Mice deficient in the interferon consensus sequence binding protein (ICSBP) develop a disease resembling chronic myeloid leukemia (CML), which in humans is caused by the BCR/ABL oncoprotein. Interferon-α (IFN-α) induces ICSBP expression and is an effective therapy for CML. This study examined whether enforced expression of ICSBP might antagonize BCR/ABL-induced leukemia; results demonstrated that ICSBP-modified cells generated a protective CD8+ cytotoxic T-cell response against BCR/ABL-transformed BaF3 cells in a murine leukemia model. ICSBP expression represents a novel means of stimulating a host immune response to BCR/ABL+ leukemia cells and a potential strategy for immunotherapy of CML.
Introduction
Chronic myeloid leukemia (CML) is a hematopoietic stem cell malignancy caused by the BCR/ABL oncoprotein. The disease is invariably fatal but can be cured by allogeneic bone marrow transplantation (BMT). This highly morbid therapy serves only a minority of patients, primarily younger individuals with an HLA-matched donor.1 The remainder are typically treated with interferon-α (IFN-α), which induces complete cytogenetic remission, delays blast crisis, and prolongs survival in a significant percentage of patients.2 IFN-α selectively suppresses BCR/ABL+ cells in the bone marrow by restoring defective integrin function and apoptosis in CML progenitors,3 and may down-regulate BCR/ABL transcription.4 Although some patients treated with IFN sustain complete cytogenetic remission, persistence of BCR/ABL+ clones can be demonstrated by reverse transcription–polymerase chain reaction (RT-PCR), arguing that leukemia cells remain dormant.5-9 IFN-α potentiates the development of antileukemic natural killer (NK) cells in patients with CML, and some IFN-treated patients in long-term remission have demonstrable cellular immunoreactivity against BCR/ABL sequences.10,11 Specific T-cell immunity against an epitope on the proteinase 3 component of neutrophil granules has recently been demonstrated in patients who have sustained a complete cytogenetic remission on IFN-α.12 These observations suggest that IFN encourages active suppression of leukemic clones by the immune system, but the precise molecular mechanisms by which IFN modulates immunity in patients with CML have not been defined.
Treatment with IFN induces JAK-STAT1/2 signaling and activation of transcription factors called interferon regulatory factors (IRFs). IRFs have homologous DNA binding domains that recognize a consensus sequence present in both IFNs and IFN-inducible genes. IRFs modulate antiviral actions, cell proliferation, apoptosis, and integrin function and are also important in regulating hematopoiesis and immune responses.13 Genetic disruption of IRF-1 or IRF-2 results in poor responses to IFN-α, -β, and -γ and abnormal lymphocyte homeostasis.14
Another IRF family member, the interferon consensus sequence binding protein (ICSBP) was identified as a conditional regulator of IFN-inducible transcription.15,16 ICSBP expression is primarily restricted to hematopoietic tissues, where it binds to and functionally cooperates with PU.1, an ETS transcription factor involved in regulating gene expression in myeloid and lymphoid cells.17-20 Gene knock-out of ICBSP induces a CML-like disease in mice.21 Myeloid cells derived from ICSBP-deficient mice exhibit reduced spontaneous apoptosis and a significant decrease in sensitivity to apoptosis induced by DNA damage.22 In addition, ICSBP−/−hematopoietic progenitors are hypersensitive to cytokine stimulation by interleukin (IL)-3, granulocyte-macrophage colony-stimulating factor (GM-CSF), and granulocyte colony-stimulating factor (G-CSF).23 Because the myeloproliferative disease observed in ICSBP-deficient mice resembled human CML, expression patterns of ICSBP were examined in patients with CML. ICSBP transcript levels were depressed in the leukemic blood cells of patients with CML and restored in patients successfully treated with IFN-α.24Retroviral transduction of mouse bone marrow with BCR/ABL resulted in a marked decrease in ICSBP expression, whereas forced overexpression of human ICSBP attentuated BCR/ABL-induced leukemia in a murine model.25 These data suggest that ICSBP might act as a tumor suppressor gene in hematopoietic cells.
Various studies have determined that ICSBP also plays a role in modulating the immune response by influencing the differentiation and maturation of immune cells and by affecting cytokine expression.26-29 ICSBP-deficient mice have defective production of IL-12 and IFN-γ, an impaired Th1 immune response, and enhanced susceptibility to intracellular pathogens.21 27We hypothesized that ICSBP might play a role in the ability of IFN-α to antagonize BCR/ABL transformation through immune suppression. We tested whether ectopic overexpression of murine ICSBP could antagonize BCR/ABL-induced leukemia using a simple model system and have demonstrated that expression of ICSBP in the BCR/ABL-transformed BaF3 cell line completely abolished its leukemogenicity in an immunocompetent host. Furthermore, mice that survived the challenge with ICSBP-modified leukemia cells gained long-lasting immunity against parental leukemia cells. This immune rejection was associated with T-cell–mediated reactivity against cell surface antigens specific to BCR/ABL-transformed cells. The immune-mediated rejection of leukemic cells induced by ICSBP is rapid, potent, and effective against pre-established disease, suggesting that ectopic ICSBP expression may provide a novel immunotherapeutic strategy for BCR/ABL+leukemia patients.
Materials and methods
Animals
Six- to 12-week-old female Balb/c mice were purchased from Jackson Laboratories (Bar Harbor, ME). Rag-1−/− and nonobese diabetic/severe combined immunodeficiency (NOD/SCID) mice (Jackson Laboratories) were obtained at 6 weeks old and maintained in sterile conditions.
Cell lines, transfection, and infection
BaF3, a murine pro-B cell line,30 was maintained in complete RPMI supplemented with 10% conditioned medium from WEHI-3B cells, as a source of IL-3. Mycoplasma infection has been reported to alter the tumorigenicity of cell lines and the corresponding host immune responses.31 32 We have confirmed that the BaF3 cell lines we used in our experiments wereMycoplasma free by both PCR and antibody detection. ICSBP and BCR/ABL constructs were introduced into the cells by electroporation using vectors pCXN2-ICSBP (kindly provided by Dr Keiko Ozato, National Institutes of Health) and MSCV-BCR/ABL-PAC. Cells were selected in 2 μg/mL puromycin for BCR/ABL and 2 mg/mL G418 for ICSBP to achieve stable expression. Moloney murine sarcoma virus (M-MSV)–transformed 3T3 cells and Lewis lung carcinoma cells were obtained form American Type Culture Collection (ATCC, Manassas, VA) and cultured in RPMI medium supplemented with 10% fetal bovine serum.
Immunoblotting analysis and enzyme-linked immunosorbent assay
Cells were lysed in buffer containing 10% glycerol, 150 mM NaCl, 20 mM Tris (pH 7.4), 10 mM NaF, 1 mM ZnCl2, 1 mM MgCl2, and 1% NP-40. Total proteins (40 μg) were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (10%) and transferred to nitrocellulose membrane. Both polyclonal α-ICSBP (kindly provided by Dr Keiko Ozato) and α-ABL antibodies were used at 1:5000 dilution. Horseradish peroxidase (HRP)-conjugated goat antirabbit IgG secondary antibody (Santa Cruz Biotechnology, Santa Cruz, CA) and enhanced chemoluminescence (ECL) were used to detect protein expression. Conditioned media from BaF3 and its derived cell lines were collected and concentrated with Centricon filters (YM-10; Millipore, Bedford, MA). Enzyme-linked immunosorbent assays (ELISAs) for IL-12, IFN-γ, and GM-CSF were carried out using Quantikine immunoassay reagents according to manufacturer's protocols (R & D Systems, Minneapolis, MN).
CD8/CD4 fractionation
The CD8+ or CD4+cells were purified or depleted from single spleen cell suspensions using Dynalbeads conjugated with CD8 (Lyt2) or CD4 (L3T4) antibodies according to the manufacturer's protocol (Dynal, Lake Success, NY). The purity of positively isolated cells and the efficiency of negative depletions were analyzed by flow cytometric analysis.
Flow cytometric analysis
Cells (106) were resuspended in phosphate-buffered saline (PBS) and incubated with phycoerythrin (PE)-conjugated antibodies against major histocompatibility complex (MHC) class I, MHC class II, CD8, and CD4. For B7-1 and B7-2 detection, purified primary antibodies were used, followed by a biotinylated secondary antibody and PE-conjugated streptavidin staining. All antibodies were purchased from Pharmingen (San Diego, CA), and cells were analyzed on the Becton Dickinson FACSCalibur immunocytometry system (San Jose, CA).
Mice injection and analyses
The BaF3-derived cells were injected intravenously into mouse lateral tail vein. Mice were closely monitored and were killed if moribund. Peripheral blood, spleen, and bone marrow samples were subsequently isolated from the mice for cell morphology analysis by staining centrifuged cells with the Hema 3 stain set (Biochemical Sciences, Swedesboro, NJ). Genomic DNA and messenger RNA (mRNA) were also isolated using DNA extraction reagent (Qiagen, Valencia, CA) and RNA STAT-60 reagent (Tel-Test “B,” Friendswood, TX).
Cell-mediated cytotoxicity and cold target inhibition assays
Fresh spleen cells were isolated from either naive or Ba-P210–ICSBP immunized mice 2 weeks after the immunization and were cultured in complete K medium (RPMI 1640, 10% fetal calf serum, with 50μM β-mercaptoethanol, 1 mM nonessential amino acids, 10 mM sodium pyruvate; Gibco BRL, Rockville, MD). Irradiated Ba-P210–ICSBP cells (20 000 cGy) were cocultured with the spleen cells for 6 days. On day 7, cytotoxic activities of stimulated cells were determined in a standard 4-hour 51Cr-release assay with the following modifications.33 Five thousand target cells were labeled with 100μCi sodium [51Cr] chromate and incubated for 1 hour before use. Cytotoxic T lymphocytes (CTLs) were added to achieve different effector-target ratios of 5:1, 25:1, 50:1, and 100:1. Specific lysis was determined as follows: percent specific release = 100 × (release by effector cells − spontaneous release)/(maximum release − spontaneous release). Spontaneous release was less than 20% of maximum release in all experiments. Competitive inhibition studies were carried out by measuring the specific lysis of 51Cr-labeled target cells by a fixed number of effector cells in the presence of various number of unlabeled tester cells. Unlabeled cells were added to the reaction wells at the same time as the 51Cr-loaded target cells.
Results
Expression of ICSBP did not alter BCR/ABL transformation of BaF3 cells in vitro
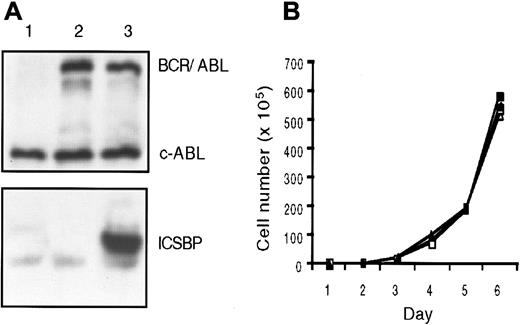
The IL-3–dependent murine bone marrow–derived cell line BaF3 lacks endogenous ICSBP expression and is readily transformed by BCR/ABL, and therefore serves as a model to investigate the effect of ectopically expressed ICSBP on BCR/ABL-induced leukemia. We created the Ba-P210 cell line by stably transfecting BaF3 cells with a BCR/ABL vector. Subsequently, ICSBP was transfected into Ba-P210 cells to generate the Ba-P210–ICSBP cell line. Both cell lines expressed a high level of the BCR/ABL (P210) oncoprotein; Ba-P210–ICSBP also expressed a high level of murine ICSBP (Figure 1A). The expression level of ICSBP in Ba-P210–ICSBP cells was comparable to the endogenous ICSBP level in the human lymphoid cell lines Namalwa, Daudi, and Ramos (data not shown).
ICSBP expression in BCR/ABL transformed BaF3 cells does not alter their growth characteristics in vitro.
(A) Immunoblot analysis of ICSBP and BCR/ABL protein expression in cell lines. Lane 1: BaF3; lane 2: Ba-P210; lane 3: Ba-P210–ICSBP. (B) Growth kinetics of Ba-P210 and Ba-P210–ICSBP cells. ■, Ba-P210; ▪, Ba-P210–ICSBP.
ICSBP expression in BCR/ABL transformed BaF3 cells does not alter their growth characteristics in vitro.
(A) Immunoblot analysis of ICSBP and BCR/ABL protein expression in cell lines. Lane 1: BaF3; lane 2: Ba-P210; lane 3: Ba-P210–ICSBP. (B) Growth kinetics of Ba-P210 and Ba-P210–ICSBP cells. ■, Ba-P210; ▪, Ba-P210–ICSBP.
The expression of BCR/ABL transforms BaF3 cells to IL-3 independence and resistance to apoptosis induced by γ-irradiation.34There was no obvious effect of ICSBP expression on the morphology or proliferation characteristics of the BCR/ABL-transformed BaF3 cells. The Ba-P210–ICSBP cell line remained growth factor–independent and resistant to γ-irradiation in culture. Two independent Ba-P210–ICSBP cell lines proliferated with the same growth kinetics as their parental cell line Ba-P210 (Figure 1B), demonstrating that ICSBP expression did not exhibit an antiproliferative effect on Ba-P210 cells in vitro.
Expression of ICSBP antagonized BCR/ABL induction of leukemia in vivo
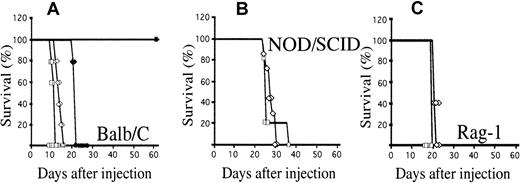
When injected intravenously into healthy syngeneic Balb/c mice, Ba-P210 cells induced a rapidly progressive leukemia that killed the host in 3 weeks (Figure 2A). Spleens from injected animals were massively enlarged (10- to 20-fold above normal), and all mice had a pathologic burden of Ba-P210 cells in their peripheral blood, spleen, and bone marrow. BCR/ABL expression was detected by RT-PCR in involved tissues such as bone marrow and spleen (data not shown). In contrast, all mice receiving Ba-P210-ICSBP cells survived (maximal follow-up for 1 year; Figure 2A). Peripheral blood from mice injected with Ba-P210–ICSBP cells was monitored weekly and leukemia was not observed at any time even when up to 5 × 107 Ba-P210–ICSBP cells were injected. The expression of ICSBP altered the leukemic behavior of BCR/ABL (P210)-transformed BaF3 cells in a syngeneic host.
Ba-P210–ICSBP cells fail to induce leukemia in immunocompetent mice, but remain leukemogenic in irradiated and immunodeficient mice.
(A) A total of 106 Ba-P210 (⋄, ♦) or Ba-P210–ICSBP cells (■, ▪) was injected intravenously into normal (▪, ♦) or sublethally irradiated (450 cGy; ■, ⋄) Balb/c mice. Ten mice were used in each arm. (B) NOD/SCID mice, 5 to 6 mice were used in each arm. (C) Rag-1–deficient mice; 5 mice were used in each arm.
Ba-P210–ICSBP cells fail to induce leukemia in immunocompetent mice, but remain leukemogenic in irradiated and immunodeficient mice.
(A) A total of 106 Ba-P210 (⋄, ♦) or Ba-P210–ICSBP cells (■, ▪) was injected intravenously into normal (▪, ♦) or sublethally irradiated (450 cGy; ■, ⋄) Balb/c mice. Ten mice were used in each arm. (B) NOD/SCID mice, 5 to 6 mice were used in each arm. (C) Rag-1–deficient mice; 5 mice were used in each arm.
Intact host immunity was necessary for ICSBP-mediated protection
We reasoned that the failure of Ba-P210–ICSBP cells to induce a lethal leukemia in Balb/c mice might be due to a cell-intrinsic proliferation defect apparent only in vivo or an indirect activation of host defense mechanisms. To see if impairment of host defenses altered tumorigenicity in vivo, we injected 106 Ba-P210 or Ba-P210–ICSBP cells into Balb/c mice exposed to a sublethal dose of irradiation (450 cGy). Both mouse groups developed lethal leukemia and died with comparable disease latencies (Figure 2A). This observation demonstrated that ICSBP expression in Ba-P210 cells did not alter the intrinsic tumorigenicity in vivo. Thus, the inability of Ba-P210–ICSBP cells to induce leukemia in healthy Balb/c mice was due to an inhibitory mechanism that could be destroyed by γ-irradiation. Because a major effect of γ-irradiation is compromise of host immunity, we investigated the importance of an intact immune system using immunodeficient mice. We injected 106 Ba-P210 and Ba-P210–ICSBP cells into NOD/SCID and Rag-1−/− mice. NOD/SCID mice are severely deficient in T/B cells and NK cells,35 and Rag-1−/− mice have no T/B cell function, but maintain relatively normal function of NK cells.36 Similar to γ-irradiated Balb/c mice, the disease latencies induced by both Ba-P210 and Ba-P210–ICSBP cell lines were comparable in NOD/SCID and Rag-1−/− mice (Figure2B,C). This experiment strongly suggested that the protective function of ICSBP was dependent on an intact immune system in the recipient mice. Furthermore, the result implicated T/B cells and not NK cells in the process.
Ba-P210–ICSBP immunization led to resistance against subsequent rechallenge with Ba-P210 cells
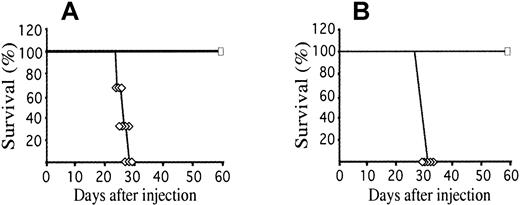
To assess whether the induced immunity was directed against ICSBP-specific antigens or antigens unrelated to ICSBP, we tested whether mice that survived Ba-P210–ICSBP injection could survive subsequent challenge with parental Ba-P210 cells. Thirty days after a single-dose immunization with Ba-P210–ICSBP cells, mice were reinjected with 106 Ba-P210 cells. All mice tested achieved long-term leukemia-free survival (Figure3A, maximal follow-up for 1 year). This result indicated that tumor rejection was not directed against ICSBP-derived antigens on the tumor cells. Immunized mice rechallenged with Ba-P210 cells 90 days after Ba-P210–ICSBP immunization also survived, demonstrating that the immunity induced by ICSBP expression in Ba-P210 cells was potent and long-lasting. Mice injected with a sublethal dose of Ba-P210 cells (< 5 × 105 cells) failed to develop resistance to subsequent challenge with lethal doses of Ba-P210 cells, indicating that expression of ICSBP was critical to the development of protective immunity (data not shown). A single dose of γ-irradiated Ba-P210–ICSBP cells (up to 107) also failed to induce immunity (data not shown). Irradiation with doses higher than 2 Gy inhibits antigen presentation by B cells,37 but the failure of irradiated cells to induce immunity also suggests that proliferating cells expressing ICSBP are required to stimulate a protective immune response.
Injection of ICSBP modified cells (Ba-P210–ICSBP) generates immunologic memory for parental leukemia cells (Ba-P210).
(A) Survival of naive Balb/c mice (⋄) or Balb/c mice preimmunized with 106 Ba-P210–ICSBP cells (■) following intravenous injection with 106 Ba-P210 cells. Ten mice were used in each arm. (B) Balb/c mice received an intravenous injection of 7 × 106 spleen cells from naive (⋄) or Ba-P210–ICSBP immunized mice (■), along with 106 Ba-P210 cells on the following day. Five mice were used in each arm.
Injection of ICSBP modified cells (Ba-P210–ICSBP) generates immunologic memory for parental leukemia cells (Ba-P210).
(A) Survival of naive Balb/c mice (⋄) or Balb/c mice preimmunized with 106 Ba-P210–ICSBP cells (■) following intravenous injection with 106 Ba-P210 cells. Ten mice were used in each arm. (B) Balb/c mice received an intravenous injection of 7 × 106 spleen cells from naive (⋄) or Ba-P210–ICSBP immunized mice (■), along with 106 Ba-P210 cells on the following day. Five mice were used in each arm.
ICSBP-induced immunity can be transferred to naive mice
The spleen is a primary immune organ in an adult mouse. To determine whether immunologic memory could be adoptively transferred from one animal to another, donor Balb/c mice were immunized with a single dose of 106 Ba-P210–ICSBP cells. Four weeks after the immunization, spleens were removed from the donor mice, single-cell suspensions were made, and 6 × 107 mononuclear spleen cells were injected intravenously into nonirradiated recipient mice (representing about half the total cells from a single spleen). At the time of spleen cell transfer, there was no residual Ba-P210–ICSBP cells detectable by genomic PCR (data not shown). Recipient mice were then challenged with 106 Ba-P210 cells on the second day. All mice receiving ICSBP-immunized spleen cells were protected from Ba-P210-induced leukemia, whereas control mice receiving naive spleen cells died of leukemia with the expected latency (Figure 3B).
Ba-P210–ICSBP injection protected mice with pre-established leukemia
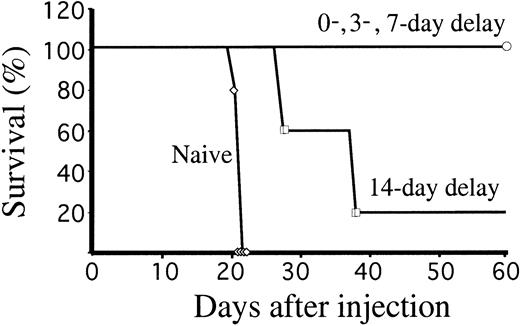
We have demonstrated that ectopic expression of ICSBP in BCR/ABL-transformed BaF3 cells induced potent cellular immunity. To test whether ICSBP-induced immunity could eradicate pre-existing disease, we first injected 106 Ba-P210 cells into naive Balb/c mice to induce leukemia. A single dose of 106Ba-P210-ICSBP cells was injected simultaneously into the same hosts, or following a delay of 3, 7, or 14 days. Simultaneous injection of both cell lines allowed survival of all mice (Figure4). More strikingly, all mice survived even when Ba-P210–ICSBP cells were injected 3 or 7 days after the injection of the leukemic Ba-P210 cells. When leukemia was allowed to develop for 14 days, equivalent to two thirds of the disease latency, all mice achieved prolonged survival and 20% of the mice survived disease free. The results demonstrated that the antileukemic effect of the immunized cells could be initiated rapidly and that ectopic ICSBP expression in leukemic cells was potent enough to eradicate established disease.
Ba-P210–ICSBP immunization rescues mice with pre-established leukemia.
Survival of Balb/c mice injected with 106 Ba-P210 cells, followed by 106 Ba-P210–ICSBP cells injected on the same day or after a delay of 3, 7, or 14 days. Control mice (naive) received no Ba-P210–ICSBP cells. Five mice were used in each arm.
Ba-P210–ICSBP immunization rescues mice with pre-established leukemia.
Survival of Balb/c mice injected with 106 Ba-P210 cells, followed by 106 Ba-P210–ICSBP cells injected on the same day or after a delay of 3, 7, or 14 days. Control mice (naive) received no Ba-P210–ICSBP cells. Five mice were used in each arm.
CTLs were induced by Ba-P210–ICSBP immunization
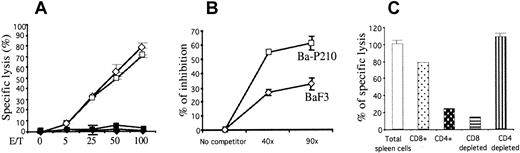
To determine whether mice immunized with Ba-P210–ICSBP cells generated CTLs against BCR/ABL transformed BaF3 cells, spleens from naive or immunized Balb/c mice were isolated 2 weeks after immunization and tested in a cytotoxicity assay. Single-cell suspensions were cultured for 6 days in the presence of γ-irradiated Ba-P210–ICSBP cells to stimulate specific CTL proliferation in vitro. A51Cr-release assay was performed on day 7. Spleen cells from immunized mice lysed both Ba-P210 and Ba-P210–ICSBP cells at comparable levels (maximum of 94.1% and 84.9%, respectively; Figure5A). No cytolytic activity was observed when naive spleen cells were used as effector cells, or when M-MSV–transformed 3T3 (Balb/c derived) or LL2 Lewis lung carcinoma (C57BL/6 derived) cells were used as targets. This result not only demonstrated the generation of specific CTLs from in vivo immunization, but also confirmed that ICSBP-derived antigens were unlikely to be the targets recognized by CTLs. Cytotoxicity was also observed when BaF3 cells were used as targets, though at a lower level, indicating that antigens on BaF3 cells were also targets of the immune response initiated by ICSBP expression (data not shown).
Immunization with Ba-P210–ICSBP cells activates CD8+ CTLs, which exhibit specific cytolytic activity against BCR/ABL-associated cell surface antigens.
(A) Cytolytic activity of Ba-P210–ICSBP immunized spleen cells against different cell targets. Five thousand 51Cr-labeled target cells were used: ⋄, Ba-P210; ■, Ba-P210–ICSBP; ♦, 3T3-MSV; ▪, LL2. (B) Cytotoxicity competition assay; 5000 51Cr labeled Ba-P210 cells were used as target cells, and 125 000 Ba-P210–ICSBP immunized spleen cells were used as effector-cells (effector-target ratio of 25:1). An excess of unlabeled BaF3 or Ba-P210 cells were added to the target-effector mixture (40-fold or 90-fold as indicated). CTL activity was measured as described in “Materials and methods.” Graph represents the percentage of inhibition of cytolytic activity in the presence of unlabeled cells normalized against total cytolytic activity in the absence of unlabeled cells. (C) Cytolytic activities of CD8+/CD4+ cells or the corresponding CD8/CD4-depleted Ba-P210–ICSBP immunized spleen cells. Five thousand Ba-P210 cells were used as target and the effector-target cell ratio was 25:1.
Immunization with Ba-P210–ICSBP cells activates CD8+ CTLs, which exhibit specific cytolytic activity against BCR/ABL-associated cell surface antigens.
(A) Cytolytic activity of Ba-P210–ICSBP immunized spleen cells against different cell targets. Five thousand 51Cr-labeled target cells were used: ⋄, Ba-P210; ■, Ba-P210–ICSBP; ♦, 3T3-MSV; ▪, LL2. (B) Cytotoxicity competition assay; 5000 51Cr labeled Ba-P210 cells were used as target cells, and 125 000 Ba-P210–ICSBP immunized spleen cells were used as effector-cells (effector-target ratio of 25:1). An excess of unlabeled BaF3 or Ba-P210 cells were added to the target-effector mixture (40-fold or 90-fold as indicated). CTL activity was measured as described in “Materials and methods.” Graph represents the percentage of inhibition of cytolytic activity in the presence of unlabeled cells normalized against total cytolytic activity in the absence of unlabeled cells. (C) Cytolytic activities of CD8+/CD4+ cells or the corresponding CD8/CD4-depleted Ba-P210–ICSBP immunized spleen cells. Five thousand Ba-P210 cells were used as target and the effector-target cell ratio was 25:1.
To investigate whether there were specific CTLs directed against BCR/ABL-associated antigens, we performed competition assays with unlabeled target cells. Either BaF3 or Ba-P210 cells were added in excess as unlabeled cells to compete against 51Cr-labeled target Ba-P210 cells in cytotoxicity assays. The addition of excess unlabeled Ba-P210 cells was significantly more effective at inhibition of ICSBP-induced spleen cell cytotoxicity than a comparable number of unlabeled BaF3 cells (Figure 5B). This result confirmed that a proportion of spleen CTLs were directed against antigens associated with BCR/ABL expression, whereas other CTLs recognized antigens associated with the BaF3 cells.
CD8+ cells were the primary effector cells mediating ICSBP-induced immunity
To characterize which T-cell subtype mediated the cytotoxic effect we observed, we isolated CD8+ or CD4+ cells from the immunized total spleen cell population by immunoaffinity fractionation. Both positively selected CD8+ or CD4+ cells and the corresponding depleted cell populations were used for CTL assays. As determined by flow cytometry, positively selected cells were more than 85% pure and depletions for both CD4+ and CD8+ cells were more than 97% efficient (data not shown). Consistently, purified CD8+cells exhibited about 80% CTL activity, whereas CD8+depletion resulted in more than 85% loss of CTL activity (Figure 5C). CD4+ depletion had no effect on CTL activity, whereas purified CD4+ cells elicited only 20% activity. These results suggested that CD8+ cells were the primary cytotoxic effector cells in vitro and therefore likely to account for ICSBP-induced cellular immunity in vivo.
Discussion
Previous studies linked ICSBP deficiency to the pathogenesis of myeloproliferative disease in mice and humans and correlated restoration of ICSBP expression with the action of IFN against CML.21,24 25 In the present study we have made the surprising observation that ectopic expression of ICSBP, an IFN-regulated transcription factor, induces a rapid, potent, and long-lasting vaccine-like effect against BCR/ABL-transformed BaF3 cells in a murine leukemia model. A single-dose injection with ICSBP-modified cells can eradicate pre-established leukemia in this model, and in vitro cellular cytotoxicity assays implicate CD8+ T cell immunoreactivity against leukemic targets. As a modulator of IFN signaling, ICSBP might activate or repress genes that contribute to the therapeutic effect of IFN-α by enhancing immune recognition of malignant cells. Unraveling the mechanism by which ICSBP expression stimulates protective immune surveillance against BCR/ABL-transformed BaF3 cells may lead to novel approaches to leukemia immunotherapy.
Allogeneic BMT and donor lymphocyte infusion induce a potent graft-versus-leukemia effect, a form of immunotherapy that has a major therapeutic benefit in CML.38 Philadelphia chromosome– positive cells persist in bone marrow and blood samples of patients with CML who are in clinical remission after transplant or chronic IFN therapy, supporting the notion that active mechanisms of immunosuppression play a role in maintaining leukemia in a dormant state.5-9 Given that BCR/ABL represents a novel tumor-specific antigen, BCR/ABL peptides have been intensively studied for their ability to trigger specific immunity. Indeed, peptides from the fusion sequences of BCR/ABL are bound by HLA molecules, and BCR/ABL peptide-sensitized dendritic cells can generate cellular immune reactivity.38-40 Hints of specific immunity against CML cells have been gleaned from clinical trials in which patients have been immunized with BCR/ABL fusion peptides.39,40 In this study, we demonstrated that cytotoxic T cells generated by Ba-P210–ICSBP immunization were directed against antigens associated with BCR/ABL expression on leukemia cells. It is therefore tempting to speculate that ICSBP enhances the presentation of BCR/ABL-derived peptides, but confirmation of this requires the isolation of specific CTL clones and definition of the target epitopes. It is possible that antigens whose expression is induced by BCR/ABL may also contribute to active immune suppression. A recent report documented that some patients with CML in remission harbor antigen-specific T cells targeted against epitopes of the proteinase-3 component of neutrophil granules.12
The molecular basis for the immunostimulatory effect of ICSBP expression in BCR/ABL-transformed BaF3 cells is unclear, but we have considered 2 possible mechanisms. First, ICSBP may enhance antigen presentation or immune effector cell recognition by modulating expression of MHC or costimulatory molecules. ICSBP can negatively regulate MHC class I gene expression,41 which might render cells susceptible to clearance by NK cells. We examined both MHC class I (H-2Dd and H-2Kd) and class II (I-Ad and I-Ed) expression in our cell lines by flow cytometry. BaF3 cells expressed relatively high levels of cell surface MHC class I, which was increased modestly on transformation by BCR/ABL. ICSBP overexpression did not alter the cell surface–expression of MHC class I. MHC class II expression was not detected in any of our BaF3 cell lines. Because costimulatory proteins facilitate the interaction between antigen-presenting cells and T cells, and the costimulatory molecule B7 has been shown to induce cellular immunity against BCR/ABL-induced leukemia,42 we examined B7 expression in our ICSBP-modified cells. BaF3 cells express low levels of B7-1 and undetectable levels of B7-2, neither of which were altered by expression of ICSBP. Therefore, we could not find evidence that the ICSBP-induced immune response against BCR/ABL-transformed BaF3 cells was due to altered MHC or B7 function.
Second, we investigated whether ICSBP induced the secretion of cytokines that stimulate dendritic or T-cell function. ICSBP-deficient mice are markedly impaired in their production of the T-helper type 1 cytokines IL-12 and IFN-γ and have compromised Th-1–driven immunity.21,28 Both IL-12 and IFN-γ are among the most potent antitumor cytokines. IL-12–modified tumor cells activate CD8+ cytotoxic T cells and tumor rejection in animal models,43 whereas IFN-γ possesses both direct antiproliferative activity and immunomodulating properties, including activation of macrophages and enhancement of T-cell–mediated immunity.44 Recently, it was shown that overexpression of ICSBP stimulates IL-12 p40 promoter activity in macrophages.45 We tested whether enhanced secretion of IL-12 or IFN-γ or both could be responsible for the immunoprotective activity of ICSBP-expressing cells in vivo. We measured the levels of IL-12, IFN-γ, and GM-CSF, another potent antitumor cytokine,46 in conditioned culture medium of ICBSP-modified BaF3 cells. None of these cytokines was detected by ELISA from Ba-P210 or Ba-P210–ICSBP cells. Moreover, we failed to detect altered mRNA expression for these cytokines by microarray expression profiling (data not shown). Therefore, the mechanism by which ICSBP induces antileukemia immunity does not appear to be a direct consequence of increased secretion of these cytokines by ICSBP-modified cells.
The vaccine-like effect in our system appeared to require coexpression of ICSBP and BCR/ABL in BaF3 cells. We attempted to determine whether ICSBP expression would stimulate immune rejection of cytokine-independent leukemogenic subclones of BaF3 that arise spontaneously after prolonged liquid culture. The ICSBP-expressing, spontaneously transformed BaF3 cells were incapable of triggering an effective immune response on their own. However, once the immune response was established by Ba-P210-ICSBP injection, mice were resistant to challenges from both Ba-P210 or spontaneously transformed BaF3 clones (data not shown). This result was consistent with our CTL assays that showed that CTLs derived from Ba-P210–ICSBP–immunized mice also lysed BaF3 target cells. Presentation of BCR/ABL-associated epitopes may be particularly effective in ICSBP-expressing BaF3 cells, which are derived from the Balb/c strain, because we have been unable to demonstrate that ICSBP expression stimulates the same protective immunity against BCR/ABL-transformed 32D cells in syngeneic C3H/HeJ mice. Future efforts will be aimed at determining specific peptide epitopes from BCR/ABL that can bind to and be effectively presented in the context of the Balb/c class I molecules (H-2Dd, H-2Kd).
Ren and colleagues have demonstrated that enforced coexpression of ICSBP attenuates and alters the disease phenotype induced by BCR/ABL in a murine model of CML,25 but the precise mechanism for this effect or whether an immune phenomenon plays any role is unclear. Our data using a distinct syngeneic leukemia model suggest that ectopic ICSBP expression can stimulate a CD8+ cytotoxic T-cell–mediated antileukemia immune response that is active even against pre-established disease. To our knowledge, this represents the first demonstration that expression of an intracellular transcriptional regulator, rather than a cell surface or secreted protein, can elicit a protective antileukemic immune response.
We thank Haiyan Zhang for assistance with animal care and Dr Tomohiko Tamura and Dr Keiko Ozato for providing critical reagents, including the ICSBP cDNA and antibody. We also thank Professor Herman Eisen for critical guidance, Carol Mckinley for expert technical advice on the cytotoxicity assays, and Herman Eisen, Jianzhu Chen, and Eugene Koh for discussions and critical reading of the manuscript.
Supported by grants from the National Cancer Institute CA76418 and CA86991 (G.Q.D.). G.Q.D. is a recipient of the Burroughs Wellcome Career Development Award and is the Birnbaum Scholar of the Leukemia and Lymphoma Society of America. M.D. is a recipient of a fellowship from the Cancer Research Institute (New York).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
George Q. Daley, Whitehead Institute, 9 Cambridge Center, Cambridge, MA 02142; e-mail: daley@wi.mit.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal