Abstract
Autoimmune lymphoproliferative syndrome (ALPS) is an inherited disorder in which genetic defects in proteins that mediate lymphocyte apoptosis, most often Fas, are associated with enlargement of lymph nodes and the spleen and a variety of autoimmune manifestations. Some patients with ALPS have relatives with these same apoptotic defects, however, who are clinically well. This study showed that the circulating levels of interleukin 10 (IL-10) were significantly higher (P < .001) in 21 patients with ALPS than in healthy controls. Moreover, the peripheral blood mononuclear cells (PBMCs) and lymphoid tissues of these patients with ALPS contained significantly higher levels of IL-10 messenger RNA (mRNA;P < .001 and P < .01, respectively). By fractionating PBMC populations, disproportionately high concentrations of IL-10 mRNA were found in the CD4−CD8−T-cell population, expansion of which is virtually pathognomonic for ALPS. Immunohistochemical staining showed intense IL-10 protein signals in lymph node regions known to contain CD4−CD8− T cells. Nonetheless, in vitro studies showed no influence of IL-10 on the survival of CD4−CD8− T cells. Overexpression of IL-10 in patients with inherited apoptotic defects is strongly associated with the overt manifestations of ALPS.
Introduction
Since 1990, we have evaluated patients with a constellation of features, including chronic nonmalignant, noninfectious lymphadenopathy; hepatosplenomegaly, and autoimmune phenomena. This condition, subsequently named by us autoimmune lymphoproliferative syndrome (ALPS),1-4 and lymphoproliferation with autoimmunity or the Canale-Smith syndrome by others5-7 was characterized by a marked expansion of α/β T-cell receptor (TCR)+CD4−CD8− T cells (double-negative T cells; DNTCs). Thus, the condition appeared clinically and immunologically similar to that in mice bearing thelpr and gld gene mutations that have defects in the lymphocyte apoptosis pathway mediated by the cell surface receptor Fas (CD95; APO1).8 Recognition of the common phenotype oflpr mice and ALPS patients enabled us to establish Fas defects as a primary genetic and cellular basis of the disorder.
Currently, we recognize 3 genetically distinct subtypes of ALPS. Patients with ALPS type Ia are genetically similar to thelpr mice and possess mutations in TNFRSF6, the gene encoding Fas itself.9-14 Patients with ALPS type Ib, like the gld mice, possess mutations in the gene for the Fas ligand.15 Patients designated as having ALPS type II have mutations in caspase 10,16 and patients with ALPS type III have lymphocyte apoptotic defects, but the mutations responsible for their disorder have not yet been identified.9 17 Although these forms of ALPS differ genetically, they share defects in crucial homeostatic apoptotic mechanisms for controlling mature lymphocytes, leading to the features of ALPS.
Ongoing studies have revealed a broad clinical spectrum of ALPS and its major complications, including hypersplenism, autoimmune hemolytic anemia, thrombocytopenia, and neutropenia.4,14,18Possession of a particular mutation or defective apoptosis is not sufficient to produce ALPS; many relatives of ALPS patients possess the same Fas mutations or apoptotic defects but remain in good health.2,3,5,9-14 18 Thus, there must be additional factors that determine whether overt clinical features of ALPS occur.
In an earlier study of the immunologic features of ALPS, we showed that activated T cells that expressed DR antigens, when stimulated in vitro, manifested a T helper 2 (Th2) phenotype, with impaired production of interleukin (IL) 2 and interferon (IFN)-γ and increased IL-4 and IL-5 release.19 Additionally, stimulated monocytes/macrophages produced subnormal amounts of IL-12 and increased amounts of IL-10. Mean serum levels of IL-4, IFN-γ, and IL-2 as well as tumor necrosis factor (TNF)-α, IL-1β, and IL-6 were generally normal or mildly elevated. Serum IL-10 concentrations, however, were strikingly elevated in 9 patients with ALPS (a median increase of 26-fold).
On the basis of these observations, we hypothesized that an elevated IL-10 level could promote some clinical features of ALPS and, thus, intensify disease expression in individuals with defective lymphocyte apoptosis.19 In this report, we explored the origins and nature of IL-10 production in ALPS patients and their family members. We have established that individuals with ALPS have elevated levels not only of IL-10 protein in serum and in lymphoid tissues, but also of IL-10 messenger RNA (mRNA) in tissues and in peripheral blood mononuclear cells (PMBCs), with disproportionate elevations in DNTCs. The elevations in IL-10 protein and mRNA correlated strongly with disease expression and not with the presence of defective in vitro apoptosis or a Fas mutation.
Patients, materials, and methods
Subjectenrollment
Patients and their families were evaluated for unexplained lymphadenopathy, splenomegaly, or both, at the Warren Grant Magnuson Clinical Center of the National Institutes of Health (Bethesda, MD). Peripheral blood specimens were obtained by standard phlebotomy or by leukopheresis from patients with ALPS, their relatives, and normal controls under approved clinical research protocols with written informed consent. Patients were classified as having ALPS based on the following case criteria: clinical history of chronic nonmalignant lymphadenopathy, evidence of defective apoptosis in vitro, and elevated percentages (≥ 1%) of DNTCs. Fas gene sequencing was performed, as described.2,9,11 14
Cellpreparation
The PBMCs were obtained from all subjects by standard Ficoll-Hypaque centrifugation of the product of a leukopheresis or standard phlebotomy. The cells were washed 3 times in Dulbecco phosphate-buffered saline, resuspended in RPMI 1640, 20% heat-inactivated fetal calf serum (FCS), and 50 μg/mL gentamicin (MA Whittaker Bioproducts, Walkersville, MD) and counted. The cells were frozen in RPMI 1640, 10% FCS, and 7.5% DMSO (final concentration; Sigma Chemical, St Louis, MO) using a controlled-rate freezer (Cryomed, New Baltimore, MI) and stored in vapor-phase liquid nitrogen.
IL-10 determination
Levels in serum of human IL-10 were determined on coded specimens using a commercially available enzyme-linked immunosorbent assay kit (Endogen, Cambridge, MA). The limit of sensitivity of the assay as supplied by the manufacturer was 3 pg/mL.
RNA preparation and processing
For quantitative reverse transcriptase-polymerase chain reactions (RT-PCR) on PBMCs, RNA was extracted from cryopreserved cells using Qiagen RNeasy 96 and RNeasy mini kits with QIAshredder homogenizers (Qiagen, Santa Clara, CA) and suspended in DEPC-treated dH2O. RNA was divided into 4 aliquots, coded, and frozen at −80°C until use. Immediately prior to RT-PCR, each RNA sample was rendered free of contaminating DNA by incubation with 20 U RNase-free DNase I (Stratagene, La Jolla, CA), 1 × PCR buffer (no Mg2+), and 40 U of recombinant ribonuclease inhibitor (RNasin; Promega, Madison WI) for 30 minutes at 37°C, followed by a 15-minute heat-inactivation step at 65°C.
The KMM-1 cells were chosen as a control standard for the PBMC IL-10 mRNA studies. The KMM-1 cell line was developed by Dr A. Togawa20 and provided to us by Dr Takemi Otsuki. It constitutively synthesizes high levels of IL-10 mRNA, as detected on Atlas human complementary DNA (cDNA) expression array filters (Clontech Laboratories, Palo Alto, CA) (M.R., October 3, 1997). The IL-10 mRNA content of all patient samples was compared with a dilution series of total KMM-1 RNA.
For semiquantitation of IL-10 mRNA in tissues, paraffin-embedded archival specimens from 15 lymph nodes and spleens of 9 ALPS patients and from 8 cases with reactive lymphoid hyperplasia. Total RNA was extracted from tissue slices by guanidine thiocyanate/CsCl centrifugation.
RT-PCR
Initial semiquantitative reactions with PBMCs were done using separate RT and PCR reactions. RT reactions were done with a Superscript-RT kit (Gibco-BRL, Gaithersburg, MD) using oligo-dT, according to the manufacturer's instructions. Primers for PCR amplification of human IL-10 (IL-10) were: 10For(ward) = CTTCGAGATCTCCGAGA TGCCTTC; 10Rev(erse) = ATTCTTCACCTGCTCCACGGCCTT. Primers for human glyceraldehyde phosphate dehydrogenase (GAPDH) amplification were described previously.12 The thermal cycler program consisted of 95°C for 3 minutes, 80°C for 10 minutes, followed by 35 cycles of 95°C for 1 minute/61°C for 1 minute/72°C for 2 minutes, followed by 72°C for 1 minute and a 4°C hold.
Quantitative fluorescent (QF) RT-PCR in a real-time assay was done on coded PBMC specimens using sequence-specific primers and probes for IL-10 and GAPDH. The GAPDH primers were designed to match the Perkin Elmer GAPDH control reagent kit (Perkin Elmer, Foster City, CA). RT-PCR reactions for IL-10 and 18S human ribosomal RNA (rRNA) were carried out in a single tube with probes and primers for 18S rRNA (Perkin Elmer) and the IL-10 probe and the forward and reverse primers shown below.
The QF RT-PCR reactions were carried out in a 25-μL final volume using the following reaction mixture: 1∥× Taqman buffer A, 5.5 mM MgCl2, 0.025 U/μL Amplitaq Gold, 300 μM dATP, 300 μM dCTP, 300 μM dGTP, 600 μM dUTP, and 0.025 U/μL SuperscriptII reverse transcriptase (Life Technologies, Gaithersburg, MD). For reactions in which IL-10 and GAPDH mRNAs were quantitated individually in separate wells, 400 nM each of the forward and reverse IL-10 primers (IL-10 For: TCAAGGCGCATGTGAACTC; IL-10 Rev: CGGCCT TGCTCTTGTTTTC), and 75 nM FAM-labeled IL-10 probe (FAM-CGGCGCTGTCATCGAT TTCTTCC-TAMRA (Bioserve Biotechnologies, Laurel MD) were used in the IL-10 reactions, whereas 200 nM GAPDH primers and 100 nM TET-labeled GAPDH probe were used in the GAPDH reactions. Studies in which IL-10 and 18S rRNA were quantitated in the same tube required 900 nM forward and reverse IL-10 primers (10.2For = GTGATGCCCCAAGCTG AGA, 10.2Rev = GGCCTTGCTCTTGTTTTCACA), with 200 nm IL-10 probe (the same probe was used in both sets of reactions). The primers IL-10For and IL-10.2For are homologous to sequence in exon 3 of the IL-10gene; primers IL-10Rev and IL-10.2Rev are in exon 4. The IL-10 probe straddles the exon 3/4 boundary. 18S rRNA primers were added at 50 nM each, with 50 nM probe. Thermal cycler conditions were 48°C for 45 minutes, 95°C for 10 minutes, followed by 40 cycles of 95°C for 0.5 minutes/60°C for 2 minutes for all IL-10 and GAPDH reactions. For IL-10 and 18S rRNA reactions, the reactions conditions were 49°C for 30 minutes, 95°C for 10 minutes, followed by 40 cycles of 95°C for 0.5 minutes/60°C for 2 minutes.
In all QF RT-PCR experiments, each sample was assayed in triplicate. Fluorescent PCR reactions were done in an ABI Model 7700 Sequence Detector (PE Applied Biosystems, Foster City, CA). Reactions were controlled by a Power Macintosh 4400 (Apple Computer, Santa Clara, CA) on which the data analysis was performed using Sequence Detector v1.6 software (PE Applied Biosystems).
RNA extracted from lymphoid tissues was reverse transcribed using RNase H-RT (Superscript; Gibco-BRL) as previously described with some modifications.21 The resultant cDNA was amplified using radiolabeled dNTP by a semiquantitative PCR. The number of amplification cycles was selected for each primer to provide the maximum signal intensity within the linear portion of a product versus the template amplification curve. Aliquots from each amplification reaction were electrophoresed through 6% acrylamide (Long Ranger; AT Biochem, Malvern, PA) Tris borate EDTA gels, followed by autoradiography and quantification by PhosphoImager using ImageQuant v3.3 software (Molecular Dynamics, Sunnyvale, CA). The amount of cDNA used in each amplification reaction was based on the results of a preliminary PCR for GAPDH to achieve equivalent amounts of product amplified from all samples. The primers used in these studies spanned one splice junction and were designed for amplification of short amplicons (80-130 bp) from tissue RNA that is often highly degraded RNA. In these tissue studies, the forward and reverse IL-10 primers were CTTCGAGATCTCCGAGATGCCTTC and GGATCATCTCAGACAAGGCTTGGC, respectively. The forward and reverse GAPDH primers were GCCACATCGCTAAGACACC ATGGG and CCTGGTGACCAGGCGCCCAAT.
PCR of tissue IL-10 cDNA was performed in a Robocycler thermocycler (Stratagene) with 1.25 U Taq polymerase (Gibco-BRL) after heating at 94°C for 3 minutes (“hot start”). It was then followed by serial amplification cycles (94°C, 45 seconds; primer annealing temperature and extension at 72°C) and maintained at 4°C until analysis. The number of amplification cycles was determined to remain within the linear part of the sigmoid curve reflecting the relationship between the number of amplification cycles and the amount of PCR product. PCR products were quantitated by incorporating α32P dCTP, 3000 Ci/mmol (Amersham, Arlington Heights, IL). The entire amplification reaction (50 μL) was analyzed by electrophoresis on 8% acrylamide gels, followed by autoradiography and PhophorImage analysis. The quantity of the PCR product was estimated by integrating the number of pixels in the area around each PCR band and subtracting pixels in the background. The results of RT-PCR analysis are presented as absolute numbers of normalized arbitrary units (pixels)/sample. The ability of the RT-PCR assay to detect quantitative differences in mRNA for each gene product was assessed in experiments where the input DNA derived from RNA extracted from paraffin-embedded tissues was serially diluted (100-1 ng) and then subjected to PCR amplification. Using paraffin-embedded tissues positive for a given gene product along with appropriate negative controls, we verified that the intensity of the PCR product correlated with the dilution of input cDNA in the range used for PCR (25-100 ng). Variability of results from different experiments was minimized by use of standard control RNA preparations in parallel PCR. Experiments were considered evaluable only if standard control PCR results were within 15% of the mean.
Cellfractionation
The PBMCs from patients and control subjects were fractionated by negative selection to identify relative IL-10 mRNA concentrations in B, T, and monocyte/macrophage populations. Magnetic beads directly conjugated to antibodies against cell surface markers (Dynal, Lake Success, NY) were washed twice with phosphate-buffered saline/2% FCS. Cryopreserved patient PBMCs were thawed in 50% FCS, then washed 3 times in RPMI with 10% FCS and brought to a final concentration of approximately 106 cells/mL. Cells (1 mL) were incubated with each set of washed beads (using the manufacturer's suggested concentrations) to remove specific cell populations. Cells were incubated at 4°C for 30 minutes either with anti-CD3, CD4, CD8, CD14, or CD19 beads individually or with anti-CD2, CD3, CD4, CD8, CD14, and CD19 beads simultaneously. Beads and attached cells were then magnetically isolated. The unbound cells in the supernatant were then aliquoted: one third for RT-PCR analysis of cytokine mRNA levels, and two thirds for FACS analysis to determine extent of lineage depletion on a FACScan (Becton Dickinson, San Jose, CA) using the Cellquest software package. Cell staining was done at an antibody dilution of 1:50 as follows: CD3 depletion: phycoerythrin (PE)–anti-CD3, fluorescein isothiocyanate (FITC)–anti-CD4, FITC–anti-CD8, FITC–anti-CD19; CD4 depletion: PE–anti-CD3, FITC–anti-CD4; CD8 depletion: PE–anti-CD3, FITC–anti-CD8; CD14 depletion: PE–anti-CD14, FITC–anti-CD4, FITC–anti-CD8; CD19 depletion: PE–anti-CD3, FITC–anti-CD19; DNTC enrichment: PE–anti-CD3, FITC–anti-CD4, FITC–anti-CD8, FITC–anti-CD19. All antibodies were purchased from Pharmingen (San Diego, CA).
Measurement of spontaneous apoptosis
The PBMCs were obtained by standard Ficoll-Hypaque from 2 ALPS patients with documented mutations in Fas gene and who fulfilled the case criteria for ALPS, and from 3 healthy controls. The cells were washed and cultured at a concentration of 106cells/mL in RPMI 1640, 10% heat-inactivated FCS, 100 μg/mL streptomycin, 100 U/mL penicillin G, and 2 mmol/mLl-glutamine (Life Technologies, Grand Island, NY) at 37°C and in 5% CO2. PBMCs were cultured with and without 400 ng/mL recombinant IL-10 (R&D Systems, Minneapolis, MN). Cells were harvested at 0, 24, 48, or 72 hours later and examined for apoptosis using the annexin-V/propidium iodide (PI) detection kit (Pharmingen) according to the manufacturer's instructions, in combination with cell surface staining with anti-CD3, anti-CD4, and anti-CD8 with the appropriate controls. Data were acquired with a dual laser FACSCalibur flow cytometer (Becton Dickinson) and analyzed with BD CellQuest software.
Immunohistochemistry
Immunohistochemical detection of IL-10 was performed using the avidin/biotinylated horseradish peroxidase complex (ABC) method on paraffin sections, as previous described.22 Briefly, 5-μm thick paraffin sections were mounted on Fisherbrand/plus Superfrost precleaned slides (Fisher Scientific, Pittsburgh, PA) and attached by overnight heating at 58°C. After deparaffinization and rehydration, the slides were placed in a microwave oven set at 800 W for 25 minutes. Sections were then cooled and washed in Tris-buffered saline (pH 7.6) containing 5% FCS (Life Technologies, Grand Island, NY) for 30 minutes. Primary antibody incubation with a rat neutralizing monoclonal antibody (dilution 1:100) reacting with human IL-10 (Pharmingen) was carried out overnight at room temperature. The sections were then washed and incubated with rabbit antirat IgG (dilution 1:400; Vector Laboratories, Burlingame, CA) for 30 minutes at room temperature. The color reaction was developed by overlaying ABC for 30 minutes (Vector Laboratories) and 3,3′-diaminobenzidine tetrachloride (Vector Laboratories) for 10 minutes at room temperature.
Statisticalmethods
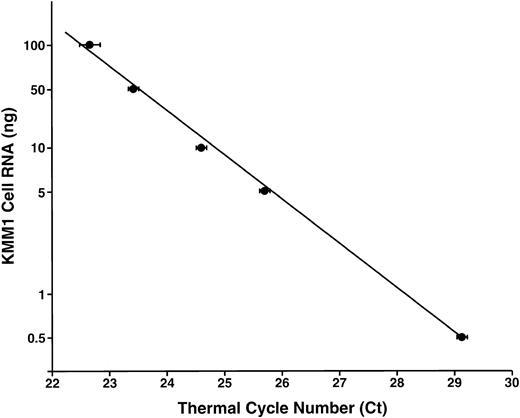
For QF RT-PCR analyses, the computed Ct values (the PCR thermal cycle number at which reaction fluorescence exceeded a threshold set at 10 SD above the mean baseline determined in the early cycles) were exported into an Excel spreadsheet (Microsoft Excel Analysis Tools, Seattle, WA). Standard curves were calculated for IL-10 and GAPDH (Figure 1), or IL-10 and 18S rRNA, levels using data from serial dilutions of KMM-1 samples run in parallel with unknowns. IL-10 and GAPDH mRNA (or 18S rRNA) data from the coded clinical samples were fit to the standard curve for subsequent quantitation relative to the standard curve created using the serially diluted total KMM-1 RNA. Computed IL-10 RNA levels were then divided by GAPDH or 18S rRNA levels to normalize for variations in quantity and quality of input RNA.
Serum IL-10 concentrations.
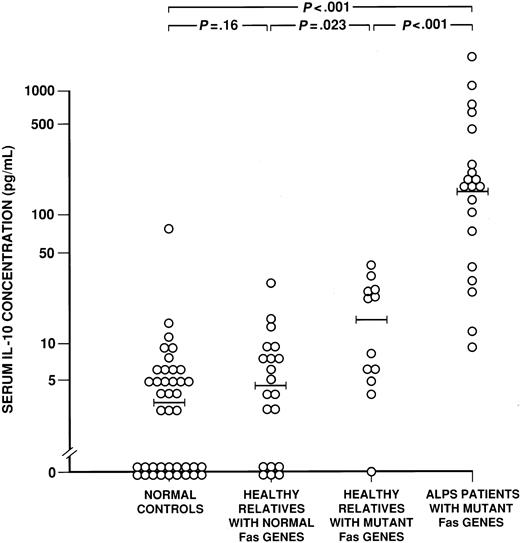
The serum IL-10 concentrations (pg/mL) are shown for 20 patients with ALPS associated with Fas mutations, 12 of their healthy relatives with Fas mutations, 20 of their relatives withoutFas mutations, and 40 healthy, nonrelated control subjects. The wide horizontal bars denote the median values for each cohort.P values for differences between cohorts are shown above the data (Wilcoxon rank sum test).
Serum IL-10 concentrations.
The serum IL-10 concentrations (pg/mL) are shown for 20 patients with ALPS associated with Fas mutations, 12 of their healthy relatives with Fas mutations, 20 of their relatives withoutFas mutations, and 40 healthy, nonrelated control subjects. The wide horizontal bars denote the median values for each cohort.P values for differences between cohorts are shown above the data (Wilcoxon rank sum test).
For each sample, the average normalized quantity of IL-10 for each triplicate reaction was found. For samples run on multiple plates, the average of all normalized values was then computed. After the samples were decoded, a mean and median normalized IL-10 value was found for the ALPS patient population and the control populations: first, healthy, related individuals with the same TNFRSF6 mutations as ALPS probands; second, relatives without TNFRSF6mutations; and third, healthy, unrelated controls. The unitless median-normalized IL-10 RNA level from each control set was then compared against patient sample values (IL-10/GAPDH or IL-10/18S rRNA) using the JMP statistical software package (SAS, Cary, NC). All samples were described by their fold difference from the median healthy, non–Fas mutant control value. Populations were compared using the Wilcoxon rank sum test. When log normalization of relative IL-10 mRNA levels allowed, parametric comparisons of populations with the Student t test were done. Relative IL-10 mRNA levels in enriched lymphocyte populations were compared using Tukey-Kramer analysis.
Results
ALPS patients have elevated circulating IL-10 levels
In a previous study, we found that 9 ALPS patients with functionalFas mutations had significantly elevated serum levels of IL-10 protein as compared with both healthy individuals and individuals with various autoimmune or lymphoproliferative diseases.19Here we confirmed and extended these observations in a study of 21 ALPS patients, including 20 with defined Fas mutations (ALPS type Ia; Table 1) and one without a Fas mutation (ALPS type III); 12 relatives with a Fas mutation (Table2); 20 relatives of ALPS type Ia patients and one relative of the ALPS type III patient, all with normal Fas (Table 3); and 40 healthy unrelated controls. Figure 1 shows that 20 ALPS patients with Fasmutations had serum IL-10 levels from 10 to more than 1000 pg/mL, being significantly higher (median, 166 pg/mL) than the levels in their healthy relatives with (n = 12) or without (n = 20) Fasmutations (median levels of 15.0 and 4.7 pg/mL, respectively) or the panel of 40 controls (median of 3.4 pg/mL; all differences,P < .001). The one ALPS type III patient with normal Fas had elevated serum IL-10 (295 pg/mL; Family 14, Table1), whereas her healthy relative, also with normal Fas, did not (4.6 pg/mL). IL-10 levels in the 12 healthy relatives who had Fas mutations and defective in vitro apoptosis (Table 2) were also increased, but to a smaller degree than seen in ALPS patients, when compared with levels in the 40 healthy unrelated normal controls (15.0 versus 3.4 pg/mL;P = .001) as well as when compared with levels in 20 healthy relatives with normal Fas (Table 3; median level of 4.7 pg/mL;P = .023). The healthy relatives with normal Fas, however, did not manifest IL-10 elevations (P = .16). The serum protein IL-10 concentration in all subjects correlated with the percentage of DNTCs (P < .001; Tables 1-3).
Clinical and laboratory features of 21 autoimmune lymphoproliferative syndrome patients
| Family* . | Race . | Gender . | Age (y) . | Age of onset . | Fas mutant . | LN size (1 to 4+)† . | Splenomegaly/ splenectomy . | α/β DNTCs (%) (no./μL3)‡ . | Serum IgG (mg/dL)1-153 . | Serum IL-10 (pg/mL) . | Autoantibodies . | Autoimmunity . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | W | M | 11 | 4 m | Y | 3+ | Y/Y | 1615 (14) | 1480 | 1761 | + | ITP |
| 2 | W | F | 12 | 18 m | Y | 4+ | Y/Y | 2144 (20) | 4020 | 719 | + | ITP, GN, PBC |
| 3 | W | M | 16 | 5 y | Y | 2+ | Y/N | 517 (8) | 1690 | 199 | + | AIHA, ITP, AIN |
| 3 | W | M | 17 | 9 y | Y | 2+ | Y/Y | 44 (2) | 1110 | 26 | + | ND |
| 3 | W | M | 41 | 3 y | Y | 2+ | Y/Y | 233 (6) | 1930 | 30 | + | AIHA, ITP |
| 4 | W | M | 14 | 2 y | Y | 3+ | Y/N | 50 (2) | 1870 | 683 | NT | ND |
| 6 | B | F | 6 | 4 m | Y | 4+ | Y/N | 1566 (13) | 780 | 467 | + | AIHA |
| 14 | W | F | 16 | 9 m | N | 4+ | Y/Y | 1067 (17) | 2090 | 295 | + | AIHA |
| 17 | W | F | 26 | 4 m | Y | 4+ | Y/Y | 395 (11) | 1380 | 135 | + | AIHA, AIN |
| 17 | W | M | 29 | 5 y | Y | 2+ | Y/Y | 93 (3) | 1200 | 39 | NT | AIN |
| 20 | W | F | 15 | 3 y | Y | 2+ | Y/N | 88 (5) | 1650 | 212 | + | GB |
| 24 | W | M | 8 | 11 m | Y | 3+ | Y/Y | 790 (18) | 2790 | 224 | NT | ITP |
| 26 | W | M | 16 | 1 m | Y | 3+ | Y/N | 367 (20) | 4910 | 1025 | + | ND |
| 26 | W | F | 37 | 5 y | Y | 2+ | Y/N | 245 (10) | 3340 | 104 | NT | ND |
| 27 | W | M | 10 | 5 y | Y | 2+ | Y/Y | 640 (8) | 1530 | 174 | + | ND |
| 30 | W | M | 9 | 12 m | Y | 1+ | Y/Y | 557 (17) | 2140 | 160 | + | AIHA, ITP, AIN, GN |
| 31 | W | F | 16 | 5 y | Y | 2+ | Y/Y | 2105 (36) | 1890 | 9 | + | AIHA, ITP, AIN |
| 31 | W | M | 37 | 18 m | Y | 1+ | Y/Y | 87 (3) | 1120 | 166 | + | ND |
| 31 | W | F | 41 | 2 y | Y | 1+ | Y/Y | 106 (4) | 1460 | 12 | + | ITP, AIN |
| 34 | W | M | 6 | 2 y | Y | 2+ | Y/N | 196 (5) | ND | 73 | + | AIHA |
| 45 | W | M | 11 | 4 y | Y | 3+ | Y/Y | 1137 (12) | 1590 | 135 | + | ITP |
| Family* . | Race . | Gender . | Age (y) . | Age of onset . | Fas mutant . | LN size (1 to 4+)† . | Splenomegaly/ splenectomy . | α/β DNTCs (%) (no./μL3)‡ . | Serum IgG (mg/dL)1-153 . | Serum IL-10 (pg/mL) . | Autoantibodies . | Autoimmunity . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | W | M | 11 | 4 m | Y | 3+ | Y/Y | 1615 (14) | 1480 | 1761 | + | ITP |
| 2 | W | F | 12 | 18 m | Y | 4+ | Y/Y | 2144 (20) | 4020 | 719 | + | ITP, GN, PBC |
| 3 | W | M | 16 | 5 y | Y | 2+ | Y/N | 517 (8) | 1690 | 199 | + | AIHA, ITP, AIN |
| 3 | W | M | 17 | 9 y | Y | 2+ | Y/Y | 44 (2) | 1110 | 26 | + | ND |
| 3 | W | M | 41 | 3 y | Y | 2+ | Y/Y | 233 (6) | 1930 | 30 | + | AIHA, ITP |
| 4 | W | M | 14 | 2 y | Y | 3+ | Y/N | 50 (2) | 1870 | 683 | NT | ND |
| 6 | B | F | 6 | 4 m | Y | 4+ | Y/N | 1566 (13) | 780 | 467 | + | AIHA |
| 14 | W | F | 16 | 9 m | N | 4+ | Y/Y | 1067 (17) | 2090 | 295 | + | AIHA |
| 17 | W | F | 26 | 4 m | Y | 4+ | Y/Y | 395 (11) | 1380 | 135 | + | AIHA, AIN |
| 17 | W | M | 29 | 5 y | Y | 2+ | Y/Y | 93 (3) | 1200 | 39 | NT | AIN |
| 20 | W | F | 15 | 3 y | Y | 2+ | Y/N | 88 (5) | 1650 | 212 | + | GB |
| 24 | W | M | 8 | 11 m | Y | 3+ | Y/Y | 790 (18) | 2790 | 224 | NT | ITP |
| 26 | W | M | 16 | 1 m | Y | 3+ | Y/N | 367 (20) | 4910 | 1025 | + | ND |
| 26 | W | F | 37 | 5 y | Y | 2+ | Y/N | 245 (10) | 3340 | 104 | NT | ND |
| 27 | W | M | 10 | 5 y | Y | 2+ | Y/Y | 640 (8) | 1530 | 174 | + | ND |
| 30 | W | M | 9 | 12 m | Y | 1+ | Y/Y | 557 (17) | 2140 | 160 | + | AIHA, ITP, AIN, GN |
| 31 | W | F | 16 | 5 y | Y | 2+ | Y/Y | 2105 (36) | 1890 | 9 | + | AIHA, ITP, AIN |
| 31 | W | M | 37 | 18 m | Y | 1+ | Y/Y | 87 (3) | 1120 | 166 | + | ND |
| 31 | W | F | 41 | 2 y | Y | 1+ | Y/Y | 106 (4) | 1460 | 12 | + | ITP, AIN |
| 34 | W | M | 6 | 2 y | Y | 2+ | Y/N | 196 (5) | ND | 73 | + | AIHA |
| 45 | W | M | 11 | 4 y | Y | 3+ | Y/Y | 1137 (12) | 1590 | 135 | + | ITP |
LN indicates lymph node; DNTCs, double-negative T cells; IgG, immunoglobulin G; IL-10, interleukin-10; ITP, idiopathic thrombocytopenic purpura; GN, glomerulonephritis; PBC, primary biliary cirrhosis; AIHA, autoimmune hemolytic anemia; AIN, autoimmune neutropenia; ND, none documented; NT, not tested; GB, Guillain-Barre.
The number refers to the family in the NIH ALPS research cohort from whom samples were available for this study.
Lymph node sizes as defined in reference 2.
Normal range of 0 to 18 cells/μL3, or less than 1% of all lymphocytes.
Normal range of 523 to 1482 μg/dL.
Clinical and laboratory features of 12 healthy relatives with Fas defects
| Family* . | Race . | Gender . | Age (y) . | LN size (1 to 4+)† . | Splenomegaly/ splenectomy . | α/β DNTCs (%) (no./μL3)‡ . | Serum IgG (mg/dL)2-153 . | Serum IL-10 (pg/ml) . | Autoantibodies . | Autoimmunity . |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | W | F | 48 | 1+ | N/N | 11 (0.8) | NT | 26 | NT | NDf |
| 1 | W | F | 28 | 1+ | N/N | 18 (0.7) | 782 | 6 | − | ND |
| 2 | W | M | 70 | 1+ | N/N | 7 (0.5) | 688 | 8 | − | ND |
| 2 | W | F | 41 | 1+ | N/N | 8 (0.6) | 1010 | 4 | + | ND |
| 4 | W | F | 34 | 1+ | N/N | 36 (1.2) | NT | 25 | NT | ND |
| 4 | W | F | 62 | 1+ | N/N | 7 (0.3) | 1300 | 5 | − | ND |
| 4 | W | F | 37 | 1+ | N/N | 6 (0.3) | 844 | 0 | + | ND |
| 5 | W | F | 37 | 1+ | N/N | 9 (0.7) | 909 | 6 | + | ND |
| 20 | W | M | 45 | 1+ | N/N | 16 (1.2) | 1380 | 22 | − | ND |
| 20 | W | F | 10 | 1+ | N/N | 32 (2.0) | 1350 | 35 | − | ND |
| 30 | W | F | 38 | 1+ | N/N | 27 (2.1) | 1400 | 11 | + | ND |
| 31 | W | M | 33 | 1+ | N/N | 22 (2.1) | 1450 | 23 | − | ND |
| Family* . | Race . | Gender . | Age (y) . | LN size (1 to 4+)† . | Splenomegaly/ splenectomy . | α/β DNTCs (%) (no./μL3)‡ . | Serum IgG (mg/dL)2-153 . | Serum IL-10 (pg/ml) . | Autoantibodies . | Autoimmunity . |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | W | F | 48 | 1+ | N/N | 11 (0.8) | NT | 26 | NT | NDf |
| 1 | W | F | 28 | 1+ | N/N | 18 (0.7) | 782 | 6 | − | ND |
| 2 | W | M | 70 | 1+ | N/N | 7 (0.5) | 688 | 8 | − | ND |
| 2 | W | F | 41 | 1+ | N/N | 8 (0.6) | 1010 | 4 | + | ND |
| 4 | W | F | 34 | 1+ | N/N | 36 (1.2) | NT | 25 | NT | ND |
| 4 | W | F | 62 | 1+ | N/N | 7 (0.3) | 1300 | 5 | − | ND |
| 4 | W | F | 37 | 1+ | N/N | 6 (0.3) | 844 | 0 | + | ND |
| 5 | W | F | 37 | 1+ | N/N | 9 (0.7) | 909 | 6 | + | ND |
| 20 | W | M | 45 | 1+ | N/N | 16 (1.2) | 1380 | 22 | − | ND |
| 20 | W | F | 10 | 1+ | N/N | 32 (2.0) | 1350 | 35 | − | ND |
| 30 | W | F | 38 | 1+ | N/N | 27 (2.1) | 1400 | 11 | + | ND |
| 31 | W | M | 33 | 1+ | N/N | 22 (2.1) | 1450 | 23 | − | ND |
Clinical and laboratory features of 20 healthy relatives without Fas defects
| Family3-150 . | Race . | Gender . | Age (y) . | LN size (1 to 4+)3-151 . | Splenomegaly/ splenectomy . | α/β DNTCs (%) (no./μL3)3-152 . | Serum IgG (mg/dL)3-153 . | Serum IL-10 (pg/ml) . | Autoantibodies . | Autoimmunity . |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | W | M | 30 | 1+ | N/N | NT′ | 1370 | 0 | − | ND |
| 2 | W | F | 69 | 1+ | N/N | 2 (0.1) | 819 | 3 | − | ND |
| 2 | W | F | 44 | 1+ | N/N | NT | NT | 9 | NT | ND |
| 2 | W | M | 46 | 1+ | N/N | 5 (0.2) | 1060 | 0 | + | ND |
| 2 | W | F | 13 | 1+ | N/N | 16 (0.8) | 927 | 0 | + | ND |
| 2 | W | F | 43 | 1+ | N/N | NT | NT | 4 | NT | ND |
| 3 | W | M | 14 | 1+ | N/N | 14 (0.7) | 928 | 0 | − | ND |
| 3 | W | F | 65 | 1+ | N/N | 4 (0.2) | 949 | 0 | − | ND |
| 3 | W | F | 35 | 1+ | N/N | 19 (0.6) | 708 | 4 | − | ND |
| 4 | W | M | 45 | 1+ | N/N | 7 (0.8) | NT | 29 | NT | ND |
| 4 | W | M | 36 | 1+ | N/N | 9 (0.6) | 950 | 3 | + | ND |
| 4 | W | M | 12 | 1+ | N/N | NT | 954 | 7 | − | ND |
| 4 | W | F | 7 | 1+ | N/N | NT | 818 | 6 | + | ND |
| 4 | W | M | 9 | 1+ | N/N | NT | 887 | 0 | − | ND |
| 5 | W | M | 38 | 1+ | N/N | 10 (0.7) | 708 | 14 | − | ND |
| 6 | B | M | 36 | 1+ | N/N | 4 (0.2) | 990 | 15 | − | ND |
| 6 | B | F | 35 | 1+ | N/N | 24 (0.7) | 1450 | 5 | + | ND |
| 11 | B | F | 37 | 1+ | N/N | 5 (0.4) | 1110 | 5 | + | ND |
| 14 | W | F | 36 | 1+ | N/N | 9 (0.5) | 886 | 5 | − | ND |
| 27 | W | F | 42 | 1+ | N/N | 6 (0.3) | 824 | 9 | − | ND |
| Family3-150 . | Race . | Gender . | Age (y) . | LN size (1 to 4+)3-151 . | Splenomegaly/ splenectomy . | α/β DNTCs (%) (no./μL3)3-152 . | Serum IgG (mg/dL)3-153 . | Serum IL-10 (pg/ml) . | Autoantibodies . | Autoimmunity . |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | W | M | 30 | 1+ | N/N | NT′ | 1370 | 0 | − | ND |
| 2 | W | F | 69 | 1+ | N/N | 2 (0.1) | 819 | 3 | − | ND |
| 2 | W | F | 44 | 1+ | N/N | NT | NT | 9 | NT | ND |
| 2 | W | M | 46 | 1+ | N/N | 5 (0.2) | 1060 | 0 | + | ND |
| 2 | W | F | 13 | 1+ | N/N | 16 (0.8) | 927 | 0 | + | ND |
| 2 | W | F | 43 | 1+ | N/N | NT | NT | 4 | NT | ND |
| 3 | W | M | 14 | 1+ | N/N | 14 (0.7) | 928 | 0 | − | ND |
| 3 | W | F | 65 | 1+ | N/N | 4 (0.2) | 949 | 0 | − | ND |
| 3 | W | F | 35 | 1+ | N/N | 19 (0.6) | 708 | 4 | − | ND |
| 4 | W | M | 45 | 1+ | N/N | 7 (0.8) | NT | 29 | NT | ND |
| 4 | W | M | 36 | 1+ | N/N | 9 (0.6) | 950 | 3 | + | ND |
| 4 | W | M | 12 | 1+ | N/N | NT | 954 | 7 | − | ND |
| 4 | W | F | 7 | 1+ | N/N | NT | 818 | 6 | + | ND |
| 4 | W | M | 9 | 1+ | N/N | NT | 887 | 0 | − | ND |
| 5 | W | M | 38 | 1+ | N/N | 10 (0.7) | 708 | 14 | − | ND |
| 6 | B | M | 36 | 1+ | N/N | 4 (0.2) | 990 | 15 | − | ND |
| 6 | B | F | 35 | 1+ | N/N | 24 (0.7) | 1450 | 5 | + | ND |
| 11 | B | F | 37 | 1+ | N/N | 5 (0.4) | 1110 | 5 | + | ND |
| 14 | W | F | 36 | 1+ | N/N | 9 (0.5) | 886 | 5 | − | ND |
| 27 | W | F | 42 | 1+ | N/N | 6 (0.3) | 824 | 9 | − | ND |
For abbreviations, see Table 1.
The number refers to the family in the NIH ALPS research cohort from whom samples were available for this study.
Lymph node sizes as defined in reference 2.
Normal range of 0 to 18 cells/μL3, or less than 1% of all lymphocytes.
Normal range of 523 to 1482 μg/dL.
IL-10 mRNA is elevated in ALPS patient PBMCs
After establishing that ALPS patient sera contained high levels of IL-10, we sought to determine its cellular source and its relationship to clinical status and Fas mutations by analyzing PBMC samples from patients and controls with 2 sensitive and specific QF RT-PCR assays for IL-10 mRNA.
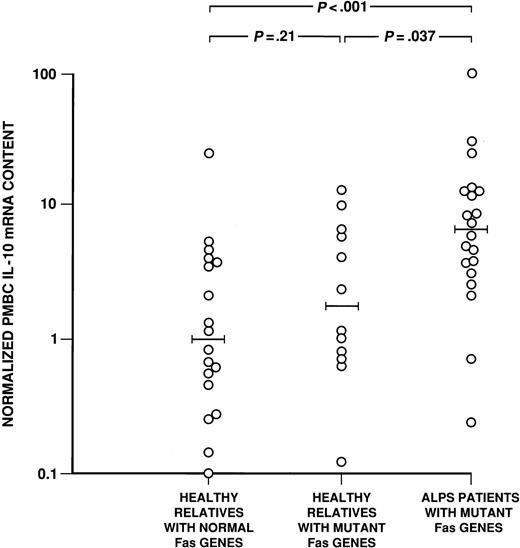
In the first set of QF RT-PCRs, the IL-10 mRNA level for each sample was normalized to that sample's GAPDH mRNA content, determined in a parallel reaction tube, to control for quality and quantity of input RNA. Samples were compared by calibration of their IL-10 mRNA content against a standard curve created by serially diluting RNA from IL-10–producing KMM-1 cells (Figure 2). By this assay, PBMCs from 20 ALPS patients with Fasmutations were found to contain more IL-10 mRNA than did PBMCs from their 12 healthy relatives with Fas mutations (median normalized level of 6.5 vs. 1.7; P = .037) or PBMCs from 20 relatives without Fas mutations (levels of 6.5 versus 1.0; P < .001) (Figure 3). Moreover, 12 healthy relatives with Fas mutations had similar IL-10 mRNA levels to those of 20 healthy relatives withoutFas mutations (median normalized levels of 1.7 versus 1.0;P = .21; Figure 3). Thus, Fas status alone did not determine whether an individual had increased IL-10 mRNA. It should be noted that the PBMCs from patient 14 with ALPS type III also contained higher levels of IL-10 RNA (normalized content of 54.6) than did PBMCs from her healthy relative without a Fas defect and other controls.
IL-10 mRNA concentration.
The IL-10 mRNA concentration in total input KMM-1 mRNA is shown as a function of the mean (± SD) thermal cycle number (Ct) of triplicate reactions. The results demonstrate linearity over 2 logs. This creates the standard curve for estimation of the IL-10 mRNA content in sample PBMCs.
IL-10 mRNA concentration.
The IL-10 mRNA concentration in total input KMM-1 mRNA is shown as a function of the mean (± SD) thermal cycle number (Ct) of triplicate reactions. The results demonstrate linearity over 2 logs. This creates the standard curve for estimation of the IL-10 mRNA content in sample PBMCs.
The mRNA levels in PBMCs.
The log distribution of average fold differences between median GAPDH-normalized IL-10 mRNA levels in 20 ALPS PBMCs is compared with the values for PBMCs from 12 healthy relatives with Fasmutations and 20 healthy relatives with normal Fas genes. The horizontal bars denote the median values for each cohort.P values for differences between these cohorts are shown above the data (Wilcoxon rank sum test).
The mRNA levels in PBMCs.
The log distribution of average fold differences between median GAPDH-normalized IL-10 mRNA levels in 20 ALPS PBMCs is compared with the values for PBMCs from 12 healthy relatives with Fasmutations and 20 healthy relatives with normal Fas genes. The horizontal bars denote the median values for each cohort.P values for differences between these cohorts are shown above the data (Wilcoxon rank sum test).
To confirm these observations, we designed a second QF RT-PCR assay in which IL-10 mRNA levels were normalized relative to 18S rRNA levels in the same reaction tube. This ensured that differences in GAPDH levels in various cell subpopulations would not skew our results, as they might have done in the prior experiment. In these experiments, PBMC samples were assayed from 14 ALPS type I patients, 8 healthy relatives with Fas mutations, 14 relatives with normal Fas, and 21 healthy unrelated controls. The median normalized IL-10 mRNA level for 14 ALPS Type Ia patients (7.6) was higher than that of the 21 unrelated controls (1.0; P = .03) and that of 8 relatives withFas mutations (1.0; P = .007). The median normalized level of IL-10 mRNA in these 8 relatives with mutations (1.0) was similar to that of 14 relatives with normal Fas (0.5;P = .13) and to that of the 21 unrelated controls (1.0;P = .7). The results obtained using this approach correlated well with those of the previous assays (Spearman rho = 0.54; P < .001).
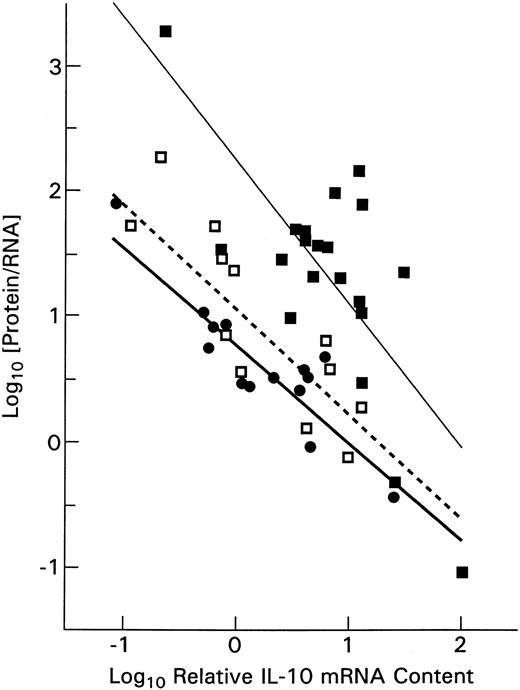
The IL-10 mRNA levels in PBMCs correlated directly with serum IL-10 protein levels (P < .001) whether GAPDH or 18S rRNA was used for assay normalization. Furthermore, we calculated that higher IL-10 mRNA levels were associated with decreasing amounts of IL-10 protein per unit of IL-10 mRNA (Figure4). This is consistent with the reported down-regulation of IL-10 mRNA transcription by higher IL-10 protein concentrations.23 Of interest, ALPS patient PBMCs not only contained elevated levels of IL-10 mRNA but, as can also be seen in Figure 4, the upward shift in the (thin continuous) regression line fitting their data indicated that the ALPS patients' PBMCs contained relatively more IL-10 protein per unit of IL-10 mRNA than did PBMCs of relatives with Fas mutations (dashed line in Figure 4) or healthy controls (heavy continuous line). This ratio was only slightly higher for relatives with Fas mutations than for the healthy controls.
The distribution of serum IL-10 protein concentration per unit of PBMC mRNA plotted against the log of relative IL-10 mRNA level.
The ALPS patients (▪ ; fitted by a light linear regression line) can be seen to produce more protein per unit of IL-10 mRNA than do the healthy subjects (●; heavier regression line). Healthy relatives who had Fas mutations show slightly more IL-10 protein per unit of RNA (■; dashed regression line) than did healthy, unrelated controls.
The distribution of serum IL-10 protein concentration per unit of PBMC mRNA plotted against the log of relative IL-10 mRNA level.
The ALPS patients (▪ ; fitted by a light linear regression line) can be seen to produce more protein per unit of IL-10 mRNA than do the healthy subjects (●; heavier regression line). Healthy relatives who had Fas mutations show slightly more IL-10 protein per unit of RNA (■; dashed regression line) than did healthy, unrelated controls.
Elevated IL-10 mRNA in ALPS patient tissues
The median circulating levels of IL-10 protein were nearly 50-fold higher in ALPS patients than in healthy controls (Figure 1), whereas median IL-10 mRNA levels in PBMCs were only 6-to 8-fold higher than in healthy relatives without Fas mutations (Figure 3) and, as indicated above, the ratio of IL-10 protein to RNA was also higher for ALPS patients (Figure 4). Among the possible explanations for this trend is that a large fraction of the IL-10 protein detected in ALPS patient serum represents release into the circulation of cytokine produced by cells in lymphoid tissues. This led us to investigate IL-10 mRNA content in 15 archival tissue lymph node and spleen blocks from 9 ALPS patients and 8 archival lymph node blocks from individuals with reactive lymphoid hyperplasia. Figure 5shows an example of the radioactive blots from which these data were derived. The relative quantity of IL-10 mRNA was estimated using a phosphorimager and normalized according to the quantity of GAPDH cDNA.
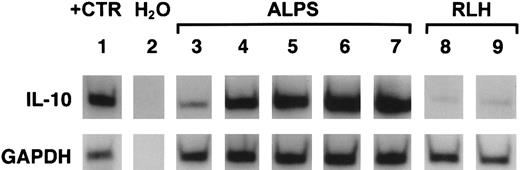
Relative IL-10 and GAPDH mRNA content in patient tissues.
Shown are autoradiographs of gels containing radiolabeled IL-10 (upper panel) and GAPDH (lower panel) cDNAs prepared by RT-PCR on tissue extracts. Lane 1 shows IL-10 cDNA and GAPDH cDNA in IL-10–producing positive control cells. Lane 2 shows the result of an RT-PCR reaction on water without added cell RNA extracts. Lanes 3 to 7 show RT-PCR products from tissues of 5 ALPS patients. Lanes 8 and 9 show products of reactions with RNAs from tissues showing reactive lymphoid hyperplasia (RLH). The relative GAPDH signals (lower panel) are similar for all specimens, but the IL-10 signals (upper panel) are markedly more intense for ALPS specimens.
Relative IL-10 and GAPDH mRNA content in patient tissues.
Shown are autoradiographs of gels containing radiolabeled IL-10 (upper panel) and GAPDH (lower panel) cDNAs prepared by RT-PCR on tissue extracts. Lane 1 shows IL-10 cDNA and GAPDH cDNA in IL-10–producing positive control cells. Lane 2 shows the result of an RT-PCR reaction on water without added cell RNA extracts. Lanes 3 to 7 show RT-PCR products from tissues of 5 ALPS patients. Lanes 8 and 9 show products of reactions with RNAs from tissues showing reactive lymphoid hyperplasia (RLH). The relative GAPDH signals (lower panel) are similar for all specimens, but the IL-10 signals (upper panel) are markedly more intense for ALPS specimens.
The median tissue IL-10 mRNA content was 441-fold higher for 9 ALPS patients than for 8 individuals with various forms of reactive lymphoid hyperplasia (P < .01). There were no gross differences in IL-10 mRNA content between lymph node and spleen tissues or between replicate sections from the same tissues. Thus, cells in lymphoid tissues could be a major source of the circulating IL-10 protein in ALPS patients.
ALPS patient DNTCs contain disproportionately high IL-10 mRNA levels
We next sought to determine which lymphocyte subpopulations in PBMCs and tissues were potential sources of the IL-10. In these studies, we used negative selection by immunomagnetic beads coated with specific antibodies to enrich for DNTCs without altering their activation state. The enrichment procedure was successful in that 29.3% ± 7.9% of cells from 4 ALPS patients were double-negative before selection and 69.7% ± 11.6% afterward; similarly, in 4 normal subjects, 3.0% ± 1.5% of cells were double-negative before selection and 12.5% ± 8.6% afterward.
It was anticipated that depletion of a cell type not producing high levels of IL-10 would increase the relative IL-10 mRNA level detected in the remaining population. Conversely, fractions depleted of high IL-10–producing cells would have low relative IL-10 mRNA levels. The effect of each depletion on residual IL-10 mRNA content was normalized to the endogenous IL-10 mRNA levels in each subject's PBMCs that had undergone sham depletions using anti-IgG beads.
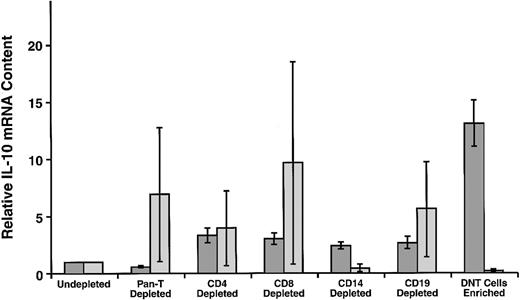
In normal controls, IL-10 mRNA levels fell substantially in the residual cell populations after CD14+ cells were depleted, indicating that CD14+ monocytes are relatively high in IL-10 mRNA (Figure 6). The CD3+ T cells contributed little, and DNTCs, in particular, provided a negligible overall contribution to IL-10 mRNA levels in these samples, as the IL-10 mRNA content increased in the residual cells after their depletion. In contrast, in the ALPS patient samples, IL-10 mRNA decreased in rough proportion to the decrease in CD3 cells (data not shown). Moreover, when patient DNTCs were enriched through depletion of all other major cell subsets, the relative IL-10 mRNA levels increased about 6-fold as compared with undepleted controls. Although all subpopulations of ALPS patient PBMCs contained elevated levels of IL-10 mRNA compared with all populations of cells from normal subjects (data not shown), the levels were disproportionately and significantly greater in the double-negative enriched T-cell subpopulation (P < .05 using Tukey-Kramer analysis).
Relative IL-10 mRNA content in PBMC subpopulations.
Cells were negatively selected to yield fractions depleted of either CD3+ cells, CD2+ and CD3+ cells, CD4+ cells, CD8+ cells, B cells, monocytes/macrophages, or were enriched for CD4−CD8− double-negative (DN) T cells by depleting all of the above cells. The mean (± SEM) results for 4 ALPS patients (shaded bars) are compared with 4 controls (lighter bars) consisting of 3 unrelated healthy controls and one related (family 3, 17-year-old boy; family 4, 14-year-old boy; family 14, 16 year-old girl; family 31, 16-year-old girl), Fas-normal, healthy control.
Relative IL-10 mRNA content in PBMC subpopulations.
Cells were negatively selected to yield fractions depleted of either CD3+ cells, CD2+ and CD3+ cells, CD4+ cells, CD8+ cells, B cells, monocytes/macrophages, or were enriched for CD4−CD8− double-negative (DN) T cells by depleting all of the above cells. The mean (± SEM) results for 4 ALPS patients (shaded bars) are compared with 4 controls (lighter bars) consisting of 3 unrelated healthy controls and one related (family 3, 17-year-old boy; family 4, 14-year-old boy; family 14, 16 year-old girl; family 31, 16-year-old girl), Fas-normal, healthy control.
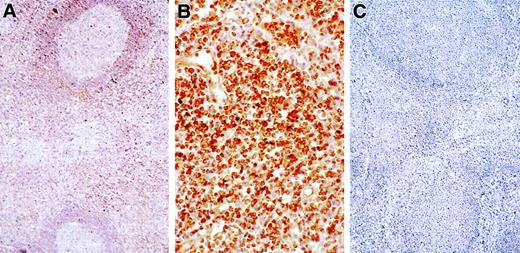
Next, we sought evidence of IL-10 protein production in lymph nodes and attempted to discern the cell types involved using immunohistochemical staining. Figure 7 demonstrates intense IL-10 staining in lymph nodes from an ALPS patient. None of 3 control nodes with reactive lymphoid hyperplasia demonstrated IL-10 signals (not shown), whereas 8 of 10 nodes tested from 9 ALPS patients showed IL-10 staining, with over 50% of cells appearing positive in 4 of them. Virtually all of the IL-10 staining was associated with lymphocytes and histiocytes in parafollicular and interfollicular regions and not in germinal centers. A large fraction of cells in the parafollicular and interfollicular regions of ALPS patient lymph nodes are known to be DNTCs,10 suggesting that at least some of the cells staining intensely for IL-10 are DNTCs, consistent with the elevated IL-10 mRNA observed in DNT-enriched peripheral leukocytes.
Immunohistochemical detection of IL-10 protein in tissues.
Positive cells contain brown pigment (ABC immunoperoxidase, hematoxylin counterstsain). (A) Lymph node from a patient with ALPS shows staining for IL-10 concentrated in the paracortex. Cells in the germinal center are not stained (× 100). (B) At higher power, intense positivity for IL-10 is observed in most of the interfollicular lymphocytes (× 400). (C) No signal for IL-10 is observed in a reactive lymph node from a normal control (× 100).
Immunohistochemical detection of IL-10 protein in tissues.
Positive cells contain brown pigment (ABC immunoperoxidase, hematoxylin counterstsain). (A) Lymph node from a patient with ALPS shows staining for IL-10 concentrated in the paracortex. Cells in the germinal center are not stained (× 100). (B) At higher power, intense positivity for IL-10 is observed in most of the interfollicular lymphocytes (× 400). (C) No signal for IL-10 is observed in a reactive lymph node from a normal control (× 100).
IL-10 does not alter DNTC survival
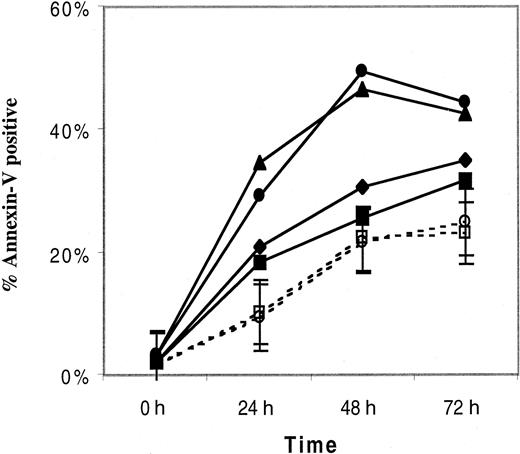
Given the detection of high levels of IL-10 in ALPS patients and its reported effects on lymphocyte proliferation and survival,24-29 it was logical to inquire whether IL-10 has any influence on spontaneous lymphocyte apoptosis in vitro. PBMCs from 2 patients with ALPS type Ia, with elevated plasma levels of IL-10 in vivo, displayed increased spontaneous apoptosis as measured by the percentage of annexin-V+ cells, when compared with 3 healthy controls. This was almost entirely the result of increased spontaneous apoptosis in the DNTCs (data not shown). The patient with the highest percentage of annexin-V+ cells had the greatest expansion of α/β TCR DNTCs (13.9% of lymphocytes [667 cells/μL] versus 6% [311 cells/μL] in the other patient, and < 1% [< 20 cells/μL] in the 3 controls). As illustrated in Figure 8, addition of 400 ng/mL recombinant IL-10 to the cultures had no effect on spontaneous in vitro apoptosis in either of the 2 patients or controls.
The influence of IL-10 on lymphocyte survival.
The percentage of apoptotic PBMCs, as measured by annexin-V staining, in 2 patients with ALPS (listed here as Pt 1 and 2, but these are not their ALPS family numbers) and the mean percentage (± SEM) of apoptotic cells of 3 healthy controls (HC), cultured with and without 400 ng/mL recombinant IL-10 and harvested after 0, 24, 28, or 72 hours. Annexin-V+ PBMCs represent the cumulative percentage of annexin-V+/propidium iodide (PI−) cells and annexin-V+/PI+ cells. ■, HC with IL-10; ○, HC without IL-10; ▪, Pt1 with IL-10; ♦, Pt1 without IL-10; ▴, Pt2 with IL-10; ●, Pt2 without IL-10.
The influence of IL-10 on lymphocyte survival.
The percentage of apoptotic PBMCs, as measured by annexin-V staining, in 2 patients with ALPS (listed here as Pt 1 and 2, but these are not their ALPS family numbers) and the mean percentage (± SEM) of apoptotic cells of 3 healthy controls (HC), cultured with and without 400 ng/mL recombinant IL-10 and harvested after 0, 24, 28, or 72 hours. Annexin-V+ PBMCs represent the cumulative percentage of annexin-V+/propidium iodide (PI−) cells and annexin-V+/PI+ cells. ■, HC with IL-10; ○, HC without IL-10; ▪, Pt1 with IL-10; ♦, Pt1 without IL-10; ▴, Pt2 with IL-10; ●, Pt2 without IL-10.
Discussion
An inherited defect of lymphocyte apoptosis, usually the result of a functional Fas mutation, is a defining characteristic of ALPS. The apoptotic defect alone, however, is not sufficient to produce the clinical manifestations of ALPS, because healthy relatives with defective in vitro apoptosis are frequently identified.2-5We therefore sought additional cofactors that might determine the clinical expression of ALPS. Previously we found that activated DR+ ALPS patient CD4+ T cells manifest a Th2 cytokine profile.19 In addition, LPS-stimulated patient macrophages produced increased amounts of IL-10 in vitro, and patients' serum had elevated IL-10 levels. These early findings in ALPS patients, and the known effects of IL-10 on survival of B and T cells, suggested IL-10 as a cofactor that determines the clinical expression of ALPS.
Here, we confirmed with sera from 20 patients with type Ia ALPS and one with type III ALPS that ALPS is associated with moderate to extreme elevations of circulating IL-10. In addition, we showed that healthy relatives with defective apoptosis may have serum IL-10 protein elevations, but these increases are modest compared with those in the ALPS patients (Figure 1). We documented that PBMCs from both type Ia and type III ALPS patients express supranormal levels of IL-10 mRNA using a highly sensitive linear RT-PCR assay based on detection of a fluorescent reporter displaced from a dual-labeled fluorogenic DNA probe specific to the amplified target cDNA. Unfractionated ALPS PBMCs contained 6- to 9-fold higher levels of IL-10 mRNA than did cells from healthy controls and cells from relatives with the same apoptotic defects.
That mean IL-10 protein levels in ALPS patient serum were nearly 50-fold higher than in serum from normal controls (medians of 166 pg/mL versus 3.4 pg/mL), whereas patient PBMCs contained only 6- to 8-fold more IL-10 mRNA than did healthy control PBMCs suggested that PBMCs are not the only source for circulating IL-10. Semiquantitative RT-PCR and immunohistochemical studies confirmed this suspicion, showing marked increases in IL-10 mRNA and IL-10 in ALPS patient lymph nodes and spleens (Figure 5).
A striking finding is that much of the IL-10 from ALPS patients was associated with DNTCs. This was true both for PBMCs IL-10 mRNA (Figure4) and for tissue IL-10 protein (Figure 7). This finding appears at odds with our prior study wherein DNTCs could not be activated in vitro and did not produce increased IL-10 on in vitro culture.19The discrepancy may be understood, however, by considering that in the present study we measured IL-10 mRNA and protein levels of unstimulated cells, reflecting in vivo induction of IL-10 synthesis. We propose that DNTCs were likely to have undergone stimulation to produce IL-10 in vivo prior to assuming an unresponsive state to subsequent in vitro challenge. If DNTCs had been stimulated in vivo, it seems likely that the high circulating IL-10 arose in large measure from cells in tissues, particularly the DNTCs that are abundant in spleen and lymph node perifollicular areas.10
As alluded to earlier, elevations in IL-10 production have ramifications for B- and T-cell survival and for the development of autoimmune disease in ALPS.24-31 First, IL-10 directly stimulates B-cell growth.25 Second, IL-10 induces the antiapoptotic protein Bcl-2 in B and T cells24-28 and may therefore exacerbate the inherent apoptotic defects resulting from defects in Fas or other apoptosis mediators.29-31 This additional interference with apoptotic processes may further enhance the survival of autoreactive cell clones. In this regard, however, our demonstration of increased spontaneous in vitro apoptosis of DNTCs of ALPS patients, despite increased IL-10 levels in vivo and the lack of an effect of recombinant IL-10 in vitro (Figure 8), does not support the concept that IL-10 dysregulation in ALPS patients exerts an influence on ALPS pathogenesis through direct interference with apoptosis. Demonstration of spontaneous in vitro apoptosis (without added recombinant IL-10) of DNTCs in an ALPS patient (who likely shares the abnormality of increased IL-10 levels in vivo with our extensive ALPS population, regardless of subtype) by Haas and colleagues32 is also not supportive of this concept.
Rather than affecting the survival of particular cell populations, IL-10 appears to be altering the Th1/Th2 balance in patients with ALPS. Specifically, IL-10 antagonizes the development of Th1 cells and thus indirectly enhances Th2 development. The Th2-oriented lymphocyte profile generated by IL-10 would promote B-cell production of antibodies, including autoantibodies. In this regard, the finding that ALPS patients have an expanded population of predominantly CD5+ B cells that correlates with plasma levels of IL-10 (manuscript submitted) is consistent with the reported influence of IL-10 on B cell homeostasis.25,26 The net result expected from IL-10 elevation would be greater lymphoid expansion and autoantibody production than would occur with a single, genetically defined apoptotic defect alone (Tables 1 and 2). The elevation of IL-10 and its contributory role in development of autoimmunity in ALPS is consistent with observations in various mouse models of autoimmunity and other human autoimmune diseases.32-38
A major question is whether the elevation of IL-10 in ALPS is a normal physiologic response to supernormal levels of apoptotically impaired cells, or whether the elevated IL-10 levels reflect a second point of dysregulation in a subset of individuals. That healthy relatives with Fas mutations had slightly increased serum IL-10 levels (Figure 1) and slightly higher IL-10 protein/mRNA ratios in PBMCs (Figure 4) suggests that the defect in apoptosis may be sufficient by itself to increase IL-10 levels, perhaps because these individuals might manifest a subclinical increase in lymphoid mass that secretes IL-10. Nevertheless, it is also possible that a subset of people with an inherited apoptotic defect also possess an independent defect in IL-10 regulation. The apoptotic defect associated with elevated IL-10 cannot be attributable to Fas alterations alone, because similar IL-10 elevations were documented in a patient with ALPS type III, in which Fas is normal. Moreover, relatively higher levels of circulating IL-10 were detected in an important subset of ALPS patients. Defects in the extracellular Fas domain are significantly less likely to result in the clinical features of ALPS.39 40 In the present series, 3 of 4 patients with extracellular Fas defects had circulating IL-10 levels of more than 200 pg/mL, whereas only 3 of 16 patients with defects in the intracellular segment of Fas had elevations to this extent (P = .06; ANOVA). This strong trend suggests that IL-10 dysregulation may be particularly important for the clinical expression of ALPS in individuals with extracellular Fas defects who possess only a modest impairment of apoptosis.
Dysregulation of IL-10 has been postulated in systemic lupus erythematosus.41 42 In individuals with impaired lymphocyte apoptosis, even modest degrees of IL-10 up-regulation may exacerbate the diminished capacity of lymphocytes to undergo apoptosis, potentiating an increase in IL-10–producing cells including a rare cell subset (the DNTCs) most dependent on the pathways being altered. The increased number of such cells raises further the ambient IL-10 level, compounding the drive for autoreactive cellular proliferation.
In conclusion, we have confirmed an overproduction of IL-10 in ALPS patients and identified circulating and tissue DNTCs as major sources of this IL-10. Thus, these data suggest that DNTCs are involved in the pathogenesis of ALPS. The elevated IL-10 must derive, at least in part, through the failure of lymphocytes that produce it to die, such as the DNTCs, affording the highest levels in individuals with clinically apparent adenopathy and splenomegaly. Although we suspected initially that there may also be an independently inherited abnormality inIL-10 gene expression in those who manifest ALPS, the detection of modest increases in IL-10 in their otherwise healthy relatives with Fas mutations but normal levels in their relatives without Fas mutations indicates this to be unlikely. The full reasons for IL-10 dysregulation in ALPS and, in particular, the cofactors that distinguish those with full-blown ALPS from those without remain unknown.
The authors thank the referring physicians and patients for their participation in this research, Dr Rona LeBlanc for statistical help, and Ms Brenda Rae Marshall for editorial assistance.
U.L. was supported by the NIH Clinical Research Training Program.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Stephen E. Straus, LCI, NIAID, 10 Center Dr, Rm 10/11N228, NIH, Bethesda, Md 20892; e-mail: sstraus@nih.gov.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal