Interaction between von Willebrand factor (vWF) and glycoprotein Ib (GPIb) stimulates tyrosine kinases and subsequent tyrosine phosphorylation events in human platelets. This study found that the combination of vWF and botrocetin, by interacting with GPIb, induced tyrosine phosphorylation of Fc receptor γ-chain (FcR γ-chain), Syk, linker for activation of T cells (LAT), and phospholipase C γ2 (PLCγ2). Pretreatment of platelets with 10 μM PP1 completely inhibited these tyrosine phosphorylation events. On GPIb stimulation, Src and Lyn formed a complex with FcR γ-chain and Syk, suggesting that Src and Lyn are involved in FcR γ-chain tyrosine phosphorylation and downstream signals. In spite of the PLCγ2 tyrosine phosphorylation, however, there was no intracellular calcium release and inositol 1,4,5-trisphosphate production. In Brij 35 lysates, FcR γ-chain was found to constitutively associate with GPIb. The number of GPIb expressed on FcR γ-chain–deficient platelets was comparable to that of the wild-type, as assessed by flow cytometry. However, tyrosine phosphorylation of Syk, LAT, and PLCγ2 in response to vWF plus botrocetin was significantly suppressed, suggesting that FcR γ-chain mediates activation signals related to GPIb. Compared with the aggregation response of wild-type platelets, that of FcR γ-chain–deficient platelets in response to vWF plus botrocetin was impaired, implying that FcR γ-chain is required for the full activation of platelets mediated by GPIb.
Introduction
von Willebrand factor (vWF) is a multimeric protein that mediates platelet adhesion to exposed subendothelium at sites of vascular injury. The adhesive property of vWF is tightly regulated so that plasma vWF does not normally interact with circulating platelets. However, after vWF is activated by binding to damaged vessel walls, it serves to bridge the constituents of subendothelium to glycoprotein Ib (GPIb) on the membrane of circulating platelets.1 2 Although much is known about the molecular basis of the interaction between vWF and GPIb, little has been clarified about the intracellular signal transduction pathway in GPIb-mediated platelet activation.
Events related to protein-tyrosine phosphorylation have emerged as important signals mediated by GPIb.3-7 GPIb-mediated platelet activation in response to vWF plus botrocetin, shear stress, or vWF from patients with von Willebrand disease type IIb induces tyrosine phosphorylation of multiple proteins,3-5suggesting that the binding of vWF to GPIb causes the activation of tyrosine kinases. Studies have shown that the activation of Syk and Src and their association can be induced by GPIb stimulation.6-10 To date, there is no report showing that tyrosine residues within the GPIb molecule can be phosphorylated, nor has GPIb itself intrinsic kinase activity. Thus, how clustering of GPIb induced by vWF mediates the activation of tyrosine kinases such as Src and Syk has become an important issue. Because Syk is activated by engagement of its tandem SH2 domains with phosphorylated tyrosine residues in proteins containing the immunoreceptor tyrosine-based activation motif (ITAM),11 it is of interest whether ITAM-containing transmembrane molecules are also involved in GPIb signaling. Two ITAM-containing proteins have been identified in platelets, the low-affinity receptor for immunoglobulin (Ig) G, FcγRIIA, and the Fc receptor γ-chain (FcR γ-chain). Of particular note is the finding that FcγRIIA is physically associated with GPIb and that it mediates some signaling events.12 13 However, the level of FcγRIIA tyrosine phosphorylation induced by GPIb clustering is much lower than that inducible by FcγRIIA clustering. Further, murine platelets lacking FcγRIIA on their membrane appear to have normal responses to GPIb-related signals. Thus, to what extent FcγRIIA contributes to the GPIb signaling pathway remains to be determined.
The other ITAM-containing protein, FcR γ-chain, has been recognized for its role in signaling related to the high-affinity receptors for IgE (FcεRI)14 and IgG (FcγRI),15 and the low-affinity IgG receptor (FcγRIII).16 In platelets, FcR γ-chain is colocalized with GPVI and is critical for GPVI-mediated platelet activation.17,18 Clustering and activation of GPVI induces tyrosine phosphorylation of FcR γ-chain ITAM,19 which then facilitates the recruitment of Syk, resulting in its activation.20 Moreover, FcR γ-chain is required for tyrosine phosphorylation of phospholipase C γ2 (PLCγ2).20 Recently, Falati et al6 reported that alboaggregin A, by interacting with GPIb, induces tyrosine phosphorylation of FcR γ-chain, PLCγ2 activation, and Ca++ mobilization in platelets. On the basis of their studies, we sought to further define the role of FcR γ-chain in the GPIb-related signal transduction pathway.
Materials and methods
Materials
FcR γ-chain–deficient C57BL/6 mice and control mice were kindly provided by Dr Toshiyuki Takai (Tohoku University, Japan). The following materials were obtained from the indicated suppliers: monoclonal antibodies (MoAbs) against Lyn and Syk (Wako Pure Chemical Industries, Tokyo, Japan); anti-integrin β1 MoAb, antiphosphotyrosine MoAb, PY 20 (Transduction Laboratories, Lexington, KY); antiphosphotyrosine MoAb, 4G10, anti-Src MoAb, anti-FcR γ-chain, anti-Lck, anti-LAT (linker for activation of T cells) polyclonal Abs (Upstate Biotechnology, Lake Placid, NY); anti-Fyn MoAb, polyclonal anti-PLCγ2, anti-Syk, peroxidase-conjugated goat antirabbit IgG Abs (Santa Cruz, CA); control fluorescence-labeled monoclonal rat antimouse antibody (Exalpha Biologicals, Boston, MA); thrombin (Green Cross, Osaka, Japan); collagen (Hormon-Chemie, Munich, Germany); PP1 (Biomol, Plymouth Meeting, PA); Gly-Arg-Gly-Asp-Ser (GRGDS) peptide (Peptide Institute, Osaka, Japan); glutathione-Sepharose 4B and protein A-Sepharose 4B (Pharmacia Biotech, Uppsala, Sweden); peroxidase-conjugated goat antimouse IgG Ab (Cappel, Durham, NC); D-myo-inositol 1,4,5-trisphosphate (IP3) assay kit (Amersham, Buckinghamshire, United Kingdom); bovine serum albumin (BSA), Tween-80, prostaglandin I2, phenylmethylsulfonyl fluoride (PMSF), Na3VO4, Triton X-100, Brij 35 (Sigma, St Louis, MO); and Fura-2 am (Dojindo Laboratories, Kumamoto, Japan).
vWF and botrocetin were purified as described previously.7 21 Nonfunctional anti-GPIb MoAb, WGA3, was provided by Dr M. Handa (Keio University, Tokyo, Japan). Fluorescence-labeled rat antimouse GPIbα (p0p4), GPIX (p0p6), and GPIIb/IIIa (JON1) MoAbs were supplied by Dr Bernhard Nieswandt (University of Witten-Herdecke, Wuppertal, Germany). GlutathioneS-transferase (GST) fusion protein containing the tandem SH2 domains of Syk was obtained from Dr C.-L. Law (University of Washington, Seattle, WA). Jararaca GPIb-binding protein was donated by Dr Y. Fujimura (Nara Medical University, Nara, Japan). Convulxin was a gift from Dr T. Morita (Meiji Pharmaceutical University, Tokyo, Japan).
Preparation and stimulation of human and murine platelets
Murine blood was taken by cardiac puncture immediately after death. Human venous blood was obtained from healthy, drug-free volunteers on the day of the experiment, using acid citrate dextrose (120 mM sodium citrate, 110 mM glucose, 80 mM citric acid) as anticoagulant. Washed platelets were prepared as previously described7 and were suspended in modified HEPES-Tyrode buffer (134 mM NaCl, 0.34 mM Na2HPO4, 2.9 mM KCl, 12 mM NaHCO3, 20 mM HEPES, 5 mM glucose, 1 mM MgCl2, pH 7.3). Unless otherwise stated, murine platelets were resuspended at a count of 2.0 × 108 cells/mL, and human platelets were adjusted to 1.0 × 109 cells/mL. Platelets were stimulated at 37°C under constant stirring at 1000 rpm in an AG-10 aggregation analyzer (Kowa, Tokyo, Japan). In preliminary experiments, we confirmed that human vWF alone does not induce aggregation of washed murine platelets but that aggregation is induced in the copresence of botrocetin. This finding is in agreement with previous reports that GPIb of murine platelets can interact with human vWF.22 23
Platelet aggregation study
Platelet aggregation was monitored by measuring light transmission with the use of an AG-10 aggregation analyzer (Kowa, Tokyo, Japan). The instrument was calibrated with either the washed platelet suspension or platelet-rich plasma (PRP) for zero light transmission and with buffer or platelet-poor plasma (PPP) for 100% transmission, respectively. Aggregation was initiated by addition of agonists under constant stirring at 1000 rpm at 37°C.
Platelet aggregation of mice was measured not only by changes in light transmission but also by a particle-counting technique using light scatter.24 Briefly, a diode laser light beam (width: 40 μm; wavelength: 675 nm) was passed through mouse PRP in a cylindrical glass cuvette maintained at 37°C with constant stirring. The light scatter by particles in a limited volume (33 × 65 × 65 μm) was measured with an optical system that minimized multiple light scatter. The signals were digitized to quantitate the number and size of platelet aggregates. Light-scattering method provides a sensitive, in situ continuous measurement of platelet aggregation by counting the number and size of aggregates. On the basis of the level of scattered light intensity, platelet aggregates can be divided into small aggregates (0.2 to 2.0 V/min, consisting of less than 500 platelets) and large aggregates (2.0 to 10 V/min).24
Immunoprecipitation and GST-fusion protein precipitation
After the indicated time intervals of activation, platelets were solubilized with an equal volume of 2 × ice-cold lysis buffer (100 mM Tris/HCl, pH 7.4, 5.0 mM EGTA, 2.0 mM PMSF, 2.0 mM Na3VO4, 100 μg/mL leupeptin, and 2% Triton X-100) and kept on ice for 30 minutes. All subsequent steps were performed at 4°C. The lysates were centrifuged at 15 000gfor 5 minutes, and the resulting supernatants were precleared by incubation for 30 minutes with protein A-Sepharose for immunoprecipitation experiments or with glutathione-Sepharose for GST-fusion protein precipitation. For immunoprecipitation, the supernatants were then incubated with the appropriate antibodies, followed by the addition of protein A-Sepharose. For GST-fusion protein precipitation, the supernatants were incubated with GST-Syk-SH2 (20 μg/sample), followed by the addition of glutathione-Sepharose beads. The precipitates obtained after centrifugation were washed 3 times in 1 × lysis buffer before the addition of Laemmli sample buffer.
As indicated elsewhere, the precipitates with GST-Syk-SH2 were subjected to re-immunoprecipitation in some experiments. Briefly, the precipitated proteins with GST fusion protein bound to glutathione-Sepharose beads were eluted with Glutathione Elution Buffer (10 mM reduced glutathione in 50 mM Tris/HCl, pH 8.0). The eluates were collected, dialyzed against 50 mM Tris/HCl (pH 8.0) containing 1 mM PMSF, 50 μg/mL leupeptin, and 1 mM Na3VO4, and subjected to immunoprecipitation with the indicated antibody.
Immunoblotting
Precipitated proteins were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto PVDF membranes. The membranes were blocked with 1% BSA in phosphate-buffered saline (PBS). After extensive washing with PBS containing 0.4% Tween-80, the immunoblots were incubated with the appropriate antibodies for 2 hours at room temperature, or overnight at 4°C. Antibody binding was detected by using peroxidase-conjugated secondary antibodies diluted at 1:7500 and was visualized with the enhanced chemiluminescence reaction reagent. For reprobing with other antibodies, the antibody bound to PVDF membranes was removed with a stripping buffer (2% SDS, 62.5 mM Tris/HCl, pH 6.8, 100 μM 2-mercaptoethanol) at 50°C for 20 minutes. After washing, the membranes were blocked with 1% BSA and reprobed with the indicated antibodies. When indicated, the level of proteins as detected by immunoblotting was quantified by using a PDI420oe scanner (PDI, New York, NY).
Flow cytometry
Washed murine platelets (8 × 107 cells/mL) in a volume of 25 μL were incubated with fluorescence-labeled rat antimouse GPIbα (p0p4), antimouse GPIX (p0p6), or antimouse GPIIb/IIIa (JON1) MoAbs at the final concentration of 10 μg/mL for 15 minutes at 37°C, respectively. After dilution with 400 μL PBS, the samples were analyzed immediately on a FACScan (Becton Dickinson, San Jose, CA).
Measurement of IP3 production
Platelets were suspended in HEPES-Tyrode buffer at a count of 3 × 109/mL. After stimulation, an equal volume of ice-cold 15% trichloroacetic acid (TCA) was added to the platelet suspension to terminate reactions, and the mixtures were kept on ice for 30 minutes. The mixtures were centrifuged at 4000g for 15 minutes at 4°C, and the resultant supernatant was treated 5 times with 5 mL water-saturated diethyl ether to extract trichloroacetic acid. Residual ether was further removed under vacuum for 1 hour. The samples were neutralized by titration with 0.2 N NaOH, and the production of IP3 was measured with an IP3assay kit (Amersham) according to the manufacturer's instructions.
Measurement of the intracellular Ca++ concentration
Measurement of intracellular Ca++ concentration ([Ca++]i) was performed with the use of Ca++-sensitive fluorophore, fura2, as described previously.25 The fura2-loaded platelets were adjusted to a count of 2 × 108/mL, and 200 μM EGTA was added to the platelet suspension to inhibit Ca++ influx, as GPIb-mediated platelet activation appears to induce Ca++influx.5 26 After stimulation, changes in [Ca++]i were measured with a Hitachi 2000 fluorescence spectrophotometer (Hitachi, Tokyo, Japan). The [Ca++]i values were determined from the ratio of the fura2 fluorescence intensity at 340 nm excitation wavelength to that at 380 nm.
Statistical analysis
The data are expressed as the mean ± SEM. With the use of Statworks statistical software (Cricket Software, Philadelphia, PA), statistical analysis was done by Student t test withP < .05 taken to indicate significance.
Results
vWF plus botrocetin stimulates tyrosine phosphorylation of FcR γ-chain
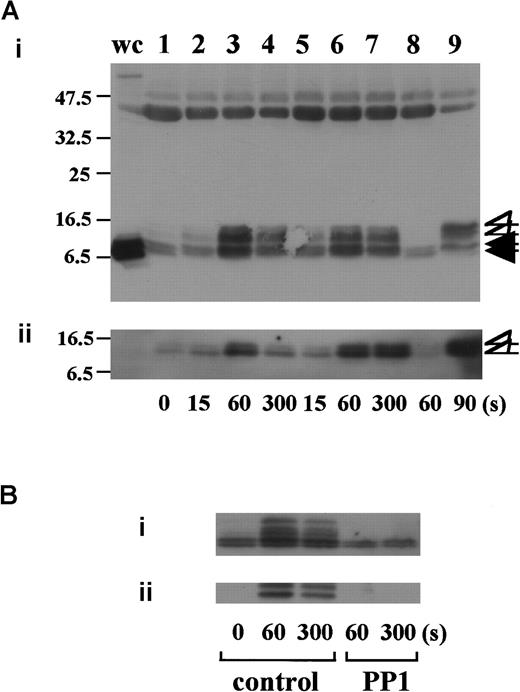
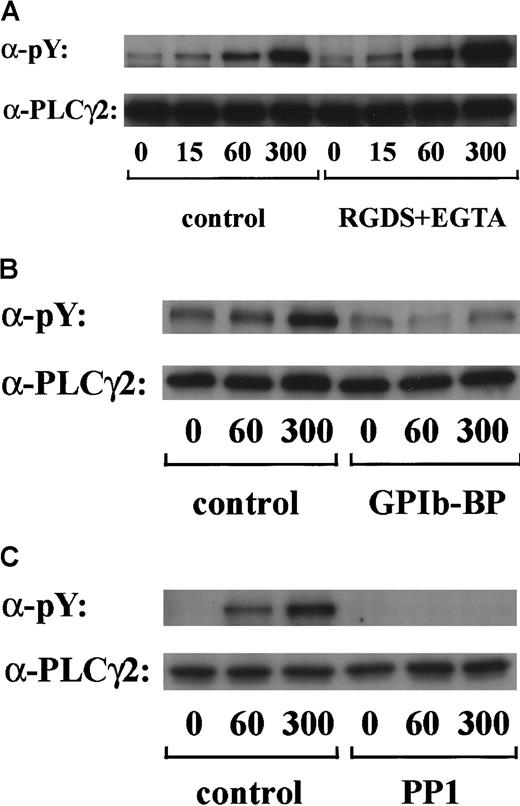
In preliminary experiments, we used 2 commercially available anti-FcR γ-chain polyclonal antibodies to detect tyrosine phosphorylation of FcR γ-chain in GPIb-mediated or collagen-induced platelet activation. However, there was little detectable change, if any. These antibodies appeared to be unsuitable for immunoprecipitation. Thus, we sought another way to address this issue. Human platelets have only 2 ITAM-containing proteins, FcγRIIA and FcR γ-chain. Because the tandem SH2 domains of Syk bind specifically to the ITAM-containing proteins, they are often employed to detect the tyrosine-phosphorylated ITAMs.19,20 Thus, we used a GST-fusion protein expressing the tandem SH2 domains of Syk, GST-Syk-SH2. Human platelets were stimulated with 10 μg/mL vWF plus 6 μg/mL botrocetin, or 50 μg/mL collagen, the subsequent platelet lysates were incubated with GST-Syk-SH2, and proteins precipitated by GST-Syk-SH2 were checked for the presence of FcR γ-chain by using anti-FcR γ-chain polyclonal antibodies (Figure1A). Approximately 4 proteins of 13, 11, 8.5, and 7 kd were detected as FcR γ-chain in platelets stimulated with vWF plus botrocetin [Figure 1Ai, lanes 1-4]. The anti-FcR γ-chain polyclonal antibodies were thus suitable for Western blotting but not for immunoprecipitation. Immunoblotting with antiphosphotyrosine antibodies revealed that the upper 2 bands (13 and 11 kd) were heavily tyrosine-phosphorylated, indicating that tyrosine phosphorylation of FcR γ-chain leads to a mobility shift on SDS-PAGE [Figure 1Aii, lanes 1-4]. Because only polyclonal antibodies against FcR γ-chain are available, whether the lower 2, nonphosphorylated bands correspond to the nonphosphorylated FcR γ-chain awaits elucidation. The pattern of FcR γ-chain tyrosine phosphorylation, including those of nonphosphorylated bands, is similar to that in GPVI-stimulated platelets, observed by Tsuji et al.18Tyrosine phosphorylation of FcR γ-chain reached its peak 60 seconds after stimulation, and partial dephosphorylation occurred at 300 seconds [Figure 1Aii, lanes 1-4]. Precipitates with GST-Syk-SH2 from collagen-activated platelets showed patterns similar to vWF plus botrocetin [Figure 1Ai,ii, lane 9]. It is established that vWF contains not only a binding site for GPIbα, the A1 domain, but also a C1 domain peptide sequence, Arg-Gly-Asp (RGD), which mediates vWF binding to integrin αIIbβ3.27 To rule out the possibility that integrin αIIbβ3 signaling is involved in tyrosine phosphorylation of FcR γ-chain, the platelets were pretreated with RGDS and EGTA and then stimulated with vWF plus botrocetin. As shown in Figure 1Aii, lanes 5-7, blockage of integrin αIIbβ3 by RGDS and EGTA did not inhibit tyrosine phosphorylation of FcR γ-chain. We next used jararaca GPIb-binding protein (GPIb-BP) to confirm that FcR γ-chain tyrosine phosphorylation is mediated by GPIb-vWF interaction. Jararaca GPIb-BP itself induced neither platelet aggregation nor serotonin release from platelets, and it completely inhibited vWF binding to GPIb in the presence of botrocetin or ristocetin.28 In jararaca GPIb-BP–pretreated platelets, tyrosine phosphorylation of FcR γ-chain was abolished [Figure 1Aii, lane 8]. These findings indicate that GPIb stimulation specifically induces tyrosine phosphorylation of FcR γ-chain. Falati et al6 also found that both ristocetin plus normal vWF and ristocetin plus RGGS-vWF, a mutant vWF able to bind to GPIb but not to integrin αIIbβ3, induced both tyrosine phosphorylation of FcR γ-chain and its association with Syk. Our findings are in good accord with their report and confirm that tyrosine phosphorylation of FcR γ-chain indeed takes place in the GPIb-mediated signal transduction pathway. Recently, FcγRIIA, which also contains one ITAM, has been shown to be physically proximal to the GPIb-IX-V complex and functionally related to it.13 In our study, however, tyrosine phosphorylation of FcγRIIA was not detectable in the GST-Syk SH2 precipitates (data not shown).
Stimulation of platelets with vWF plus botrocetin induces tyrosine phosphorylation of FcR γ-chain.
(A) Human platelets were preincubated for 10 minutes at 37°C with a vehicle solution (lanes 1-4, 9), 1 mM RGDS plus 1 mM EGTA (lanes 5-7), or 20 μg/mL jararaca GPIb-BP (lane 8). After stimulation with 6 μg/mL botrocetin plus 10 μg/mL vWF (lanes 1-8) or 50 μg/mL collagen (lane 9) for the indicated time periods, the reactions were terminated by the addition of lysis buffer. The platelet lysates were then precipitated with GST-Syk-SH2. (B) After preincubation with 0.25% dimethyl sulfoxide (DMSO) as control or 10 μM PP1 for 5 minutes at 37°C, the human platelets were activated by the addition of 6 μg/mL botrocetin plus 10 μg/mL vWF for the indicated time frames. The cells were processed for precipitation with GST-Syk-SH2 as described in the legend for A. For A and B, precipitated proteins were then separated by SDS-PAGE (15% polyacrylamide) and immunoblotted with anti-FcR γ-chain (i) and 4G10 plus PY20 (ii). The numbers below the panels show time in seconds. The data are representative of 3 experiments. The open arrowheads indicate the proteins of 13, 11 kd. The closed arrowheads indicate the proteins of 8.5, 7 kd. wc indicates whole cell lysates.
Stimulation of platelets with vWF plus botrocetin induces tyrosine phosphorylation of FcR γ-chain.
(A) Human platelets were preincubated for 10 minutes at 37°C with a vehicle solution (lanes 1-4, 9), 1 mM RGDS plus 1 mM EGTA (lanes 5-7), or 20 μg/mL jararaca GPIb-BP (lane 8). After stimulation with 6 μg/mL botrocetin plus 10 μg/mL vWF (lanes 1-8) or 50 μg/mL collagen (lane 9) for the indicated time periods, the reactions were terminated by the addition of lysis buffer. The platelet lysates were then precipitated with GST-Syk-SH2. (B) After preincubation with 0.25% dimethyl sulfoxide (DMSO) as control or 10 μM PP1 for 5 minutes at 37°C, the human platelets were activated by the addition of 6 μg/mL botrocetin plus 10 μg/mL vWF for the indicated time frames. The cells were processed for precipitation with GST-Syk-SH2 as described in the legend for A. For A and B, precipitated proteins were then separated by SDS-PAGE (15% polyacrylamide) and immunoblotted with anti-FcR γ-chain (i) and 4G10 plus PY20 (ii). The numbers below the panels show time in seconds. The data are representative of 3 experiments. The open arrowheads indicate the proteins of 13, 11 kd. The closed arrowheads indicate the proteins of 8.5, 7 kd. wc indicates whole cell lysates.
Tyrosine phosphorylation of FcR γ-chain, in response to the activation of immune receptors in lymphocytes or of GPVI in platelets, is catalyzed by Src family tyrosine kinases.29,30 Because previous reports suggest the involvement of Src in GPIb-mediated platelet activation,7,8 the platelets were pretreated with a specific Src family kinase inhibitor, PP1, to examine whether FcR γ-chain is also tyrosine-phosphorylated by Src family kinases. PP1 is selective to Src family kinase over Syk by 10 000-fold in both in vitro and functional studies.31,32 As shown in Figure 1B, PP1 at 10 μM completely inhibited tyrosine phosphorylation of FcR γ-chain, suggesting that Src kinase(s) is responsible for FcR γ-chain tyrosine phosphorylation, a finding in agreement with that of Falati et al.6
FcR γ-chain tyrosine-phosphorylated by Src kinases recruits Syk
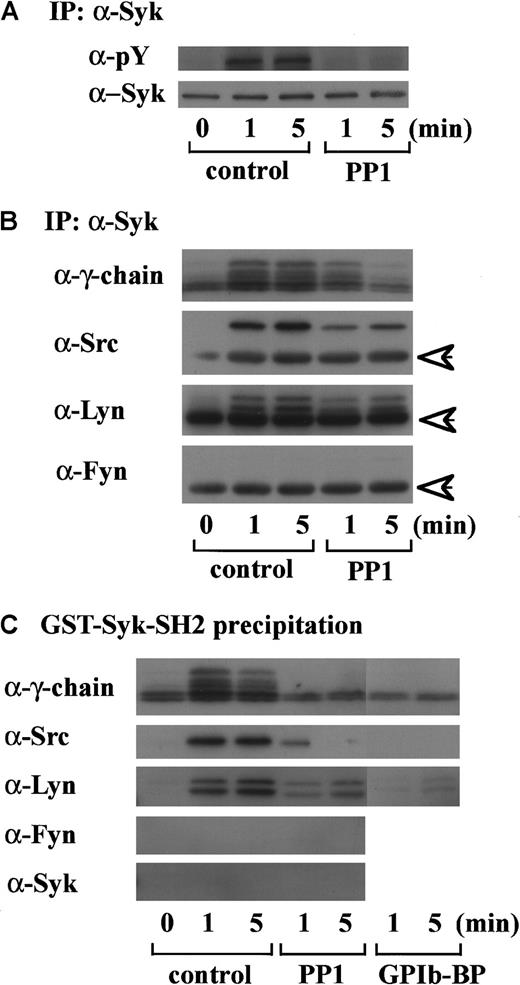
An accumulating body of evidence suggests that tyrosine phosphorylation of FcR γ-chain catalyzed by Src kinases is required for the membrane recruitment and activation of Syk in signal transduction related to immune receptors and GPVI.11,20Because we found in a previous report that GPIb mediates tyrosine phosphorylation of Syk and its association with Src,7 we then asked if Syk locates downstream of Src kinase activation and FcR γ-chain tyrosine phosphorylation in GPIb signaling. As shown in Figure 2A, PP1 pretreatment completely suppressed tyrosine phosphorylation of Syk, suggesting that Src family kinase activation lies upstream of Syk tyrosine phosphorylation. To determine which member(s) of the Src family tyrosine kinases is responsible for Syk activation, the proteins precipitated with anti-Syk MoAb were probed with the antibodies against various Src kinases. As shown in Figure 2B, in platelets stimulated with vWF plus botrocetin, not only Src but also Lyn were found to associate in vivo with Syk, which is also complexed with tyrosine-phosphorylated FcR γ-chain. Fyn and other kinases of the Src family were not detected (Figure 2B and data not shown). To investigate whether Src and Lyn associate with Syk via the SH2 domain of Syk, the platelet lysates were precipitated with GST-Syk-SH2, instead of anti-Syk MoAb. As shown in Figure 2C, the pattern of association of Src/Lyn, FcR γ-chain with GST-Syk-SH2 was consistent with their association with Syk (Figure 2B), and the precipitates were also negative for Fyn. Pretreatment of platelets with jararaca GPIb-BP inhibited association of Src, Lyn, FcR γ-chain with GST-Syk-SH2 (Figure 2C), implying that their association is specifically mediated by GPIb stimulation. Additionally, PP1 pretreatment also inhibited the association of Src/Lyn, FcR γ-chain with Syk or GST-Syk-SH2. This inhibition was not complete at an early time point; the reason remains elusive. In platelets, Src kinases and FcR γ-chain are membrane-associated proteins, and this complex formation may serve to recruit Syk to the membrane, thereby resulting in its tyrosine phosphorylation.
Tyrosine phosphorylation of Syk and its association with FcR γ-chain, Src, and Lyn are dependent on Src kinase activity.
(A) After incubation with 0.25% DMSO as the vehicle control or 10 μM PP1 for 5 minutes at 37°C, human platelets were stimulated with 6 μg/mL botrocetin plus 10 μg/mL vWF for the indicated time periods. The lysates were immunoprecipitated with anti-Syk MoAb. Precipitated proteins were then separated by SDS-PAGE and immunoblotted with 4G10 plus PY20 and anti-Syk MoAb. (B) Precipitated proteins with anti-Syk MoAb obtained in A were separated by SDS-PAGE and analyzed by immunoblotting with anti-FcR γ-chain, anti-Src, anti-Lyn, and anti-Fyn Abs as indicated in the left of each panel. (C) After pretreatment with 0.25% DMSO (control), 10 μM PP1 or 20 μg/mL jararaca-GPIb-BP, human platelets were stimulated with vWF plus botrocetin. The lysates were precipitated with GST-Syk-SH2, and the precipitated proteins were separated by SDS-PAGE and analyzed by immunoblotting with anti-FcR γ-chain, anti-Src, anti-Lyn, anti-Fyn, and anti-Syk Abs. The data are representative of 3 experiments. The arrows represent the band derived from the heavy chains of IgG. pY indicates phosphotyrosine.
Tyrosine phosphorylation of Syk and its association with FcR γ-chain, Src, and Lyn are dependent on Src kinase activity.
(A) After incubation with 0.25% DMSO as the vehicle control or 10 μM PP1 for 5 minutes at 37°C, human platelets were stimulated with 6 μg/mL botrocetin plus 10 μg/mL vWF for the indicated time periods. The lysates were immunoprecipitated with anti-Syk MoAb. Precipitated proteins were then separated by SDS-PAGE and immunoblotted with 4G10 plus PY20 and anti-Syk MoAb. (B) Precipitated proteins with anti-Syk MoAb obtained in A were separated by SDS-PAGE and analyzed by immunoblotting with anti-FcR γ-chain, anti-Src, anti-Lyn, and anti-Fyn Abs as indicated in the left of each panel. (C) After pretreatment with 0.25% DMSO (control), 10 μM PP1 or 20 μg/mL jararaca-GPIb-BP, human platelets were stimulated with vWF plus botrocetin. The lysates were precipitated with GST-Syk-SH2, and the precipitated proteins were separated by SDS-PAGE and analyzed by immunoblotting with anti-FcR γ-chain, anti-Src, anti-Lyn, anti-Fyn, and anti-Syk Abs. The data are representative of 3 experiments. The arrows represent the band derived from the heavy chains of IgG. pY indicates phosphotyrosine.
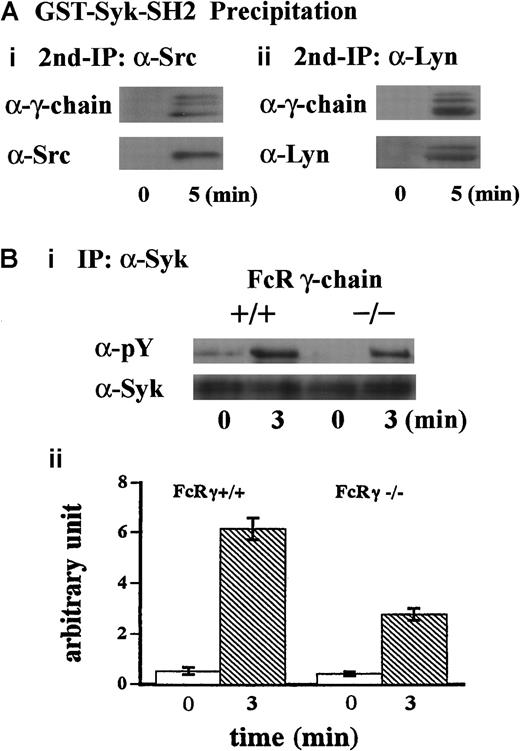
To further clarify the mechanism of this complex formation, the lysates were first precipitated with GST-Syk-SH2. The precipitates with GST fusion proteins were eluted with glutathione, then the resultant eluates were subjected to immunoprecipitation with anti-Src or anti-Lyn MoAb. As shown in Figure 3A, FcR γ-chain was recovered with anti-Src and anti-Lyn immunoprecipitates, suggesting that Src and Lyn bind to FcR γ-chain. Additionally, Syk was not present in GST-Syk-SH2 precipitates (Figure 2C), suggesting that association of Src/Lyn with FcR γ-chain is not mediated by Syk associated with FcR γ-chain. To verify that FcR γ-chain is required for tyrosine phosphorylation of Syk, we performed similar experiments with FcR γ-chain knockout mice. As shown in Figure 3B (i and ii), tyrosine phosphorylation of Syk was reduced by approximately 60% in the FcR γ-chain–deficient platelets. Although FcR γ-chain plays an important role in Syk activation, there may be an additional signal transduction pathway(s) for Syk activation other than FcR γ-chain in GPIb-mediated platelet activation. Collectively, these findings suggest that Src and Lyn first bind to FcR γ-chain or an FcR γ-chain–associated protein and tyrosine-phosphorylate FcR γ-chain and that Syk subsequently joins in this complex.
FcR γ-chain associated with Src and Lyn is required for tyrosine phosphorylation of Syk.
(A) Samples were prepared as described in the legend for Figure 2C. The precipitates with GST-Syk-SH2 from human platelet lysates were eluted with Glutathione Elution Buffer (10 mM glutathione in50 mM Tris/HCl, pH 8.0), and the resultant eluates were subjected to re-immunoprecipitation with anti-Src and anti-Lyn MoAbs, respectively. Precipitated proteins were separated by SDS-PAGE and were analyzed by immunoblotting with anti-FcR γ-chain (i and ii), anti-Src (i), and anti-Lyn (ii) Abs. (Bi) Washed platelets prepared from wild-type C57BL/6 and FcR γ-chain–deficient mice were stimulated with 6 μg/mL botrocetin plus 10 μg/mL vWF for 3 minutes. Syk was immunoprecipitated from the lysates, and precipitates were separated by SDS-PAGE and analyzed with 4G10 plus PY20 and anti-Syk immunoblotting. pY indicates phosphotyrosine. (Bii) Level of Syk tyrosine phosphorylation shown in Bi was quantified by densitometry. Columns and error bars represent means ± SEM (n = 3).
FcR γ-chain associated with Src and Lyn is required for tyrosine phosphorylation of Syk.
(A) Samples were prepared as described in the legend for Figure 2C. The precipitates with GST-Syk-SH2 from human platelet lysates were eluted with Glutathione Elution Buffer (10 mM glutathione in50 mM Tris/HCl, pH 8.0), and the resultant eluates were subjected to re-immunoprecipitation with anti-Src and anti-Lyn MoAbs, respectively. Precipitated proteins were separated by SDS-PAGE and were analyzed by immunoblotting with anti-FcR γ-chain (i and ii), anti-Src (i), and anti-Lyn (ii) Abs. (Bi) Washed platelets prepared from wild-type C57BL/6 and FcR γ-chain–deficient mice were stimulated with 6 μg/mL botrocetin plus 10 μg/mL vWF for 3 minutes. Syk was immunoprecipitated from the lysates, and precipitates were separated by SDS-PAGE and analyzed with 4G10 plus PY20 and anti-Syk immunoblotting. pY indicates phosphotyrosine. (Bii) Level of Syk tyrosine phosphorylation shown in Bi was quantified by densitometry. Columns and error bars represent means ± SEM (n = 3).
Association of FcR γ-chain with GPIb in platelets
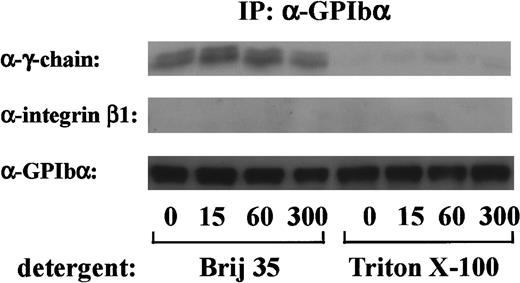
FcR γ-chain, which is tyrosine-phosphorylated on GPVI stimulation, binds constitutively to GPVI as a coreceptor.18 Because tyrosine phosphorylation of FcR γ-chain occurs early in GPIb-mediated platelet activation, it is possible that FcR γ-chain also locates proximal to GPIb. To determine whether there was a constitutive association between FcR γ-chain and GPIb, the GPIb immunoprecipitates obtained from Triton X-100 lysates were immunoblotted with anti-FcR γ-chain antibody. As shown in Figure4, there was little, if any, FcR γ-chain associated with GPIb in Triton X-100 lysates. When a weaker detergent, Brij 35, was used instead of Triton X-100, FcR γ-chain association with GPIb was clearly detected, suggesting that FcR γ-chain is loosely linked with GPIb. In contrast, integrin β1, another integrate transmembrane protein, could not be recovered in any lysates (Figure 4). There was no significant change in the level of FcR γ-chain associated with GPIb, irrespective of stimulation. As described above, tyrosine phosphorylated FcR γ-chain is distinguished from the nonphosphorylated form by its mobility shift. However, no band shift was detectable in GPIb-associated FcR γ-chain. Although our findings hitherto suggest that Src and/or Lyn tyrosine-phosphorylates FcR γ-chain with subsequent binding of Syk, we were unable to detect Src/Lyn or Syk in GPIb immunoprecipitates. This discrepancy needs to be addressed in the future.
FcR γ-chain is associated with GPIb.
After stimulation with 6 μg/mL botrocetin plus 10 μg/mL vWF for the indicated time periods, human platelets were solubilized with an equal volume of lysis buffer containing 2% Brij 35 (vol/vol) or 2% Triton X-100 (vol/vol). GPIb was immunoprecipitated with WGA3, an anti-GPIb MoAb. Precipitated proteins were separated by SDS-PAGE and were analyzed by anti-FcR γ-chain, anti-integrin β1, and anti-GPIb immunoblotting, respectively. The data are representative of 4 experiments. The numbers below the panels show time in seconds.
FcR γ-chain is associated with GPIb.
After stimulation with 6 μg/mL botrocetin plus 10 μg/mL vWF for the indicated time periods, human platelets were solubilized with an equal volume of lysis buffer containing 2% Brij 35 (vol/vol) or 2% Triton X-100 (vol/vol). GPIb was immunoprecipitated with WGA3, an anti-GPIb MoAb. Precipitated proteins were separated by SDS-PAGE and were analyzed by anti-FcR γ-chain, anti-integrin β1, and anti-GPIb immunoblotting, respectively. The data are representative of 4 experiments. The numbers below the panels show time in seconds.
Tyrosine phosphorylation of PLCγ2 by botrocetin plus vWF is sensitive to PP1 pretreatment and independent of integrin αIIbβ3 signaling
PLCγ2 is the major PLCγ isoform expressed in platelets33 and is the only type reported to undergo tyrosine phosphorylation on stimulation.34 Tyrosine phosphorylation of PLCγ2 has been recently reported to occur in response to alboaggregin A, which presumably interacts with GPIb.6 We confirmed in this study that stimulation of platelets by vWF plus botrocetin caused marked tyrosine phosphorylation of PLCγ2 in a time-dependent manner. Densitometric analysis revealed that the level of PLCγ2 tyrosine phosphorylation increased 2-fold at 15 seconds and reached a maximum level at 300 seconds (Figure5A and data not shown). As shown in Figure 5B, tyrosine phosphorylation of PLCγ2 was markedly inhibited by jararaca GPIb-BP pretreatment. Moreover, the pretreatment of platelets with RGDS and EGTA did not suppress the level of PLCγ2 tyrosine phosphorylation (Figure 5A). These findings suggest that tyrosine phosphorylation of PLCγ2 induced by vWF plus botrocetin is specifically mediated by GPIb and is not dependent on integrin αIIbβ3 signaling. Tyrosine phosphorylation of PLCγ2 induced by vWF plus botrocetin was completely inhibited by PP1 (Figure 5C), suggesting the contribution of Src family kinases.
GPIb-vWF interaction induces tyrosine phosphorylation of PLCγ2, independently of integrin αIIbβ3 signaling.
Human platelets were preincubated with a vehicle solution as the control (A-C, left panels), 1 mM RGDS peptide plus 1 mM EGTA (A, right panel), 20 μg/mL jararaca GPIb-BP (B, right panel), or 10 μM PP1 (C, right panel) for 5 minutes and then stimulated with 6 μg/mL botrocetin plus 10 μg/mL vWF for the indicated time periods. After cells were solubilized, PLCγ2 was isolated by immunoprecipitation with the corresponding Ab. Precipitated proteins were then separated by SDS-PAGE and analyzed by immunoblotting with 4G10 plus PY20. The membranes were reprobed by immunoblotting with anti-PLCγ2. The data are representative of 3 experiments. The numbers below the panels show time in seconds. pY indicates phosphotyrosine.
GPIb-vWF interaction induces tyrosine phosphorylation of PLCγ2, independently of integrin αIIbβ3 signaling.
Human platelets were preincubated with a vehicle solution as the control (A-C, left panels), 1 mM RGDS peptide plus 1 mM EGTA (A, right panel), 20 μg/mL jararaca GPIb-BP (B, right panel), or 10 μM PP1 (C, right panel) for 5 minutes and then stimulated with 6 μg/mL botrocetin plus 10 μg/mL vWF for the indicated time periods. After cells were solubilized, PLCγ2 was isolated by immunoprecipitation with the corresponding Ab. Precipitated proteins were then separated by SDS-PAGE and analyzed by immunoblotting with 4G10 plus PY20. The membranes were reprobed by immunoblotting with anti-PLCγ2. The data are representative of 3 experiments. The numbers below the panels show time in seconds. pY indicates phosphotyrosine.
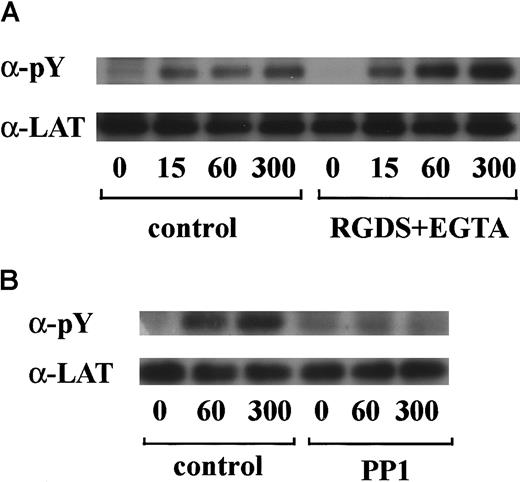
vWF plus botrocetin stimulates tyrosine phosphorylation of LAT
LAT is a 36- to 38-kd transmembrane protein that is found in glycolipid-enriched membrane domains.35 Recent studies have shown that LAT plays an essential role in regulation of PLCγ2 following stimulation of GPVI in platelets.36 Hence, we asked whether LAT is also involved in the GPIb-mediated signal transduction pathway. As shown in Figure6A, stimulation with vWF plus botrocetin induced tyrosine phosphorylation of LAT in a time-dependent manner. Densitometric analysis revealed that tyrosine phosphorylation of LAT increased rapidly by 7-fold as early as 15 seconds after stimulation. Because pretreatment with RGDS and EGTA failed to inhibit phosphorylation of LAT (Figure 6A), it is suggested that integrin αIIbβ3 signaling is not involved. The potent inhibition of LAT tyrosine phosphorylation by PP1 suggests that this is also downstream of Src family kinase activation (Figure 6B).
LAT is tyrosine-phosphorylated in GPIb-mediated platelet activation.
Human platelets were preincubated with a vehicle solution as the control (A and B, left panels), 1 mM RGDS peptide plus 1 mM EGTA (A, right panel), or 10 μM PP1 (B, right panel) for 5 minutes and then activated with 6 μg/mL botrocetin plus 10 μg/mL vWF for the indicated time periods. After cell lysis, LAT was isolated by immunoprecipitation with the corresponding Ab. Precipitated proteins were then separated by SDS-PAGE and analyzed by immunoblotting with 4G10 plus PY20. The membranes were reprobed for anti-LAT by immunoblotting. The data are representative of 3 experiments. The numbers below the panels show time in seconds. pY indicates phosphotyrosine.
LAT is tyrosine-phosphorylated in GPIb-mediated platelet activation.
Human platelets were preincubated with a vehicle solution as the control (A and B, left panels), 1 mM RGDS peptide plus 1 mM EGTA (A, right panel), or 10 μM PP1 (B, right panel) for 5 minutes and then activated with 6 μg/mL botrocetin plus 10 μg/mL vWF for the indicated time periods. After cell lysis, LAT was isolated by immunoprecipitation with the corresponding Ab. Precipitated proteins were then separated by SDS-PAGE and analyzed by immunoblotting with 4G10 plus PY20. The membranes were reprobed for anti-LAT by immunoblotting. The data are representative of 3 experiments. The numbers below the panels show time in seconds. pY indicates phosphotyrosine.
Stimulation with vWF plus botrocetin fails to induce IP3 production and calcium release
In platelets, stimulation with collagen or FcγRIIA cross-linking induces activation of PLCγ2, which leads to IP3production and intracellular calcium mobilization.37 38Because vWF plus botrocetin also stimulates tyrosine phosphorylation of PLCγ2, we then asked whether PLCγ2 is activated in GPIb-mediated platelet activation. Activation of PLCγ2 can be monitored by measurement of IP3 production and intracellular Ca++ release. As shown in Figure7A, although collagen induced a rapid and prominent increase in IP3, platelet stimulation with vWF plus botrocetin failed to induce IP3 production. In addition, vWF plus botrocetin did not induce intracellular calcium release, whereas collagen was able to induce calcium release as early as 20 seconds after stimulation (Figure 7B), which is in good correlation with the time course of IP3 production. These findings suggest that PLCγ2 is not activated in vWF/botrocetin GPIb-mediated platelet activation in vitro despite its tyrosine phosphorylation.
IP3 production and calcium release are not observed in platelets stimulated with vWF plus botrocetin.
(A) IP3: after human platelets were stimulated with 75 μg/mL collagen (▪) or 6 μg/mL botrocetin plus 10 μg/mL vWF (▧) for the indicated time periods, the production of IP3was measured as described in “Materials and methods.” The data are expressed as means ± SEM of 3 separate experiments. (B) Ca++: stimulation of human platelets with 75 μg/mL collagen (i) or 6 μg/mL botrocetin plus 10 μg/mL vWF (ii) was initiated at the time indicated by the arrow. The [Ca++]i elevation in the absence of extracellular calcium was monitored as changes in fura2 fluorescence for 300 seconds. The ordinate represents the ratio of fura2 fluorescence. The results are representative of 3 experiments.
IP3 production and calcium release are not observed in platelets stimulated with vWF plus botrocetin.
(A) IP3: after human platelets were stimulated with 75 μg/mL collagen (▪) or 6 μg/mL botrocetin plus 10 μg/mL vWF (▧) for the indicated time periods, the production of IP3was measured as described in “Materials and methods.” The data are expressed as means ± SEM of 3 separate experiments. (B) Ca++: stimulation of human platelets with 75 μg/mL collagen (i) or 6 μg/mL botrocetin plus 10 μg/mL vWF (ii) was initiated at the time indicated by the arrow. The [Ca++]i elevation in the absence of extracellular calcium was monitored as changes in fura2 fluorescence for 300 seconds. The ordinate represents the ratio of fura2 fluorescence. The results are representative of 3 experiments.
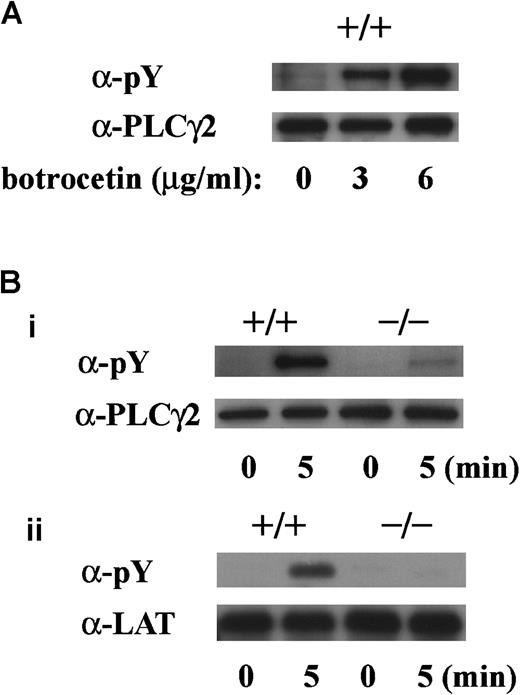
GPIb-mediated tyrosine phosphorylation of PLCγ2 and LAT is absent in murine platelets lacking FcR γ-chain
To determine whether tyrosine phosphorylation of PLCγ2 and LAT is dependent on FcR γ-chain, we performed experiments with platelets obtained from FcR γ-chain knockout mice. The level of tyrosine phosphorylation of PLCγ2 by vWF plus botrocetin stimulation was dependent on the botrocetin dose in platelets of wild-type C57BL/6 mice (Figure 8A). In platelets lacking FcR γ-chain, tyrosine phosphorylation of PLCγ2 on botrocetin plus vWF stimulation was reduced by approximately 80%, and tyrosine phosphorylation of LAT was virtually absent (Figure 8Bi,ii).
GPIb-stimulated tyrosine phosphorylation of LAT and PLCγ2 are absent in platelets lacking FcR γ-chain.
(A) Washed platelets from wild-type C57BL/6 mice were stimulated with 10 μg/mL vWF plus 0, 3, or 6 μg/mL botrocetin for 5 minutes. After lysis, platelets were immunoprecipitated with anti-PLCγ2 Ab. (B) Washed platelets from wild-type C57BL/6 and FcR γ-chain–deficient mice were stimulated with 6 μg/mL botrocetin plus 10 μg/mL vWF for the indicated time periods. After lysis, PLCγ2 (i) and LAT (ii) were immunoprecipitated with the corresponding Ab, respectively. The precipitated proteins in A and B were separated by SDS-PAGE and were analyzed by 4G10 plus PY20 immunoblotting. The loaded proteins were confirmed to be at comparable levels by immunoblotting with the corresponding Ab. The data are representative of 3 experiments. pY indicates phosphotyrosine.
GPIb-stimulated tyrosine phosphorylation of LAT and PLCγ2 are absent in platelets lacking FcR γ-chain.
(A) Washed platelets from wild-type C57BL/6 mice were stimulated with 10 μg/mL vWF plus 0, 3, or 6 μg/mL botrocetin for 5 minutes. After lysis, platelets were immunoprecipitated with anti-PLCγ2 Ab. (B) Washed platelets from wild-type C57BL/6 and FcR γ-chain–deficient mice were stimulated with 6 μg/mL botrocetin plus 10 μg/mL vWF for the indicated time periods. After lysis, PLCγ2 (i) and LAT (ii) were immunoprecipitated with the corresponding Ab, respectively. The precipitated proteins in A and B were separated by SDS-PAGE and were analyzed by 4G10 plus PY20 immunoblotting. The loaded proteins were confirmed to be at comparable levels by immunoblotting with the corresponding Ab. The data are representative of 3 experiments. pY indicates phosphotyrosine.
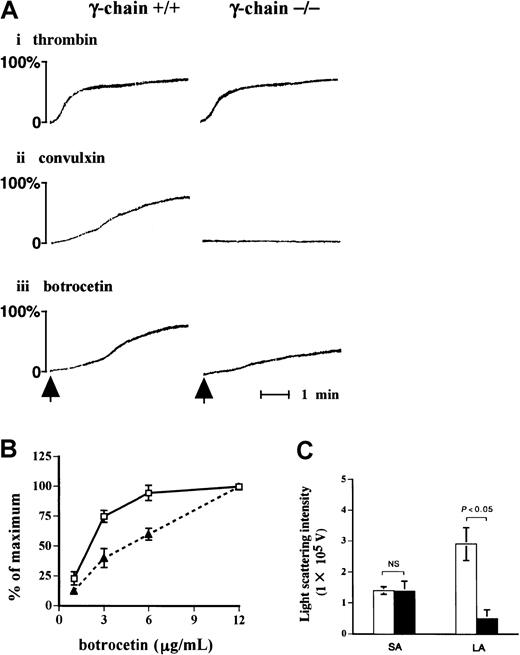
Impaired large aggregate formation of FcR γ-chain–deficient platelets
Because the findings hitherto point to an important role of FcR γ-chain in the signal transduction pathway in GPIb-mediated platelet activation, we evaluated the functional role of FcR γ-chain in GPIb signaling. As detected by the light-transmission method (Figure9A), platelets lacking FcR γ-chain failed to respond to convulxin, but they aggregated normally on thrombin stimulation as was the case with the platelets from wild-type C57BL/6 mice. Platelet aggregation in response to vWF plus botrocetin with FcR γ-chain–deficient mice was partially impaired compared with that of wild-type mice (Figure 9A,B). Platelet aggregation was further evaluated with a particle-counting method using light scattering. This method is unique in that it distinguishes the size of aggregates, based on the intensity of light scattering in PRP. Although the formation of small aggregates with FcR γ-chain–deficient mice was detected at a level comparable to that of the control, the formation of large aggregates was markedly reduced in FcR γ-chain–deficient platelets (Figure 9C).
Formation of large aggregates is suppressed in platelets from FcR γ-chain–deficient mice.
(A) Platelets from wild-type or FcR γ-chain–deficient mice were challenged with 1 U/mL thrombin (i), 10 ng/mL convulxin (ii), or 6 μg/mL botrocetin (iii), respectively. In the case of thrombin, washed platelets were used instead of platelet-rich plasma (PRP). The aggregation was evaluated for 5 minutes by the light transmission method. The arrows show the time points for the addition of the reagents. (B) PRPs from wild-type (■) or FcR γ-chain–deficient mice (▴) were stimulated with increasing amounts of botrocetin for 5 minutes. The maximal light transmission of platelet aggregation induced by 12 μg/mL was set as 100%. The ordinate represents the percentage maximal magnitude of platelet aggregation induced by various concentrations of botrocetin. The data are expressed as means ± SEM (n = 3). (C) The formation of small and large aggregates was assessed by a light-scattering method, as described in “Materials and methods.” ■, platelet aggregate formation from wild-type mice (n = 7); ▪, FcR γ-chain–deficient mice (n = 5). The data are expressed as means ± SEM. NS indicates not significant; SA, small aggregates; LA, large aggregates.
Formation of large aggregates is suppressed in platelets from FcR γ-chain–deficient mice.
(A) Platelets from wild-type or FcR γ-chain–deficient mice were challenged with 1 U/mL thrombin (i), 10 ng/mL convulxin (ii), or 6 μg/mL botrocetin (iii), respectively. In the case of thrombin, washed platelets were used instead of platelet-rich plasma (PRP). The aggregation was evaluated for 5 minutes by the light transmission method. The arrows show the time points for the addition of the reagents. (B) PRPs from wild-type (■) or FcR γ-chain–deficient mice (▴) were stimulated with increasing amounts of botrocetin for 5 minutes. The maximal light transmission of platelet aggregation induced by 12 μg/mL was set as 100%. The ordinate represents the percentage maximal magnitude of platelet aggregation induced by various concentrations of botrocetin. The data are expressed as means ± SEM (n = 3). (C) The formation of small and large aggregates was assessed by a light-scattering method, as described in “Materials and methods.” ■, platelet aggregate formation from wild-type mice (n = 7); ▪, FcR γ-chain–deficient mice (n = 5). The data are expressed as means ± SEM. NS indicates not significant; SA, small aggregates; LA, large aggregates.
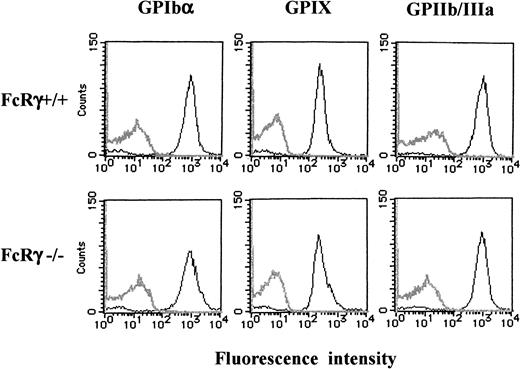
GPIb expression in wild-type and FcR γ-chain–deficient platelets
Because FcR γ-chain is coexpressed with many receptors, we then asked whether it is also required for the expression of GPIb-IX complex. As shown in Figure 10, flow cytometric studies indicated that the expression of GPIb, IX, or GPIIb/IIIa in FcR γ-chain–deficient platelets was comparable to those of the wild-type.
Comparable platelet GPIb-IX expression between FcR γ-chain–deficient and wild-type mice.
Platelets from wild-type (upper panels) or FcR γ-chain–deficient (lower panels) mice were stained with phycoerythrin-labeled p0p4 (α-mouse GPIbα), fluorescein isothiocyanate–labeled p0p6 (α-mouse GPIX), or phycoerythrin-labeled JON1 (α-mouse GPIIb/IIIa), respectively. The fluorescence was analyzed by flow cytometry (solid lines). Gray lines represent the control, the fluorescence of rat α-mouse Ab. The results shown are representative of 4 experiments.
Comparable platelet GPIb-IX expression between FcR γ-chain–deficient and wild-type mice.
Platelets from wild-type (upper panels) or FcR γ-chain–deficient (lower panels) mice were stained with phycoerythrin-labeled p0p4 (α-mouse GPIbα), fluorescein isothiocyanate–labeled p0p6 (α-mouse GPIX), or phycoerythrin-labeled JON1 (α-mouse GPIIb/IIIa), respectively. The fluorescence was analyzed by flow cytometry (solid lines). Gray lines represent the control, the fluorescence of rat α-mouse Ab. The results shown are representative of 4 experiments.
Discussion
The FcR γ-chain constitutes an integral part of several Fc receptors and is coexpressed with GPVI, forming a platelet collagen receptor.18 On stimulation of this complex, Lyn and Fyn, Src family kinases, are activated and phosphorylate the ITAM of FcR γ-chain.29,30 Subsequently, Syk binds to the tyrosine-phosphorylated FcR γ-chain and sends downstream signals, leading to PLCγ2 activation.20 Recently, Falati et al6 reported that the GPIb-mediated activation signal, elicited by alboaggregin A, induces tyrosine phosphorylation of FcR γ-chain, a complex formation between Lyn, Fyn, FcR γ-chain, and Syk, as well as activation of PLCγ2 with resultant Ca++release. Their findings imply that the GPIb-mediated signal transduction pathway has several features in common with that of the GPVI collagen receptor. In this report, we sought to define changes in FcR γ-chain–related signaling molecules in response to GPIb-vWF interaction.
By using GST-Syk-SH2, we confirmed that vWF plus botrocetin specifically stimulates tyrosine phosphorylation of FcR γ-chain, an event completely blocked by jararaca GPIb-BP, a specific GPIb inhibitor. Tyrosine phosphorylation of FcR γ-chain was also independent of integrin αIIbβ3. Our findings confirm those of Falati et al6 and suggest that the activation signal mediated by GPIb induces FcR γ-chain tyrosine phosphorylation. Furthermore, we showed that Src/Lyn and FcR γ-chain associate with Syk by its tandem SH2 domains, suggesting that a complex of Src/Lyn, FcR γ-chain, and Syk is formed in vivo on GPIb stimulation. This complex formation is specifically mediated by GPIb, as jararaca GPIb-BP significantly abrogated its formation. In the present study, we also found that PP1 significantly inhibits the association of Src/Lyn, FcR γ-chain with Syk, as well as Syk phosphorylation. This finding suggests that the complex formation is dependent of Src kinase activity and that Syk is downstream of activated Src kinase(s). However, the incomplete inhibition by PP1 implies that there may be an additional mechanism of the complex formation, distinct for Src kinase activation. By re-immunoprecipitation experiments, we demonstrated that Src and Lyn either bind directly to FcR γ-chain or to an FcR γ-chain–associated protein. On the basis of these findings, it is most likely that Src/Lyn first phosphorylates the tyrosine residues of FcR γ-chain, which then results in Syk association via its tandem SH2 domains, thereby inducing autophosphorylation of Syk. However, our findings are not in accord with the previous report of Falati et al6 who showed that Lyn, Fyn, FcR γ-chain, and Syk form a complex in alboaggregin-A–stimulated platelets. We could not detect the presence of Fyn in the complex, whereas they apparently could not detect Src. Although an anti-GPIb MoAb blocked alboaggregin A-induced platelet aggregation, those researchers did not show any effects of specific GPIb blockade on complex formation.6 It, therefore, remains possible that alboaggregin A interacts with membrane molecules other than GPIb, inducing the association between FcR γ-chain and Lyn/Fyn. Indeed, the mode of complex formation reported by them is quite reminiscent of that mediated by GPVI, the collagen receptor.11
In the present study, we show that phosphorylation of Syk is significantly suppressed in platelets of FcR γ-chain–deficient mice, suggesting that FcR γ-chain is located upstream of Syk and is required for phosphorylation of Syk in GPIb-mediated platelet activation, similar to that of GPVI. However, the incomplete suppression of Syk tyrosine phosphorylation (approximately 60%) in the absence of FcR γ-chain suggests the existence of an FcR γ-chain–independent pathway for Syk activation. This situation may be compatible with the fact that mice lacking Syk suffer severe hemorrhage during fetal development,39 40 whereas FcR γ-chain–deficient mice present no hemorrhagic diathesis. In contrast with incomplete suppression of Syk phosphorylation in FcR γ-chain–deficient platelets, tyrosine phosphorylation of LAT, known as a substrate of Syk, is completely abrogated, suggesting that LAT phosphorylation is more strictly dependent on FcR γ-chain. It is, therefore, possible that FcR γ-chain–bound Syk is responsible for tyrosine phosphorylation of LAT, whereas FcR γ-chain–independent Syk activation exists in GPIb-mediated platelet activation.
The precise mechanism by which the GPIb-vWF interaction activates Src/Lyn with resultant FcR γ-chain tyrosine phosphorylation is still elusive. In a previous report, we found that GPIb-vWF interaction induces an increase in GPIb-associated tyrosine kinase activity.7 However, we were unable to identify the kinase responsible for this increase at that time. In this study, we found that FcR γ-chain coprecipitated with GPIb in Brij 35 lysates but not in Triton X-100 lysates. Thus, FcR γ-chain appears to be constitutively but loosely associated with GPIb. However, we could neither detect tyrosine-phosphorylated FcR γ-chain associated with GPIb nor Src family kinases associated with GPIb. It is possible that a portion of FcR γ-chain undergoes tyrosine phosphorylation and that the phosphorylated form rapidly dissociates from GPIb. In collagen-stimulated platelets, tyrosine phosphorylated FcR γ-chain does not detectably coprecipitate with GPVI, suggesting that FcR γ-chain after being phosphorylated dissociates from GPVI.18 Further investigation is needed to address this issue.
Falati et al6 reported that alboaggregin A, a putative GPIb activator, induces PLCγ2 tyrosine phosphorylation in platelets. In the present study, we have confirmed that GPIb stimulation by vWF plus botrocetin is able to induce tyrosine phosphorylation of PLCγ2. In hematopoietic cells, including platelets, LAT, a transmembrane protein, and PLCγ2 have been shown to be downstream of Syk and FcR γ-chain.11,35,36 Stimulation of platelets with collagen induces LAT tyrosine phosphorylation,36 and tyrosine-phosphorylated LAT serves as a docking site to recruit PI 3-K to the membrane.41 These events are required for tyrosine phosphorylation and activation of PLCγ2.36 In the present study, we found that LAT is also tyrosine-phosphorylated by vWF plus botrocetin. Because PP1 completely blocks tyrosine phosphorylation of LAT and PLCγ2, it is suggested that these events are also catalyzed by a Src family kinase, as with FcR γ-chain and Syk. In platelets lacking FcR γ-chain, LAT and PLCγ2 tyrosine phosphorylation are significantly suppressed, indicating that FcR γ-chain plays a critical role in these signaling events mediated by GPIb-vWF interaction. Whereas tyrosine phosphorylation of LAT was entirely absent in FcR γ-chain–deficient platelets, PLCγ2 tyrosine phosphorylation still partially occurred on GPIb stimulation, suggesting the existence of an alternative pathway for PLCγ2 tyrosine phosphorylation. A previous report suggested that PLCγ2 becomes activated and induces calcium release in platelets activated by alboaggregin A and that these phenomena are mediated by GPIb.6 However, we found in this study that tyrosine phosphorylation of PLCγ2 induced by the GPIb-vWF interaction is not sufficient for its activation, because there was no IP3production and subsequent calcium release. Although platelet aggregation induced by alboaggregin A was blocked by an anti-GPIb MoAb, there was no corresponding blocking experiment on Ca++release.6 Thus, it cannot be entirely ruled out that alboaggregin A induces Ca++ release by binding to some membrane molecule(s) other than GPIb. There has been an accumulating body of evidence to suggest that, in addition to its tyrosine phosphorylation, activation of PLCγ2 requires additional factors, such as PLCγ2 translocation to the membrane,42 the production of PI(3,4,5)P3 and phosphatidic acid, or the presence of AHNAK (a Hebrew term for a giant, presumably required for PLCγ activation in presence of arachidonic acid).43-46Further studies are necessary to understand what is specifically lacking to allow PLCγ2 activation in GPIb stimulation.
It is, therefore, reasonable to ask whether the FcR γ-chain–related signaling molecules, Syk, LAT, and PLCγ2, play any role at all in GPIb-mediated platelet activation. There is an increasing body of evidence indicating that tyrosine-phosphorylated FcR γ-chain and its downstream signal, LAT, play an important role in cell activation.36,47 They facilitate the membrane recruitment of multiple signaling molecules, including Grb2, Grb2-related adaptor downstream of Shc, the p85 subunit of PI 3-K, Vav, SLP-76, and others.48-50 Of particular interest is that LAT can also serve as a docking site to recruit PI 3-K to the membrane,41 the function of which appears to be important in GPIb-mediated platelet activation.8 51 This issue needs to be addressed in the future. We then sought to determine whether there is any functional impairment in platelets deficient in FcR γ-chain. In response to a maximal amount of botrocetin, 12 μg/mL, there was no difference in the magnitude of aggregation between control and FcR γ-chain–deficient platelets. However, at lower doses of botrocetin, there was a slight, but distinct, difference in the magnitude of aggregation. By using a light-scattering method, we found that the formation of large aggregates is selectively impaired in FcR γ-chain–deficient mice. The factors that determine the size of platelet aggregates remain elusive. However, it is conceivable that FcR γ-chain–related signals consolidate GPIb-mediated aggregate formation. Because flow cytometric analysis demonstrated that the expression of GPIb-IX complex is not distinguishable between the wild-type and FcR γ-chain–deficient platelets, it is most likely that there is certain functional impairment with FcR γ-chain–deficient platelets.
In conclusion, the present study has demonstrated that FcR γ-chain, which is constitutively associated with GPIb, is critical for the signal transduction of activated Src kinases and downstream tyrosine phosphorylation of Syk, LAT, and PLCγ2 in GPIb-stimulated platelets. In platelets lacking FcR γ-chain, large aggregate formation is significantly impaired, suggesting that FcR γ-chain is required for the full activation of platelets mediated by GPIb.
We are grateful to Dr T. Takai for providing FcR γ-chain–knockout and wild-type mice; Prof Y. Fujimura for kindly supplying jararaca- GPIb–binding protein; Dr M. Handa for the generous donation of anti-GPIb MoAb, WGA3; Dr B. Nieswandt for providing antimouse GPIb, GPIX, and GPIIb/IIIa MoAbs; Dr C.-L. Law for the kind gift of GST-Syk-SH2; and Prof T. Morita for providing convulxin.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Yukio Ozaki, Department of Clinical and Laboratory Medicine, Yamanashi Medical University, 1110 Shimokatoh, Tamaho, Nakakoma, Yamanashi 409-3898, Japan; e-mail:yozaki@res.yamanashi-med.ac.jp.

![Fig. 7. IP3 production and calcium release are not observed in platelets stimulated with vWF plus botrocetin. / (A) IP3: after human platelets were stimulated with 75 μg/mL collagen (▪) or 6 μg/mL botrocetin plus 10 μg/mL vWF (▧) for the indicated time periods, the production of IP3was measured as described in “Materials and methods.” The data are expressed as means ± SEM of 3 separate experiments. (B) Ca++: stimulation of human platelets with 75 μg/mL collagen (i) or 6 μg/mL botrocetin plus 10 μg/mL vWF (ii) was initiated at the time indicated by the arrow. The [Ca++]i elevation in the absence of extracellular calcium was monitored as changes in fura2 fluorescence for 300 seconds. The ordinate represents the ratio of fura2 fluorescence. The results are representative of 3 experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/97/12/10.1182_blood.v97.12.3836/6/m_h81211191007.jpeg?Expires=1769083496&Signature=KmDm3yN4Mq2vzvgjUtfCA2YRgJXD40~Aou4Z9HeGoOWirnTqdhCfKyaE-DeidROzRSk0GN3YxscuRrS37cRilKgn9WTc8baR44bVLp9rUOkkFg4g6I-Vv3rIvVFzTrSLmAawMKjnFor~QQr8mO5mCYb8T3m7xc4gxwLv~zrcq5ir00XoQaeuSXBD4cyI6LeL8Eyvb0rVxFHYLW7cB1D9IJQeFGgmhIsVrMXwE6tplYOQetE3TgvxU7PflZm9JgLp0QmnY00gT6g6c9q2FSeD174vlDvpyv8SIUuZ3K9S55xi7SIGRMlI~j7eBAE~Ns6SynTNn9AiRI4MCAfMJiQd-g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal