A complementary DNA expression library derived from marrow samples from myeloma patients was recently screened and human macrophage inflammatory protein-1α (hMIP-1α) was identified as an osteoclastogenic factor expressed in these samples. hMIP-1α enhanced osteoclast (OCL) formation in human marrow cultures and by highly purified OCL precursors in a dose-dependent manner (5-200 pg/mL). Furthermore, hMIP-1α enhanced OCL formation induced by human interleukin-6 (IL-6), which is produced by marrow stromal cells when they interact with myeloma cells. hMIP-1α also enhanced OCL formation induced by parathyroid hormone-related protein (PTHrP) and receptor activator of nuclear factor κB ligand (RANKL), factors also implicated in myeloma bone disease. Time-course studies revealed that the hMIP-1α acted during the last 2 weeks of the 3-week culture period. Reverse transcription–polymerase chain reaction analysis showed that the chemokine receptors for hMIP-1α (CCR1 and CCR5) were expressed by human bone marrow and highly purified early OCL precursors. Furthermore, hMIP-1α did not increase expression of RANKL. These data demonstrate that hMIP-1α is an osteoclastogenic factor that appears to act directly on human OCL progenitors and acts at the later stages of OCL differentiation. These data further suggest that in patients with myeloma, MIP-1α produced by myeloma cells, in combination with RANKL and IL-6 that are produced by marrow stromal cells in response to myeloma cells, enhances OCL formation through their combined effects on OCL precursors.
Introduction
Bone destruction is a common manifestation of multiple myeloma and results from increased osteoclastic bone resorption in areas of bone adjacent to myeloma cells.1,2Many efforts have been made to identify the osteoclast stimulatory factor (OSF) in myeloma. In vitro studies have implicated several cytokines as responsible for bone destruction in myeloma, including interleukin-1β (IL-1β),3,4 interleukin-6 (IL-6),5 and lymphotoxin.6 However, none of these cytokines is consistently elevated in peripheral blood or marrow of patients with multiple myeloma. Recently, we screened a human myeloma complementary DNA (cDNA) expression library derived from marrow samples of myeloma patients and identified human macrophage inflammatory protein-1α (hMIP-1α), also termed CCL3,7as an osteoclastogenic factor expressed in these specimens.8 Levels of hMIP-1α were elevated in the bone marrow supernatants of 62% of patients with active myeloma, whereas increased concentrations of hMIP-1α protein were detected in only 17% of patients with stable myeloma and were undetectable in normal marrow plasma samples. Furthermore, addition of a neutralizing antibody to hMIP-1α to human bone marrow cultures treated with freshly isolated marrow supernatants from myeloma patients blocked the stimulatory effects of these bone marrow supernatants on osteoclast (OCL) formation but had no effect on control levels of OCL formation.8 These data suggested that high levels of hMIP-1α are present in marrow samples from patients with multiple myeloma, and that hMIP-1α may be responsible for the increased OCL stimulatory activity present in marrow supernatants from patients with active myeloma. In this study, we evaluated the osteoclastogenic activity of hMIP-1α in normal human bone marrow cultures to further investigate the potential mechanism of action of hMIP-1α in multiple myeloma.
Materials and methods
Human bone marrow cultures
Nonadherent normal human marrow mononuclear cells were prepared as previously described9 and resuspended in α-minimum essential media (αMEM)/20% horse serum (αMEM, Gibco, Grand Island, NY; horse serum, Hyclone, Logan, UT) at 106 cells/mL in quadruplicate determinations. The marrow cells (1 × 105cells/well) were plated in 96-well plates in the presence or absence of varying concentrations of recombinant human MIP-1α, IL-6, parathyroid-related protein (PTHrP), receptor activator of nuclear factor κB ligand (RANKL) and/or 1,25-dihydroxyvitamin D3(1,25-(OH)2D3) (hMIP-1α and IL-6 from R&D Systems, Minneapolis MN; PTHrP from Bachem, Torrance, CA; RANKL from Immunex, Seattle, WA; and 1,25-(OH)2D3 from Calbiotec, San Diego, CA). Cultures were maintained in an atmosphere of 4% CO2 and air at 37°C for 3 weeks. The cultures were fed weekly by replacing half the media with an equal volume of fresh media containing the cytokine of interest. In selected experiments, MIP-1α was only added for the first week, the first 2 weeks, the last 2 weeks, or the last week of culture. After 3 weeks of culture, cells were fixed with 2% formaldehyde in phosphate-buffered saline (PBS), and the number of OCL-like multinucleated cells (nuclei > 3) that cross-reacted with the 23c6 monoclonal antibody, which identifies OCL-like cells (generously provided by Dr Michael Horton, St Bartholomew's Hospital, London, England) were scored.10Binding of the 23c6 monoclonal antibody was assessed with biotin-conjugated rabbit antimouse IgG coupled to alkaline phosphatase (Vector Laboratories, Burlingame, CA). The cells were counterstained with methylgreen.11
Isolation of osteoclast precursors, colony-forming units-granulocyte/macrophage
Nonadherent human bone marrow cells were cultured at 5 × 103 cells/well in αMEM containing 1.2% methylcellulose, 30% fetal bovine serum (FBS), 1% deionized bovine serum albumin (BSA; Sigma Chemical, St Louis, MO), and 100 pg/mL recombinant human granulocyte/macrophage colony-stimulating factor (GM-CSF) (Immunex). These cells were plated in a volume of 1 mL in 35-mm culture dishes (Corning, Corning, NY) as described previously.12 The dishes were incubated at 37°C in a humidified atmosphere of 4% CO2 for 7 days. Colonies were scored after 7 days of culture using an inverted microscope. Early OCL precursors were isolated by diluting the methylcellulose with media, washing the cells 3 times with αMEM, and then immunopanning of early OCL precursors using the M01 antibody (Boehringer-Mannheim, Indianapolis, IN) as previously described.12
The OCL precursors (1 × 104/mL) were cultured in microtiter plates for 3 weeks in αMEM/20% horse serum with MIP-1α (200 pg/mL) with or without 1,25-(OH)2D310−8 M or IL-6 (10-100 pg/mL) to induce OCL formation. The cultures were then processed as described above to score OCLs. In selected experiments, sperm whale dentin slices (generously provided by the US Fish and Wildlife Service) were added at the start of the cultures and then examined for resorption lacunae as previously described.13
Reverse transcription–polymerase chain reaction analysis of the expression of human chemokine receptors and RANKL
RNA preparation.
Nonadherent human bone marrow cells, CFU-GM–derived cells or PSV-10 human marrow stromal cells were incubated with hMIP-1α (200 pg/mL), hIL-6 (100 pg/mL) and both cytokines, respectively. RNA was extracted with RNAzol (Tel Test, Friendswood, TX) according to the manufacturer's protocol.
Polymerase chain reaction analysis.
The reverse transcription–polymerase chain reaction (RT-PCR) analysis was performed using the PerkinElmer RNA PCR CORE kit (Branchburg, NJ). After RT, the PCR was carried out under the following conditions: 94°C for 30 seconds, 58°C for 30 seconds, and 72°C for 1 minute for 28 cycles. The glyceraldehyde-3-phosphate dehydrogenase (GAPDH) primer sets were used with the same PCR condition as a control.14 The PCR primers for MIP-1α receptors (CCR1, CCR5) and GAPDH used were as follows: (1) CCR1 SS: 5′-CTA AGT GTA CCA GAG AAG GG-3′; (2) CCR1 AS: 5′-GGA AAT GAT GAG TCC CTC CC-3′; (3) CCR5 SS: 5′-TGA GAA GAA GAG GCA CAG GG-3′; (4) CCR5 AS: 5′-CGT TTG GCA ATG TGC TTT TGG-3′; (5) GAPDH SS: 5′-ACC ACA GTC CAT GCC ATC AC-3′; and (6) GAPDH AS: 5′-TCC ACC ACC CTG TTG CTG TA-3′.
Western blot analysis of RANKL expression in human bone marrow cultures treated with hMIP-1α and hIL-6
Human bone marrow cells from normal human donors were cultured with recombinant hMIP-1α (200 pg/mL) or recombinant hlL-6 (100 pg/mL) or both for 1 week. At day 7, fresh media containing cytokines were added to the cultures, and the cultures were continued for 48 hours. At day 9, cells were harvested and the cells lysed in polyacrylamide gel electrophoresis (PAGE) loading buffer, loaded into 7.5% premade ready gels (Biorad, Hercules, CA) and electrophoresed. Electrophoretic transfer of proteins from polyacrylamide to nitrocellulose (S&S, Dassel, Germany) was performed using a Semi-Dry-Blotting Unit (Fisher, Madison, WI) at 20 V for 45 minutes. After protein transfer, the nitrocellulose membrane was blocked with 5% skim milk and then blotted with the hRANKL polyclonal antibody (Immunex) or the anti–β-actin monoclonal antibody (Sigma Chemical). The nitrocellulose membrane was then washed and reacted with horseradish peroxidase–conjugated antirabbit goat antibody (Sigma Chemical) and visualized with the enhanced chemiluminescence (ECL) system (Amersham Life Sciences, Buckinghamshire, England) on the Kodak X-AR5, according to the manufacturer's protocol. Positive controls were cultured with 10−8 M 1,25-(OH)2D3 and negative control was cultured without osteoclastogenic factors. Each lane was loaded with the same amount of total protein.
Statistical analysis
Results are represented as the mean ± SEM for quadruplicate experiments and compared by paired t test. Results were considered significantly different atP < .05.
Results
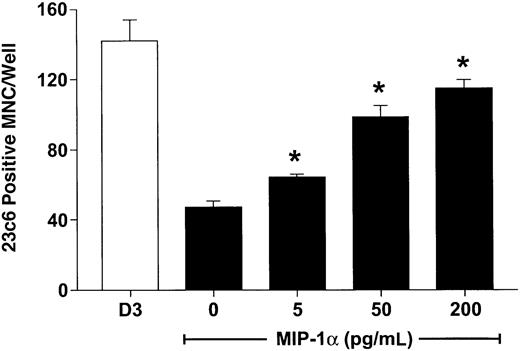
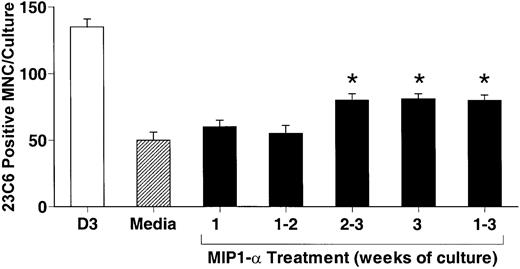
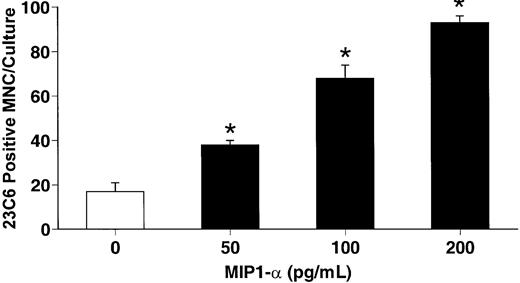
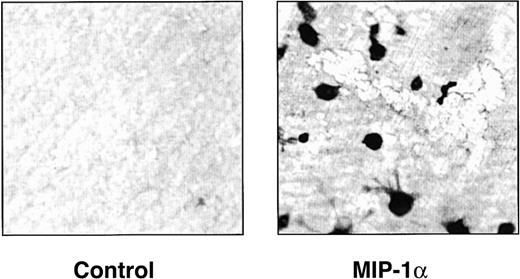
hMIP-1α increased the OCL formation in a dose-dependent fashion (5-200 pg/mL) (Figure 1) in human marrow cultures. Concentrations of MIP-1α as low as 5 pg/mL significantly increased OCL formation. Addition of MIP-1α to marrow cultures only significantly increased OCL formation if it was present for the last 2 weeks of culture (Figure 2). We then determined if MIP-1α was acting directly or indirectly on OCL precursors. As shown in Figure 3, MIP-1α increased OCL formation by highly purified OCL precursors (> 95% pure) in a dose-dependent manner. Furthermore, the OCLs that formed resorbed dentin (Figure 4).
MIP-1α induces osteoclast formation in human marrow cultures.
Long-term human marrow cultures were established and treated with varying concentrations of recombinant hMIP-1α. Controls for these experiments were cultures treated with 1,25-(OH)2D3 (10−8 M). MIP-1α at concentrations of 5 to 200 pg/mL significantly increased osteoclast formation in these cultures. Results represent the mean ± SEM for quadruplicate determinations for a typical experiment. Similar results were seen in 3 independent experiments. *P < .05 compared to culture lacking MIP-1α.
MIP-1α induces osteoclast formation in human marrow cultures.
Long-term human marrow cultures were established and treated with varying concentrations of recombinant hMIP-1α. Controls for these experiments were cultures treated with 1,25-(OH)2D3 (10−8 M). MIP-1α at concentrations of 5 to 200 pg/mL significantly increased osteoclast formation in these cultures. Results represent the mean ± SEM for quadruplicate determinations for a typical experiment. Similar results were seen in 3 independent experiments. *P < .05 compared to culture lacking MIP-1α.
MIP-1α acts at the later stages of osteoclast formation.
Human bone marrow cultures were treated with hMIP-1α (200 pg/mL) for varying periods of time. hMIP-1α significantly increased OCL formation only when present during the last 2 weeks of the 3-week culture period. Results represent the mean ± SEM for quadruplicate determinations for a typical experiment. Similar results were seen in the 3 independent experiments. *P < .05 compared to media alone.
MIP-1α acts at the later stages of osteoclast formation.
Human bone marrow cultures were treated with hMIP-1α (200 pg/mL) for varying periods of time. hMIP-1α significantly increased OCL formation only when present during the last 2 weeks of the 3-week culture period. Results represent the mean ± SEM for quadruplicate determinations for a typical experiment. Similar results were seen in the 3 independent experiments. *P < .05 compared to media alone.
hMIP-1α stimulates osteoclast formation in human CFU-GM cultures.
Human CFU-GM–derived cells were prepared and then isolated using the MO1 monoclonal antibody. Highly purified CFU-GM cells were then cultured in the presence of varying concentrations of recombinant hMIP-1α. hMIP-1α significantly increased osteoclast-like cell formation in these cultures in a dose-dependent fashion. The results represent the mean ± SEM for quadruplicate determinations for a typical experiment. Similar results were seen in 4 independent experiments. *P < .05 compared to culture lacking MIP-1α.
hMIP-1α stimulates osteoclast formation in human CFU-GM cultures.
Human CFU-GM–derived cells were prepared and then isolated using the MO1 monoclonal antibody. Highly purified CFU-GM cells were then cultured in the presence of varying concentrations of recombinant hMIP-1α. hMIP-1α significantly increased osteoclast-like cell formation in these cultures in a dose-dependent fashion. The results represent the mean ± SEM for quadruplicate determinations for a typical experiment. Similar results were seen in 4 independent experiments. *P < .05 compared to culture lacking MIP-1α.
hMIP-1α induces osteoclasts that resorb dentin.
Human CFU-GM–derived cells were cultured in the presence of dentin slices in the presence or absence of MIP-1α (200 pg/mL). At the end of the cultures, the dentin slices were stained for tartrate-resistant acid phosphatase activity and then photographed. Similar results were seen in 2 independent experiments (original magnification × 100).
hMIP-1α induces osteoclasts that resorb dentin.
Human CFU-GM–derived cells were cultured in the presence of dentin slices in the presence or absence of MIP-1α (200 pg/mL). At the end of the cultures, the dentin slices were stained for tartrate-resistant acid phosphatase activity and then photographed. Similar results were seen in 2 independent experiments (original magnification × 100).
To confirm that MIP-1α could act directly on OCL precursors, we then determined if OCL precursors expressed MIP-1α receptors. RT-PCR analysis showed that the chemokine receptors (CCR1 and CCR5) were expressed by human bone marrow cells and highly purified OCL precursors, but were not expressed or were expressed at very low levels (CCR5) by the PSV10 human marrow stromal cell line (Figure5).
Osteoclast precursors express MIP-1α receptors.
Isolated human bone marrow and CFU-GM–derived cells were prepared and subjected to RT-PCR analysis for CCR1 and CCR5 expression. Human bone marrow (HBM) and CFU-GM–derived cells both expressed CCR1 and CCR5. Human marrow stromal cells (PSV10) only expressed the CCR5 receptor in low concentrations. Controls for these experiments were expression of GAPDH messenger RNA.
Osteoclast precursors express MIP-1α receptors.
Isolated human bone marrow and CFU-GM–derived cells were prepared and subjected to RT-PCR analysis for CCR1 and CCR5 expression. Human bone marrow (HBM) and CFU-GM–derived cells both expressed CCR1 and CCR5. Human marrow stromal cells (PSV10) only expressed the CCR5 receptor in low concentrations. Controls for these experiments were expression of GAPDH messenger RNA.
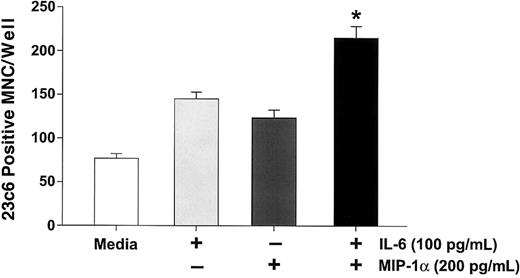
Because IL-6 is expressed by marrow stromal cells in response to myeloma cells15 and myeloma cells from a subgroup of patients express PTHrP,16 we then determined if MIP-1α enhanced the effects of IL-6 or PTHrP on OCL formation in normal marrow cultures. In addition, we have examined the effects of MIP-1α on RANKL-induced OCL formation, because RANKL mediates the effects of PTHrP on OCL formation.17 As shown in Figure6, MIP-1α enhanced OCL formation induced by either suboptimal (10 pg/mL) or optimal (100 pg/mL) concentrations of IL-6 in human bone marrow cultures, as well as in cultures of highly purified OCL precursors (Figure7). Similarly, MIP-1α enhanced OCL formation induced by PTHrP (Figure 8A), and RANKL (Figure 8B).
MIP-1α enhances IL-6 stimulated osteoclast formation in human marrow cultures.
Long-term human marrow cultures were treated with varying concentrations of MIP-1α, IL-6, and a combination of IL-6 and MIP-1α. MIP-1α enhanced the effects of low (10 pg/mL) or high (100 pg/mL) concentrations of IL-6 on osteoclast formation. Results represent the mean ± SEM for quadruplicate determinations for a typical experiment. Similar results were seen in 3 independent experiments (*P < .05).
MIP-1α enhances IL-6 stimulated osteoclast formation in human marrow cultures.
Long-term human marrow cultures were treated with varying concentrations of MIP-1α, IL-6, and a combination of IL-6 and MIP-1α. MIP-1α enhanced the effects of low (10 pg/mL) or high (100 pg/mL) concentrations of IL-6 on osteoclast formation. Results represent the mean ± SEM for quadruplicate determinations for a typical experiment. Similar results were seen in 3 independent experiments (*P < .05).
MIP-1α enhances IL-6–stimulated osteoclast formation in human CFU-GM–derived cell cultures.
CFU-GM–derived cells were prepared and treated with MIP-1α (200 pg/mL) or IL-6 (100 pg/mL) or their combination. Control cultures were treated with 10−8 M 1,25-(OH)2D3. MIP-1α or IL-6 alone significantly stimulated osteoclast formation. However, the combination enhanced osteoclast formation to levels that were greater than either IL-6 or MIP-1α alone. Results represent the mean ± SEM for quadruplicate determinations for a typical experiment. Similar results were seen in 3 independent experiments. *P < .05 compared to IL-6 or MIP-1α alone.
MIP-1α enhances IL-6–stimulated osteoclast formation in human CFU-GM–derived cell cultures.
CFU-GM–derived cells were prepared and treated with MIP-1α (200 pg/mL) or IL-6 (100 pg/mL) or their combination. Control cultures were treated with 10−8 M 1,25-(OH)2D3. MIP-1α or IL-6 alone significantly stimulated osteoclast formation. However, the combination enhanced osteoclast formation to levels that were greater than either IL-6 or MIP-1α alone. Results represent the mean ± SEM for quadruplicate determinations for a typical experiment. Similar results were seen in 3 independent experiments. *P < .05 compared to IL-6 or MIP-1α alone.
Effects of MIP-1α on osteoclast-like cell formation induced by PTHrP or RANKL.
Long-term marrow cultures were treated with either PTHrP (0-50 ng/mL) or RANKL (0-50 ng/mL) (■), or with a combination of MIP-1α (100 pg/mL) and PTHrP or RANKL (▪). MIP-1α enhanced the effects of PTHrP (A) or RANKL (B) on osteoclast-like cell formation in long-term human marrow cultures. Results represent the mean ± SEM for quadruplicate determinations for a typical experiment. Similar results were seen in 2 independent experiments. *P < .05 compared to cultures lacking MIP-1α.
Effects of MIP-1α on osteoclast-like cell formation induced by PTHrP or RANKL.
Long-term marrow cultures were treated with either PTHrP (0-50 ng/mL) or RANKL (0-50 ng/mL) (■), or with a combination of MIP-1α (100 pg/mL) and PTHrP or RANKL (▪). MIP-1α enhanced the effects of PTHrP (A) or RANKL (B) on osteoclast-like cell formation in long-term human marrow cultures. Results represent the mean ± SEM for quadruplicate determinations for a typical experiment. Similar results were seen in 2 independent experiments. *P < .05 compared to cultures lacking MIP-1α.
Previous studies have demonstrated that most osteoclastogenic factors induce OCL formation by up-regulating marrow stromal cell production of RANKL, a potent stimulator of OCL formation.17 Therefore, we determined if MIP-1α and IL-6 were also inducing RANKL expression. Unfractionated human marrow mononuclear cells were treated with 1,25-(OH)2D3 (10−8 M), MIP-1α (200 pg/mL), or IL-6 (100 pg/mL) for 24 hours and RANKL expression examined by Western blot. In contrast to 1,25-(OH)2D3, a factor known to enhance RANKL expression, MIP-1α or IL-6 did not enhance RANKL expression (Figure9A). Furthermore, treatment of human marrow cultures with RANK-Fc, a soluble form of the RANK receptor18 that inhibits RANKL-induced OCL formation,19 failed to inhibit MIP-1α–stimulated OCL formation (Figure 9B), except at very high concentrations. This concentration of RANK-Fc also inhibited basal OCL formation (Figure9B).
MIP-1α and IL-6 do not increase RANKL expression in human bone marrow and RANK-Fc does not inhibit MIP-1α–stimulated osteoclast formation.
(A) Cells were incubated for 48 hours with media, 1,25-(OH)2D3 (10−8 M), IL-6 (100 pg/mL), MIP-1α (200 pg/mL), or a combination of MIP-1α and IL-6. The cells were then collected and the lysate subjected to Western blot analysis. 1,25-(OH)2D3 increased RANKL expression in human bone marrow cultures. IL-6, MIP-1α, or IL-6 plus MIP-1α did not significantly increase RANKL expression in human bone marrow. Expression of β-actin is shown as a control. There was no effect on β-actin expression. (B) CFU-GM–derived cells were prepared and then treated with RANKL (50 ng/mL) or MIP-1α (200 pg/mL) in the presence of varying concentrations of recombinant RANK-Fc (0-100 ng/mL). RANK-Fc significantly decreased RANKL-stimulated osteoclast formation in human marrow cultures. In contrast, RANK-Fc modestly affected or did not affect osteoclast-like cell formation stimulated by MIP-1α. High concentrations of RANK-Fc (100 ng/mL) decreased basal osteoclast formation in these cultures. Results represent the mean ± SEM for quadruplicate determinations for a typical experiment. Similar results were seen in 2 independent experiments.
MIP-1α and IL-6 do not increase RANKL expression in human bone marrow and RANK-Fc does not inhibit MIP-1α–stimulated osteoclast formation.
(A) Cells were incubated for 48 hours with media, 1,25-(OH)2D3 (10−8 M), IL-6 (100 pg/mL), MIP-1α (200 pg/mL), or a combination of MIP-1α and IL-6. The cells were then collected and the lysate subjected to Western blot analysis. 1,25-(OH)2D3 increased RANKL expression in human bone marrow cultures. IL-6, MIP-1α, or IL-6 plus MIP-1α did not significantly increase RANKL expression in human bone marrow. Expression of β-actin is shown as a control. There was no effect on β-actin expression. (B) CFU-GM–derived cells were prepared and then treated with RANKL (50 ng/mL) or MIP-1α (200 pg/mL) in the presence of varying concentrations of recombinant RANK-Fc (0-100 ng/mL). RANK-Fc significantly decreased RANKL-stimulated osteoclast formation in human marrow cultures. In contrast, RANK-Fc modestly affected or did not affect osteoclast-like cell formation stimulated by MIP-1α. High concentrations of RANK-Fc (100 ng/mL) decreased basal osteoclast formation in these cultures. Results represent the mean ± SEM for quadruplicate determinations for a typical experiment. Similar results were seen in 2 independent experiments.
Discussion
In the present study, we demonstrated that MIP-1α increased OCL formation in a dose-dependent manner in human bone marrow cultures and stimulated OCL formation by highly purified OCL precursors. The data suggest that MIP-1α is a potent osteoclastogenic factor that acts directly on OCL precursors. Consistent with these observations are the results of other investigators.20-23 Fuller and colleagues20 reported that MIP-1α was chemotactic for isolated osteoclasts, and Votta and coworkers21 showed that MIP-1α was chemotactic for purified human OCL precursors. Kukita and associates22 demonstrated the expression of MIP-1α messenger RNA in rat marrow eosinophilic myelocytes, as well as in osteoblasts in active bone remodeling sites and showed that MIP-1α induced OCL differentiation in rat marrow cultured on a calcified matrix. Scheven and coworkers23 demonstrated that MIP-1α enhanced preosteoclast differentiation in porcine marrow cultures. In contrast to the results reported here, Fuller and coworkers20 found that MIP-1α inhibited bone resorption by isolated OCLs. However, these workers used very high concentrations of MIP-1α (10-100 ng/mL), which also inhibited OCL formation (J.-H.H., unpublished results, January 2000).
MIP-1α is a member of beta chemokine family that can interact with 3 types of chemokine receptors (CCR1, CCR5, and CCR9).24 In this study, we demonstrated by RT-PCR analysis that CCR1 and CCR5 were expressed in human bone marrow and highly purified CFU-GM cells, but not or minimally in the PSV10 human marrow stromal cell line, consistent with our findings that MIP-1α acts directly on OCL precursors. In preliminary studies, we have found that CCR1 appears to be mediating the effects of MIP-1α on OCL formation (Y.O., unpublished results, October 2000).
MIP-1α enhanced OCL formation induced by IL-6, PTHrP, and RANKL. We have previously reported that MIP-1α is produced by myeloma cells and correlated with the activity of the disease.8 IL-6 is produced by marrow stromal cells in response to myeloma cells15 and can stimulate proliferation and prevent apoptosis of myeloma cells. Furthermore, we have previously shown that IL-6 is a potent osteoclastogenic factor for human OCL precursors25 and induces bone resorption by human OCLs.26,27 Taken together, these data suggest that MIP-1α, produced by myeloma cells in combination with IL-6 secreted by marrow stromal cells in response to myeloma cells, can markedly enhance OCL formation in patients with myeloma. These data further suggest that small amounts of IL-6 may be sufficient to increase OCL formation stimulated by MIP-1α because concentrations of IL-6 as low as 10 pg/mL were sufficient to enhance the effects of MIP-1α on OCL formation. We have previously reported that the primary effect of IL-6 on OCL formation is to increase the size of the early OCL precursor pool (CFU-GM). These OCL precursors can then be induced to form large numbers of OCLs, as occurs in mice treated with IL-6 and PTHrP in vivo.28 Because MIP-1α also acts at the later stage of OCL formation, these data suggest that the enhanced effects of IL-6 on MIP-1α–induced OCL formation most likely result from IL-6 increasing the size of the pool of early OCL precursors and MIP-1α inducing the differentiation and fusion of these precursors to form large numbers of OCLs.
MIP-1α also enhanced the effects of PTHrP and RANKL on OCL formation, suggesting that it can enhance OCL formation by factors that also act at the later stage of OCL precursor differentiation,28-30and are produced by myeloma cells31 or marrow stromal cells in response to myeloma cells.
MIP-1α or IL-6 did not increase RANKL expression in human bone marrow. RANKL is a recently described osteoclastogenic factor17 that is expressed on the surface of marrow stromal cells and osteoblasts and mediates the effects of most osteoclastogenic factors such as PTHrP, IL-1, IL-11, and 1,25-(OH)2D3.17 Furthermore, RANK-Fc did not inhibit OCL formation induced by MIP-1α except at very high concentrations. These data are consistent with our observations that MIP-1α is a potent osteoclastogenic factor that acts directly on OCL precursors.8 IL-6 also did not up-regulate RANK ligand expression in contrast to results in murine systems.32 Riggs and coworkers33 have also reported that IL-6 does not induce RANKL expression in human osteoblasts.
In summary, these data demonstrate that hMIP-1α, which is produced by myeloma cells, is an osteoclastogenic factor in normal human bone marrow cultures that acts directly on OCL progenitor cells and enhances the osteoclastogenic effects of IL-6, PTHrP, and RANKL. The combination of these factors may be responsible for the severe bone destruction seen in patients with myeloma.
Supported by Research Funds from the Veterans Administration (G.D.R.), National Institutes of Health NCI grant CA40035 (G.D.R.), and the Multiple Myeloma Research Foundation (G.D.R.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
G. David Roodman, Research Service (151), Audie L. Murphy Veterans Administration Hospital, 7400 Merton Minter Blvd, San Antonio, TX 78284; e-mail: roodman@uthscsa.edu.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal