Abstract
Red blood cells (RBCs) are known to perform one prominent function: to carry and deliver oxygen to the tissues. Earlier studies, however, suggested a role for RBCs in potentiating T-cell proliferation in vitro. Here it is shown that the presence of RBCs in cultures of stimulated human peripheral blood lymphocytes strengthens T-cell proliferation and survival. Analysis of phosphatidylserine externalization and DNA fragmentation showed that RBCs inhibit T-cell apoptosis. This inhibition correlated with a reduction in CD71 but not CD95 expression. RBCs enhanced T-cell proliferation and survival upon activation with phytohemagglutinin and with OKT3 antibodies. Studies aimed at characterizing the cellular and molecular basis of the protection afforded to T cells by RBCs showed that (1) optimal protection required intact RBCs and red cell/T-cell contact but not monocytes; (2) RBCs markedly reduced the level of intracellular reactive oxygen species; and (3) RBCs inhibited the formation of protein-bound acrolein, a peroxidation adduct in biologic systems. Overall, these data indicate that human RBCs protect T cells from activation-induced cell death, at least in part by reducing the pro-oxidant state, and suggest a role for RBCs as conceivable modulators of T-cell homeostasis.
Introduction
Programmed cell death, or apoptosis, is a physiological process that contributes to the homeostasis of multicellular organisms. Apoptosis resulting from activation of T cells—ie, activation-induced cell death (AICD)—is thought to serve as a feedback mechanism that deletes activated T cells.1Contrary to immature thymocytes and transformed T-cell lines, resting T cells are highly resistant to apoptosis after initial activation but then become highly susceptible.2-4 Accordingly, most studies on AICD have focused on the study of interactions between the death receptors, CD95 (Fas) and tumor necrosis factor (TNF)-α receptor, with their agonists, CD95L (Fas ligand) and TNF-α, on activated T cells and T-cell hybridomas5,6 (for review, see7). Quiescent normal T cells express low or nondetectable levels of CD95 and CD95L, but mitogenic activation of primary T cells markedly increases their expression.8,9 Other members of the TNF-α receptor family, such as TRAIL-R1 and TRAIL-R2, can also trigger apoptosis in susceptible cells after binding of their ligands.7
Despite the important role played by the death receptors, growing evidence indicates that environmental constituents, such as nonlymphoid secreted factors and cytokines, can modulate apoptosis of activated T cells, thus emphasizing the significance of the environment in the maintenance of T-cell homeostasis.10-12 Any imbalance in the apoptotic process may result in either of 2 possible scenarios: lymphocyte accumulation or lymphocyte depletion. In the former, lymphoproliferative disorders—now designated as autoimmune lymphoproliferative syndrome—result from down-regulated in vivo apoptosis, develop in mice and in humans, result from genetic mutations in CD95 or its ligand, and are associated with autoimmune phenomena.13-16 In the latter scenario, lymphopenia resulting from up-regulated in vivo apoptosis is the clinical finding in human immunodeficiencies, such as idiopathic CD4+ T lymphocytopenia, and it correlates with increased levels of CD95 and CD95L, a finding also observed in patients with CD8+ T lymphopenia.17 Lymphocytopenias found in adults could also result from adenosine deaminase deficiency.18
Other factors implicated in different forms of apoptosis are reactive oxygen species (ROS). Several studies have provided evidence for the involvement of ROS in apoptosis of T-cell blasts and hybridomas by using antioxidants such as N-acetyl cysteine and glutathione.19,20 Apoptosis in these cells is the consequence of changes in mitochondrial permeability and the subsequent release of ROS.21 Studies performed with primary T cells, however, indicate that the formation of intracellular ROS is necessary for T-cell activation and IL-2 secretion but also regulates activation-induced T-cell apoptosis, therefore suggesting that intracellular ROS could play a role in peripheral T-cell homeostasis.22-24 Nevertheless, studies with primary T cells are usually performed in cultures lacking other “nonlymphoid” cells, albeit activation-induced T-cell apoptosis is thought to occur in organs and tissues where other cell types, such as red blood cells (RBCs), are present (for review, see 25). In addition to their main function in oxygen and CO2transport,26 RBCs also have the capacity to scavenge exogenous ROS because of the permeability of their membranes to oxygen radicals and the presence of high levels of intracellular antioxidant enzymes27-29 (for review, see 30). In addition, RBCs are also known to increase T lymphocyte proliferation upon stimulation in vitro.31-33 These features confer RBCs the potential to function as ROS scavengers produced within their milieu, thereby protecting neighboring cells from ROS-mediated damage.34-37 Whether the potentiating effect of RBCs on T-cell proliferation is related to their capacity to scavenge ROS is unknown. The main goal of the present study was to perform a comprehensive study to examine the effect of RBCs on T lymphocytes after activation. Results showed that RBCs enhance T-cell expansion and survival by inhibiting activation-induced T-cell death, a finding associated with a decrease in oxidative stress within the activated T cells.
Materials and methods
Reagents and monoclonal antibodies
Phytohemagglutinin (PHA-P), leuco-agglutinin (PHA-L), antibiotic-antimicotic solution, propidium iodide (PI), Triton X-100, and Biomax MR-1 Kodak films were obtained from Sigma-Aldrich (Madrid, Spain). RPMI 1640, Hanks balanced salt solution (HBSS), fetal calf serum (FCS), and L-glutamine were from Gibco BRL (Paisley, United Kingdom). Methyl-3H-thymidine ([3H]TdR), Hybond C-super membranes, and enhanced chemiluminescence reagents (ECL) were obtained from Amersham-Pharmacia Biotech (Buckinghamshire, United Kingdom). The following mouse fluorochrome-conjugated monoclonal antibodies (mAbs) were used: CD3 (clone UCHT1) and CD14 (clone TüK4) were from Dakopatts (Glostrup, Denmark); CD71 (clone JT.01) was from ImmunoSource (Los Altos, CA); and HLA-DR (clone Tü36), CD25 (clone m-A251), and CD69 (clone FN50) were from Becton Dickinson (Mountain View, CA). Annexin V–fluorescein isothiocyanate (FITC) was from Pharmingen (San Diego, CA). Pure CD95 (clone DX2) and CD71 (clone H68.4) were from Pharmingen and Zymed (San Francisco, CA), respectively. OKT3 is an anti-CD3 antibody.38 5F6 is a mouse mAb against protein-bound acrolein, a marker of oxidative stress.39 Mouse and rabbit immunoglobulins were from Dakopatts.
Cell preparation and culture
Fresh peripheral blood mononuclear cells (PBMCs) were obtained from buffy coats or heparinized blood of healthy blood donors after centrifugation over Lymphoprep (Nycomed, Oslo, Norway). PBMCs were washed with HBSS and contaminating RBCs—always present at varying levels in the PBMC preparations—and were lysed in lysis solution (10 mM Tris, 150 mM NH4Cl, pH 7.4) at 37°C for 10 minutes. RBCs were obtained from the pellet region after Lymphoprep centrifugation and were washed twice with HBSS. Partially purified peripheral blood T lymphocytes were obtained after culturing mononuclear cells in culture media (CM) at 37°C, 5% CO2, and 99% humidity from 1 hour to overnight. Recovered nonadherent cell suspensions, referred to as PBLs, were usually more than 80% CD3+. Adherent cells were collected after incubation in PBS-EDTA solution and were routinely more than 70% CD14 and less than 20% CD3, as determined by flow cytometry. T cells were obtained after rosetting of PBLs with sheep red blood cells (ProBiologica, Lisbon, Portugal) for 2 hours in ice, subsequent centrifugation over Lymphoprep, and lysis of SRBC. Rosetted T cells were routinely more than 95% CD3+ and contained less than 2% CD14+ cells. CM was RPMI-1640 supplemented with 1% FCS, 1% L-glutamine, and 1% antibiotic-antimicotic solution.
PBLs were cultured at a final concentration of 0.25 × 106 cells/mL either in 96- or 6-well plates (TPP, Trasadingen, Switzerland) in a final volume of 0.2 mL or 5 mL CM, respectively, giving a density of 1650 cells/mm2in either culture plate. PBLs were either left unstimulated or stimulated with 5 μg/mL PHA-P and cultured in the absence or presence of autologous RBCs in an incubator at 37°C, 5% CO2, and 95% humidity for 1 to 5 days. In some experiments PBLs were stimulated with 5 μg/mL PHA-L and with 0.5 μg/mL OKT3. For proliferation studies, 5 × 104 cells were cultured in triplicate in 96-well flat-bottom microplates. For other studies 1.5 × 106 cells were cultured in 6-well macroplates. RBC suspensions were prepared in CM and adjusted to give an RBC:PBL ratio of 100:1 after addition to the PBL cultures. In some experiments, RBCs were lysed by brief sonication before they were added to the PBL cultures. Sonic cell disruption was performed by 10 bursts of 1 second each at 0.1 W power output. In experiments with cell culture inserts (pore size, 0.2 μm; Nunc, Roskilde, Denmark), PBLs were placed in the lower chamber and RBCs in the upper chamber.
Red cell lysis procedure
For the determination of parameters that required the use of flow cytometry—(eg, T-cell activation or death, intracellular ROS production)—only activated T cells that received RBCs were treated with lysis solution (10 mM Tris, 150 mM NH4Cl, pH 7.4) to remove RBCs. This treatment did not significantly alter the activation parameters studied when compared with nontreated activated T cells. In some experiments, however, flow cytometry determinations (eg, Annexin V and ROS) were performed without the removal of RBCs.
Determination of T-cell activation and proliferation
T-cell activation and proliferation was studied by 3 methods: (1) thymidine uptake; (2) determination of cell size; and (3) examination of activation markers. For thymidine uptake, 0.5 μCi [3H]-TdR (specific activity, 5.0 Ci/mmol; Amersham-Pharmacia Biotech, United Kingdom) was added 4 hours before the end of the culture, and cells were harvested on glass fiber filters (Filter MAT; Skatron Instruments, Suffolk, United Kingdom), using a semi-automatic cell harvester (Skatron, Norway). The incorporated [3H]-TdR was measured in a Beckman liquid scintillation counter, and results were expressed as counts per minute (cpm). For determination of cell size and activation, forward and side scatter characteristics (FSC/SSC) and expression of CD69, CD25, CD71, CD95, and HLA-DR, respectively, were analyzed by flow cytometry. Proliferation index was defined as follows: cpm in the presence of RBC/cpm in the absence of RBC.
Detection of lymphocyte apoptosis and death
Apoptosis was studied by characterizing phosphatidylserine expression and DNA fragmentation. T cells were harvested 1 to 5 days after activation, washed with PBS, and counted. For phosphatidylserine determination, cells were washed twice with binding buffer (10 mM HEPES, 140 mM NaCl, and 2.5 mM CaCl2, pH 7.4) and incubated with Annexin V–FITC for 15 minutes at room temperature. Cells were immediately harvested and analyzed by flow cytometry. For DNA fragmentation, cells were lysed by overnight incubation at 37°C in lysis buffer (100 mM Tris, pH 8, 5 mM EDTA, 200 mM NaCl, 0.2% SDS, 0.2 mg/mL proteinase K). After centrifugation at 13 000g for 15 minutes, the supernatant containing fragmented DNA was transferred to another tube and precipitated with an equal volume of 100% ethanol. The pellet was washed twice with cold 70% ethanol and then dissolved in 20 μL Tris-EDTA buffer containing 0.2 mg/mL RNAse A. After incubation at 37°C for 2 hours, DNA was resolved on 2% agarose gels and visualized with ethidium bromide staining.
T-cell death was determined by PI and trypan blue (TB) staining (PI and TB are 2 dyes that enter cells with damaged plasma membranes) and by a decrease in cell size (shrinking). For PI staining, 2 μL/sample PI (25 μg/mL) was added to each cell sample before flow cytometry analysis. For TP staining, aliquots of activated lymphocytes were resuspended in PBS-containing trypan blue. Dead and alive cells were counted in a Neubauer chamber under a light microscope. For cell shrinking, FSC/SSC characteristics of activated lymphocytes were analyzed by flow cytometry.
Measurement of oxidative stress
Oxidative stress in T cells induced by PHA was measured by the detection of ROS and the detection of protein-bound acrolein. ROS produced within activated T cells were detected with the membrane-permeant probe 2′,7′-dichlorofluorescein-diacetate (DCFH-DA). The probe freely enters the cell and is incorporated into hydrophobic regions, and the acetate moiety is cleaved off by cellular esterases leaving a nonfluorescent and impermeant form of DCFH.40,41ROS produced by the cell oxidize DCFH to DCF, which, after excitation at 488 nm, emits fluorescence at 530 nm (FL1 channel). Resting PBLs were incubated with 100 μM DCFH-DA in CM for 30 minutes at 37°C and were washed 3 times with the same media. Then PBLs were left unstimulated or were stimulated with 5 μg/mL PHA-P. At time intervals after activation, cells were harvested, washed with PBS, and directly analyzed by flow cytometry. Protein-bound acrolein was also detected by flow cytometry using the mouse mAb 5F6 (see 38 for a detailed description of the mAb), followed by the proper fluorochrome-conjugated rabbit antimouse immunoglobulins.
Flow cytometry analysis
Cells were stained on day 0 (resting cells) and at different time points after mitogenic stimulation (T-cell blasts), as described previously.42 Briefly, cells were harvested from cultures by gentle pipetting and washed twice with PBS. In cultures with RBCs, erythrocytes were first lysed in lysis solution as indicated above. Staining was performed at 4°C for 30 minutes in staining solution (PBS, 0.2% BSA, 0.1% NaN3) in 96-well round-bottom plates (Greiner, Nürtingen, Germany) with approximately 0.5 × 106 cells/well. Cells were stained in one (direct), 2 (indirect), or 3 steps depending on the combination of mAbs and the antigens studied. Irrelevant mouse mAbs were used as negative controls to define background staining. Rabbit antimouse FITC or R-phycoerythrin (RPE)-conjugated F(ab')2 fragments were used as second-step antibodies. After they were stained, cells were washed 3 times with staining buffer and were immediately acquired without fixation in a FACSort (Becton Dickinson, Mountain View, CA) equipped with an argon laser that emitted at 488 nm. For each sample 10 000 cells were usually acquired using FSC/SSC characteristics and analyzed using the Lysys II software.
Western blot analysis
Immunodetection was performed as previously described.43 Briefly, resting and activated T cells were solubilized in 1% Triton X-100 lysis buffer, and cell debris was removed by centrifugation at 11 000g. Cell lysates were boiled for 5 minutes in 2× SDS buffer and resolved by 12% SDS-PAGE. Proteins were transferred to nitrocellulose membranes (Hybond C-super, Amersham), and filters were blocked with TBS-T containing 5% (wt/vol) nonfat dry milk for 1 hour. After washing with TBS-T, filters were incubated for 1 hour with a 1:1000 dilution of an mAb against the transferrin receptor (clone H68.4). Filters were washed and incubated with horseradish peroxidase-conjugated goat-antimouse antibodies (Transduction Laboratories, Lexington, United Kingdom) for 1 additional hour. All incubations were performed at room temperature. Finally, membranes were extensively washed, and detection was accomplished using enhanced chemiluminescence (Amersham) and exposure to Biomax MR-1 Kodak films (Sigma-Aldrich).
Statistical analysis
The Student t test was used to test the significance of the differences between group means. Statistical significance was defined as P < .05. Kolgomorov-Smirnov statistics were used for analysis of the statistical significance of differences seen between histograms.44,45 Two histograms were considered significantly different when D/s(n) was greater than 15, as described.46
Results
Activation-induced T-cell death in normal human PBLs
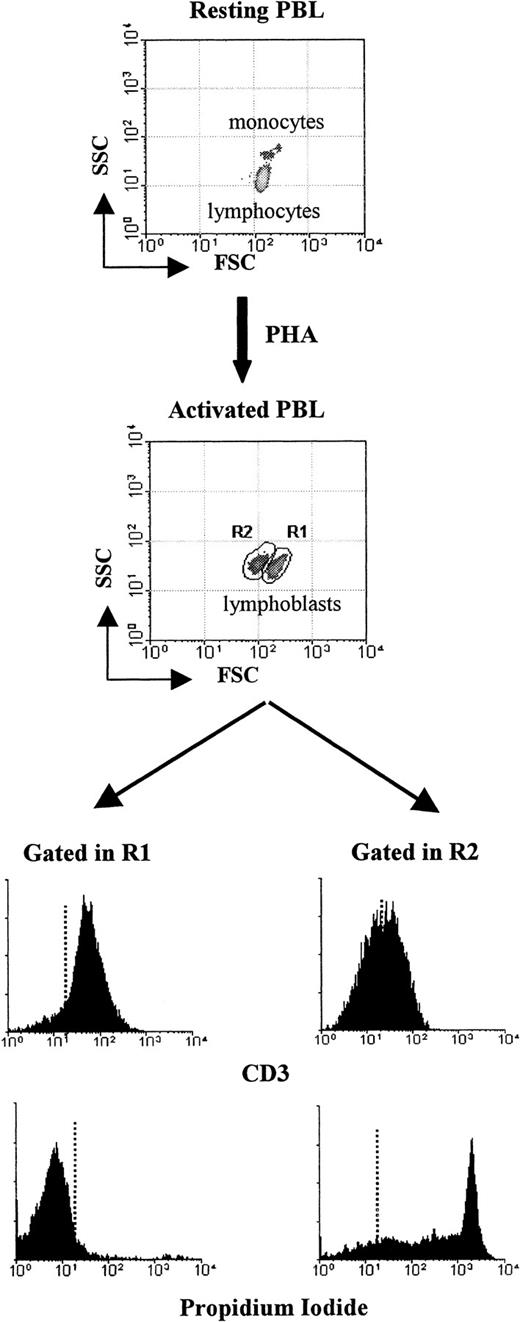
Activation of resting human PBLs with the T-cell mitogen PHA-P increased thymidine uptake (141 ± 105 in resting vs 4606 ± 1914 in activated; mean cpm ± 1 SD; P < .0005), a measure of cell division. Morphologically, however, PHA-P stimulation was characterized by the appearance of 2 populations of activated T cells with an overall increase in cell size when compared to resting lymphocytes (Figure 1, gates R1 and R2). Blasts within gate R1 were usually more than 95% CD3+ and PI−. On the contrary, the characteristics of the cells within gate R2 clearly show that they were undergoing cell death. They had a reduced size (cell shrinking), showed reduced CD3 expression, and a high percentage (more than 90%) were PI+, all features of T cells that have lost plasma membrane integrity. Activation-induced T-cell death was observed in all PBL samples studied and started as early as 1 hour after mitogenic stimulation (data not shown). The data illustrate the T-cell specificity of the mitogen used and that AICD takes place in primary cultures of human peripheral blood T cells. Characterization of early (eg, CD69) and late (eg, HLA-DR) T-cell activation markers after activation by flow cytometry further demonstrated that, under the culture conditions used, human PBLs responded optimally to PHA-P stimulation (see below).
Activation-induced T-cell death among normal human PBLs.
Freshly collected human PBLs were stimulated in 6-well plates in CM with PHA-P (5 μg/mL) and cultured for 5 days. Blasts cells were harvested and stained with CD3-FITC and PI, as indicated in “Materials and methods.” Ten thousand events were acquired in a FACSsort and analyzed using the Lysis II program. Results show dot blots (FCS vs SSC) before (upper dot blot) and 5 days after (lower dot blot) PHA activation. Histograms show the results of CD3 and PI staining of lymphoblasts in regions R1 and R2. Dotted lines represent background staining. Data from 1 of 5 separate experiments with similar results are shown.
Activation-induced T-cell death among normal human PBLs.
Freshly collected human PBLs were stimulated in 6-well plates in CM with PHA-P (5 μg/mL) and cultured for 5 days. Blasts cells were harvested and stained with CD3-FITC and PI, as indicated in “Materials and methods.” Ten thousand events were acquired in a FACSsort and analyzed using the Lysis II program. Results show dot blots (FCS vs SSC) before (upper dot blot) and 5 days after (lower dot blot) PHA activation. Histograms show the results of CD3 and PI staining of lymphoblasts in regions R1 and R2. Dotted lines represent background staining. Data from 1 of 5 separate experiments with similar results are shown.
RBCs enhance T-cell proliferation by inhibiting activation-induced T-cell death
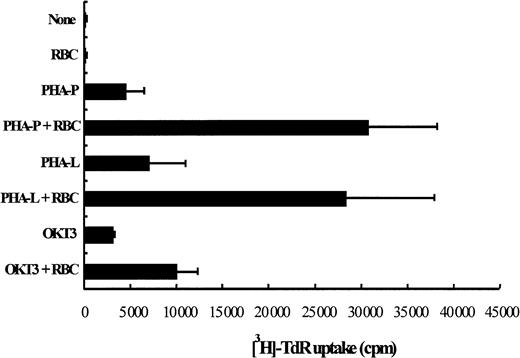
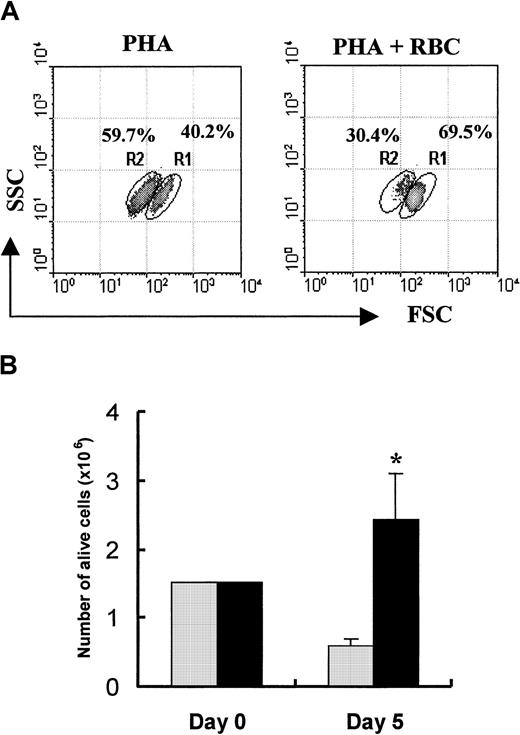
To examine the effect of RBCs on PHA-P–driven T-cell proliferation and survival, PBL cultures were stimulated with or without the presence of RBCs for 5 days, as indicated in “Materials and methods.” As illustrated in Figure2, T cells proliferated approximately 5 times better when cocultured with RBCs. This enhanced T-cell proliferation was observed at low (1% FCS) and high (10% FCS) serum concentrations (data not shown). In the absence of the mitogenic stimulus, RBCs were unable to drive T cells into cell division. The significantly enhanced proliferation observed in cultures with RBCs (P < .05) was also observed with 2 other mitogens, PHA-L (ie, leuco-agglutinin) and OKT3 antibodies (Figure 2). Flow cytometry analysis showed that the percentage of T-cell blasts undergoing activation-induced cell death was significantly decreased by the presence of RBCs (59.7 ± 6.9 vs 30.4 ± 8.7;P < .002; n = 7). An illustration of the inhibition of AICD in the presence of RBCs is shown in Figure3A and is further corroborated by the impact that an enriched RBC environment had on total cell recovery. Thus, T-cell activation of human PBLs in an enriched RBC environment resulted in a 5-fold increase in viable cell recovery 5 days after activation when compared with cultures of PBL alone (Figure 3B,P < .01).
RBCs enhance mitogen-driven T lymphocyte proliferation.
PBLs were stimulated in triplicate in 96-well plates with PHA-P (5 μg/mL), PHA-L (5 μg/mL), and OKT3 (0.5 μg/mL) and were cultured for 5 days in the presence or absence of RBCs (RBC:PBL ratio of 100:1). In control cultures, mitogen was omitted. Tritiated thymidine (0.5 μCi/well) was added 4 hours before the end of the culture, cells were harvested on fiber filters, and incorporated thymidine was determined by scintillation counting. Results show thymidine incorporation (cpm, mean ± SD, n = 3) in the different cultures conditions.
RBCs enhance mitogen-driven T lymphocyte proliferation.
PBLs were stimulated in triplicate in 96-well plates with PHA-P (5 μg/mL), PHA-L (5 μg/mL), and OKT3 (0.5 μg/mL) and were cultured for 5 days in the presence or absence of RBCs (RBC:PBL ratio of 100:1). In control cultures, mitogen was omitted. Tritiated thymidine (0.5 μCi/well) was added 4 hours before the end of the culture, cells were harvested on fiber filters, and incorporated thymidine was determined by scintillation counting. Results show thymidine incorporation (cpm, mean ± SD, n = 3) in the different cultures conditions.
RBCs inhibit activation-induced T-cell death.
(A) PBLs were stimulated in 6-well plates with PHA-P (5 μg/mL) and cultured for 5 days in the absence or presence of RBCs. After the culture period, activated T-cell blasts were stained with PI, and 10 000 cells were acquired in a FACSort and analyzed using the Lysis II program. Based on morphologic (FSC/SSC) and PI labeling characteristics (Figure 1), regions R1 and R2 represent live and dead cells, respectively. The mean percentage of cells gated within each region from 7 separate experiments is indicated. (B) Total number of live blast cells in cultures with (▪) and without (░) RBCs was determined by trypan blue exclusion, as indicated in “Material and methods.” Results show the mean ± SD of 3 independent experiments. *P < .03.
RBCs inhibit activation-induced T-cell death.
(A) PBLs were stimulated in 6-well plates with PHA-P (5 μg/mL) and cultured for 5 days in the absence or presence of RBCs. After the culture period, activated T-cell blasts were stained with PI, and 10 000 cells were acquired in a FACSort and analyzed using the Lysis II program. Based on morphologic (FSC/SSC) and PI labeling characteristics (Figure 1), regions R1 and R2 represent live and dead cells, respectively. The mean percentage of cells gated within each region from 7 separate experiments is indicated. (B) Total number of live blast cells in cultures with (▪) and without (░) RBCs was determined by trypan blue exclusion, as indicated in “Material and methods.” Results show the mean ± SD of 3 independent experiments. *P < .03.
Enriched RBC environment reduces T-cell apoptosis and CD71 expression
The above data showed that the presence of RBCs inhibited the death of activated human T cells. To ascertain whether the decreased T-cell death was associated with changes in the expression of early markers of T-cell apoptosis, phosphatidylserine externalization was studied by flow cytometry. Kinetic experiments showed that the presence of RBCs reduced the percentage of Annexin V+“apoptotic” T-cell blasts along the culture period after mitogenic activation (Figure 4). Despite interindividual variation in Annexin V labeling, RBCs consistently reduced phosphatidylserine externalization. The protection of T-cell apoptosis by RBCs was substantiated by results of DNA fragmentation (data not shown).
RBCs reduce the percentage of apoptotic T cells after mitogenic activation.
PBLs were stimulated with PHA and cultured in the absence or presence of RBCs for 1 to 3 days. Cell surface expression of externalized phosphatidylserine by activated T cells was determined by Annexin V-FITC binding. Histograms show Annexin V+ cells gated among PI− cells at days 1, 2, and 3 after activation of PBLs in the absence or presence of RBCs. Annexin V labeling of resting PBL was less than 2%. Data from 1 of 4 separate experiments with similar results are shown.
RBCs reduce the percentage of apoptotic T cells after mitogenic activation.
PBLs were stimulated with PHA and cultured in the absence or presence of RBCs for 1 to 3 days. Cell surface expression of externalized phosphatidylserine by activated T cells was determined by Annexin V-FITC binding. Histograms show Annexin V+ cells gated among PI− cells at days 1, 2, and 3 after activation of PBLs in the absence or presence of RBCs. Annexin V labeling of resting PBL was less than 2%. Data from 1 of 4 separate experiments with similar results are shown.
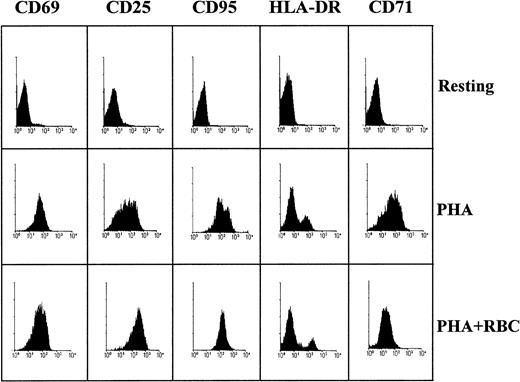
Considering that high expression of CD95 has been associated with accelerated lymphocyte apoptosis and that triggering of CD95 on activated T cells and T-cell lines leads to apoptosis, we tested the possibility that the protection provided to T lymphocytes by RBCs was associated with a decrease in CD95 expression. However, the expression of CD95 in activated T cells cultured either in the absence or the presence of RBCs was not significantly different. In contrast, the presence of RBCs markedly reduced the expression of CD71, the transferrin receptor (Figure 5). Thus, a 2-fold reduction in the mean fluorescence intensity of CD71 in activated T cells was observed in cultures of PBLs stimulated in the presence of RBCs when compared with cultures of PBLs alone (40.9 ± 12.3 vs 96.7 ± 20.3, respectively;P < .001; n = 5). Kinetic experiments showed that the RBC-mediated decrease in cell surface CD71 expression takes place along the entire culture period. CD71 down-modulation in the presence of RBCs was confirmed by Western blot analysis (data not shown). Analysis of other T-cell activation markers showed that RBCs slightly enhanced the expression of CD25 and HLA-DR but not of the early activation marker CD69 (Figure 5).
Phenotypic characterization of T cells after activation.
Human PBLs were stimulated with PHA and cultured for 1 to 5 days in the absence or presence of RBCs, as indicated. Activated T cells were stained with FITC- or RPE-conjugated antibodies against CD69, CD25, CD71, HLA-DR, and unlabeled anti-CD95, followed by FITC-conjugated rabbit-antimouse immunoglobulins. Labeled cells were acquired in a FACSort and analyzed using the Lysis II program. Histograms show results of resting PBL (day 0) and activated (day 5) T cells for all markers except CD69, for which day 1 is shown. Data are representative of 3 separate experiments.
Phenotypic characterization of T cells after activation.
Human PBLs were stimulated with PHA and cultured for 1 to 5 days in the absence or presence of RBCs, as indicated. Activated T cells were stained with FITC- or RPE-conjugated antibodies against CD69, CD25, CD71, HLA-DR, and unlabeled anti-CD95, followed by FITC-conjugated rabbit-antimouse immunoglobulins. Labeled cells were acquired in a FACSort and analyzed using the Lysis II program. Histograms show results of resting PBL (day 0) and activated (day 5) T cells for all markers except CD69, for which day 1 is shown. Data are representative of 3 separate experiments.
Inhibition of T-cell apoptosis by RBCs correlates with a reduction in oxidative stress
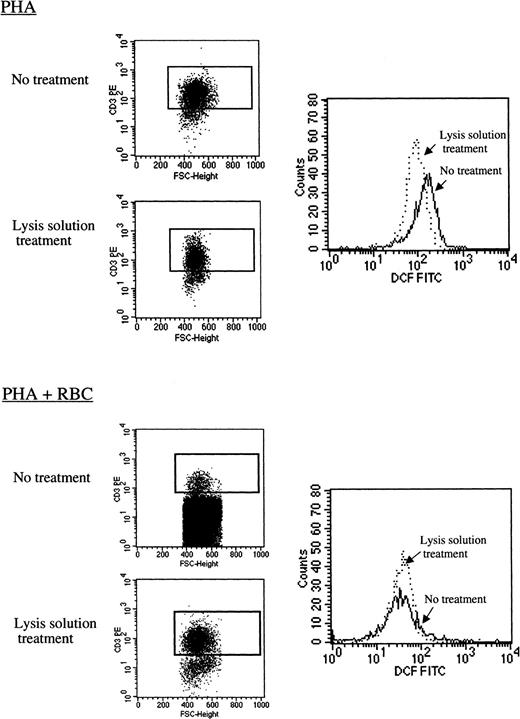
ROS have been implicated in the apoptotic process in a number of systems, including activated T lymphocytes, and previous studies have shown that T cells produce intracellular ROS after mitogenic stimulation.22-24 In our hands, human peripheral blood CD3+ T cells activated in vitro with PHA also produced intracellular ROS, as demonstrated by a marked increase in DCF fluorescence (data not shown). Importantly, the presence of RBCs significantly reduced ROS levels. As illustrated in Figure6, RBCs decreased by approximately 4-fold the level of intracellular ROS among activated CD3+ T cells (D/s(n) = 39.07;D = 0.83). This decrease was observed regardless of the use of lysis solution to remove RBCs before analysis. However, in cultures not treated with the lysis solution, the high number of RBCs present interfered with the detection of activated CD3+ T cells by the flow cytometer (Figure 6 and data not shown). In 7 separate experiments and 16 different determinations performed within the first 24 hours after stimulation, the presence of RBCs decreased DCF mean fluorescence intensity of PHA-activated T cells on average by 3.5-fold (range, 1.4-9.7; P = .0013). Kinetic experiments demonstrated that intracellular ROS are detectable as soon as 30 minutes after mitogenic activation of PBLs with PHA and that RBCs are capable of counteracting ROS production (data not shown).
RBCs reduce ROS production in activated human T cells.
Resting human PBLs were labeled with 100 μM DCFH-DA as indicated in “Materials and methods.” T cells were left unstimulated or were activated with PHA-P (5 μg/mL) in CM in the absence (PHA) or presence (PHA+RBC) of RBCs for 24 hours. After harvesting, duplicates of the cell cultures were treated with lysis solution or were left untreated and were subsequently stained with CD3-RPE, and labeled cells were acquired in a FACSort. Dot blots show FSC versus CD3 staining in cultures treated with lysis solution and in nontreated cultures. Histograms show DCF fluorescence on gated CD3+ T cells in both conditions. The decrease in DFC mean fluorescence intensity in the presence of RBCs was statistically significant according to K/S statistics (D/s(n) = 39.07).
RBCs reduce ROS production in activated human T cells.
Resting human PBLs were labeled with 100 μM DCFH-DA as indicated in “Materials and methods.” T cells were left unstimulated or were activated with PHA-P (5 μg/mL) in CM in the absence (PHA) or presence (PHA+RBC) of RBCs for 24 hours. After harvesting, duplicates of the cell cultures were treated with lysis solution or were left untreated and were subsequently stained with CD3-RPE, and labeled cells were acquired in a FACSort. Dot blots show FSC versus CD3 staining in cultures treated with lysis solution and in nontreated cultures. Histograms show DCF fluorescence on gated CD3+ T cells in both conditions. The decrease in DFC mean fluorescence intensity in the presence of RBCs was statistically significant according to K/S statistics (D/s(n) = 39.07).
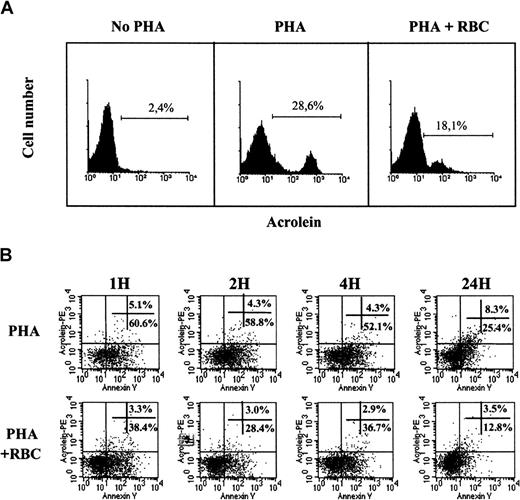
Next, we examined whether intracellular ROS production by activated T cells led to the expression of protein-bound acrolein, a marker of oxidative stress. Acrolein was indeed detected in activated, but not in resting, PBLs. We observed maximal expression of acrolein adducts by activated T cells 3 days after mitogenic activation (Figure7A). The presence of RBCs decreased both the percentage of acrolein-positive cells and the amount of reactive acrolein adducts, as indicated by a marked (approximately 3-fold) decrease in the mean fluorescence intensity (Figure 7A). These results suggested that acrolein expression among activated T cells was a late event in T-cell apoptosis. To clarify this possibility, double labeling (ie, Annexin V vs acrolein) kinetic experiments were performed. As illustrated in Figure 7B, cells expressing cell surface phosphatidylserine were observed as soon as 1 hour after T-cell activation, and they constituted more than half the activated T cells for 24 hours, when this percentage dropped drastically. In marked contrast, the percentage of Annexin V+ “apoptotic” cells expressing cell surface acrolein adducts was very low during the first 4 hours after activation. Cell surface acrolein expression started to increase 24 hours after activation and was maximal by day 3 (see Figure 7A). RBCs markedly reduced the percentage of both Annexin V and acrolein-positive cells after T-cell activation at all time points studied. Characterization of acrolein adducts among activated T cells undergoing T-cell death (ie, PI+) showed that acrolein expression during the early phases of T-cell activation is mainly intracellular (data not shown).
RBCs reduce oxidative stress in activated human T cells.
(A) Resting human PBLs were activated with PHA for 3 days in the absence or presence of RBCs, as indicated. Cells were collected and stained with 5F6 mAb followed by rabbit-antimouse RPE immunoglobulins, and cells were acquired in a FACSort. Histograms show acrolein staining in resting (day 0, no PHA) and 3-day activated PBLs in the absence (PHA) or presence (PHA+RBC) of RBC. The percentage of acrolein-positive cells is indicated. (B) Resting human PBLs were activated with PHA-P (5 μg/mL) in CM in the absence or presence of RBCs for the times indicated. Activated T cells were harvested and stained with 5F6 mAb plus rabbit-antimouse RPE immunoglobulins and with Annexin V–FITC. Cells were immediately acquired in a FACSort. Dot blots show Annexin V versus acrolein fluorescence in activated T cells 1, 2, 4, and 24 hours after activation in the absence (PHA) or presence (PHA+RBC) of RBCs. One representative of at least 3 separate experiments is shown.
RBCs reduce oxidative stress in activated human T cells.
(A) Resting human PBLs were activated with PHA for 3 days in the absence or presence of RBCs, as indicated. Cells were collected and stained with 5F6 mAb followed by rabbit-antimouse RPE immunoglobulins, and cells were acquired in a FACSort. Histograms show acrolein staining in resting (day 0, no PHA) and 3-day activated PBLs in the absence (PHA) or presence (PHA+RBC) of RBC. The percentage of acrolein-positive cells is indicated. (B) Resting human PBLs were activated with PHA-P (5 μg/mL) in CM in the absence or presence of RBCs for the times indicated. Activated T cells were harvested and stained with 5F6 mAb plus rabbit-antimouse RPE immunoglobulins and with Annexin V–FITC. Cells were immediately acquired in a FACSort. Dot blots show Annexin V versus acrolein fluorescence in activated T cells 1, 2, 4, and 24 hours after activation in the absence (PHA) or presence (PHA+RBC) of RBCs. One representative of at least 3 separate experiments is shown.
Enhanced proliferation induced by RBCs requires T-cell contact and intact RBCs
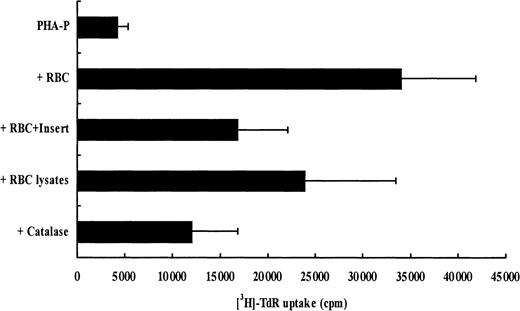
The latter results suggested that the enhancement of T-cell proliferation and protection from apoptosis by RBCs could be due, at least in part, to the ROS-scavenging properties of RBCs. Thus, we first investigated whether the effect of RBCs on T-cell expansion and survival required cell contact by using cell culture inserts. As illustrated in Figure 8, RBCs had their highest enhancing effect on T-cell proliferation and survival when they were cultured with the activated PBLs. When activated PBLs and RBCs were cultured separately using cell inserts, T-cell proliferation was reduced by 50%. In accordance with the proliferation results, AICD in cultures with cell inserts was also decreased (data not shown). Next, we examined whether intact red blood cells were required. Proliferation in the presence of red cell lysates was reduced by 30% when compared to intact RBCs (Figure 8). Finally, the addition of purified catalase, an abundant erythrocyte antioxidant enzyme, to stimulated cultures of PBLs did not reproduce the marked enhancing effect of RBCs, either in T-cell proliferation or in survival (Figure 8 and data not shown).
Intact RBCs and RBC/T-cell contacts are required for optimal T-cell expansion and survival.
T-cell proliferation was measured 5 days after stimulation in the following conditions: PHA-P, PHA-P plus intact RBCs, PHA-P plus intact RBCs separated by a cell insert, PHA-P plus sonicated RBCs, and PHA-P plus catalase (10 μg/mL). Results show thymidine incorporation (cpm, mean ± SD, n = 3) in the different culture conditions. PHA-P was used at 5 μg/mL.
Intact RBCs and RBC/T-cell contacts are required for optimal T-cell expansion and survival.
T-cell proliferation was measured 5 days after stimulation in the following conditions: PHA-P, PHA-P plus intact RBCs, PHA-P plus intact RBCs separated by a cell insert, PHA-P plus sonicated RBCs, and PHA-P plus catalase (10 μg/mL). Results show thymidine incorporation (cpm, mean ± SD, n = 3) in the different culture conditions. PHA-P was used at 5 μg/mL.
Monocytes are not involved in the enhancing effect of RBCs in T-cell proliferation
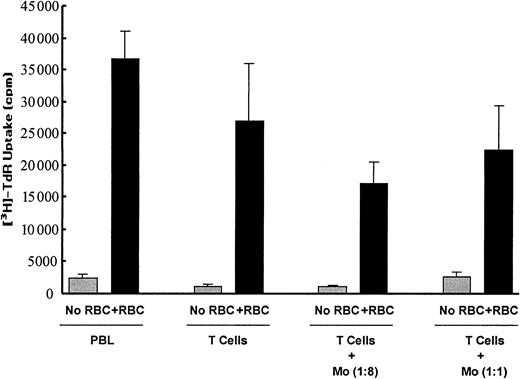
To ascertain whether the RBC-enhancing effect on T-cell proliferation and survival was dependent on the presence of accessory cells such as monocytes, experiments with highly enriched T-cell preparations (more than 95% CD3+, less than 2% CD14+) were performed. As shown in Figure9, the depletion of monocytes resulted in a very low proliferative response by T cells to mitogen stimulation. However, the proliferative response by purified T cells was restored by the addition of increasing amounts of monocyte-enriched populations (more than 70% CD14+). Adding RBCs not only increased T-cell proliferation in cultures of PBLs, as expected, but also in cultures of purified T cells (Figure 8). Interestingly, adding RBCs to the purified T cells resulted in a significantly higher proliferation index than that seen with PBLs (26.9% ± 5.8% vs 15% ± 3.1%;P < .01; n = 3 ). Adding monocytes to the activated T-cell/RBC cultures had no further effects on T-cell proliferation.
Enhancing effect of RBCs on T-cell expansion does not require monocytes.
PBLs and T cells were stimulated in 96-well plates and cultured for 5 days in the absence or presence of RBCs. Enriched monocytes (Mo, more than 70% CD14+) were added to the cultures at monocyte:PBL/T-cell ratios of 1:8 and 1:1. Results show thymidine uptake (mean ± SD of triplicates) in the different culture conditions. One representative of 3 separate experiments is shown.
Enhancing effect of RBCs on T-cell expansion does not require monocytes.
PBLs and T cells were stimulated in 96-well plates and cultured for 5 days in the absence or presence of RBCs. Enriched monocytes (Mo, more than 70% CD14+) were added to the cultures at monocyte:PBL/T-cell ratios of 1:8 and 1:1. Results show thymidine uptake (mean ± SD of triplicates) in the different culture conditions. One representative of 3 separate experiments is shown.
Discussion
The maintenance of accurate peripheral T-lymphocyte homeostasis is crucial to a patient's health, and a balance between T-cell death and survival is central to the homeostatic process.47 Most of the research performed on T-cell apoptosis has focused on the characterization of cytoplasmic, secreted, and membrane-anchored molecules. Less attention has been paid to the role that nonlymphoid cells play in this process. This is a relevant point if one takes into consideration that T-cell death or survival in the periphery takes place in organs and tissues populated by different cell types,25,48 some of which—such as fibroblasts and macrophages—modulate activation-induced T-cell death.10,49 In the present study, we have evaluated T-cell proliferation, death, and survival in the presence of a nonlymphoid cell, the circulating erythrocyte. Data showing that mature RBCs strengthen the proliferation of mitogen-activated human peripheral blood T cells confirm previous findings pointing to RBCs as enhancers of T-cell proliferation.31-33 In addition, we showed that the RBC-mediated effect on T-cell proliferation did not require the presence of monocytes. It is well known that phagocytosis of erythrocytes by monocyte/macrophages represents a major mechanism to remove senescent or damaged RBCs from the circulation and also plays a central role in iron homeostasis by recycling heme iron.50,51 Recent studies have shown that iron compounds released after erythrophagocytosis have immunomodulatory properties.52
The main finding of this study was that RBCs inhibit the T-cell apoptotic process started after mitogenic activation of resting human peripheral blood T cells. RBCs reduced exposure of phosphatidylserine on the outer leaflet of the plasma membrane of T lymphocytes, a hallmark of apoptosis. Several studies have shown that intracellular oxidative stress, as a result of activation, also initiates apoptotic processes in primary T cells.22-24,53 More recently, it has been proposed that selective oxidation of phosphatidylserine during oxidative stress promotes its externalization.54 By using dichlorofluorescein, an indicator of generalized oxidative stress, we have shown that human peripheral blood T cells activated in vitro produced intracellular ROS. It is likely that intracellular ROS reduction in activated T cells was in part due to the removal of ROS by RBCs. Numerous studies have shown that RBCs can scavenge radicals such as H2O2 and ONOO−, the product of the reaction between O2− and NO.30 In addition, hydrogen peroxide, peroxynitrite, and other oxygen radicals are capable of diffusing across erythrocyte membranes.27-29 Therefore, by inhibiting ROS production, RBCs could have interfered with the apoptotic process, phosphatidylserine externalization, and, therefore, contributed to the increased T-cell survival.
The mechanism by which RBCs counteract intracellular ROS production in T cells, and hence enhance T-cell proliferation, could depend on the high antioxidant activity of the RBCs.30 Yet, exogenous added catalase (10 μg/mL), though increasing T-cell proliferation by approximately 2-fold, did not completely reproduce the proliferation levels observed with RBCs. Previous studies with human PBLs have also shown that catalase had no significant influence, either in the proliferative response of T cells to PHA or in the inhibition of monocyte-dependent T-cell death.55,56 These findings are in accordance with the reported inability of catalase to cross the lymphocyte membrane and to inhibit the intracellular production of ROS.23,57 Optimal T-cell proliferation and survival was only observed with intact RBCs and when RBCs were in close contact or proximity with the activated T cells. The need for proximity and for RBC integrity might imply the involvement of interactions between receptors present on each cell type, as suggested by some authors.33 58 The overall results, however, do not support that scenario. First, though reduced by 50%, the proliferative responses with RBCs in cell inserts were still much higher than with PHA alone. Second, red cell lysates were almost as efficient as intact RBCs in enhancing T-cell proliferation. Third, preliminary results indicate that RBCs are not required during the early phases of T-cell activation to fully exert their protective effect (Fonseca et al, unpublished data).
Among the T-cell receptors studied, RBCs significantly modulated only the expression of CD71, the transferrin receptor. Thus, the presence of RBCs in cultures of stimulated PBLs resulted in a decrease in the level of expression of CD71. The transferrin receptor is considered a marker of T-cell activation that is expressed by activated T lymphocytes to import extracellular iron for metabolic needs (reviewed in59). However, earlier studies demonstrated that the expression of CD71 in mitogen-activated human peripheral blood T cells is mainly regulated by the intracellular iron level rather than the rate of proliferation.60 It is now well established that CD71 expression is regulated at the post-transcriptional level not only by iron availability but also by extracellular and intracellular oxidative stress induced by reactive oxygen species such as H2O2 and NO.61,62 Therefore, CD71 down-regulation in activated T cells cultured in the presence of RBCs may reflect a reduction of the pro-oxidant state generated after T-cell activation, an increase in the intracellular iron pool, or a combination of both.60-62 In this context, the possibility that the enhancing effect on T-cell proliferation and survival is due to the costimulatory effect of RBC components, such as heme and iron, released during the culture period cannot be ruled out. Iron compounds are costimulatory agents of T-cell proliferation and induce a decrease in CD71 expression.50,60,63,64 Although we do not have evidence that hemolysis takes place in the cultures, some authors have pointed out the possibility of a T-cell–dependent RBC elimination.65 Given the existence of a close interplay between the intracellular iron pool and sensitivity to oxidative stress,30 66 the intracellular iron status of the resting human PBLs before stimulation could also have influenced the final outcome of the RBC effect.
Finally, we have demonstrated that human T cells activated in vitro generated protein-bound acrolein. Acrolein is a lipid-derived product that functions as a marker of oxidative stress and that has recently been found in several human diseases such as diabetic nephropathy and Alzheimer disease.39,67 A recent report showed that acrolein inhibits glucose and glutamate uptake in primary neuronal cultures.68 Whether acrolein also inhibits glucose uptake by activated T cells is presently unknown. Acrolein adducts were only significantly detected 24 hours after stimulation and were maximal by day 3. This suggests that acrolein formation in activated T cells is a late event during the apoptotic process. The presence of RBCs reduced the expression of acrolein adducts among activated T cells, therefore reinforcing the view that RBCs play an important role in reducing oxidative stress and apoptosis in activated T cells.
Overall, these results are in agreement with a recent study showing that RBCs inhibit apoptosis of human neutrophils.69 They add to the growing evidence indicating that hemoglobin-containing RBCs may perform unforeseen functions in addition to O2 and CO2 transport. For example, RBCs can regulate vascular vasodilatation by reacting with endothelial NO, a process regulated by intravascular flow.70 In a series of elegant studies, Jain et al71,72 have demonstrated that RBCs are crucial elements that facilitate the engagement of circulating lymphocytes with the vascular endothelium. It is well known that during inflammation and local immune response, capillary diameter and blood flow increase.73 This may allow the extravasation of RBCs, together with activated T cells, to inflammatory places, which would favor T-cell survival and enhance T-cell effector functions. Moreover, the reported increase in natural killer cytotoxicity against tumors after interaction with intact RBCs74 adds further support to the data presented here.
In summary, we have presented evidence that red blood cells can function as modulators of T-cell apoptosis in vitro, a finding associated with a reduction in intracellular oxidative stress. Whether protection from T-cell apoptosis also takes place, or is hampered, in vivo if erythrocyte anomalies exist remains the topic of future studies. In our view, the correlation found between anomalies in erythrocyte antioxidant defense and abnormalities in T-cell phenotype and function in certain chronic disorders, such as thalassemia,75-77 emphasizes the potential that mature red blood cells may have in regulating T-cell death or survival in vivo.
We thank the personnel of the Blood Bank of the Santo António General Hospital for their help in collecting blood samples. We thank Dr Alexandre M. Carmo for helpful comments during the performance of this work, Dr Rui Appelberg for reviewing the manuscript, and Dr Angelo Cardoso for valuable help in providing antibodies used in this study and for critical reading of the manuscript. We also thank Prof Maria de Sousa for continuous support. We thank the University of Porto for supporting the publication costs of this manuscript.
This work is part of the PhD thesis of A.M.F., who is the recipient of a Praxis XXI fellowship from Fundaçâo para a Ciêucia e a Tecnologia (BD 18503/98).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Fernando A. Arosa, Laboratory of Molecular Immunology, Institute for Molecular and Cell Biology, Rua do Campo Alegre, 823, 4150 Porto, Portugal; e-mail:farosa@ibmc.up.pt.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal