implicated in many studies as a direct mediator of cellular resistance to bifunctional alkylating agents and anthracyclines.28,29Furthermore, preliminary results of phase I clinical trials combining alkylating agents with modulation of glutathione levels supported the proposal that tumor response to alkylating agents is enhanced by glutathione depletion17 30-32 However, there are as yet no studies reporting the effect of glutathione modulation on treatment response that use agents important to the treatment of ALL.
Although blast glutathione level and presenting WBC had independent effects on the risk of relapse, the correlation between them was highly significant. This correlation was independent of immunophenotype. An increase in glutathione either may directly promote a high WBC or may act as a marker for another process that leads to increased blasts in the peripheral circulation. The majority of childhood leukemias present with nearly 100% marrow infiltration regardless of the peripheral WBC. The peripheral blood WBC can be considered as a balance between the rate of blast proliferation in the marrow and the duration of blast survival in the circulation. If the rate of blast cell proliferation significantly exceeds the rate of cell death, whether by apoptosis or necrosis, a high number of blasts will appear in the peripheral circulation. Intracellular glutathione has been implicated in the control of both cell proliferation33-35and apoptosis.13-16 Bone marrow proliferation rate at presentation has been reported as an indicator of prognosis.36-38 A strong correlation exists between lymphoblast expression of cyclin D1, the cyclin-dependent kinase CDK4, and relapse-free survival in childhood ALL.39 Glutathione has been shown to affect cellular activation and proliferation,33,40-42 especially in lymphocytes where modulation of glutathione levels can control T-cell activation–dependent proliferation.34,35 One study demonstrated enhanced T-lymphocyte proliferation if glutathione levels were increased and inhibition of proliferation when levels were reduced by means of the gamma-glutamyl cysteine synthetase inhibitor, buthionine sulfoximine (BSO).35 Thus, it is possible that a high blast glutathione level may promote a high blast proliferation rate.
In addition to the implication of glutathione in cell proliferation, there is also increasing evidence supporting a number of roles for glutathione in regulation of cell survival. Several studies have demonstrated a fall in glutathione levels in association with the onset of apoptosis.43 Apoptosis can be induced by depletion of glutathione or, conversely, inhibited by elevation of glutathione levels.13-16 Evidence that glutathione homeostasis may be more specifically involved in cell survival was suggested by studies investigating regulation of proteins crucial to cell signaling, eg, the transcription factors AP-1 and NFκB.44,45 There is evidence that the DNA-binding capacity of these proteins is redox status dependent and can be modified by changes in intracellular glutathione concentrations.41 More recently, attention has focused on the importance of the intracellular redox status in maintaining the integrity of the mitochondrial membrane. Disruption of the mitochondrial trans-membrane potential has been shown to immediately precede the nuclear changes associated with apoptosis.46 Depletion of GSH and disruption of the mitochondrial trans-membrane potential are early interdependent events in the apoptotic cascade.13 Thus, there is increasing evidence to implicate glutathione homeostasis in the regulation of both cell proliferation and apoptosis. It would therefore be reasonable to propose that blast glutathione levels are directly involved in the mechanism by which leukemic blasts become resistant to treatment, perhaps by determining the propensity for a cell to proliferate or apoptose. Further investigation of the role of glutathione metabolism in leukemic blast proliferation and apoptosis would be valuable.
It might be expected that blasts from relapse patients would have a higher glutathione level, but no difference was demonstrated. There were no matched presentation and relapse samples in this study. To accurately assess the mechanisms of resistance at relapse, it would be optimal to study paired samples, ie, pretreatment and at relapse.
In conclusion, blast glutathione levels are related to clinical outcome in childhood ALL and to a number of established indicators of prognosis, most significantly, the WBC at presentation. It would be interesting to further explore the proposal that glutathione influences leukemia cell biology by determining the propensity for the cell to proliferate or undergo apoptosis. The modulation of intracellular glutathione levels by nontoxic agents such as BSO may be of therapeutic value. Recently, BSO was shown to be toxic to some myeloid cell lines and cells isolated from patients with acute myeloid leukemia47 and has been shown in a number of clinical studies to be well tolerated at doses in excess of the levels required to produce these in vitro effects.30 Therefore, glutathione reduction in patients presenting with high levels may represent an important new treatment modality.
Supported by a Leukaemia Research Fund Clinical Fellowship grant.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Andrew G. Hall, The LRF Molecular Pharmacology Specialist Programme, Cancer Research Unit, Medical School, Newcastle Upon Tyne, NE2 4HH, UK; e-mail: a.g.hall@ncl.ac.uk.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal