Abstract
The chemokine stromal cell-derived factor-1α (SDF-1α) and its G-protein–linked receptor CXCR4 are involved in hematopoietic progenitor cell and lymphocyte migration. The integrin VLA-4 is a cell adhesion receptor for CS-1/fibronectin and VCAM-1 and constitutes one of the main adhesion receptors mediating myeloma cell adhesion to bone marrow (BM) stroma in multiple myeloma (MM). It is shown here that MM CD38hiCD45RA− BM cells and myeloma-derived cell lines expressed CXCR4 and displayed a moderate chemotactic response to SDF-1α. Because cell migration in response to SDF-1α might require a dynamic regulation of integrin function, it was investigated whether SDF-1α can modulate VLA-4 function on myeloma cells. SDF-1α rapidly and transiently up-regulated VLA-4–mediated myeloma cell adhesion to both CS-1/fibronectin and VCAM-1, which was inhibited by pertussis toxin and cytochalasin D, indicating the involvement of Gi protein downstream signaling and an intact cytoskeleton. Modulation of VLA-4–dependent myeloma cell adhesion by SDF-1α could contribute to the trafficking and localization of these cells in the BM microenvironment.
Introduction
SDF-1α is a CXC chemokine first cloned from mouse bone marrow (BM) stromal cells and originally characterized as a pre–B-cell growth-stimulating factor1,2 that potently attracts different cell types, including CD34+hematopoietic progenitor cells (HPC),3-5 and B-cell and megakaryocyte progenitors,6-9 and T lymphocytes.10 In addition to mediating cell migration, SDF-1α can act in synergy with other cytokines to promote cell proliferation.11,12 SDF-1α performs its functions after binding to its 7-transmembrane G-protein–linked receptor CXCR4.13,14 Studies on mice genetically lacking both SDF-1 and CXCR4 revealed that the SDF-1/CXCR4 system is required for B lymphopoiesis and myelopoiesis in the bone marrow.15-18
The integrin VLA-4 is a receptor for VCAM-1 and for the CS-1 region of fibronectin,19 and it plays key roles during hematopoiesis.20,21 VLA-4 is involved in the adhesion of HPC to BM stroma22-24 and participates in the retention and homing of HPC to bone marrow,25,26 and VLA-4–dependent adhesion modulates the proliferation of HPC.27 28
Multiple myeloma (MM) is a neoplasia of terminally differentiated B cells that are mainly found in the BM in close association with stromal cells, except at the final stages of disease, when they proliferate in the extramedullary area.29,30VLA-4–mediated cell attachment to stroma represents a key adhesion pathway used by myeloma cells to localize to the BM in multiple myeloma.31,32 In addition, VLA-4–dependent myeloma adhesion has recently been shown to play an important role in the resistance of these cells to apoptosis due to chemotherapy.33 Modulation of VLA-4 activity by factors in the BM in MM might contribute to the predominant localization of the myeloma cells. In the current study we have analyzed whether BM myeloma cells express functional CXCR4, and, because cell migration involves a dynamic regulation of cell adhesion, we also investigated the potential of SDF-1α to modulate VLA-4 function on these cells.
Materials and methods
Myeloma cell lines and multiple myeloma bone marrow cells—antibodies
The multiple myeloma-derived cell lines NCI-H929 and RPMI 8226 were cultured in RPMI 1640 (Biowhittaker, Verviers, Belgium) supplemented with 10% fetal bovine serum. For selection of myeloma cells, the mononuclear fractions of BM samples from patients with active MM disease were subjected to negative selection using anti-CD33, CD13 (Caltag, San Francisco, CA), CD14, CD3, CD5, CD16, CD19, and glycophorin A (Pharmingen, San Diego, CA) monoclonal antibody (mAb). Cell-bound antibodies were collected with immunobeads coated with antimouse IgG (Dynal A. S., Oslo, Norway), and nonbound cells were recovered and resuspended in appropriate media. Between 80% and 92% of the selected cell population expressed high levels of CD38, was consistently negative for the expression of CD45RA and CD19 (MM–CD38hiCD45RA−), and expressed variable levels of CD56, whereas most cells expressed the α4 and β1 integrin subunits (not shown). Antibodies to CD38 and CD56 were from Pharmingen. The integrin anti-α4 HP1/2 and anti-β1 TS2/16, the P3X63 mAb, and mAb to CD45RA were gifts of Dr F. Sánchez-Madrid (Hospital de la Princesa, Madrid, Spain). Anti–SDF-1α receptor (CXCR4) antibodies 12G5 and 44.717.111 were from R&D Systems (Abingdon, United Kingdom).
VLA-4 ligands and cell adhesion assays
The recombinant FN-H89 fragment of fibronectin containing the CS-1 site was generated as described.34 For adhesion to VCAM-1, the soluble 7-extracellular domain recombinant human VCAM-1 was used (R&D Systems). Before adhesion, cell lines were starved in adhesion medium (DMEM/0.5% BSA) without a detectable loss of viability, as determined by trypan blue exclusion. MM–CD38hiCD45RA− BM cells resuspended in adhesion medium were used directly after their isolation. Cells were labeled with BCECF-AM (Molecular Probes, The Netherlands), preincubated with or without inhibitors (cytochalasin D, wortmannin, Ly294002, Sigma; pertussis toxin, Calbiochem; PD98059, New England Biolabs, Beverly, MA) or antibodies, and finally incubated with rhSDF-1α (R&D Systems). Cells (6 × 104 in 100 μL) were added in triplicate to 96-well dishes (High-binding; Costar, Cambridge, MA) coated with 5 μg/mL FN-H89 or 1 μg/mL sVCAM-1, as described.23 Plates were centrifuged for 15 seconds at 400 rpm and placed at 37°C for 2 minutes for myeloma cell lines or 5 minutes for MM–CD38hiCD45RA− BM cells, and unbound cells were removed by 3 washes with DMEM medium. Bound cells were quantified using a fluorescence analyzer (CytoFluor 2300; Millipore, Bedford, MA).
Actin polymerization and chemotactic assays
To determine the content of F-actin, cells were permeabilized, fixed, and stained in a single step by the addition of a 2× solution containing 0.5 mg/mL L-α–lysophosphatidyl-choline, 8% formaldehyde, and 4 U/mL fluorescein isothiocyanate–phalloidin (Molecular Probes, Eugene, OR). Cells were incubated at 22°C for 10 minutes, washed, and subjected to flow cytometry. For chemotactic assays, starved myeloma cell lines or MM–CD38hiCD45RA− BM cells were placed in adhesion medium in the upper chamber of Transwells (5-μm pore size; Costar, Cambridge, MA). Then 600 μL adhesion medium with or without 100 ng/mL SDF-1α was added to the lower chamber, and transwells were incubated at 37°C for 3 hours. Viable migrated cells were counted in the flow cytometer by passing each sample in the same predetermined time and flow conditions. Where indicated, cells were pretreated with 200 ng/mL pertussis toxin for 2 hours at 37°C.
Western blotting
For p44/42 analysis, 107 cells incubated in the absence or presence of SDF-1α were solubilized in 1 mL RIPA lysis buffer (0.5% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate) in the presence of 1 mM phenylmethylsulfonyl fluoride, 100 μg/mL soybean trypsin inhibitor, 50 μg/mL aprotinin, 10 μg/mL leupeptin and antipain, 10 mM sodium fluoride, and 1 mM sodium orthovanadate (all from Sigma) in phosphate-buffered saline (PBS). Proteins were separated by SDS-PAGE and transferred to nitrocellulose membranes (Millipore). Immunoblots were blocked with 5% skim milk in PBS, incubated for 2 hours at room temperature with anti-phospho-p44/42 antibodies (New England Biolabs), washed in T-PBS (0.05% Tween 20 in PBS), and further incubated with a horseradish peroxidase-conjugated secondary antirabbit antibody (New England Biolabs) in blocking buffer. After washing as above, blots were developed by a chemiluminescence reaction and exposed to radiographic films. After stripping and blocking, the same blots were reprobed with anti-p44/42 antibodies (New England Biolabs) to test for total p44/42 protein content.
Results and discussion
Expression and functionality of CXCR4 on MM bone marrow cells
To investigate whether BM myeloma cells expressed CXCR4, we performed flow cytometry on the MM-CD38hiCD45RA− cell population. First we used both 12G5 and 44.717.111 anti-CXCR4 mAbs, but because the latter reacted better with myeloma CXCR4, we then used only this antibody. The FACS profiles revealed heterogenous CXCR4 expression from sample to sample. Several (n = 7) contained a low percentage (10%-35%) of cells positive for CXCR4 expression, and 3 samples expressed medium levels (35%-70%); in other samples (n = 7), a high percentage (70%-100%) of the myeloma cells expressed CXCR4 (Figure1A). In addition, the multiple myeloma-derived cell lines NCI-H929 and RPMI 8226 expressed CXCR4. There were apparently no correlations between CXCR4 expression, isotypes of M-protein, and clinical features among the different samples (all patients were symptomatic and had high-stage disease (stage II or III), according to Durie and Salmon35).
Expression and functionality of CXCR4 on BM myeloma cells.
(A) Myeloma CD38hiCD45RA− BM cells and myeloma-derived cell lines were incubated with the anti-CXCR4 44.717.111 or control [C] P3X63 mAb and analyzed by flow cytometry. Results from 3 different CD38hiCD45RA− BM samples (upper panels) expressing low (left), medium (middle), or high (right) levels of CXCR4 is shown. (B) Myeloma CD38hiCD45RA− BM and NCI-H929 cells were preincubated without or with pertussis toxin (PTx) in DMEM/BSA 0.5% and allowed to migrate in Transwell chemotaxis chambers to medium alone or an SDF-1α–containing lower chamber. Results from 3 different MM-CD38hiCD45RA− BM samples expressing medium or high levels of CXCR4 are shown. Data represent the mean ± SD of duplicate samples. (C) Cells were incubated for the indicated times with SDF-1α (solid circles) or adhesion medium (open circles), stained with fluorescein isothiocyanate–phalloidin, and subjected to flow cytometry.
Expression and functionality of CXCR4 on BM myeloma cells.
(A) Myeloma CD38hiCD45RA− BM cells and myeloma-derived cell lines were incubated with the anti-CXCR4 44.717.111 or control [C] P3X63 mAb and analyzed by flow cytometry. Results from 3 different CD38hiCD45RA− BM samples (upper panels) expressing low (left), medium (middle), or high (right) levels of CXCR4 is shown. (B) Myeloma CD38hiCD45RA− BM and NCI-H929 cells were preincubated without or with pertussis toxin (PTx) in DMEM/BSA 0.5% and allowed to migrate in Transwell chemotaxis chambers to medium alone or an SDF-1α–containing lower chamber. Results from 3 different MM-CD38hiCD45RA− BM samples expressing medium or high levels of CXCR4 are shown. Data represent the mean ± SD of duplicate samples. (C) Cells were incubated for the indicated times with SDF-1α (solid circles) or adhesion medium (open circles), stained with fluorescein isothiocyanate–phalloidin, and subjected to flow cytometry.
CD38hiCD45RA− BM myeloma samples expressing low CXCR4 did not display a chemotactic response to SDF-1α (not shown). Most MM-CD38hiCD45RA− BM samples (7 of 10) in which medium-high CXCR4 expression was detected exhibited a moderate chemotactic response to SDF-1α; the other 3 samples showed no or very low response (Figure 1B). The presence of myeloma CD38hiCD45RA− cells in the migrated cell population was corroborated by FACS and showed similar expression levels of CD38 before and after chemotaxis to SDF-1α (not shown). Pertussis toxin (PTx), an inhibitor of the Gαi subunit of G proteins, abolished the SDF-1α–induced chemotaxis. NCI-H929 (Figure 1B) and RPMI 8226 (not shown) cells showed consistently stronger chemotaxis to SDF-1α than BM CD38hiCD45RA− myeloma cells. Additional confirmation of the functionality of CXCR4 on myeloma cells was obtained in experiments in which SDF-1α triggered a rapid and transient increase in the polymerization of F-actin on MM-CD38hiCD45RA− BM and NCI-H929 cells (Figure1C), which was blocked by cytochalasin D (Cyt D, 0.5 μg/mL) (not shown).
We do not yet have an experimental explanation for the occasional absence or very low chemotactic response of CXCR4+CD38hiCD45RA− BM myeloma cells to SDF-1α. There were no clinical or laboratory features distinguishing these samples from the samples that responded to SDF-1α. Whether these results reflect differences in SDF-1α binding to CXCR4 or alterations in downstream signaling pathways represents an important issue for study. Diminished chemotaxis toward SDF-1α has been reported as B-cell differentiation advances, even if CXCR4 expression does not substantially change.6,7,36,37 This fact seems to be independent of SDF-1α binding to unresponsive and responsive cells,36 suggesting that elements downstream of CXCR4 regulate responsiveness to SDF-1α on B cells. Potential mechanisms include the participation of RGS proteins or the cross-desensitization of the chemokine receptor.38-40 It has been hypothesized that SDF-1α–CXCR4 interaction might play an important role in the retention in the BM of early B-cell progenitors, and the decrease in responsiveness to SDF-1α in mature B cells might contribute to their release into the blood circulation.37 41 Therefore, it could also be speculated that retention or egress of myeloma cells from the bone marrow might involve alterations in their responsiveness to SDF-1α, which could then have important therapeutic consequences.
SDF-1α modulates VLA-4–mediated myeloma cell adhesion
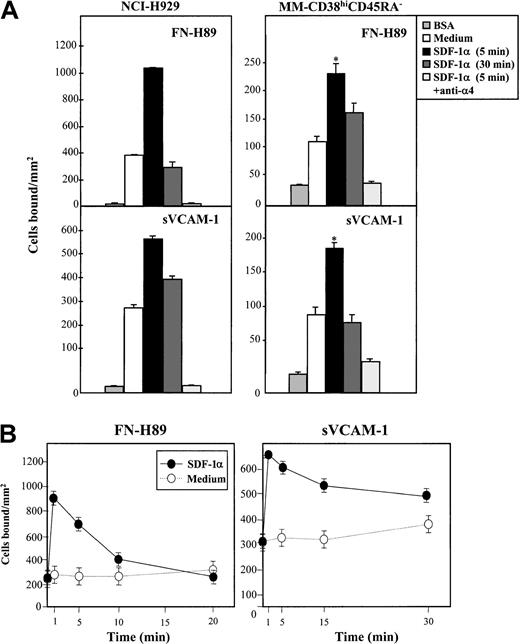
Because cell migration in response to SDF-1α might require dynamic regulation of integrin function, we studied whether SDF-1α influenced VLA-4–dependent myeloma adhesion. For this, we subjected cells to adhesion assays to FN-H89, a CS-1–containing fragment of fibronectin, and to sVCAM-1. Incubation for 5 minutes with SDF-1α substantially up-regulated VLA-4–mediated adhesion of NCI-H929 (n = 4) and MM–CD38hiCD45RA− BM cells to both FN-H89 (5 of 6 samples tested) and to sVCAM-1 (5 of 5 samples tested), which was still detected in some cases at 30 minutes, though to lower degrees (Figure 2A). The specificity of the SDF-1α up-regulated adhesion was confirmed by the ability of anti-α4 HP1/2 mAb to block cell adhesion to both VLA-4 ligands. The increase of NCI-H929 cell adhesion to both FN-H89 and sVCAM-1 by SDF-1α was rapid and transient, peaking at 1 minute and returning to basal levels by 30 minutes (Figure 2B).
Effect of SDF-1α on VLA-4–dependent myeloma cell adhesion.
BCECF-AM–labeled cells were preincubated in adhesion medium in the absence or in the presence of SDF-1α (100 ng/mL) for 5 or 30 minutes (A) or for the times indicated (NCI-H929 cells) (B) and were allowed to adhere to FN-H89– or sVCAM-1–coated wells for 2 minutes. Some samples were preincubated with the anti-α4 HP1/2 mAb before exposure to SDF-1α. Adhesions were quantified in a fluorescence analyzer. Adhesion data represent the mean ± SD of triplicate samples from one representative result of at least 4 independent experiments. Basal adhesion to wells coated with BSA alone is also shown. *Statistical significance compared with control and assessed asP < .05.
Effect of SDF-1α on VLA-4–dependent myeloma cell adhesion.
BCECF-AM–labeled cells were preincubated in adhesion medium in the absence or in the presence of SDF-1α (100 ng/mL) for 5 or 30 minutes (A) or for the times indicated (NCI-H929 cells) (B) and were allowed to adhere to FN-H89– or sVCAM-1–coated wells for 2 minutes. Some samples were preincubated with the anti-α4 HP1/2 mAb before exposure to SDF-1α. Adhesions were quantified in a fluorescence analyzer. Adhesion data represent the mean ± SD of triplicate samples from one representative result of at least 4 independent experiments. Basal adhesion to wells coated with BSA alone is also shown. *Statistical significance compared with control and assessed asP < .05.
Percentage averages of MM-CD38hiCD45RA− BM cell adhesion to FN-H89 and sVCAM-1 were 5.2% ± 1.7% (n = 6) and 6.0% ± 2.2% (n = 5), respectively, and they increased to 9.8% ± 2.8% and 11.8% ± 2.5% in the presence of SDF-1α (P < .05). Similar to chemotaxis, analysis of FN-H89– and sVCAM-1–adhered cells revealed that expression levels of CD38 closely correlated with those of the input cells in the adhesion assay, both when adhesions were performed in the absence and in the presence of SDF-1α (not shown). In contrast to the chemotactic assays, SDF-1α up-regulation of VLA-4–dependent adhesion was already obtained with MM–CD38hiCD45RA− BM cell populations expressing CXCR4 levels as low as 20%. In spite of the fact that most myeloma cells express VLA-4, the percentages of MM-CD38hiCD45RA− BM cells adhering to FN-H89 and sVCAM-1 were low, even when these adhesions were enhanced by SDF-1α. Instead, the levels of adhesion of NCI-H929 myeloma cells in the absence or presence of the chemokine were at least 3-fold compared with those obtained with fresh myeloma BM cells. First, the mean fluorescence intensity values of α4 integrin expression on MM-CD38hiCD45RA− BM cells were always substantially lower than on NCI-H929 cells (not shown), which could be one factor contributing to low adhesion. Second, VLA-4 on MM-CD38hiCD45RA− BM cells might be expressed in a low activation state that could be turned into a higher activation state by means of inside-out signaling by SDF-1α, which will be discussed below. Therefore, low levels of VLA-4 expression on MM-CD38hiCD45RA− BM cells or the low activation state of this integrin could be responsible for the poor adhesion displayed by these cells.
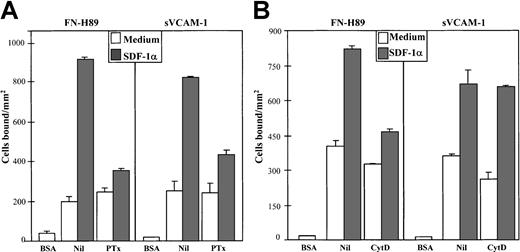
Expression of both α4 and β1 subunits on NCI-H929 cells preincubated for 1, 5, and 30 minutes with SDF-1α showed no significant alterations (data not shown). Pertussis toxin strongly inhibited the up-regulation in cell adhesion to both FN-H89 and sVCAM-1 triggered by SDF-1α (Figure 3), indicating the participation of receptor-linked Gi-protein downstream signaling pathways in the up-regulation of VLA-4 function. Inhibition by PTx was generally higher in SDF-1α–stimulated cell adhesion to FN-H89 than adhesion to sVCAM-1. To study whether the rapid (1 minute) and transient increase of F-actin polymerization was associated with the up-regulation in VLA-4 adhesive activity by SDF-1α, we tested the effect of Cyt D on the increased adhesion. Cyt D (0.5-1 μg/mL) substantially inhibited the SDF-1α–up-regulated VLA-4–dependent NCI-H929 adhesion to FN-H89 (Figure 3). We did not detect a significant effect of Cyt D on the increased cell adhesion to sVCAM-1, and we did not use higher concentrations of the inhibitor because basal cell adhesion to the VLA-4 ligands was dramatically reduced (data not shown).
Effect of pertussis toxin and cytochalasin D on SDF-1α–up-regulated VLA-4–dependent myeloma cell adhesion.
BCECF-AM–labeled NCI-H929 cells were preincubated in medium without (Nil) and with pertussis toxin (PTx) (200 ng/mL, 2 hours) (A) or with cytochalasin D (Cyt D) (0.5 μg/mL, 30 minutes) (B). SDF-1α (100 ng/mL, 1 minute) was added, and cells were allowed to adhere to FN-H89– or sVCAM-1–coated wells for 2 minutes. Adhesions were quantified in a fluorescence analyzer. Adhesion data represent the mean ± SD of triplicate samples from one representative result of at least 4 independent experiments.
Effect of pertussis toxin and cytochalasin D on SDF-1α–up-regulated VLA-4–dependent myeloma cell adhesion.
BCECF-AM–labeled NCI-H929 cells were preincubated in medium without (Nil) and with pertussis toxin (PTx) (200 ng/mL, 2 hours) (A) or with cytochalasin D (Cyt D) (0.5 μg/mL, 30 minutes) (B). SDF-1α (100 ng/mL, 1 minute) was added, and cells were allowed to adhere to FN-H89– or sVCAM-1–coated wells for 2 minutes. Adhesions were quantified in a fluorescence analyzer. Adhesion data represent the mean ± SD of triplicate samples from one representative result of at least 4 independent experiments.
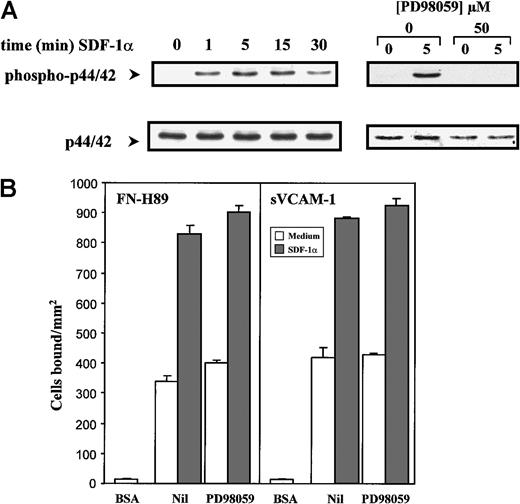
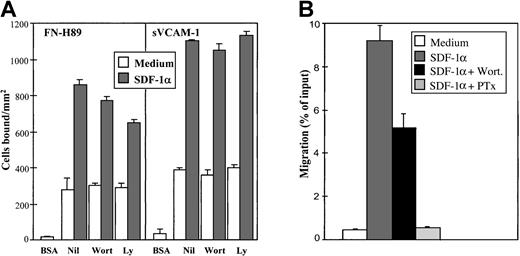
It has been reported in studies with model progenitor cell lines and T lymphocytes that SDF-1α rapidly activates the p44/42 (Erk1/2) mitogen-activated protein (MAP) kinase and the phosphatidylinositol 3-kinase (PI3-K).42-44 SDF-1α induced a transient up-regulation in the phosphorylation of the p44/42 MAP kinase in NCI-H929 myeloma cells, which was clearly detected after 1 minute of pretreatment with the chemokine, peaked at 5 minutes, and diminished with longer incubations (30 minutes) (Figure4A). The increase in phosphorylation of p44/42 was due to the activation of MEK1 by SDF-1α because it was completely blocked by the specific MEK1 inhibitor PD98059. However, the enhancement of VLA-4–dependent adhesion of NCI-H929 cells to FN-H89 and sVCAM-1 by SDF-1α was not significantly influenced by cell preincubation with PD98059 at concentrations up to 100 μM (Figure4B), suggesting that the activation of p44/42 MAP kinase was not necessary for the SDF-1α–triggered increase in adhesion. In addition, preincubation with PD98059 did not inhibit the in vitro chemotaxis of NCI-H929 cells to SDF-1α (not shown). To study whether PI3-K activity was involved in the increase in VLA-4–dependent cell adhesion because of SDF-1α, we tested whether 2 chemically distinct inhibitors of this enzyme, wortmannin and Ly294002, could influence the enhanced adhesion. Although the up-regulation of adhesion of NCI-H929 cells to sVCAM-1 by SDF-1α was not affected by these inhibitors, inhibition (range, 15%-25%) was obtained in cell adhesion to FN-H89 with NCI-H929 cells (Figure 5A). In addition, wortmannin also partially inhibited the chemotaxis of these cells to SDF-1α (Figure 5B), in agreement with previous data obtained with lymphocytes, hematopoietic progenitor cells, and B-CLL cells.43-46
Activation of the p44/42 MAP kinase by SDF-1α is not required for the up-regulation of VLA-4–mediated cell adhesion.
(A) NCI-H929 cells were pre-incubated in the absence or in the presence of PD98059 before the addition of SDF-1α (100 ng/mL) for the indicated times. Cells were solubilized and subjected to immunoblotting using an anti-phospho–p44/42 antibody. After stripping and blocking, the same blots were reprobed with anti-p44/42 antibodies to check for total p44/42 protein content. Blots were developed by a chemiluminescence reaction and exposed to radiographic film. (B) Cells were labeled with BCECF-AM and treated for 30 minutes in adhesion medium without (Nil) or with PD98059 (50 μM). SDF-1α (100 ng/mL, 1 minute) was added, and cells were allowed to adhere to FN-H89– or sVCAM-1–coated wells for 2 minutes. Adhesions were quantified in a fluorescence analyzer. Adhesion data represent the mean ± SD of triplicate samples from one representative result of 4 independent experiments.
Activation of the p44/42 MAP kinase by SDF-1α is not required for the up-regulation of VLA-4–mediated cell adhesion.
(A) NCI-H929 cells were pre-incubated in the absence or in the presence of PD98059 before the addition of SDF-1α (100 ng/mL) for the indicated times. Cells were solubilized and subjected to immunoblotting using an anti-phospho–p44/42 antibody. After stripping and blocking, the same blots were reprobed with anti-p44/42 antibodies to check for total p44/42 protein content. Blots were developed by a chemiluminescence reaction and exposed to radiographic film. (B) Cells were labeled with BCECF-AM and treated for 30 minutes in adhesion medium without (Nil) or with PD98059 (50 μM). SDF-1α (100 ng/mL, 1 minute) was added, and cells were allowed to adhere to FN-H89– or sVCAM-1–coated wells for 2 minutes. Adhesions were quantified in a fluorescence analyzer. Adhesion data represent the mean ± SD of triplicate samples from one representative result of 4 independent experiments.
Effect of inhibitors of phosphatidylinositol 3-kinase on SDF-1α–up-regulated VLA-4–dependent myeloma cell adhesion and chemotaxis.
(A) BCECF-AM–labeled NCI-H929 cells were treated for 30 minutes in adhesion medium without (Nil) or with wortmannin (Wort) (0.2 μM) or Ly294002 (Ly) (20 μM). After cell treatment with the inhibitors, SDF-1α (100 ng/mL, 1 minute) was added, and cells were allowed to adhere to FN-H89– or sVCAM-1–coated wells for 2 minutes. Adhesions were quantified in a fluorescence analyzer. Each panel shows data representing the mean ± SD of triplicate samples from one representative result of at least 4 separate experiments. (B) Comparison of the effects of wortmannin and pertussis toxin on the chemotaxis of myeloma NCI-H929 cells in response to SDF-1α. Cells were preincubated with or without the inhibitors and allowed to migrate for 3 hours at 37°C to an SDF-1α (100 ng/mL)-containing lower chamber. Data represent the mean ± SD of duplicate samples from a representative result of 3 separate experiments.
Effect of inhibitors of phosphatidylinositol 3-kinase on SDF-1α–up-regulated VLA-4–dependent myeloma cell adhesion and chemotaxis.
(A) BCECF-AM–labeled NCI-H929 cells were treated for 30 minutes in adhesion medium without (Nil) or with wortmannin (Wort) (0.2 μM) or Ly294002 (Ly) (20 μM). After cell treatment with the inhibitors, SDF-1α (100 ng/mL, 1 minute) was added, and cells were allowed to adhere to FN-H89– or sVCAM-1–coated wells for 2 minutes. Adhesions were quantified in a fluorescence analyzer. Each panel shows data representing the mean ± SD of triplicate samples from one representative result of at least 4 separate experiments. (B) Comparison of the effects of wortmannin and pertussis toxin on the chemotaxis of myeloma NCI-H929 cells in response to SDF-1α. Cells were preincubated with or without the inhibitors and allowed to migrate for 3 hours at 37°C to an SDF-1α (100 ng/mL)-containing lower chamber. Data represent the mean ± SD of duplicate samples from a representative result of 3 separate experiments.
Collectively, inhibition of the SDF-1α–triggered up-regulation of VLA-4–dependent myeloma cell adhesion obtained with pertussis toxin, cytochalasin D, and wortmannin show differences with respect to the VLA-4 ligand tested. In general, the increased adhesion to FN-H89 was more inhibitable than the adhesion to sVCAM-1. As shown above, a more striking effect was observed with the use of Cyt D, which did not affect the SDF-1α–induced myeloma adhesion to sVCAM-1. A previous study47 also reported that Cyt D did not inhibit IL-8–induced up-regulation of α4β7-dependent cell adhesion to VCAM-1.47 It is likely that both avidity and affinity of VLA-4 for ligands are transiently up-regulated by SDF-1α. A transient increase in the polymerization of the actin cytoskeleton because of SDF-1α might cluster VLA-4 molecules on the myeloma cell surface, providing optimal interactions between the integrin, cytoskeleton, and signaling components, which could represent one mechanism contributing to an enhancement in the avidity of VLA-4 for its ligands. Members of the Ras superfamily of small guanosine triphosphates, such as RhoA, Rac1, and Cdc42, regulate the assembly of actin filaments.48 We have obtained preliminary data showing that SDF-1α rapidly and transiently activates Rac1 on NCI-H929 cells (Wright et al, manuscript in preparation), raising the possibility that it might be involved in the up-regulation of VLA-4–mediated myeloma adhesion by SDF-1α. Investigation of SDF-α–induced VLA-4 clustering at the myeloma cell membrane is under way, and, in this regard, it has been demonstrated that expression of an activated form of Rac1 results in α4 integrin clustering and increased T-lymphocyte adhesion.49 Additionally, participation of Rho GTP-ases in SDF-1α-triggered cell responses has been documented for T lymphocytes.50 51
The p85 subunit of PI3-K has been shown to bind Rac-GTP,52and, given that activation of this kinase is associated with Rac-related signaling and motility,53 54 the partial effect of PI3-K inhibitors on the SDF-1α triggered VLA-4–dependent cell adhesion to FN-H89, and in the chemotaxis toward this chemokine, might reflect alterations of this signaling.
As pointed out above, changes in the affinity of VLA-4 for its ligands might also be associated with the SDF-1α stimulation of VLA-4–mediated myeloma adhesion. We have recently shown that the affinity of VLA-4 on myeloma cells can be a target of modulation by stimuli that modify its conformation to a high-affinity state leading to increased adhesion, such as with the TS2/16 anti-β1 mb.55 It had been reported that VLA-4 affinity for VCAM-1 is higher than for CS-1/FN.56 If SDF-1α is increasing both the avidity and the affinity of VLA-4 for its ligands, then a possible explanation for the lack of effect of Cyt D on SDF-1α–triggered up-regulation of cell adhesion to sVCAM-1 would be that high-affinity VLA-4 will remain bound to sVCAM-1 even by altering the avidity of VLA-4 with Cyt D. In the case of adhesion to FN-H89, the disruption by Cyt D of a high-avidity state of VLA-4 induced by SDF-1α might produce a more drastic effect because of the lower affinity of VLA-4 for this ligand.
An SDF-1α triggered rapid up-regulation of VLA-4-mediated adhesion has also been reported for BM CD34+ HPC57 (and Hidalgo et al, manuscript submitted), which could modulate their trafficking within and to the bone marrow. The myeloma cells in MM are localized to the BM in close association with stromal cells, using several adhesion receptors including VLA-4.29-32 We have found that plasma supernatants of MM bone marrow samples (n = 4) contained SDF-1α chemotactic activity for NCI-H929 cells that was inhibitable by pertussis toxin and anti-CXCR4 antibodies (Sanz-Rodrı́guez et al, manuscript in preparation), indicating the presence of SDF-1α in the MM bone marrow. Modulation of VLA-4–mediated myeloma cell adhesion by SDF-1α could contribute to the trafficking and localization of these cells in the BM microenvironment and might also be involved in the homing of myeloma cells to the BM because it has been shown that SDF-1 can be exposed on BM endothelium.57 In addition, this modulation might influence the survival of myeloma cells given that VLA-4–mediated adhesion has been described to mediate growth control.27 28 The current results should lead to further investigations to identify the inside-out signaling components involved in the up-regulation of VLA-4 adhesive function by SDF-1α in myeloma cells.
Acknowledgments
We thank Drs Francisco Sánchez-Madrid and Martin J. Humphries for reagents. We thank Drs Adrián Alegre (Hospital de la Princesa, Madrid), Felipe Prósper (Hospital Clı́nico, Valencia), Carmen Larrocha (Hospital La Paz, Madrid), and Rafael Borstein (Hospital 12 de Octubre, Madrid) for providing the myeloma bone marrow samples.
Supported by grants SAF99-0057 from Ministerio de Educación y Ciencia and by a grant from Fundación Ramón Areces. F.S.R. and A.H. are recipients of predoctoral fellowships from Fundación Ramón Areces and the Comunidad de Madrid, respectively.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Joaquin Teixidó, Department of Immunology, Centro de Investigaciones Biológicas, Velázquez 144, 28006 Madrid, Spain; e-mail joaquint@cib.csic.es.

![Fig. 1. Expression and functionality of CXCR4 on BM myeloma cells. / (A) Myeloma CD38hiCD45RA− BM cells and myeloma-derived cell lines were incubated with the anti-CXCR4 44.717.111 or control [C] P3X63 mAb and analyzed by flow cytometry. Results from 3 different CD38hiCD45RA− BM samples (upper panels) expressing low (left), medium (middle), or high (right) levels of CXCR4 is shown. (B) Myeloma CD38hiCD45RA− BM and NCI-H929 cells were preincubated without or with pertussis toxin (PTx) in DMEM/BSA 0.5% and allowed to migrate in Transwell chemotaxis chambers to medium alone or an SDF-1α–containing lower chamber. Results from 3 different MM-CD38hiCD45RA− BM samples expressing medium or high levels of CXCR4 are shown. Data represent the mean ± SD of duplicate samples. (C) Cells were incubated for the indicated times with SDF-1α (solid circles) or adhesion medium (open circles), stained with fluorescein isothiocyanate–phalloidin, and subjected to flow cytometry.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/97/2/10.1182_blood.v97.2.346/5/m_h80210597001.jpeg?Expires=1764957058&Signature=s1VXkskJVOr1RMlIMcMllQWmUj2UJvVjwALKEkIGABkSF-SlNLXFrxTWeo0mnXDqOa6LBUQ77c0kcn-pcdBLCv4V8ZLzBZjQEszvkRVddej4vxOCZuTmH~m2fHz5ek7-XpCI51tX4UpJkW1XgBVUWADoR-dHbwtf62GN9w8xCqe-81J85jLg4REeO7FLl-4golVjvV3h~FWhE6ppd8RnEkRNeVE~XYTBMspW9DfjiPQAj8AAKF2VRpJguzVPZA4uxgGGKNif-YBKRsdKp5h3KLKnnvD90FRUwt1UvnTXdSJ1ECbIaU-05AjVbPj8kngtFbG0YJOsudFuSRyicK87TQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal