Interleukin-12 p70 (IL-12p70) heterodimer, composed of p35 and p40 subunits, is a major Th1-driving cytokine, promoting cell-mediated immunity. In contrast, IL-12p40 homodimer, secreted by APC in the absence of p35 expression, and free p40 monomer do not mediate IL-12 activity but act as IL-12 antagonists. Here it is reported that prostaglandin E2 (PGE2), an inflammatory mediator with a previously known Th2-driving function, dose-dependently enhances the IL-12p40 mRNA expression and the secretion of IL-12p40 protein in human tumor necrosis factor-alpha (TNFα)–stimulated immature dendritic cells (DCs). This effect is selective and is not accompanied by the induction of IL-12p35 expression or by secretion of IL-12p70 heterodimer. Inability of TNFα/PGE2 to induce IL-12p70 was not compensated by interferon gamma (IFNγ), which strongly enhanced the lipopolysaccharide (LPS)–induced IL-12p70 production. In addition to the selective induction of IL-12p40 in TNFα-stimulated DCs, PGE2 inhibited the production of IL-12p70 and IL-12p40 in DCs stimulated with LPS or CD40 ligand. These data suggest an additional level of the Th2-promoting activity of PGE2, via selective induction of IL-12p40. Selective induction of IL-12p40 and suppression of bioactive IL-12p70 may have negative impact on anticancer vaccination with PGE2-matured DCs.
Introduction
Bioactive interleukin-12 p70 (IL-12p70) is a heterodimer composed of 2 subunits: p35 and p40.1 Although p35 is expressed in numerous cell types, p40 expression is more restricted and determines the ability of a given cell-type to produce bioactive IL-12.1 However, in the cells that are capable of producing both IL-12 subunits, it is the p35 subunit, produced in much lower amounts than p40, that plays the role of a limiting factor and determines the amount of bioactive IL-12p70 that is secreted.1,2 IL-12p70 heterodimer is a major Th1-driving cytokine. In contrast, p40 homodimer, secreted by antigen presenting cells (APCs) in the absence of p35 production, and to a lesser extent the p40 monomer, were shown to act as antagonists of mouse and human IL-12 receptors1,3-5 and to inhibit IL-12–dependent immune functions in vitro and in vivo.6-13
Bioactive IL-12p70 is produced by dendritic cells (DCs) during their interaction with Th cells, as a result of CD40-CD40L interaction between these 2 cell types.14-18 Although CD40-triggering alone provides a sufficient signal for the induction of IL-12p40 and several other cytokines, effective induction of IL-12p70 depends on the presence of an additional signal that can be provided by at least 2 of the Th cell–produced cytokines, interferon gamma (IFNγ) or IL-4.17-19 DCs also produce both IL-12p40 and IL-12p70 in response to many microbial stimuli, including lipopolysaccharide (LPS) and Staphylococcus aureus Cowan strain I (SAC).17 18
Prostaglandin E2 (PGE2), is a common inflammatory mediator with a Th2-driving role at several levels of immune response. PGE2 selectively impairs the production of IFNγ and promotes the production of Th2 cytokines in murine and human Th clones, acting directly20,21 and by inhibiting the responsiveness to IL-12.22 The presence of PGE2 during the priming of naive Th cells, drives their development into Th2 subset.23 At the level of APCs, PGE2 inhibits IL-12p70 production in CD40L- or LPS-stimulated cells24,25 and can induce a stable IL-12–deficient, Th2-inducing phenotype in maturing DCs.25,26 A similar IL-12 antagonistic and Th2-promoting activity is shared by other and cyclic adenosine monophosphate (cAMP)-elevating agents, including β-adrenergic agonists,27 histamine,28 or cholera toxin.29
In apparent contrast, PGE2 was recently reported to synergize with tumor necrosis factor-alpha (TNFα) in the induction of at least IL-12p40 subunit production in DCs, as determined by an IL-12p40–specific enzyme-linked immunosorbent assay (ELISA), sensitive for IL-12p70 heterodimer, p40 homodimer, and free p40 monomer.30 This led to the proposal that at the inflammatory sites PGE2 may paradoxically play the role of an IL-12 inducer, supporting cell-mediated immunity. Since PGE2 is known to enhance the TNFα-induced DC maturation,26,30,31 its potential IL-12–inducing ability could be important for anticancer vaccination protocols utilizing DCs matured in the presence of a “complete cytokine mix,” consisting of TNFα, IL-1β, IL-6, and PGE2. 31
Having in mind the opposite roles played by IL-12p70 and IL-12p40 and the different requirements for the induction of either factor in human DCs,1,2 17-19 we tested whether the enhancement of IL-12p40 production in TNFα-activated DCs by PGE2 is accompanied by the induction of p35 gene transcription and by secretion of bioactive IL-12p70. Our results indicate that although PGE2 participates in the induction of IL-12p40, this effect is selective and is not accompanied by the induction of p35 gene expression nor by the production of bioactive IL-12p70 heterodimer. In addition, PGE2 suppresses the production of IL-12p70 induced by the “classical” inducers of cytokine production, LPS or CD40L.
Materials and methods
Generation of “tissue-type” immature CD1a+CD83−DCs
Monocytes were isolated from peripheral blood of healthy volunteers and cultured at 5 × 105 cells/mL in Iscoves modified Dulbecco medium (IMDM) with 10% fetal calf serum (FCS) (Hyclone, Logan, UT) in the presence of rhu granulocyte-macrophage–colony-stimulating factor (GM-CSF) (500 U/mL; a gift of Schering-Plough, Uden, The Netherlands) and rhuIL-4 (250 U/mL; Strathman, Hannover, Germany) for 6 days, as described elsewhere.25 26 At day 6, the cultures consisted of uniformly HLA-DR+ (L243; Becton Dickinson, San Jose, CA), CD83− (HB15; Immunotech, Marseille, France) immature DCs, without detectable CD3+ cells, as analyzed by FACScan (Becton Dickinson). Over 90% of the cells expressed high levels of CD1a (OKT6; Ortho, Beerse, Belgium).
Analysis of cytokine production
At day 6, DCs were washed, counted, and stimulated (4 × 104) in a final volume of 200 mL by rhuTNFα (50 ng/mL, corresponding to 5 × 103 U/mL, Pharma Biotechnologie Hannover), LPS (250 ng/mL, Difco, Detroit, MI), or CD40L-transfected J558 plasmacytoma cells (J558-CD40L, 5 × 104 cells per well) (a gift of Dr P. Lane, Birmingham, United Kingdom), which were previously shown to induce IL-12 p70 in an IFNγ-independent way,16 alone or in combination with PGE2 (10−5 M – 10−9 M, as indicated; Sigma, St Louis, MO), or rhuIFNγ (1000 U/mL; a gift of Dr P. H. van der Meide, U-CyTech, Utrecht, The Netherlands), as indicated, for an additional period of 48 hours. The concentrations of cytokines in 48-hour supernatants were analyzed with specific solid phase sandwich ELISAs.
Cytokine measurements
IL-12p70 ELISA (sensitivity, 3 pg/mL),27 was performed with use of p70-specific mAb 20C2 (a gift from Dr M.K. Gately, Hoffmann-La Roche, Nutley, NJ) and p40-specific C8.6 mAb (a gift from Dr G. Trinchieri, The Wistar Institute, Philadelphia, PA). IL-12p40–specific ELISAs, recognizing p40 monomer, p40 homodimer, and p70 (p40 + p35) heterodimer were performed as described elsewhere. 2 25
Analysis of p35 and p40 mRNA expression
DCs (3 × 105 cells in 2 mL) were stimulated with the combination of TNFα (50 ng/mL) and PGE2(10−6 M) in the presence of IFNγ (1000 U/mL) or with LPS (250 ng/mL) and IFNγ (1000 U/mL), as indicated. Unstimulated DCs and DCs exposed to IFNγ alone were used as negative controls. Cells were lyzed after 6 hours and total RNA was isolated using the RNeasy Kit (Qiagen, Hilden, Germany). First-strand cDNA was synthesized from the total amount of RNA using Moloney murine leukemia virus (mMLV)–derived reverse transcriptase (RT) (MBI fermentas, Vilnius, Lithuania). Polymerase chain reaction (PCR) amplification of p35 (3:l of cDNA; 40 cycles) and p40 (2:l of cDNA; 35 cycles) was carried out using the p35 and p40 primers,2 resulting in the products of 533 base pair (bp) and 267 bp, respectively. PCR specific for human b2m (1:l cDNA, 35 cycles) was performed with specific primers,32resulting in the product of 286 bp. Ethidium bromide–stained PCR products were analyzed on agarose gel by Eagle-Eye (Stratagene, La Jolla, CA) and SigmaGel software (SPSS Science, Jandel Scientific Software, Chicago, IL).
Results
The induction of IL-12p40 mRNA and protein in DCs by TNFα and PGE2 is selective and is not accompanied by the induction of IL-12p35 mRNA or bioactive IL-12p70 heterodimer
PGE2 was previously shown to enhance the TNFα-induced final maturation of DCs,26,30,31 which was accompanied by the production of at least a p40 subunit of IL-12.30 To test whether the production of p40 in these conditions is accompanied by the induction of an IL-12p35 subunit and the secretion of bioactive IL-12p70, we analyzed the regulation of both IL-12 forms in TNFα-activated DCs by PGE2.
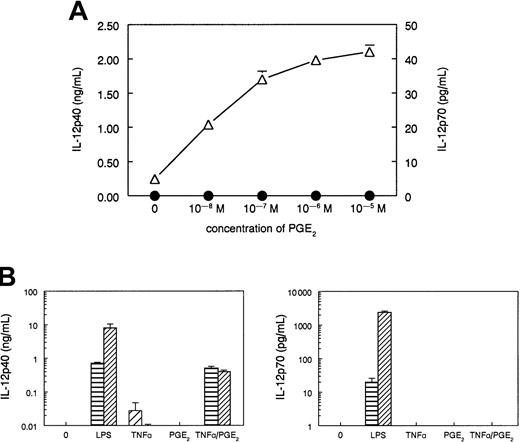
In accordance with the previous report, immature DCs exposed to TNFα (50 ng/mL) produced low, but clearly detectable, amounts of IL-12p40, found in 48-hour supernatants (Figure1A,B). Addition of increasing concentrations of PGE2 to the cultures of TNFα-stimulated DCs dose-dependently enhanced the IL-12p40 production. At all concentrations, the IL-12p40 induction was selective and was not accompanied by the induction of IL-12p70 heterodimer (Figure 1A). Although the combination of TNFα and PGE2 was as effective as LPS (250 ng/mL) in the induction of IL-12p40 production, exclusively LPS, but neither TNFα alone nor the combination of TNFα and PGE2, could induce IL-12p70 production (Figure 1B).
PGE2 selectively enhances IL-12p40 production in TNFα-stimulated DCs, but does not induce IL-12p70 production.
(A) DCs (4 × 104 cells in 0.2 mL) were stimulated with TNFα (50 ng/mL) and increasing concentrations of PGE2 for 48 hours. The data are shown as mean (± SD) of triplicate cultures and represent one experiment of 3 that gave similar results. ▵, IL-12p40; ●, IL-12p70. (B) DCs (4 × 104 cells in 0.2 mL) were stimulated for 48 hours with one of the following stimuli: TNFα (50 ng/mL), PGE2 (10−6M), or their combination, or LPS (250 ng/mL) in the absence (▤) or presence (▨) of IFNγ (1000 U/mL). The 48-hour culture supernatants were harvested and analyzed for IL-12p40 and IL-12p70 contents with specific ELISAs. The data are shown as mean (± SD) of triplicate cultures and represent one experiment of 5 that all gave similar results.
PGE2 selectively enhances IL-12p40 production in TNFα-stimulated DCs, but does not induce IL-12p70 production.
(A) DCs (4 × 104 cells in 0.2 mL) were stimulated with TNFα (50 ng/mL) and increasing concentrations of PGE2 for 48 hours. The data are shown as mean (± SD) of triplicate cultures and represent one experiment of 3 that gave similar results. ▵, IL-12p40; ●, IL-12p70. (B) DCs (4 × 104 cells in 0.2 mL) were stimulated for 48 hours with one of the following stimuli: TNFα (50 ng/mL), PGE2 (10−6M), or their combination, or LPS (250 ng/mL) in the absence (▤) or presence (▨) of IFNγ (1000 U/mL). The 48-hour culture supernatants were harvested and analyzed for IL-12p40 and IL-12p70 contents with specific ELISAs. The data are shown as mean (± SD) of triplicate cultures and represent one experiment of 5 that all gave similar results.
Previously, we have shown that the physiologic levels of CD40 triggering, by CD40L-expressing Th cells, for example, are sufficient to induce IL-12p40 production, but the efficient induction of IL-12p70 requires an additional IFNγ-mediated or IL-4–mediated signal.18-20 Similarly, IFNγ strongly enhances the IL-12p70 (and to a lesser extent IL-12p40) production in immature DCs and monocytes stimulated by bacterial products.2,18,19,25Therefore, we tested if the selective inability of TNFα and PGE2 to induce IL-12p70 production can be overcome by IFNγ. As expected,2 18-20 IFNγ (1000 U/mL) strongly enhanced the IL-12p70 (and IL-12p40) production by LPS-stimulated DCs (Figure 1B). In contrast, the addition of IFNγ to DC cultures stimulated either with TNFα alone or with the combination of TNFα and PGE2, did not induce any detectable IL-12p70 production and suppressed the production of IL-12p40 (Figure 1B).
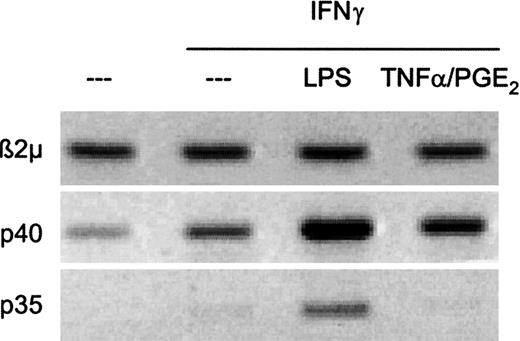
Since the secretion of bioactive IL-12p70 requires the production of both IL-12 subunits, p35 and p40, we analyzed the induction of mRNA encoding either of these subunits. As shown in Figure2, both p35 and p40 expression were observed in DCs stimulated with LPS and IFNγ. In contrast, stimulation with TNFα/PGE2 and IFNγ resulted with the selective induction of p40 in the absence of p35 induction. These results indicate that, even in the presence of IFNγ, a powerful coinducer of IL-12 production, the combination of inflammatory factors TNFα and PGE2 does not induce the bioactive IL-12p70.
The combination of TNFα and PGE2 induces IL-12p40 gene expression but fails to induce IL-12p35.
DCs (3 × 105 cells in 2 mL) were stimulated for 6 hours with one of the following stimuli: TNFα (50ng/mL) and PGE2 (10−6 M) or LPS (250 ng/mL) in the presence of IFNγ (1000 U/mL), as indicated, and lyzed for mRNA extraction. The expression of p35, p40, and β2m was analyzed with RT-PCR (see “Materials and methods”). The data shown are from a representative experiment of 3 performed.
The combination of TNFα and PGE2 induces IL-12p40 gene expression but fails to induce IL-12p35.
DCs (3 × 105 cells in 2 mL) were stimulated for 6 hours with one of the following stimuli: TNFα (50ng/mL) and PGE2 (10−6 M) or LPS (250 ng/mL) in the presence of IFNγ (1000 U/mL), as indicated, and lyzed for mRNA extraction. The expression of p35, p40, and β2m was analyzed with RT-PCR (see “Materials and methods”). The data shown are from a representative experiment of 3 performed.
PGE2 inhibits the production of both forms of IL-12 in DCs stimulated with the “classical IL-12 inducers” CD40L or LPS
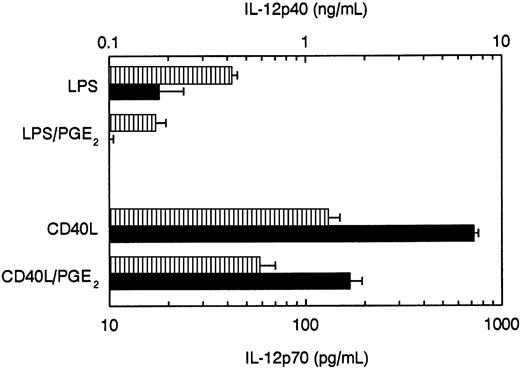
To test whether PGE2 differentially regulates the production of IL-12p40 and IL-12p70, also during the stimulation of DCs with other stimuli, we added increasing concentrations of PGE2 to DC cultures stimulated with 2 classical IL-12 inducers known to induce both IL-12p40 and bioactive IL-12p70—LPS and CD40L-transfected J558 cells (J558-CD40L) that express very high levels of CD40L. As shown in Figure 3, PGE2 inhibited the production of both IL-12p70 and IL-12p40 production induced by either of these stimuli.
PGE2 inhibits the production of IL-12p70 and IL-12p40 in DCs stimulated with CD40L or LPS.
DCs (4 × 104 cells in 0.2 mL) were stimulated with CD40L-transfected J558 cells (5 × 104 cells in 0.2 mL) or with LPS (250 ng/mL), either in the absence or in the presence of PGE2 (10−6 M). The supernatants were harvested after 48 hours and analyzed for IL-12p40 (▥) and IL-12p70 (▪) contents with specific ELISAs. The data are shown as mean (±) of triplicate cultures and represent one experiment of 4 that all gave similar results.
PGE2 inhibits the production of IL-12p70 and IL-12p40 in DCs stimulated with CD40L or LPS.
DCs (4 × 104 cells in 0.2 mL) were stimulated with CD40L-transfected J558 cells (5 × 104 cells in 0.2 mL) or with LPS (250 ng/mL), either in the absence or in the presence of PGE2 (10−6 M). The supernatants were harvested after 48 hours and analyzed for IL-12p40 (▥) and IL-12p70 (▪) contents with specific ELISAs. The data are shown as mean (±) of triplicate cultures and represent one experiment of 4 that all gave similar results.
Discussion
The current demonstration that the PGE2-assisted enhancement of IL-12p40 production in TNFα-stimulated DCs is selective and not accompanied by the induction of bioactive IL-12p70 heterodimer allows to resolve the current controversy concerning the role of PGE2 in IL-12 regulation and confirms the role of PGE2 as a factor with a multiple Th2-driving activities.
PGE2 is known to exert Th2-promoting and IL-12–antagonistic activity via several distinct mechanisms, affecting both APCs and Th cells. It suppresses the production of bioactive IL-12p70, both directly24-26 and by inhibiting the Th-cell production of IFNγ,20,21 an important coinducer of IL-12 during DC-Th cell cocultures.17-19 PGE2 also inhibits the responsiveness of T cells to IL-12 by downregulating the expression of the IL-12 receptor.22 Another example of IL-12–antagonistic and Th2-driving function of PGE2 is the induction of IL-10. PGE2 is known to enhance IL-10 production in mouse and human monocytes or in DC, when present at early stages of their development.25 35-40 However, in the current experiments, PGE2 did not enhance IL-10 production during the TNFα- or LPS-induced DC maturation, and it did not prime maturing DC for elevated IL-10 production upon subsequent CD40L stimulation (data not shown).
Human DCs matured in the presence of PGE2 showed reduced IL-12–producing capacity, compared with mature DCs obtained in the presence of high doses of IL-1β and TNFα alone, and preferentially induce Th2 cytokines in naive Th cells.26 The observation that such IL-12–deficient and control DCs showed the same expression of maturation-associated markers,26 together with the currently reported ability of PGE2 to directly inhibit the IL-12 production in LPS- or CD40L-activated DCs (Figure 3), argue that the IL-12 suppressive activity of PGE2 does not merely reflect its ability to accelerate DC maturation (a process associated with a decrease in IL-12 production 33 34), but represents a specific IL-12 inhibitory function.
The presently described selective induction of IL-12p40 suggests the existence of an additional level of Th2-promoting and IL-12–antagonistic activity of PGE2, mediated by p40 homodimer, or by free IL-12p40. Both in mouse and in human systems, it was demonstrated that p40 homodimer and to a lesser extent also the product of its dissociation, free p40 monomer, can suppress the responsiveness to IL-12 by competitively inhibiting the IL-12 receptor binding.1,3-5 This activity is very well pronounced in mice where p40 homodimer exerts the antagonistic activity with an IC50 of 1-10 ng/mL, whereas in humans concentrations at least 10-fold higher are required.3-5 Mouse p40 homodimer was shown to effectively antagonize IL-12 activity in vivo, as a factor rescuing the animals from lethal LPS-induced shock and suppressing the Th1-dominated inflammatory responses in several models of chronic inflamation, transplantation, and cancer.6-12 In humans, high levels of IL-12p40 in peritoneal fluid were postulated to play an IL-12–antagonistic role in endometriosis, as a factor locally inhibiting the activity of natural killer (NK) cells.13Unfortunately, poor stability of human p40 homodimer1constitutes a serious obstacle in analyzing its physiologic role, and its potential therapeutic in vivo use in transplantation and autoimmune diseases. For the same reason, we could not determine if the PGE2-induced IL-12p40 was released from DCs as p40 homodimer or as p40 monomer, a less potent IL-12 antagonist.1
Current demonstration of the selective induction of inactive/antagonistic IL-12p40 argues against the proposed Th1-promoting role for PGE2 at the inflammatory sites.30 On the contrary, selective induction of IL-12p40 by PGE2 is likely to contribute to selective suppression of Th1-type responses in chronic inflammation, and may play a role in the immune deviation induced by the PGE2-producing tumors.35-40 Although PGE2 enhances the expression of costimulatory molecules on DCs and increases their ability to stimulate CD4+ and CD8+ T cells,26,30,31 the ability of PGE2 to induce IL-12p40 production and to suppress the IL-12p70–producing ability of DCs25,26 may impair the tumoricidal functions of Th1, NK cells, and cytotoxic T lymphocytes. Multiple levels of IL-12 antagonism of PGE2 may have a negative impact on the effectiveness of immunotherapeutic protocols that use PGE2in combination with IL-1β, TNFα, and IL-6 to induce mature DCs for anticancer vaccination.31 It remains to be tested whether the benefit of an enhanced stimulatory capacity of PGE2-matured DCs is sufficient to offset the IL-12 antagonistic effects of PGE2.
Supported by Fundação para a Ciência e a Tecnologia, Lisbon, Portugal (grant no. PRAXIS XXI/BD/9195/96 to P.L.V.).
Correspondence:Paweł Kaliński, Department of Surgery, University of Pittsburgh, W1540 BST, 200 Lothrop St, Pittsburgh, PA 15261; e-mail: kalinskip@msx.upmc.edu, or Martien L. Kapsenberg, Department of Cell Biology and Histology, Academic Medical Center, University of Amsterdam, PO Box 22700, 1100 DE Amsterdam, The Netherlands; e-mail:m.l.kapsenberg@amc.uva.nl.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal