Abstract
This study analyzed the characteristics of 257 HLA-identical sibling transplants of granulocyte colony-stimulating factor–mobilized peripheral blood progenitor cells depleted of T cells by CD34+ positive selection (allo-PBT/CD34+) for their effect on the incidence of graft failure. Twenty-four patients developed graft failure (actuarial probability, 11%; 95% confidence interval, 7.1-14.9). Prognostic factors considered were sex and age of donor and recipient, donor-recipient blood group compatibility, diagnosis, disease status at transplant, conditioning regimen, cytomegalovirus serology, number of CD34+ and CD3+ cells infused, and cryopreservation. The major factor associated with graft failure was the number of CD3+ cells in the inoculum. Twenty-three of 155 patients receiving a T-cell dose in the graft less than or equal to 0.2 × 106/kg experienced graft failure, compared with only one of 102 patients receiving more than 0.2 × 106/kg (actuarial probability 18% vs 1%, respectively; P = .0001). The actuarial probability of graft failure progressively increased as the number of CD3+ cells in the graft decreased, which was determined by grouping the number of CD3+ cells in quartiles (log-rankP = .03; log-rank for trend P = .003). In the multivariate analysis by the proportional hazard method, 2 covariates entered into regression at a significant level: CD3+ cells less than or equal to 0.2 × 106/kg (risk ratio = 17;P < .0001), and patients with chronic myelogenous leukemia (CML) conditioned with busulphan-based regimens (risk ratio = 4.8; P = .001). From these results it appears that the number of CD3+ cells in the inoculum—with a threshold of 0.2 × 106/kg or less—is the most critical factor in maintaining a sustained engraftment in allo-PBT/CD34+ from HLA-identical siblings. In addition, for patients with CML receiving 0.2 × 106/kg or less CD3+ cells, total body irradiation might be better than busulphan-based regimens.
Introduction
Allogeneic transplantation of hemopoietic stem cells (allo-SCT) has gained widespread acceptance in the treatment of hematologic malignancies.1 Graft-versus-host disease (GVHD) is a major limitation to the success of allo-SCT. In fact, one third of the patients submitted to allo-SCT require immunosuppressive treatment due to this complication.2-4 Clinical and experimental studies have shown that the presence of mature immunocompetent T lymphocytes in the graft plays an important role in the development of GVHD.3,5 Several clinical trials indicate that the incidence and severity of GVHD in engrafted patients are greatly reduced when the inoculum is T-cell depleted.6Unfortunately, T-cell depletion (TCD) also results in a high incidence of graft failure. The reported incidence of this complication among recipients of TCD HLA-identical transplants ranges from 10% to 30%,7-11 in contrast to recipients of unmodified HLA-identical marrow grafts, in which there is a 0.1% incidence of graft failure.12 Graft failure is usually a fatal complication.7 9
Multiple mechanisms have been reported to contribute to graft failure after allo-SCT, such as primary disease,13 sex pairing between donor and recipient,7 conditioning regimen,14,15 number of CD34+ cells infused,16 and number of residual T cells in the graft.17 The few studies in which factors related to graft failure have been analyzed have been performed in mice16,17 or in the context of bone marrow transplant.7 13-15 No analyses have been conducted in the growing setting of allogeneic transplantation of T-cell-depleted granulocyte colony-stimulating factor (G-CSF)–mobilized peripheral blood progenitor cells (PBPCs).
We have investigated the characteristics of 257 allogeneic transplants of G-CSF–mobilized PBPCs depleted of T cells by CD34+ positive selection (allo-PBT/CD34+) for their effect on the incidence of graft failure. In this series, 24 patients developed graft failure (actuarial probability, 11%; 95% confidence interval [CI], 7.1-14.9); the most important factor associated with this complication was the infusion to the patient of a quantity of CD3+ cells more than or equal to 0.2 × 106/kg.
Patients, materials, and methods
Patients and donors
This multicenter study performed in 13 Spanish institutions and one Portuguese center included 257 consecutive adult patients with hematological malignancies submitted to allo-PBT/CD34+ from an HLA-identical sibling donor between March 1995 and January 2000. No second transplants were included. The vast majority of patients (205 of 257; 80%) received either cyclophosphamide 120 mg/kg plus total body irradiation 13 Gy in 4 fractions or busulphan 16 mg/kg plus cyclophosphamide 120 mg/kg. Donors received G-CSF at a median dose of 10 μg/kg/d subcutaneously for 4 to 7 days. On day 5 to 8, donors underwent a median of 10 L leukapheresis with a continuous cell separator. Patient and donor characteristics of the overall group are shown in Table 1.
Patients and donors characteristics
| No. of patients | 257 |
| Dates of transplant | March 1995-January 2000 |
| Mean follow-up (range) (mo) | 12 (1-60) |
| Median age (range) (y) | 42 (16-63) |
| Diagnosis | |
| AML/ALL | 123 (47.8%) |
| CML CP1 | 65 (25.3%) |
| NHL/CLL | 29 (11.3%) |
| MM | 22 (8.6%) |
| MDS | 18 (7%) |
| Phase of disease | |
| Early | 143 (55.6%) |
| Advanced | 114 (44.4%) |
| Sex | |
| F to M | 61 (23.7%) |
| M to M | 80 (31.1%) |
| F to F | 53 (20.6%) |
| M to F | 55 (21.4%) |
| Cytomegalovirus serology | |
| D and R negative | 29 (11.3%) |
| D and/or R positive | 212 (82.5%) |
| D or R NA | 16 (6.2%) |
| ABO incompatibility | |
| Major | 48 (18.7%) |
| Myeloablative regimen | |
| TBI based | 140 (54.5%) |
| Bu based | 117 (45.5%) |
| GVHD prophylaxis | |
| CsA + PDN or MTX | 169 (65.8%) |
| CsA | 82 (31.9%) |
| None | 6 (2.3%) |
| Cell content of the graft* (mean and range) | |
| CD34+ cells | 4 (0.6-15) |
| CD3+ cells | 0.3 (0-2.8) |
| G-CSF posttransplant | 73 (28.4%) |
| Cryopreservation | 72 (28%) |
| No. of patients | 257 |
| Dates of transplant | March 1995-January 2000 |
| Mean follow-up (range) (mo) | 12 (1-60) |
| Median age (range) (y) | 42 (16-63) |
| Diagnosis | |
| AML/ALL | 123 (47.8%) |
| CML CP1 | 65 (25.3%) |
| NHL/CLL | 29 (11.3%) |
| MM | 22 (8.6%) |
| MDS | 18 (7%) |
| Phase of disease | |
| Early | 143 (55.6%) |
| Advanced | 114 (44.4%) |
| Sex | |
| F to M | 61 (23.7%) |
| M to M | 80 (31.1%) |
| F to F | 53 (20.6%) |
| M to F | 55 (21.4%) |
| Cytomegalovirus serology | |
| D and R negative | 29 (11.3%) |
| D and/or R positive | 212 (82.5%) |
| D or R NA | 16 (6.2%) |
| ABO incompatibility | |
| Major | 48 (18.7%) |
| Myeloablative regimen | |
| TBI based | 140 (54.5%) |
| Bu based | 117 (45.5%) |
| GVHD prophylaxis | |
| CsA + PDN or MTX | 169 (65.8%) |
| CsA | 82 (31.9%) |
| None | 6 (2.3%) |
| Cell content of the graft* (mean and range) | |
| CD34+ cells | 4 (0.6-15) |
| CD3+ cells | 0.3 (0-2.8) |
| G-CSF posttransplant | 73 (28.4%) |
| Cryopreservation | 72 (28%) |
AML indicates acute myeloblastic leukemia; ALL, acute lymphoblastic leukemia; CML, chronic myeloid leukemia; CP, chronic phase; NHL, non-Hodgkin lymphoma; CLL, chronic lymphocytic leukemia; MM, multiple myeloma; MDS, myelodisplastic syndrome; F, female; M, male; D, donor; R, recipient; NA, not available; TBI, total body irradiation; Bu, busulphan; GVHD, graft-versus-host disease; CsA, cyclosporine A; PDN, prednisone; MTX, methotrexate; G-CSF, granulocyte colony-stimulating factor.
×106/kg.
Positive selection of CD34+ cells
CD34+ cells were positively selected, using an immunoadsorption biotin-avidin column (Ceprate SC System; CellPro, Bothell, WA) in 105 cases (40.8%), indirect immunomagnetic beads by means of Isolex 300 (Isolex 300; Baxter, Munich, Germany) in 104 cases (40.5%), or Clinimacs (ClinimACS, Miltenyi Biotec, Bergisch Gladbach, Germany) in 48 (18.7%) cases. Immunophenotyping was performed with a FACScan flow cytometer (Becton Dickinson Immunocytometry System, San Jose, CA). The antibodies used were 8G12-PE (HPCA-2/CD34), HLe-1-FTIC (CD45), Leu-M3-PE (CD14), Leu-4-FTIC (CD3), and PE- and FTIC-conjugated irrelevant isotype-specific antibodies. CD34+ cells and CD3+ cells were quantified as previously published.18 For those centers contributing the large majority of patients, the interlaboratory variability for enumeration of peripheral blood CD3+ cells showed a high standard of reproducibility with a coefficient of variation usually less than 5%.19 The CD34+ positive fraction was infused to the patients, with previous cryopreservation in 72 cases, as the sole source of progenitor cells. The median number of CD34+cells and CD3+ cells infused to the patients was 3.6 × 106/kg (range, 0.6-15) and 0.1 × 106/kg (range, 0-2.8), respectively.
Evaluation and definitions
Engraftment was documented by increasing neutrophil and platelet counts unsupported by transfusions. Time to neutrophil engraftment was assessed by determining the number of days after day 0 for patients to achieve more than 500/μL. Time to platelet engraftment was assessed by determining the number of days after day 0 to maintain an untransfused platelet count of 20 000/μL or greater. Kinetics of engraftment was analyzed, taking into consideration only those patients who did not receive G-CSF posttransplant. The diagnosis of graft failure was made if persistently falling peripheral blood counts and progressive bone marrow hypoplasia (< 25% cellularity) occurred after documented engraftment; occurred in the absence of leukemic relapse, drug toxicity, or infection; and persisted for at least 14 days. The day of graft failure was assigned to the day that the absolute neutrophil count (ANC) declined to less than 500/μL.
Statistical methods
Actuarial curves were obtained by the Kaplan-Meier method and statistically compared using the log-rank test. The characteristics considered in this study were donor sex, patient sex, sex pairing, donor age, patient age, donor-recipient blood group compatibility, diagnosis, disease status at transplant, conditioning regimen, pretransplant cytomegalovirus (CMV) serology of donor and recipient, number of CD34+ cells and of CD3+ cells infused with the inoculum (both analyzed by median, mean, and quartiles), and cryopreservation of the CD34+ positive fraction. All prognostic variables in the univariate analysis (Kaplan-Meier method) with a P value ≤ .2 (Table 2) were included for the multivariate analysis, to eliminate the redundancy among highly correlated characteristics, each of which may be individually significant. This was performed, using the stepwise proportional hazard Cox regression model. The proportional hazard assumption of the Cox model was checked separately for each covariate before performing the regression analysis. Such checking was done by a graphical and analytical method. The graphs of loge[−loge Ŝ (t)] versus loge (t) for each dichotomous covariate were obtained to check that the curves were roughly parallel. Additionally, for each covariate a time-dependent covariate (covariate*t) was obtained and checked whether the coefficient of the latter significantly differed from 0. The proportional hazard assumption was not rejected for any one of the covariates included in the Cox model. Statistical studies were performed by means of SPSS 6.1 (1994; SPSS, Chicago, IL), SAS 6.1 (1997; SAS Institute, Cary, NC), or BMDP (1988; SPSS) statistical software.
Univariate analysis of association with graft failure
| Univariate analysis . | Actuarial probability (%) . | P value . |
|---|---|---|
| Donor's sex (male vs female) | 8 vs 14.5 | .2 |
| Recipient's sex (male vs female) | 12 vs 10.5 | .9 |
| Sex-pairing mismatched (no vs yes) | 8 vs 15 | .16 |
| Female D to Male R (no vs yes) | 9 vs 19.5 | .11 |
| Male D to Female R (no vs yes) | 11.5 vs 11 | .99 |
| Donor's median age (≤ 40 y vs > 40 y) | 13 vs 11 | .5 |
| Recipient's median age (≤ 42 y vs. > 42 y) | 10 vs 12.5 | .7 |
| ABO incompatibility (no vs yes) | 12.5 vs 9 | .64 |
| Median CD34+ cells × 106/kg (≤ 3.6 vs > 3.6) | 12.6 vs 9.9 | .44 |
| Mean CD34+ cells × 106/kg (≤ 4 vs > 4) | 12.6 vs 9.3 | .43 |
| CD34+ cells × 106/kg in quartiles | ||
| ≤ 2.5 | 10.6 | |
| > 2.5 to 3.6 | 13 | |
| > 3.6 to 5 | 12.6 | |
| > 5 | 9.1 | |
| log-rank χ2 = 0.3 | .95 | |
| log-rank for trend χ2 = 0.1 | .75 | |
| Median CD3+ cells × 106/kg (≤ 0.12 vs > 0.12) | 18 vs 4 | .002 |
| Mean CD3+ cells × 106/kg (≤ 0.28 vs > 0.28) | 16 vs 1.5 | .001 |
| CD3+ cells × 106/kg in quartiles | ||
| ≤ 0.07 | 18.5 | |
| > 0.07 to 0.12 | 16.1 | |
| > 0.12 to 0.37 | 8.3 | |
| > 0.37 | 2.1 | |
| log-rank χ2= 8.5 | .03 | |
| log-rank for trend χ2 = 8.5 | .003 | |
| CD3+ cells × 106/kg (≤ 0.2 vs > 0.2) | 18 vs 1 | .0001 |
| Conditioning regimen (TBI vs Bu) | 8 vs 18 | .04 |
| Stage of disease (early vs advanced) | 12 vs 19 | .7 |
| Cryopreservation (no vs yes) | 8 vs 18 | .03 |
| Diagnosis (CML vs others) | 21 vs 8 | .007 |
| CML + Bu (no vs yes) | 7.5 vs 32 | .0008 |
| Univariate analysis . | Actuarial probability (%) . | P value . |
|---|---|---|
| Donor's sex (male vs female) | 8 vs 14.5 | .2 |
| Recipient's sex (male vs female) | 12 vs 10.5 | .9 |
| Sex-pairing mismatched (no vs yes) | 8 vs 15 | .16 |
| Female D to Male R (no vs yes) | 9 vs 19.5 | .11 |
| Male D to Female R (no vs yes) | 11.5 vs 11 | .99 |
| Donor's median age (≤ 40 y vs > 40 y) | 13 vs 11 | .5 |
| Recipient's median age (≤ 42 y vs. > 42 y) | 10 vs 12.5 | .7 |
| ABO incompatibility (no vs yes) | 12.5 vs 9 | .64 |
| Median CD34+ cells × 106/kg (≤ 3.6 vs > 3.6) | 12.6 vs 9.9 | .44 |
| Mean CD34+ cells × 106/kg (≤ 4 vs > 4) | 12.6 vs 9.3 | .43 |
| CD34+ cells × 106/kg in quartiles | ||
| ≤ 2.5 | 10.6 | |
| > 2.5 to 3.6 | 13 | |
| > 3.6 to 5 | 12.6 | |
| > 5 | 9.1 | |
| log-rank χ2 = 0.3 | .95 | |
| log-rank for trend χ2 = 0.1 | .75 | |
| Median CD3+ cells × 106/kg (≤ 0.12 vs > 0.12) | 18 vs 4 | .002 |
| Mean CD3+ cells × 106/kg (≤ 0.28 vs > 0.28) | 16 vs 1.5 | .001 |
| CD3+ cells × 106/kg in quartiles | ||
| ≤ 0.07 | 18.5 | |
| > 0.07 to 0.12 | 16.1 | |
| > 0.12 to 0.37 | 8.3 | |
| > 0.37 | 2.1 | |
| log-rank χ2= 8.5 | .03 | |
| log-rank for trend χ2 = 8.5 | .003 | |
| CD3+ cells × 106/kg (≤ 0.2 vs > 0.2) | 18 vs 1 | .0001 |
| Conditioning regimen (TBI vs Bu) | 8 vs 18 | .04 |
| Stage of disease (early vs advanced) | 12 vs 19 | .7 |
| Cryopreservation (no vs yes) | 8 vs 18 | .03 |
| Diagnosis (CML vs others) | 21 vs 8 | .007 |
| CML + Bu (no vs yes) | 7.5 vs 32 | .0008 |
F indicates female; M, male; D, donor; R, recipient; TBI, total body irradiation; Bu, busulphan; CML, chronic myeloid leukemia.
Results
Engraftment and pattern of graft failure
All patients reached an ANC of 500/μL and an untransfused platelet count of more than 20 000/μL. The median day to achieve an ANC more than 500/μL and an untransfused platelet count of more than 20 000/μL was 13 (range, 8-32) and 13 (range, 4-127), respectively. At a median of 65 days posttransplant (range, 20-270), 24 patients (9%) developed graft failure, for an actuarial probability of this complication of 11% (95% CI, 7.1-14.9). In the 24 cases peripheral blood neutrophil counts declined to 100/μL or less, all of them required platelet and red cell transfusions and developed marrow aplasia. Chimerism analysis at the time of graft failure was available in 17 (70.8%) of the 24 patients. In 5 cases (29.4%) peripheral blood cells were 100% host origin, and in 12 cases (70.6%) a mixed chimerism was found. In 4 of the 12 cases the study was also performed by separating peripheral blood neutrophils and T cells: in all of them, neutrophils were 100% of donor origin and a fraction of T cells were of host origin. None of the evaluable patients had a complete donor chimerism at the time of graft failure.
Recipient and donor characteristics associated with graft failure
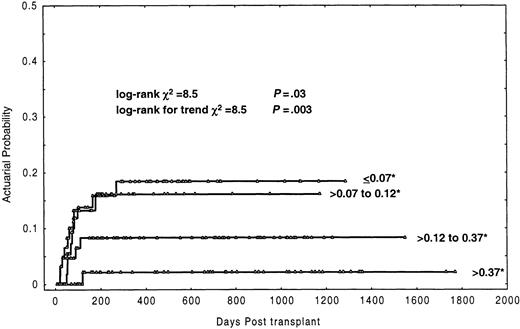
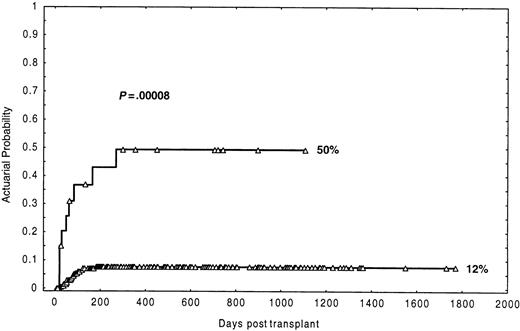
In this analysis (Table 2), the most prominent finding was the association between the number of CD3+ cells in the inoculum and the probability of graft failure. Twenty-three (14.8%) of 155 patients receiving a T-cell dose in the graft less than or equal to 0.2 × 106/kg experienced graft failure, compared with only one of 102 patients (0.8%) receiving more than 0.2 × 106/kg, the actuarial probability being 18% and 1%, respectively (P = .0001). Of note, the actuarial probability of graft failure progressively increased as the number of CD3+ cells in the graft decreased, which was determined by grouping the number of CD3+ cells in quartiles. Thus, recipients of a dose of CD3+ cells (×106/kg) of less than or equal to 0.07, more than 0.07-0.12, more than 0.12-0.37, and more than 0.37 had an actuarial probability of graft failure of 18.5%, 16.1%, 8.3%, and 2.1%, respectively (log-rankP = .03; log-rank for trend P = .003) (Figure1). In the univariate analysis (Table 2), 3 other factors were found to be associated with increased graft failure: CML patients (P = .007), conditioning regimen based on busulphan (P = .04), and cryopreservation of the graft (P = .03). Further analysis showed that the negative effect on engraftment of busulphan-based conditioning regimens only affected patients with CML. Thus, CML patients conditioned with busulphan had an actuarial probability of graft failure of 32% versus 7.5% for the other patients (P = .0008). Besides, CML patients conditioned with busulphan and receiving CD3+cells more than or equal to 0.2 × 106/kg had an actuarial probability of graft failure of 50% compared to 12% in the remaining patients (Figure 2) (P = .00008). Likewise, when the analysis was restricted to CML cases, the actuarial probability of graft failure was 50% in those conditioned with busulphan-based regimens versus 12% in those conditioned with total body irradiation–based regimens (P = .04). In this series a trend for an association of female donor to male recipients with graft failure was also observed (P = .1). Finally, the age of the patients did not influence the probability of graft failure (P = .5).
Actuarial probability of graft failure by grouping the number of CD3+ cells infused to the patients in quartiles.
* indicates ×106/kg.
Actuarial probability of graft failure by grouping the number of CD3+ cells infused to the patients in quartiles.
* indicates ×106/kg.
Actuarial probability of graft failure in the group of recipients of less than 0.2 × 106/kg CD3+cells diagnosed with CML and conditioned with busulphan.
Actuarial probability of graft failure in the group of recipients of less than 0.2 × 106/kg CD3+cells diagnosed with CML and conditioned with busulphan.
Multivariate analysis
In the first analysis, we tried to enter into regression all variables with a P value ≤ .2 (donor sex, sex-pairing mismatch, female donor and male recipient, number of CD3+ cells ≤ 0.2 × 106/kg, cryopreservation, conditioning regimen, and CML). As shown in Table3, only 2 variables entered into regression at a significant level: a quantity of CD3+ cells infused to the patients more than or equal to 0.2 × 106/kg and a diagnosis of CML. Because it was evident from the univariate analysis that the busulphan conditioning regimen acted adversely on the graft only in CML (Table 2), we performed a second multivariate analysis (Table 3) with the same covariates, adding the combined covariate of CML patients conditioned with busulphan. From the coefficient, chi-square, P value, and risk ratio, it was evident that this combined covariate contributed more significantly to the regression, whereas the variable CML alone lost its significance.
Multivariate analysis of association with graft failure
| Variable . | Regression coefficient (95% CI) . | Improvement χ2 . | P value . | Risk ratio (95% CI) . |
|---|---|---|---|---|
| First analysis | ||||
| CD3+ cells × 106/kg (≤ 0.2 vs > 0.2) | 2.8 (0.8-4.8) | 18.6 | < .0001 | 17 (2.3-126.6) |
| Diagnosis (CML vs others) | 1.1 (−0.5-2.7) | 6.4 | .01 | 2.9 (1.3-6.6) |
| Second analysis | ||||
| CD3+ cells × 106/kg (≤ 0.2 vs > 0.2) | 2.8 (0.8-4.8) | 18.6 | < .0001 | 16.9 (2.3-125) |
| CML + Bu (yes vs no) | 1.6 (0.7-2.4) | 11.3 | .001 | 4.8 (2.1-10.9) |
| Variable . | Regression coefficient (95% CI) . | Improvement χ2 . | P value . | Risk ratio (95% CI) . |
|---|---|---|---|---|
| First analysis | ||||
| CD3+ cells × 106/kg (≤ 0.2 vs > 0.2) | 2.8 (0.8-4.8) | 18.6 | < .0001 | 17 (2.3-126.6) |
| Diagnosis (CML vs others) | 1.1 (−0.5-2.7) | 6.4 | .01 | 2.9 (1.3-6.6) |
| Second analysis | ||||
| CD3+ cells × 106/kg (≤ 0.2 vs > 0.2) | 2.8 (0.8-4.8) | 18.6 | < .0001 | 16.9 (2.3-125) |
| CML + Bu (yes vs no) | 1.6 (0.7-2.4) | 11.3 | .001 | 4.8 (2.1-10.9) |
CI indicates confidence interval; CML, chronic myeloid leukemia; CML + Bu, patients diagnosed with CML and conditioned with busulphan-based regimens.
Results of secondary transplants
Twenty-three of 24 patients with graft failure received a secondary transplant with unmanipulated G–CSF-mobilized PBPCs (22 cases) or marrow (1 case). The remaining patient showed autologous reconstitution and is currently alive and free of disease 3 years posttransplant. Seven cases (30.4%) received PBPCs as the only rescue procedure, and in 16 patients (69.6%) ATG was administered immediately before the hemopoietic progenitors, either alone (n = 9) or in association with cyclophosphamide (n = 4), nodal radiotherapy (n = 1), fludarabine (n = 1), or total body irradiation (n = 1). In 3 patients (13%) engraftment was not evaluable due to early death. In the remaining 20 patients, 11 (55%) engrafted and 9 (45%) did not engraft. Fifteen (62.5%) of 24 patients died, 12 due to severe pancytopenia and 3 due to GVHD; of these 3 cases, 2 had not received prophylaxis for GVHD after the apheresis product infusion, and the third patient developed GVHD after cyclosporine A was retired. Nine (37.5%) patients of the 24 remain alive, 8 with complete donor chimerism.
Discussion
Graft failure is one of the most severe complications occurring after allo-SCT.7 In contrast to recipients of unmodified HLA-identical marrow grafts, in whom this complication is uncommon,10 allograft rejection is frequently observed in recipients of T-cell–depleted marrow.7-9,11,12 In many patients, secondary transplants cause unacceptable toxicity, and a considerable proportion of them do not reach a stable engraftment.7 In this series, graft failure developed in 24 of 257 patients (actuarial probability, 11%; 95% CI, 7.1-14.9) submitted to allo-SCT in which the leukapheresis product had been T-cell depleted by CD34+ positive selection (allo-PBT/CD34+). Following secondary transplants, 15 (62.5%) of the 24 patients died due to severe pancytopenia or to GVHD. Similar poor results have been obtained by other groups.7-9,20 Identification of risk factors associated with graft failure could be useful to recognize patients at high risk of developing this complication. Sex pairing between donor and recipient,7 intensity of conditioning regimen,8,14,15 marrow cell dose,16 and degree of TCD21 have been correlated with the risk of graft failure. However, these studies have been performed in the context of TCD of marrow graft and with a limited number of patients. No studies have yet been reported in the setting of TCD of G-CSF-mobilized PBPCs.22
The most relevant finding in our study was the strong correlation between the quantity of T cells in the graft and the engraftment. Thus, 23 (14.8%) of 155 patients receiving an amount of T cells less than or equal to 0.2 × 106/kg experienced graft failure, compared with only one of 102 patients (0.8%) receiving more than 0.2 × 106/kg, with the actuarial probability being 18% versus 1%, respectively (P = .0001). The effect of the T-cell dose on the engraftment was apparent in the univariate and in the multivariate analyses (Tables 2 and 3). This finding has not been previously reported in allo-SCT in humans and is in agreement with results in murine models, showing that rejection associated with TCD marrow is overcome by adding a small quantity of donor CD8+cells17 or thymocytes to the grafts.23,24 Our results also support clinical studies in which “partial” TCD has been found to be associated with a lower risk of graft failure than “total” TCD.25-27 The finding, in some of our cases, of a proportion of recipient T cells at the moment of graft failure supports the concept that this complication is due to small numbers of host lymphoid cells surviving the conditioning regimen.11 27-29
The optimum number of T cells that should be left in the graft to allow reliable engraftment and to prevent GVHD is presently unknown. Of note, the actuarial probability of acute GVHD clinical grade II-IV in this series of 257 patients was very similar in the group of recipients receiving 0.2 × 106/kg or less CD3+ cells than in those receiving more than 0.2 × 106/kg CD3+ cells (13% vs 20%, respectively;P = NS) (data not shown). Taking these results into account, we are considering fixing the quantity of CD3+cells in the graft at 0.3 × 106/kg for HLA-identical sibling allo-PBT/CD34+, adding to the positive fraction the necessary number of CD3+ cells to reach this quantity, and maintaining the use of cyclosporine as GVHD posttransplant prophylaxis.
The number of CD34+ cells infused to the patient is also critical for a successful allografting16,30-32 and has been considered even more important than the number of donor lymphocytes when TCD is used.16,32 Our results, however, do not support that concept. Indeed, the manipulation of the graft can produce undesirable cell losses, thereby resulting in administering a low quantity of hemopoietic progenitor cells. The use of G-CSF–mobilized PBPCs circumvents this problem; thus, in our series the mean number of CD34+ cells infused to allo-PBT/CD34+ recipients was 4 × 106/kg (range, 0.6-15), much higher than that infused after allo-SCT using TCD marrow.22 The mean number (× 106/kg) of CD34+ cells infused to the patients developing graft failure was very similar to the group of patients not having this complication (3.6 vs 4, respectively; P = NS), and the number of CD34+ cells, analyzed by median, mean, and quartiles, were not associated with graft failure (Table 2).
An additional observation in this study was that in the group of recipients of 0.2 × 106/kg or less CD3+cells, the risk of graft failure was higher in CML patients conditioned with busulphan-based regimens than in those conditioned with total body irradiation–based regimens (actuarial probability of 50% vs 12%, respectively) (Tables 2 and 3; Figure 2). It could be speculated that, because CML patients are usually transplanted after having received less immunosuppressive treatment than patients with other forms of leukemia, they could have more host residual T cells surviving the conditioning regimen. However, several studies have stressed the importance of the irradiation in the conditioning regimen to reduce the incidence of graft failure following a T-cell–depleted transplant.14 15 It would seem, therefore, that the diagnosis of CML and the type of conditioning regimen are, together, a critical factor for graft failure when the number of T cells administered to the patients is below the threshold of 0.2 × 106/kg.
To summarize, in this study a number of CD3+ cells equal to or less than 0.2 × 106/kg in the inoculum has been identified as the most critical factor to develop graft failure after allo-PBT/CD34+. In addition, our study also suggests that in patients with CML receiving 0.2 × 106/kg or less CD3+ cells, total body irradiation should be preferred to busulphan-based regimens.
Supported in part by grants FIJC-00/P-CR and FIJC-00/P-EM from the José Carreras International Foundation and grants FIS-98/0995 and FIS-98/0380 from the Fondo de Investigaciones Sanitarias de la Seguridad Social, Spanish Ministry of Health.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
A. Urbano-Ispizua, Institute of Hematology and Oncology, Department of Hematology, Hospital Clı́nic, University of Barcelona, Villarroel 170, 08036 Barcelona, Spain; e-mail:aurbano@clinic.ub.es.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal