Abstract
Opportunistic infections frequently occur in patients with adult T-cell leukemia (ATL) and human T-cell leukemia virus type I (HTLV-I) carriers. However, the underlying mechanisms of such infections remain unknown. To clarify the mechanism of immunodeficiency in those infected with HTLV-I, this study analyzed the T-cell subsets in HTLV-I carriers and patients with HTLV-I–associated myelopathy/tropical spastic paraparesis and ATL using 3-color fluorescence with CD62L and CD45RA coexpression either with CD4+ or CD8+ T cells. The number of naive T lymphocytes was markedly suppressed in patients with ATL, particularly in those with acute form, compared with uninfected control individuals. The number of naive T cells was low in HTLV-I–infected individuals under 50 years old compared with uninfected individuals, whereas the number of memory T lymphocytes was greater in HTLV-I–infected individuals. Although the increase of memory T lymphocytes correlated with HTLV-I provirus loads, no relationship was found between naive T-cell counts and provirus loads. T-cell receptor rearrangement excision circles (TRECs), which are generated by DNA recombination during early T lymphopoiesis, were quantified to evaluate thymic function in HTLV-I–infected individuals. TREC levels were lower in HTLV-I–infected individuals than in uninfected individuals. In HTLV-I carriers less than 70 years old, an increase of Epstein-Barr virus DNA in peripheral blood mononuclear cells was observed in 6 of 16 (38%) examined, whereas it was detectable in only 1 of 11 uninfected controls. These results suggested that the low number of naive T lymphocytes was due to suppressed production of T lymphocytes in the thymus, which might account for immunodeficiency observed in HTLV-I–infected individuals.
Introduction
Human T-cell leukemia virus type I (HTLV-I) is the etiologic agent of adult T-cell leukemia (ATL)1-4 and is associated with various inflammatory diseases such as HTLV-I–associated myelopathy/tropical spastic paraparesis (HAM/TSP),5,6 infective dermatitis,7 and HTLV-I uveitis.8 ATL is a highly aggressive neoplastic disease9,10; the mean survival time of patients with acute ATL is less than 1 year regardless of intensive chemotherapy.11 The major obstacles in the treatment of patients with ATL include drug resistance and development of opportunistic infections,11 caused by various organisms such as Pneumocystis carinii, cytomegalovirus,Strongyloidiasis, and a variety of fungi indicating that cell-mediated immunity is severely impaired in these patients. Although the frequency of opportunistic infections is much higher in patients with ATL than in those with other hematologic malignancies, the underlying mechanism(s) of this phenomenon remains obscure. Opportunistic malignancies, Kaposi sarcoma,12 and Epstein-Barr virus (EBV)-associated lymphoma13 have also been reported in patients with ATL, indicating a state of immunodeficiency in these patients that is similar to that observed in acquired immunodeficiency syndrome (AIDS). Impaired cell-mediated immunity such as suppressed T-cell response to EBV14,15 and seronegativity against purified protein derivative (PPD) have been reported in HTLV-I carriers,16 17 also indicating a relationship to immunodeficiency.
In individuals infected with human immunodeficiency virus type 1 (HIV-1), a low number of CD4+ T lymphocytes is one of the underlying mechanisms of immunodeficiency. In addition, CD8+ naive T cells, which are recognized by double staining with CD45RA and CD62L, are known to decrease during the early stages of HIV-1 infection, suggesting that the progressive loss of cell-mediated immunity is caused by the fall in these T cells.18 On the other hand, naive T cells, especially CD8+ cells, have been shown to decrease with age, which may account for the state of immunodeficiency often observed in elderly individuals.19Increased production of cytokines such as interleukin (IL) 6, leukemia inhibitory factor (LIF), oncostatin M, and stem cell factor occurs during aging, suggesting that such cytokines are involved in the suppression of T lymphopoiesis in the thymus.20
The T-cell receptor rearrangement excision circle (TREC) is generated during T-cell development, and its numbers represent levels of T lymphopoiesis and correlate with numbers of naive T cells.21 Reduced production of naive T cells has been reported in individuals infected with HIV-1 based on analysis of TRECs. On the other hand, in HTLV-I carriers, HTLV-I provirus has been detected in a subset of CD4+ memory T lymphocytes, but not in CD8+ cells, indicating that HTLV-I promotes the proliferation of CD4+ memory T lymphocytes.22
The present study was designed to determine the underlying immunologic and virologic mechanism(s) of opportunistic infections in patients with ATL and HAM/TSP. To this end, we analyzed the T-cell subsets in these 2 groups of patients, together with HTLV-I carriers and controls. Our results showed an increased number of memory T cells and suppression of naive T cells in HTLV-I–infected individuals. Thymic function, which was measured by TREC, was impaired in HTLV-I carriers, and increased EBV DNA levels were detected in some HTLV-I carriers. Our results imply that the reduction of naive T cells is linked to the immunodeficiency observed in HTLV-I–infected individuals.
Patients, materials, and methods
HTLV-I carriers and patients with HAM/TSP or ATL
In this study, we analyzed 31 individuals infected with HTLV-I, 9 patients with HAM/TSP, 11 patients with ATL, and 29 uninfected controls. None of the patients with HAM/TSP or ATL had received any immunosuppressive or anticancer chemotherapy at the time of analysis. Peripheral blood mononuclear cells (PBMCs) were prepared from heparinized blood of these subjects with Ficoll-Paque density centrifugation and subjected to flow cytometric analysis and extraction of genomic DNA. For measurement of TRECs, the genomic DNAs were isolated from PBMCs of 33 HTLV-I seropositive individuals and 27 seronegative healthy individuals.
Flowcytometric analysis
For analysis of CD4 and CD8 T-cell subsets, we used the following monoclonal antibodies (mAbs): PC5 labeled anti-CD4 (Immunotech, Marseilles, France), anti-CD8 (Immunotech), fluorescein isothiocyanate (FITC)–labeled anti-CD45RA (Immunotech), and phycoerythrin (PE)–labeled anti-CD62L (Pharmingen, San Diego, CA). All mAbs were pretitered for optimal and saturating staining. PBMCs were stained by anti-CD45RA, CD62L, and CD4 or CD8. Flow cytometric analysis was performed using a Coulter EPICS XL (Beckman Coulter, Fullerton, CA). In brief, lymphocytes were first gated by forward and side scatter, and CD4+ or CD8+ T cells in this population were subsequently determined. CD45RA+CD62L+ cells within CD4+ or CD8+ populations were defined as naive cells and the remaining as memory cells. Data from 3000 cells of CD4+ or CD8+ T cells were collected and analyzed by EXPO-version 2 software (Beckman Coulter, Fullerton, CA).
Quantitative analysis of HTLV-I provirus load and EBV by real-time polymerase chain reaction
The probes and primers for HTLV-I gag region and EBV DNA polymerase region were designed for quantification. The sequences of primers for HTLV-I provirus were as follows: 5′-TTATGCAGACCATCCGGCTT-3′ and 5′-TATCTAGCTGCTGGTGATGG-3′, and the probe was 5′-CGGTGCAGCAGTTTGACCCCACTGC-3′. The sequences of primers for EBV genome detection were as follows: 5′-AGTTTTCCTTTTGGGCGGC-3′ and 5′-GGACGATGGGCAGCAGATC-3′, and the probe was 5′-CCAGGAGGCAGGAGAACACGC-3′. Both probes were labeled with fluorescent 6-carboxyfluorescein (FAM) (reporter) at the 5′ end and fluorescent 6-carboxy tetramethyl rhodamine (TAMRA) (quencher) at the 3′ end. A genomic DNA (200 ng) was used for real-time polymerase chain reaction (PCR) in a 50-μL amplification reaction solution containing 1 ×TaqMan buffer A, 3.5 mM MgCl2, 200 μM each of dATP, dCTP, dGTP, 400 μM dUTP, 1.25 U AmpliTaq Gold polymerase, 0.5 U AmpErase UNG, 300 nM of each primer, and 200 nM of the probe. The reaction conditions were 95°C for 10 minutes (activation of the AmpliTaq Gold polymerase), 50 cycles of 15 seconds at 95°C (denaturing) followed by 60 seconds at 60°C (annealing and extension). All experiments were performed and analyzed by the ABI PRISM 7700 Sequence Detection Systems (Applied Biosystems, Foster City, CA). To measure cell equivalents in the input DNA, recombination activating gene 1 (RAG-1) coding sequence in each sample was quantified by the real-time PCR. The sequences of primers for RAG-1 exon 2 detection were as follows: 5′-CCCACCTTGGGACTCAGTTCT-3′ and 5′-CACCCGGAACAGCTTAAATTTC-3′, and the probe was 5′-CCCCAGATGAAATTCAGCACCCACATA-3′. This probe was labeled with FAM (reporter) at the 5′ end and TAMRA (quencher) at the 3′ end. Quantities of RAG-1 copies in same template DNAs were measured using the same protocol. In each genomic DNA sample, PBMCs were quantified as one cell per 2 RAG-1 copies, and HTLV-I provirus load and EBV genome value were calculated as the number of each per 105 PBMCs. All samples were analyzed in duplicate, and negative controls were included in each measurement.
Measurement of TRECs
To detect TRECs by real-time PCR, primers and probe were designed as follows: the primers were 5′-TCCCTTTCAACCATGCTGACA-3′ and 5′-TGCCTATGCATCACCGTGC-3′, and the probe was 5′-CTCTGGTTTTTGTAAAGGTGCCCACTCCTG-3′. The probe was designed to recognize a region containing the signal-joint of TRECs, and labeled with fluorescent FAM (reporter) at the 5′ end and fluorescent TAMRA (quencher) at the 3′ end. Genomic DNA (200 ng) was used for the template, and PCR conditions were the same as those described above. All samples were analyzed in duplicate, and negative controls were included in each measurement. To normalize for cell equivalents in the input DNA, quantities of RAG-1 copies in the same samples were measured, and the number of TRECs/105 PBMCs was calculated.
Measurement of plasma concentrations of IL-7
Concentrations of IL-7 were measured in plasma samples obtained from patients with ATL, patients with HAM/TSP, HTLV-I carriers, and uninfected controls with the sandwich enzyme-linked immunosorbent assay (ELISA) method using Quantikine HS (R&D Systems, Minneapolis, MN).
Statisticalanalysis
Data are expressed as means ± SD. Differences between groups were examined for statistical significance using the nonparametric Mann-Whitney U test. Correlations between variables were examined by the Spearman rank correlation analysis. AP value less than .05 denoted a statistically significant difference.
Results
Flow cytometric analysis of T lymphocytes in HTLV-I–infected individuals
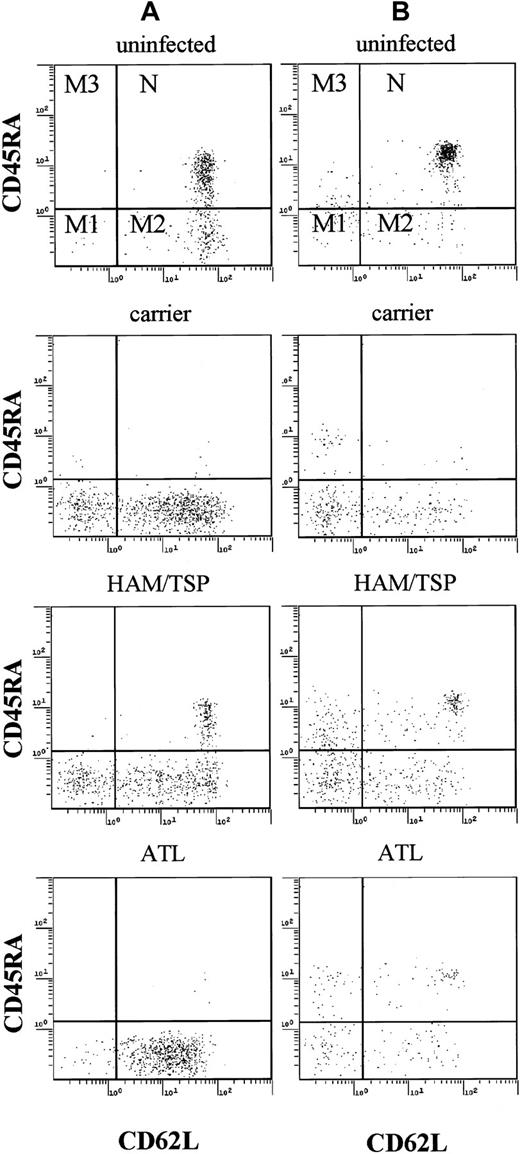
First, we analyzed the subsets of T lymphocytes in HTLV-I–infected individuals, including HTLV-I carriers, patients with HAM/TSP, and patients with ATL. As shown in Figure1, CD45RA+, CD62L+ T lymphocytes representing true naive T cells as described by Roederer and colleagues were readily identified.18 Based on this analysis, memory T cells could be divided into 3 subgroups: M1 (CD45RA−, CD62L−), M2 (CD45RA−, CD62L+), and M3 (CD45RA+, CD62L−). In patients with ATL, most lymphocytes were CD4+ memory T cells (M1 and M2 subgroups), in agreement with previous studies.23 On the other hand, naive T cells in CD4+ and CD8+subpopulations were markedly decreased in these patients (Figures 1 and2). In HTLV-I carriers, and patients with HAM/TSP, who are known to have high provirus loads,24naive T lymphocytes were also decreased, similar to patients with ATL although the extent of such decrease was milder. These findings indicate that HTLV-I–infected individuals have a low proportion of naive T lymphocytes and high memory T lymphocytes.
Flow cytometric analysis of PBMCs.
Representative data of PBMCs from one patient with ATL, one patient with HAM/TSP, and one HTLV-I carrier. PBMCs were stained by PC5-labeled anti-CD4 (A), anti-CD8 (B), FITC-labeled anti-CD45RA, PE-labeled anti-CD62L. Naive T cells (N) were CD45RA+ and CD62L+, and memory T cells can be divided into 3 groups (M1, M2, and M3).
Flow cytometric analysis of PBMCs.
Representative data of PBMCs from one patient with ATL, one patient with HAM/TSP, and one HTLV-I carrier. PBMCs were stained by PC5-labeled anti-CD4 (A), anti-CD8 (B), FITC-labeled anti-CD45RA, PE-labeled anti-CD62L. Naive T cells (N) were CD45RA+ and CD62L+, and memory T cells can be divided into 3 groups (M1, M2, and M3).
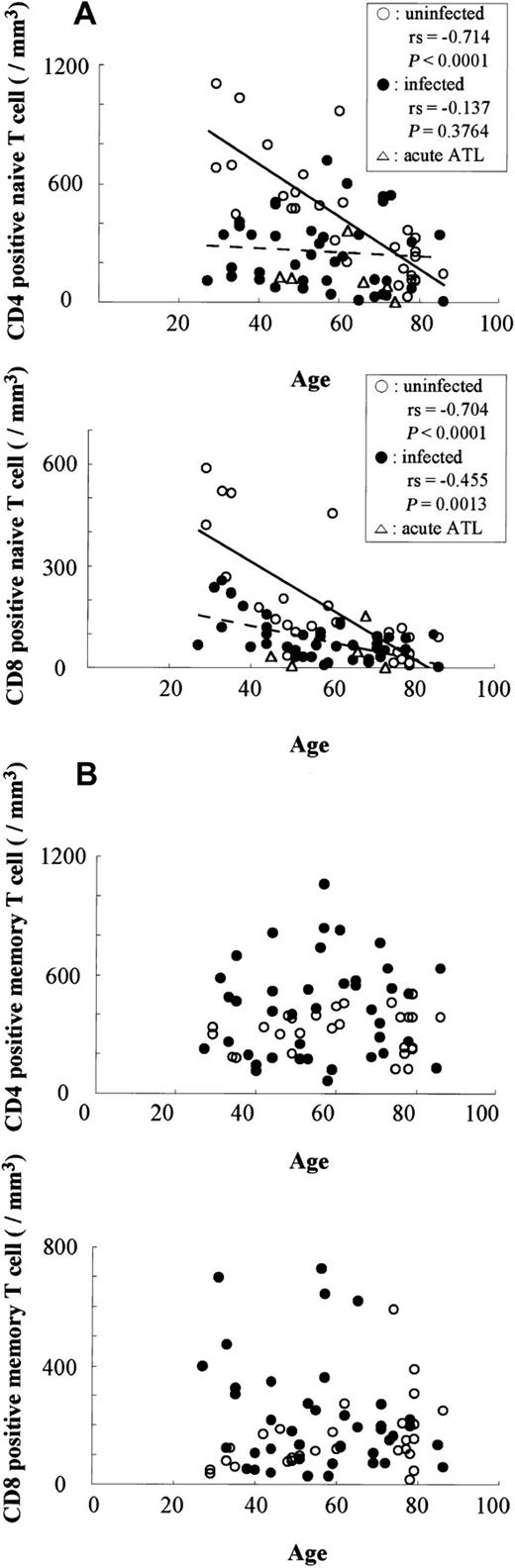
Difference in the number of naive and memory T lymphocytes in HTLV-I–infected and uninfected individuals.
The numbers of naive (A) and memory (B) T lymphocytes were compared between HTLV-I–infected (●) and uninfected (○) individuals. Correlations determined by Spearman rank order (rs).
Difference in the number of naive and memory T lymphocytes in HTLV-I–infected and uninfected individuals.
The numbers of naive (A) and memory (B) T lymphocytes were compared between HTLV-I–infected (●) and uninfected (○) individuals. Correlations determined by Spearman rank order (rs).
Figure 2 shows the correlation between ages and numbers of memory or naive T lymphocytes (CD4+ and CD8+ cells) in HTLV-I–infected and uninfected individuals. In uninfected persons, aging was associated with increased memory T lymphocytes and reduced naive T lymphocytes. Comparison of the number of T lymphocytes in HTLV-I–infected and uninfected individuals among age-matched groups showed that the number of CD4+ naive T lymphocytes was significantly lower in infected patients under 50 years of age (269 ± 149/μL, Figure 2) compared to uninfected individuals (680 ± 234/μL) (P = .0002, by Mann-Whitney). Similarly, the number of CD8+ naive T lymphocytes in those under 50 years of age is lower in HTLV-I–infected individuals (138 ± 73/μL, Figure 2) than uninfected individuals (301 ± 195/μL) (P = .0402, by Mann-Whitney). However, naive T-lymphocyte counts in infected and uninfected older patients (> 50 years of age) were not statistically different (data not shown). Numbers of memory T lymphocytes were greater in HTLV-I–infected persons, and in those under 50 years of age, whereas numbers of CD8+ memory T lymphocytes were significantly greater in the HTLV-I–infected population (246 ± 191/μL) than uninfected persons (95 ± 50/μL). However, the difference in CD4+ memory T cells between the 2 groups was not significant, which might be due to heterogeneity of provirus load among HTLV-I–infected individuals.
Determination of HTLV-I provirus load
Because the provirus load differs more than 100 times among carriers, it was critical to determine the load in HTLV-I–infected individuals we examined.25 We measured the provirus load in carriers and patients with HAM/TSP using real-time PCR. As an internal control, the copy number of genomic recombination activating gene (RAG-1) was used. As shown in Figure3, the numbers of memory T cells (both CD4+ and CD8+ subpopulations) correlated with the provirus loads (P = .0004 for CD4+ memory T cells and P = .0282 for CD8+ memory T cells, by Spearman rank correlation). An increased number of memory T cells (both CD4+ and CD8+ cell populations) suggests its relationship with provirus loads because memory T cells likely HTLV-I harbored in CD4+ memory T cells. Indeed, Richardson and coworkers22 previously showed that HTLV-I provirus was detected mainly in CD4+ memory T cells. To clarify whether the presence of HTLV-I provirus caused the proliferation of memory T cells, CD4+ and CD8+ cells were sorted out by magnetic beads and the provirus load in each subpopulation was determined. The purity of purified T lymphocytes was always greater than 97%. Although most of the provirus was detected in CD4+ cells, about 10% of total provirus load was found in CD8+ cells (Table 1). The proportion of infected cells was calculated assuming that one infected cell had one copy of provirus. The percentage of HTLV-I infected CD4+ or CD8+ cells was lower than that of memory T cells in each subpopulation, indicating that the majority of increased memory T cells were uninfected cells, which presumably proliferated through an immune response evoked by chronic HTLV-I infection. It was quite difficult to quantify HTLV-I provirus in naive T cells due to decreased number of naive T cells in vivo.
Correlation between HTLV-I provirus load and the number of memory T lymphocytes in CD4+ and CD8+subgroups.
Correlations determined by Spearman rank order (rs).
Correlation between HTLV-I provirus load and the number of memory T lymphocytes in CD4+ and CD8+subgroups.
Correlations determined by Spearman rank order (rs).
Proportions of human T-cell leukemia virus type I–infected cells and the memory T cells in CD4+ or CD8+ T cells
| Subjects . | Provirus load (copies/105CD4+ or CD8+ T cells) . | HTLV-I infected cells (%) . | Memory T cells (%) . | |
|---|---|---|---|---|
| C-1 | CD4 | 13 846 | 13.85 | 42.0 |
| CD8 | 2 115 | 2.12 | 62.6 | |
| C-2 | CD4 | 716 | 0.72 | 54.0 |
| CD8 | ND | ND | 74.0 | |
| C-3 | CD4 | 41 | 0.04 | 78.5 |
| CD8 | ND | ND | 81.2 | |
| C-4 | CD4 | 1 319 | 1.32 | 46.6 |
| CD8 | 185 | 0.19 | 66.3 | |
| C-5 | CD4 | 1 294 | 1.29 | 70.0 |
| CD8 | 92 | 0.09 | 72.0 | |
| C-6 | CD4 | 3 723 | 3.72 | 42.9 |
| CD8 | 442 | 0.44 | 45.2 | |
| C-7 | CD4 | 3 043 | 3.04 | 31.3 |
| CD8 | 440 | 0.44 | 10.1 | |
| Subjects . | Provirus load (copies/105CD4+ or CD8+ T cells) . | HTLV-I infected cells (%) . | Memory T cells (%) . | |
|---|---|---|---|---|
| C-1 | CD4 | 13 846 | 13.85 | 42.0 |
| CD8 | 2 115 | 2.12 | 62.6 | |
| C-2 | CD4 | 716 | 0.72 | 54.0 |
| CD8 | ND | ND | 74.0 | |
| C-3 | CD4 | 41 | 0.04 | 78.5 |
| CD8 | ND | ND | 81.2 | |
| C-4 | CD4 | 1 319 | 1.32 | 46.6 |
| CD8 | 185 | 0.19 | 66.3 | |
| C-5 | CD4 | 1 294 | 1.29 | 70.0 |
| CD8 | 92 | 0.09 | 72.0 | |
| C-6 | CD4 | 3 723 | 3.72 | 42.9 |
| CD8 | 442 | 0.44 | 45.2 | |
| C-7 | CD4 | 3 043 | 3.04 | 31.3 |
| CD8 | 440 | 0.44 | 10.1 | |
HTLV-I indicates human T-cell leukemia virus type I; ND, not determined.
Thymic function in HTLV-I carriers
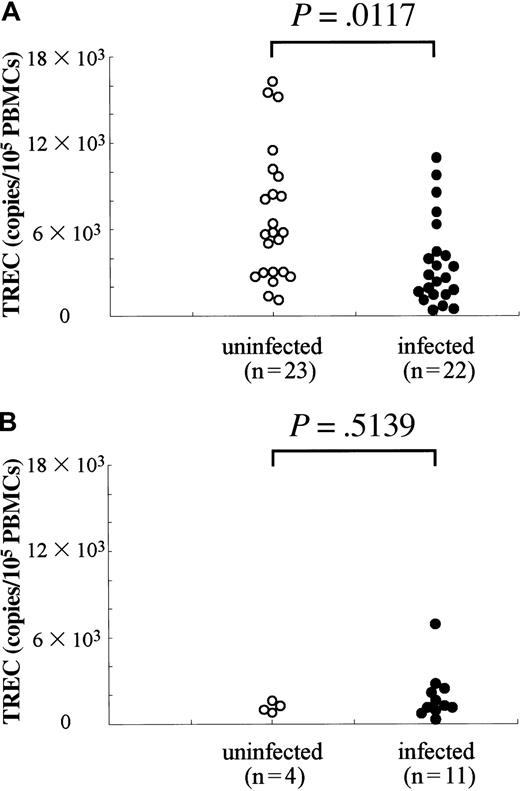
The low percentage of naive T cells in HTLV-I carriers (aged < 50 years) suggests that the function of the thymus, which generates these cells, is impaired. TRECs have been used as an indicator for measurement of thymic output in HIV-infected individuals, and reduced numbers of TRECs are known to recover after successful treatment by highly active antiretroviral therapy (HAART) in parallel with increased numbers of naive T cells.21 26 Therefore, one can infer from analysis of TRECs whether the generation of naive T cells is decreased in HTLV-I carriers or not. In uninfected individuals, TRECs diminished with age, which is consistent with the finding of age-related decrease in naive T lymphocytes. As shown in Figure4, TRECs decreased with age in HTLV-I–infected and uninfected individuals. Among individuals less than 50 years of age, TRECs in HTLV-I carriers (3701 ± 3041) was lower than in uninfected persons (6817 ± 4523) (P = .0117, by Mann-Whitney), indicating that the low count of naive T lymphocytes is due to impaired T lymphopoiesis. TRECs did not correlate with the provirus load, which was in agreement with the finding that the number of naive T lymphocytes was not influenced by provirus load (data not shown).
Quantification of TRECs in HTLV-I–infected and uninfected individuals.
(A) Individuals younger than 50, (B) individuals older than 50. Genomic DNAs (200 ng) isolated from PBMCs were used for quantification of TRECs. In each sample, the copy number of RAG-1 gene was measured as the control. Copy number of TRECs is represented as the number in 105 PBMCs.
Quantification of TRECs in HTLV-I–infected and uninfected individuals.
(A) Individuals younger than 50, (B) individuals older than 50. Genomic DNAs (200 ng) isolated from PBMCs were used for quantification of TRECs. In each sample, the copy number of RAG-1 gene was measured as the control. Copy number of TRECs is represented as the number in 105 PBMCs.
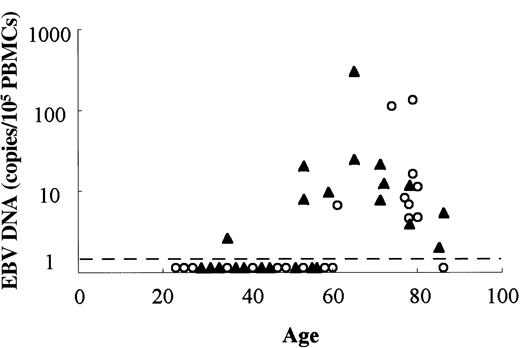
EBV load in HTLV-I carriers and patients with HAM/TSP
The above data suggest that HTLV-I infection is associated with a decrease in the number of naive T cells, which might be the underlying mechanism of immunodeficiency observed in HTLV-I carriers. Therefore, we analyzed the immunologic competence of HTLV-I carriers and patients with HAM/TSP. Although most adults are infected with EBV, EBV infection is latent in healthy individuals.27 Immunodeficiency caused by HIV infection and immunosuppressive drugs causes reactivation of EBV.28 Real-time PCR showed that the copy number of EBV DNA was greater in HTLV-I carriers and patients with HAM/TSP compared with uninfected individuals (Figure 5). EBV DNA was detected in 6 of 16 (38%) individuals less than 70 years of age, whereas it was detected in only 1 of 11 uninfected controls (9%). In individuals older than 70 years, EBV DNA was detected in infected and noninfected persons. Although EBV+lymphoma was reported in one patient with ATL, a high number of EBV-infected cells in lymph nodes of patients with ATL has been recognized.29 Thus, the increased EBV DNA is considered to reflect the immunodeficient state caused by HTLV-I infection.
Comparison of EBV load in HTLV-I–infected and uninfected individuals.
EBV DNAs in PBMCs were quantified by real-time PCR in HTLV-I infected (▴) and uninfected (○) individuals. Dashed line represents the sensitivity of this assay (1 copy/200 ng genomic DNA).
Comparison of EBV load in HTLV-I–infected and uninfected individuals.
EBV DNAs in PBMCs were quantified by real-time PCR in HTLV-I infected (▴) and uninfected (○) individuals. Dashed line represents the sensitivity of this assay (1 copy/200 ng genomic DNA).
IL-7 in HTLV-I–infected individuals
The reduced generation of naive T lymphocytes and TRECs is thought to correlate with impaired thymic functions in HTLV-I–infected individuals as mentioned above. IL-7 is the key cytokine required during the early development of T lymphocytes in the thymus.30 Therefore, we compared plasma levels of IL-7 in HTLV-I–infected individuals and age-matched controls. Plasma concentrations of IL-7 were higher in HTLV-I carriers and patients with ATL than in uninfected individuals (P = .0016, by Mann-Whitney; Figure 6). The level of IL-7 did not correlate with the number of naive T cells. These results suggest that the high levels of IL-7 may be involved in compensating for overproduction of T cells in the thymus.
Comparison of plasma IL-7 concentrations in HTLV-I–infected individuals.
Plasma concentrations of IL-7 were measured by Quantikine HS.
Comparison of plasma IL-7 concentrations in HTLV-I–infected individuals.
Plasma concentrations of IL-7 were measured by Quantikine HS.
Discussion
In this study, we found a low number of naive T lymphocytes in both CD4+ and CD8+ subpopulations in HTLV-I–infected individuals. The extent of such decrease did not correlate with provirus load but was associated with HTLV-I infection itself, whereas the number of memory T cells correlated with HTLV-I provirus load. Measurement of TRECs showed that the generation of naive T lymphocytes was impaired in HTLV-I carriers and patients with HAM/TSP. Simpson and coworkers31 demonstrated that inoculation of rabbits with a cell line infected with HTLV-I resulted in thymic atrophy, which was associated with infiltration of HTLV-I–infected cells into the thymus. Other studies have also reported thymic atrophy in pX transgenic rats.32 These findings suggest the association between HTLV-I infection and thymic atrophy. Recent studies have reported increased expression of LIF, oncostatin M, and IL-6 in the thymus during aging, a process thought to be associated with thymic atrophy, as well as decreased number of naive T cells.20 Thus, inflammatory cytokines are thought to be associated with thymic atrophy because injection of these cytokines results in a decrease in naive T cells in mice. High serum concentrations of IL-6 have been reported in HTLV-I seropositive individuals.33 Taken together, such an increased production of cytokines in HTLV-I–infected individuals might have suppressive effects on T lymphopoiesis in the thymus, resulting in reduced numbers of naive T cells.
Interleukin 7 is a critical cytokine for T lymphopoiesis, and its level does not change with aging.20 Our results revealed high plasma concentrations of IL-7 in individuals infected with HTLV-I including patients with ATL, who also showed a decrease in naive T cells. These results suggest that increased circulating levels of IL-7 may represent a compensatory mechanism to maintain the number of naive T cells in HTLV-I individuals.
The frequency of opportunistic infection in patients with ATL is higher than in patients with other hematologic malignancies.11The immunodeficient state is also recognized in HTLV-I carriers and patients with HAM/TSP without malignancies and is associated with infections caused by various fungi, viruses, and protozoa.34 Our study showed that low counts of naive T lymphocytes might be the underlying mechanism of such infections. The degree of naive T-cell depletion was more severe in patients with acute or lymphoma-type ATL than in patients with chronic and smoldering ATL, which is consistent with the clinical finding that opportunistic infections are more frequent in patients with acute or lymphoma-type ATL. Thus, the high levels of cytokines, including IL-6, in such patients are likely to be involved in the suppression of T lymphopoiesis.
Epstein-Barr virus DNA is often detected in the lymph nodes of patients with ATL,29 and we also observed a marked increase in EBV DNA in patients with ATL (unpublished observations, March 1999). In such cases, increased EBV is not considered to play an etiologic role, but rather reflects the immunodeficiency caused by HTLV-I. In our study, we showed increased EBV DNA in HTLV-I–infected individuals and uninfected individuals over 70 years of age. Low naive T-cell counts were also observed in elderly persons, some of whom had high EBV DNA in PBMCs, strongly indicating the association between EBV DNA and suppression of naive T cells. Negative reaction to PPD is reported to be common in HTLV-I–infected individuals.35These findings are considered as evidence of immunodeficiency in HTLV-I–infected individuals, which is due to suppression of naive T lymphocytes. Recent studies by Matsuyama and colleagues36have demonstrated high seropositivity among patients withMycobacterium avium complex (MAC) infection. Because defense against MAC infection depends on cell-mediated immunity, especially T lymphocytes, impaired cell-mediated immunity in HTLV-I carriers might be responsible for increased susceptibility to MAC infection.
It is noteworthy to compare the pathogenesis of immunodeficiency observed in HTLV-I–infected individuals with that of HIV-1–infected individuals. In HIV-1–infected individuals, HIV-1 itself and its products, such as gp120,37 have been considered to result in the decreased number of naive and memory T lymphocytes, whereas HTLV-I infection increased the production of inflammatory cytokines in HTLV-I–infected individuals, leading to a decrease of naive T lymphocytes. From this viewpoint, increased production of inflammatory cytokines in HIV-infected individuals might be implicated in the decrease of naive T lymphocytes in addition to the direct effect of virus.
Increased number of CD4+ T lymphocytes is considered to be due to HTLV-I, because the latter is known to cause the proliferation of this T-cell subset both in vitro and in vivo.38,39 This is consistent with our finding that HTLV-I provirus load correlated with the number of CD4+memory T lymphocytes. In our study, the data suggested that the majority of memory T cells were uninfected cells, and the increase in the number of memory T cells was caused by the immune response. HTLV-I can infect several types of cells including T cells, B lymphocytes, monocytes, myeloid cells, and fibroblasts, strongly suggesting that the receptor(s) for HTLV-I is not cell-type specific.40 The finding that HTLV-I provirus is predominantly detected in CD4+ memory T lymphocytes in vivo suggests the preferential proliferation of this subset, presumably affected by viral products, especially Tax. In fact, clonal proliferation has been reported in HTLV-I–infected individuals. The mechanism of such preferential proliferation needs further study.
We are grateful to Drs Hitoshi Suzushima and Minoru Yoshida for providing the clinical samples used in this study. The authors also thank Dr F. G. Issa (word-medex.com.au) for the careful reading and editing of the manuscript.
Department of Internal Medicine II, Department of Neurology, Kumamoto University School of Medicine, Kumamoto, Japan; Amakusa Chuo General Hospital, Kumamoto, Japan; Laboratory of Virus Immunology, Institute for Virus Research, Kyoto University, Kyoto, Japan.
Submitted October 16, 2000; accepted January 19, 2001.
Supported by a grant-in-aid for Scientific Research from the Ministry of Education, Science, Sports and Culture of Japan.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Masao Matsuoka, Laboratory of Virus Immunology, Institute of Virus Research, Kyoto University, Kyoto, Japan 606-8506; e-mail: mmatsuok@virus1.virus.kyoto-u.ac.jp.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal