In 1992, we reported the results of the first randomized trial comparing the combination of cyclophosphamide (CY) (120 mg/kg) and total body irradiation (TBI) (CYTBI) versus CY and busulfan (BU) (BUCY) as preparation for an allogeneic bone marrow transplantation (BMT) for adult patients with acute myeloblastic leukemia (AML) in first complete remission (CR1).1 With a follow-up of 23 ± 11 months, this analysis found that the BUCY regimen was associated with a significant lower overall survival and long-term leukemia-free survival (LFS) due to higher transplantation mortality and relapse rates. Since our initial publication, there have been several other reports prospectively addressing the same question but concluding that BUCY and CYTBI had similar efficacy.2-4 On this basis, it appears to be the general opinion that BUCY and CYTBI are broadly equivalent, a conclusion that our data did not support and that prompted us to update the results of our randomized trial now with a longer follow-up.
For the purposes of this update, the randomization allocation was checked and 1 patient originally reported randomized to CYTBI in fact received BUCY. As this represents a major violation of the protocol, the patient was excluded from the present analysis. He relapsed at 5 years and died 2 years later. Thus 49 patients in the CYTBI group and 51 patients in the BUCY group were part of this analysis. Patient and early transplantation characteristics have already been reported.1 No difference in acute graft-versus-host disease (a-GVHD) or chronic GVHD (c-GVHD) incidence was found between the 2 groups, though GVHD-related mortality was higher in the BUCY group (P < .05) (Table1). Finally, 23 patients died from nonleukemic death (NLD) at a median of 4 months (range, 1-112 months) after transplantation, with no statistically different cumulative incidences (CIs) (18% vs 27%; P = .21) in the 2 groups.
Kaplan-Meier estimate of survival and cumulative incidence of leukemia recurrence.
Kaplan-Meier estimates (P = .04) are represented by the top 2 curves; cumulative incidences (P = .06), by the bottom 2 curves; BUCY estimates, by the dotted lines; CYTBI estimates, by the solid lines.
Kaplan-Meier estimate of survival and cumulative incidence of leukemia recurrence.
Kaplan-Meier estimates (P = .04) are represented by the top 2 curves; cumulative incidences (P = .06), by the bottom 2 curves; BUCY estimates, by the dotted lines; CYTBI estimates, by the solid lines.
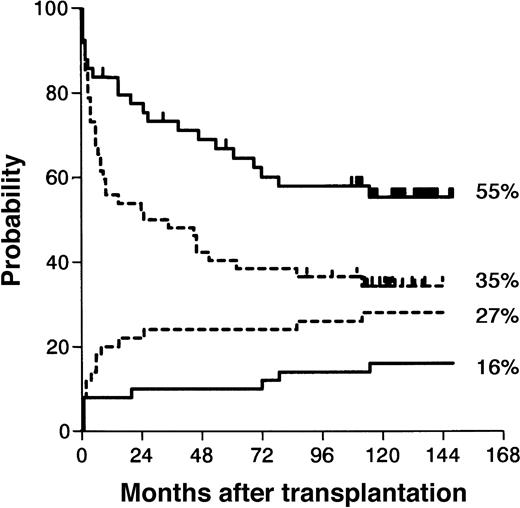
Kaplan-Meier estimate of leukemia-free survival and cumulative incidence of transplant related deaths.
Kaplan-Meier estimates (P = .02) are represented by the top 2 curves); cumulative incidences (P = .21) by the bottom 2curves; BUCY estimates, by the dotted lines; CYTBI estimates, by the solid lines.
Kaplan-Meier estimate of leukemia-free survival and cumulative incidence of transplant related deaths.
Kaplan-Meier estimates (P = .02) are represented by the top 2 curves); cumulative incidences (P = .21) by the bottom 2curves; BUCY estimates, by the dotted lines; CYTBI estimates, by the solid lines.
Outcome: occurrence of acute and chronic GVHD and causes of death
| . | CYTBI . | BUCY . |
|---|---|---|
| Number of patients | 49 | 51 |
| Acute GVHD | ||
| Grade 1 | 12 | 10 |
| Grade 2 | 12 | 5 |
| Grade 3 | 5 | 2 |
| Grade 4 | 0 | 5 |
| Chronic GVHD | ||
| Alive on day 100 | 44 | 46 |
| Patients developing c-GVHD | 20 | 17 |
| Limited/extensive | 9/11 | 12/5 |
| Causes of death | ||
| Recurrent leukemia | 11 | 16 |
| Veno-occlusive liver disease | 1 | 2 |
| Infection | 3 | 1 |
| Acute GVHD | 0 | 5 |
| Chronic GVHD | 2 | 4 |
| Secondary cancer | 2 | 2 |
| Preexistent diabetes mellitus | 1 | 0 |
| . | CYTBI . | BUCY . |
|---|---|---|
| Number of patients | 49 | 51 |
| Acute GVHD | ||
| Grade 1 | 12 | 10 |
| Grade 2 | 12 | 5 |
| Grade 3 | 5 | 2 |
| Grade 4 | 0 | 5 |
| Chronic GVHD | ||
| Alive on day 100 | 44 | 46 |
| Patients developing c-GVHD | 20 | 17 |
| Limited/extensive | 9/11 | 12/5 |
| Causes of death | ||
| Recurrent leukemia | 11 | 16 |
| Veno-occlusive liver disease | 1 | 2 |
| Infection | 3 | 1 |
| Acute GVHD | 0 | 5 |
| Chronic GVHD | 2 | 4 |
| Secondary cancer | 2 | 2 |
| Preexistent diabetes mellitus | 1 | 0 |
Entries are numbers of patients.
In fact, there was a trend toward a higher transplant related mortality (TRM) after BUCY preparation in the early period after transplantation, as within the first 18 months 11 patients died from nonleukemic causes, compared with 4 deaths after CYTBI (P = .06). Five patients died from NLD after 5 years: 1 from c-GVHD (CYTBI) and 4 from secondary cancers (Table 1) with no incidence difference in the 2 groups. Thirty-one patients relapsed at a median of 10 months (range, 2-69 months) with no statically different CI between the 2 groups (CYTBI: 12 [25%] of 49; BUCY: 19 [37%] of 51). In the BUCY group, there was a major trend toward an excess of leukemic recurrence in the early period after transplantation: 13 patients (25%) in the BUCY group relapsed within 12 months, as compared with 5 (10%) patients in the CYTBI group (P = .05). Two relapses occurred after 5 years, one in each group. Finally, with a median follow-up of 10.8 years (range, 9.5-12.7 years), the long-term outcome was significantly different between the 2 groups (overall survival [P = .04] and LFS [P = .02]) (Tables 1 and 2). Of the 49 patients treated with CYTBI, 29 survived, 28 leukemia free for a 59% (range, 42-70) 10-year actuarial overall survival and a 55% (range, 41-69) LFS. Of the 52 patients treated with BUCY, 21 survived, 18 leukemia free, for a 10-year survival probability of 43% (range, 30-57) and a LFS probability of 35% (range, 23-49). In a multivariate analysis, BUCY regimen was an independent factor that negatively influenced LFS (relative risk of failure [death or relapse]: 1.84 [1.06-3.2];P = .029).
With an extended follow-up, this analysis confirms that for patients presenting with early AML in CR1, the use of BUCY (120 mg/kg of CY) is associated with a poorer outcome than a standard CYTBI regimen. The difference is achieved early after transplantation and is due to the combination of an excess in early transplant-related deaths and leukemic recurrence while secondary and later events have a similar CI in the 2 groups. As we speculated in our initial report, one explanation of the poor results in patients treated with BUCY may be due to the wide interpatient variability in plasma BU levels, which were not monitored in this trial and which may have resulted either to a high toxicity or to a low efficacy depending on the individual patients' metabolism. It is recognized that these results may not be extrapolated to all transplantation situations and that a different outcome may be achieved if higher doses of CY are used or if CY is used in other diseases. Such differences may explain apparent divergences between observations in this report and other reports. Two of these other prospective studies included only patients with CML.2,4 First, CML patients are exposed to no or only to low-dose chemotherapy prior to transplantation. This may explain why BU may be less toxic in CML patients than in patients with AML. Second, BU is known to be an active drug in CML even at much lower doses. Its activity on AML cells may be different. Third, CML is probably the disease for which the graft-versus-leukemia (GVL) effect is the most potent. It can thus be hypothesized that the GVL effect in CML may be strong enough to overcome limitations of the antileukemic effect from the preparative regimen. Indeed, a recent encouraging report of nonmyeloablative preparative regimens in CML supports this hypothesis.5 The third trial reporting a different conclusion from ours may not be comparable, as only 51 such patients with AML in CR1 were randomized.3 A retrospective registry analysis from European Blood and Marrow Transplant group (EBMT) also failed to find a difference between BUCY and CYTBI.6 But this analysis includes many variables and markedly various doses of CY, and this may be critical. Indeed, a report in a pediatric population with AML in CR1 found a higher relapse rate in the BUCY group than in the CYTBI group, but this difference disappeared when the CY dose was increased up to 200 mg/kg.7
The long-term follow-up of our study also indicates that secondary neoplasia represent the major cause of late failures, and this may even increase with longer-term follow-up. The occurrence of secondary neoplasia emphasizes that close attention should be given to patients who may be considered cured from the initial disease. An evaluation of the quality of survival of long-term survivors is also important and is presently being addressed in a joint study of the 4 prospective trials that have been discussed.8 Finally, nonmyeloablative conditioning regimens have recently been developed with promising preliminary results indicating lower initial toxicity. But long-term results from this approach should be compared with the results achieved after a standard CYTBI preparation for patients with AML in CR1.
The authors express their gratitude to Prof Finn Bo Petersen, University of Utah, for his helpful advice.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal