Abstract
Paroxysmal nocturnal hemoglobinuria (PNH) is a clonal hematopoietic stem cell disorder characterized by complement-mediated hemolysis due to deficiencies of glycosylphosphatidylinositol-anchored proteins (GPI-APs) in subpopulations of blood cells. Acquired mutations in the X-linked phosphatidylinositol glycan–class A (PIG-A) gene appear to be the characteristic and pathogenetic cause of PNH. To develop a gene therapy approach for PNH, a retroviral vector construct, termed MPIN, was made containing the PIG-A complementary DNA along with an internal ribosome entry site and the nerve growth factor receptor (NGFR) as a selectable marker. MPIN transduction led to efficient and stable PIG-A and NGFR gene expression in a PIG-A–deficient B-cell line (JY5), a PIG-A–deficient K562 cell line, an Epstein-Barr virus–transformed B-cell line (TK-14−) established from a patient with PNH, as well as peripheral blood (PB) mononuclear cells from a patient with PNH. PIG-A expression in these cell lines stably restored GPI-AP expression. MPIN was transduced into bone marrow mononuclear cells from a patient with PNH, and myeloid/erythroid colonies and erythroid cells were derived. These transduced erythroid cells restored surface expression of GPI-APs and resistance to hemolysis. These results indicate that MPIN is capable of efficient and stable functional restoration of GPI-APs in a variety of PIG-A–deficient hematopoietic cell types. Furthermore, MPIN also transduced into PB CD34+ cells from a normal donor, indicating that MPIN can transduce primitive human progenitors. These findings set the stage for determining whether MPIN can restore PIG-A function in multipotential stem cells, thereby providing a potential new therapeutic option in PNH.
Introduction
Paroxysmal nocturnal hemoglobinuria (PNH) is an acquired clonal hematopoietic stem cell disorder characterized by intravascular hemolytic anemia.1,2 Abnormal blood cells are deficient in glycosylphosphatidylinositol-anchored proteins (GPI-APs).3-6 In the affected hematopoietic cells from patients with PNH, the first step in biosynthesis of the GPI-anchor is defective.7,8 At least 5 genes are involved in this reaction step,9 and one of them, an X-linked gene termed phosphatidylinositol glycan–class A (PIG-A),10is mutated in affected cells.11,12 The PIG-Agene is mutated in every patient with PNH reported to date, and deficiency of GPI in PNH has thus been considered to be due solely to the PIG-A mutation(s).3-6
The clinical manifestation of PNH is complex, involving primarily 3 sets of symptoms: hemolysis with acute exacerbation, cytopenia of varying severity, and a tendency for thrombosis. PNH derives its name from the episodes of brownish urine (hemoglobinuria) that frequently appear in the morning13 and that are due to intravascular hemolysis because of deficiencies of decay accelerating factor (DAF) and CD59 in erythroid cells.4,5 However, the mechanism of thrombosis and its relation to defects in PNH are not entirely clear. Many patients with PNH have evidence of deficient hematopoiesis, and the degree of bone marrow (BM) failure varies from subclinical cytopenia to the development of severe aplastic anemia (AA). Also, patients with AA have an increased risk of developing PNH.14-17 Episodes of infection occur frequently and may be attributable in part to leukopenia or to functional defects in leukocytes, which may be due to impaired migration of neutrophils18,19 and/or defective T-cell activation in PNH.20 21 GPI-APs may thus function not only as complement regulatory proteins, but also as receptors, or adhesion molecules, and they may be involved in signal transduction.
Although PNH generally lasts for many years,22,23thrombotic disease and hematopoietic failure are the major risk factors affecting survival.23,24 For example, Socie et al23 reported that patients with thrombosis at presentation had only a 40% survival rate at 4 years. Currently, allogeneic bone marrow transplantation (BMT) is the only available cure for PNH; however, this is associated with high levels of morbidity and mortality. Gene therapy involving the transduction of thePIG-A gene into autologous pluripotent stem cells is a potential alternative strategy for treating PNH. In order to develop a gene therapy approach for PNH, we have made a retroviral vector construct, termed MPIN, containing the PIG-Acomplementary DNA (cDNA) along with internal ribosome entry sites (IRES) and the nerve growth factor receptor (NGFR) as a selectable marker. In the current study, we evaluated whether MPIN could restore PIG-A function in various hematopoietic cells expressing the PNH phenotype.
Materials and methods
Vector construction
The plasmid pΔLNGFR25-27 (generously provided by Fulvio Mavilio, Instituto Scientifico H. San Raffaele, Milan, Italy) was digested by EagI (5′) and BamHI (3′) to isolate a DNA fragment containing a truncated, non–ligand-binding version of the LNGFR cDNA (termed ΔLNGFR). The resulting fragment combined 5′ with an NcoI-to-EagI linker, encoding the first 18 base pairs (bp) of the ΔLNGFR cDNA. The MN vector plasmid was generated by cloning this fragment between the NcoI and BamHI sites of the retroviral vector plasmid MFG (generously provided by S. Kaye Spratt, Somatix Therapy, Alameda, CA) as previously described26 (Figure1). The functional PIG-A cDNA was amplified from pEBPIG-A10 as a template by polymerase chain reaction (PCR) by means of the primer set of Nco-PIGA (5′-GCGGCCCATGGCCTGTAGAGGAGGA) and Bam-PIGA (5′-GCGGCGGATCCCTACCTGGTTTCAGA) to generate the unique NcoI (5′) and BamHI (3′) sites and was then digested by these enzymes. The MP vector plasmid was generated by cloning this fragment between the NcoI and BamHI sites of the retroviral vector plasmid MFG after the sequence to confirm without mutation by PCR.
Schematic representation of the MN (upper) and MPIN (lower) retroviral vectors.
Primers used for polymerase chain reaction are indicated at the corresponding positions by arrowheads. U3, R, U5 = Moloney long terminal repeat elements; SD = splice donor site; SA = splice acceptor site; Ψ = vector packaging sequence; ATG = start codon.
Schematic representation of the MN (upper) and MPIN (lower) retroviral vectors.
Primers used for polymerase chain reaction are indicated at the corresponding positions by arrowheads. U3, R, U5 = Moloney long terminal repeat elements; SD = splice donor site; SA = splice acceptor site; Ψ = vector packaging sequence; ATG = start codon.
The plasmid pCITE-1 (Novagen, Madison, WI), containing the encephalomyocarditis virus–IRES sequence,28 was digested by BalI (5′) and XbaI (3′), and then a BalI/EagI oligonucleotide and an EagI (5′)/XbaI (3′) fragment from pΔLNGFR containing the ΔLNGFR cDNA were ligated into the modified pCITE vector so that the reading frame of the IRES start codon was preserved. The plasmid was digested by EcoRI (5′) and XbaI (3′) to isolate a DNA fragment containing the IRES-LNGFR cDNA. Finally, the MPIN vector plasmid was constructed by blunt insertion of this fragment into the unique BamHI site of the MP vector plasmid (Figure 1).
The MN and MPIN amphotropic vector packaging line was generated by transfection of the ecotropic retroviral vector producer line E86, followed by infection of the amphotropic producer AM12. LNGFR-expressing AM12 cells (termed AM12/MN or MPIN) were then isolated by fluorescence-activated cell sorting (FACS), and supernatant was collected by growing AM12/MN or MPIN packaging cells to 80% confluence and replacing the media with fresh media. For both supernatants, the medium was Dulbecco's modified Eagle's medium (DMEM) (Gibco, Gaithersburg, MD) containing 10% fetal calf serum (FCS) (Gibco), and flasks with fresh media were incubated at 37°C for 16 hours, and the supernatant was collected and centrifuged to remove cell debris. All supernatants were aliquoted and frozen at −70°C.26 29
Retroviral vector gene transfer
To characterize the titer and the expression of the ΔLNGFR vectors, 1 × 105 NIH3T3 cells were grown overnight in a 60-mm dish and then exposed to 1 mL of a 1:25 dilution of vector containing supernatant in the presence of 8 μg/mL polybrene (Sigma, St Louis, MO). After 2 hours, 3 mL additional medium was added. The near-confluent infected cells were harvested by trypsinization 72 hours later and stained for expression of ΔLNGFR. The titer was determined by multiplying the proportion of cells expressing ΔLNGFR by 25 × 105.29
To introduce the PIG-A gene into various hematopoietic cells expressing the PNH phenotype, generally 2 × 106 cells were transduced by MN or MPIN supernatant, with the use of polybrene at 8 μg/mL or fibronectin (Takara, Ohtsu, Japan) at 100 μg/mL, with or without centrifugation. In some experiments, the transduction procedure was repeated 1 to 3 additional times. After transduction, cells were immunophenotyped with various monoclonal antibodies.
Isolation and culture conditions of cells expressing the PNH phenotype
The PIG-A–deficient B cell line (JY5) and a PIG-A–deficient K562 cell line30 (generously provided by Dr Shinichi Hirose, Fukuoka Medical College, Japan) were maintained in DMEM with 10% FCS. The Epstein-Barr virus (EBV)–transformed B-cell line (TK-14−) established from a patient with PNH was grown in DMEM/Ham's F12 (Gibco) with 10% FCS.31Peripheral blood mononuclear cells (PBMCs) were obtained from a patient with PNH,24 having 33.1% of CD59−cells in CD2+ MNC fraction, by Ficoll-Hypaque centrifugation (Lymphocyte Separation Solution) (American Red Cross, Rockville, MD) after informed consent. To enrich for T lymphocytes, PBMCs were cultured in RPMI 1640 (Gibco) with 10% FCS in the presence of interleukin (IL) 2 (Chiron, Emeryville, CA) at 100 U/mL, anti-CD3 (OKT3) (Ortho, Raritan, NJ) at 50 ng/mL, and anti-CD28 (Becton Dickinson, Bedford, MA) at 50 U/mL for 2 days.29 After transduction, PBMCs were maintained in RPMI 1640 with 10% FCS and 10% T-Stim (Becton Dickinson) in the presence of IL-2 at 50 μg/mL. Bone marrow mononuclear cells (BMMCs) having 49.9% of CD59− cells in the BMMC fraction were obtained from a patient with PNH32 by Ficoll-Hypaque centrifugation after informed consent. After transduction, BMMCs were maintained in Iscove's modified Dulbecco's medium (IMDM) (Gibco) with 20% BIT 9500 (Stemcell Technologies, Vancouver, British Columbia, Canada) including flt-3 ligand (Genzyme, Cambridge, MA) at 50 ng/mL, erythropoeitin (Epo) (generously provided by Chugai Pharmaceutical, Tokyo, Japan) at 1000 U/L, and IL-3 (R&D Systems, Minneapolis, MN) at 2.5 ng/mL for 10 days.33 BMMCs were also cultured in MethoCult (Stemcell Technologies) containing 0.9% methylcellulose in IMDM, 30% fetal bovine serum, 1% bovine serum albumin, 3000 U/L Epo, and optimized concentrations of stem cell factor (SCF), granulocyte-macrophage colony-stimulating factor (GM-CSF), IL-3, and granulocyte CSF (G-CSF), to generate burst-forming unit–erythroid (BFU-E), as well as colony-forming unit–granulocyte-macrophage (CFU-GM) and CFU-mix colonies as described.34 The culture dishes were incubated at 37°C, and the numbers of colonies/bursts were scored on day 12 after 3 days' transduction. PB CD34+cells were isolated from a G-CSF–primed normal donor undergoing leukapheresis for autologous stem cell transplant after informed consent; to increase the number of CD34+ cells available for analysis, the sample was depleted of lineage-committed cells by using StemSep antibody cocktail for CD34+CD38−cells (Stemcell Technologies) including anti-CD2, anti-CD3, anti-CD14, anti-CD16, anti-CD19, anti-CD24, anti-CD56, anti-CD66b, anti-CD45RA, anti-CD36, anti-CD38, and glycophorin A. After transduction, hematopoietic progenitor cells containing CD34+ cells were maintained in IMDM with 20% BIT 9500 for one more day.
FACS analysis and flow cytometric sorting
Monoclonal antibodies (mAbs) included anti-CD59–fluorescein isothiocyanate (FITC), anti-CD55–phycoerythrin (PE), and anti-CD48–FITC (Pharmingen, San Diego, CA) for the detection of GPI-APs, anti-NGFR–PE (Chromoprobe, Mountain View, CA), anti-CD3–FITC (Pharmingen), anti-CD34–FITC (Pharmingen), and anti-CD45–peridinin chlorophyll protein (Pharmingen). For the detection of erythroid cells, E6,35 an antibody to red cell surface protein band 3 (generously provided by Dr Marilyn Telen, Duke University Medical Center, Durham, NC) as a first antibody and a secondary antibody conjugated to FITC (Jackson ImmunoResearch Lab, West Grove, PA) were used for staining. Antihuman IgG2a–FITC, antihuman IgG2a–PE and antihuman IgG1–PE (Pharmingen) were used for negative isotype controls. After staining, cells were analyzed with a FacsCalibur (Becton Dickinson). To separate CD59+, CD59−, NGFR+, and/or NGFR− cells, the sample was sorted on a FACStar Plus (Becton Dickinson) or a FACS Vantage (Becton Dickinson) after staining.
Molecular analysis by PCR for PIG-A integration
DNA was isolated from transduced (MN or MPIN) or untransduced (mock) NIH3T3 cell lines (JY5, K562 mutant, and TK-14−), BMMCs (primary erythroid cells), and colonies/bursts (mixture of CFU-GM, CFU-mix, and BFU-E). A 1330-bp region including vector plus 5′PIG-A was amplified by PCR with the use of the primer set of DG1 (5′-TCTCTCCCCCTTGAACC-3′) and OX2 (5′-GCTCCCAAAAGACGCAC-3′) as shown in Figure 1. For positive control, the MPIN vector plasmid was used as a template, and Marker 6 (Takara) was loaded as a molecular marker, in each experiment.
Small-scaled Ham test
Serum was separated from whole blood of a blood-group–matched healthy volunteer. Serum was acidified with a 10% volume of 0.2 N HCl. Washed samples suspended in saline (3 × 106/0.01 mL) were incubated with 0.1 mL of the serum for 1 hour at 37°C. For 100% lysis, water was used in place of serum, and for 0% lysis, heat-inactivated serum was used. After incubation, each sample was centrifuged, and the optical density of hemoglobin in the supernatant was measured at 412 nm.36
Aerolysin assay
Cells (1 × 106) were incubated for 90 minutes at 37°C with aerolysin (1.5 × 10−8 M), which is a toxin secreted by Aeromonas hydrophila and is capable of killing target cells by binding to GPI-anchored receptors, produced by trypsin activation of pro-aerolysin (Protox Biotech, Victoria, British Columbia, Canada).37,38 For 100% lysis, water was used in place of aerolysin, and for 0% lysis, PBS was used. After incubation, each sample was centrifuged, and the optical density of hemoglobin in the supernatant was measured at 412 nm.36
Results
Retroviral vector MPIN restores PIG-A function in hematopoietic cell lines bearing PNH phenotype
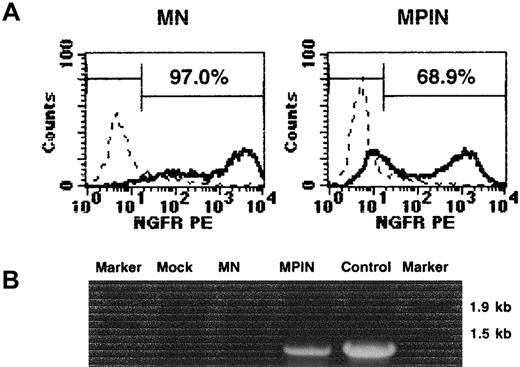
The MPIN vector was initially transduced into NIH3T3 cells. As shown in Figure 2A, 97.0% and 68.9% of NIH3T3 cells expressed LNGFR following transduction of MN and MPIN, respectively, yielding a titer of 4.8 × 106 infectious U/mL for MN and 3.4 × 106 infectious U/mL for MPIN. High levels of NGFR were expressed from MPIN, indicating that this could be a useful marker gene for analyzing gene transfer into hematopoietic cells. PCR analysis detected the region including PIG-A only in MPIN-transduced NIH3T3 cells (Figure 2B).
Characterization of MN and MPIN vectors.
(A) NGFR surface expression in the transduced NIH3T3 cells with 1:25 dilution of MN or MPIN vector supernatant. (B) Detection of the region including PIG-A in MPIN-transduced NIH3T3 cells by PCR. DNA was isolated from transduced (MN or MPIN) or untransduced (mock) NIH3T3. A region including vector plus 5′ PIG-A was amplified by PCR. For positive control, the MPIN vector plasmid was used as a template, and Marker 6 was loaded as a marker.
Characterization of MN and MPIN vectors.
(A) NGFR surface expression in the transduced NIH3T3 cells with 1:25 dilution of MN or MPIN vector supernatant. (B) Detection of the region including PIG-A in MPIN-transduced NIH3T3 cells by PCR. DNA was isolated from transduced (MN or MPIN) or untransduced (mock) NIH3T3. A region including vector plus 5′ PIG-A was amplified by PCR. For positive control, the MPIN vector plasmid was used as a template, and Marker 6 was loaded as a marker.
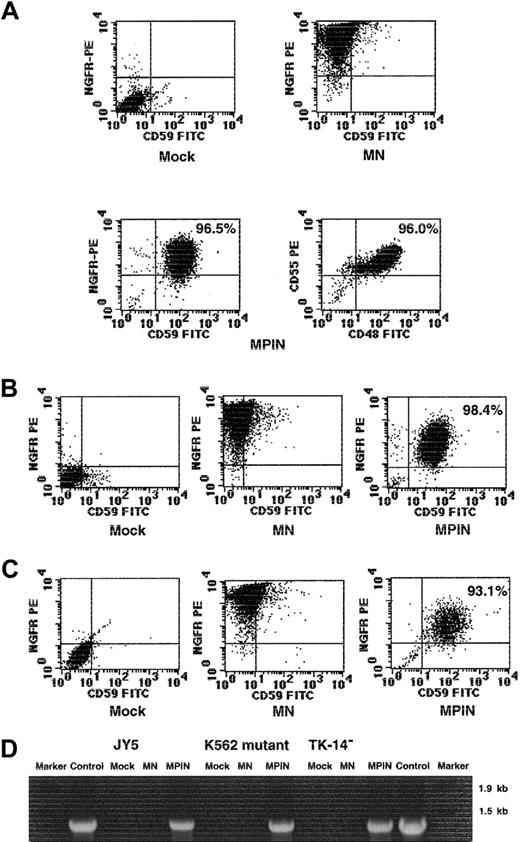
To evaluate whether MPIN would restore PIG-A function in hematopoietic cells, we transduced it into several PIG-A–deficient cell lines. MPIN was transduced into the PIG-A–deficient B-lymphoblastoid cell line JY5, and then on days 10 and 20, NGFR+CD59+cells were sorted to be enriched. On day 39, transduced cells were stained with mAbs for NGFR and GPI-APs. In the selected JY5 cells, MPIN expressed NGFR at high levels and restored stable and efficient expression (greater than 95%) of GPI-APs including CD55, CD59, and CD48 (Figure 3A). MPIN was also transduced into a PIG-A–deficient K562 mutant cell line, and an EBV-derived B cell line (TK-14−) established from a patient with PNH. After 21 days in cultures, CD59+NGFR+ cells were sorted for enrichment, and on day 27, transduced cells were stained with mAbs for NGFR and CD59. Again, MPIN expressed NGFR at high levels in these cell lines and restored stable and efficient expression of GPI-AP (greater than 93%) (Figure 3B-C). These restored expressions were still stable after repeated freezing and thawing (data not shown). These observations indicate that MPIN can restore PIG-A function in multiple hematopoietic cell types. The presence of DNA including the PIG-A sequence was also confirmed only in MPIN-transduced cell lines (Figure 3D).
Gene transfer by MPIN into PNH-phenotype hematopoietic cell lines restores PIG-A function.
(A) GPI-APs (CD55, CD59, CD48) and NGFR surface expression of PIG-A–deficient B-lymphoblastoid cell line JY5 transduced with MN or MPIN on day 39. (B) (C) GPI-AP (CD59) and NGFR surface expression of PIG-A–deficient K562 mutant cell line (panel B) and PIG-A–deficient B-lymphoblastoid cell line (TK-14−) established from a patient with PNH (panel C), transduced with MN or MPIN on day 21. (D) Detection of the region including PIG-A in MPIN-transduced cell lines (JY5, K562 mutant, and TK-14−) by PCR. DNA was isolated from transduced (MN or MPIN) or untransduced (mock) cell lines. A region including vector plus 5′ PIG-A was amplified by PCR. For positive control, the MPIN vector plasmid was used as a template, and Marker 6 was loaded as a marker.
Gene transfer by MPIN into PNH-phenotype hematopoietic cell lines restores PIG-A function.
(A) GPI-APs (CD55, CD59, CD48) and NGFR surface expression of PIG-A–deficient B-lymphoblastoid cell line JY5 transduced with MN or MPIN on day 39. (B) (C) GPI-AP (CD59) and NGFR surface expression of PIG-A–deficient K562 mutant cell line (panel B) and PIG-A–deficient B-lymphoblastoid cell line (TK-14−) established from a patient with PNH (panel C), transduced with MN or MPIN on day 21. (D) Detection of the region including PIG-A in MPIN-transduced cell lines (JY5, K562 mutant, and TK-14−) by PCR. DNA was isolated from transduced (MN or MPIN) or untransduced (mock) cell lines. A region including vector plus 5′ PIG-A was amplified by PCR. For positive control, the MPIN vector plasmid was used as a template, and Marker 6 was loaded as a marker.
Retroviral vector gene transfer by MPIN restores PIG-A function in PBMCs with PNH phenotype
To test MPIN in primary peripheral blood cells, CD59−PBMCs were isolated from a patient with PNH and transduced with MPIN after stimulation with IL-2, anti-CD3, and anti-CD28 as previously described.29 On day 19 in culture, 93.0% were positive for CD3, a T-cell marker, and negative for CD19, a B-cell marker, and the gene-transfer efficiency was 16.9% (16.8 ± 0.7%; range, 15.0% to approximately 18.4%; n = 4) as measured as a percentage of NGFR+ cells (Figure 4). MPIN restored expression of GPI-APs at 8.8% (9.7 ± 0.4%; range, 8.8% to approximately 10.6%; n = 4) as measured by NGFR+CD59+ cells (Figure 4). Even though we confirmed that the sorted PBMCs were mostly of the CD59−phenotype, 11.0% of cells in mock control expressed CD59. The most likely explanation for this paradox is that contaminated CD59dim PBMCs brightly expressed after the culture with stimulation. The CD59−NGFR+ cells may also have been observed because initiation or completion of translation did not occur in the PIG-A sequence but, for currently unknown reasons, did occur in the downstream NGFR sequence. Expression of both NGFR and GPI-APs was stable for the duration of the cell cultures (about a month). There was no significant difference in growth rates between mock, MN-infected, and MPIN-infected cells, suggesting that there was no toxicity from the virus.
Retroviral vector MPIN restores PIG-A function in PNH-phenotype PBMCs.
GPI-AP (CD59), NGFR, CD3, and CD19 surface expression of MPIN-transduced MNCs from a patient with PNH on day 19.
Retroviral vector MPIN restores PIG-A function in PNH-phenotype PBMCs.
GPI-AP (CD59), NGFR, CD3, and CD19 surface expression of MPIN-transduced MNCs from a patient with PNH on day 19.
Retroviral vector MPIN restores PIG-A function and confers resistance to hemolysis in PNH-phenotype primary erythroid cells from a patient with PNH
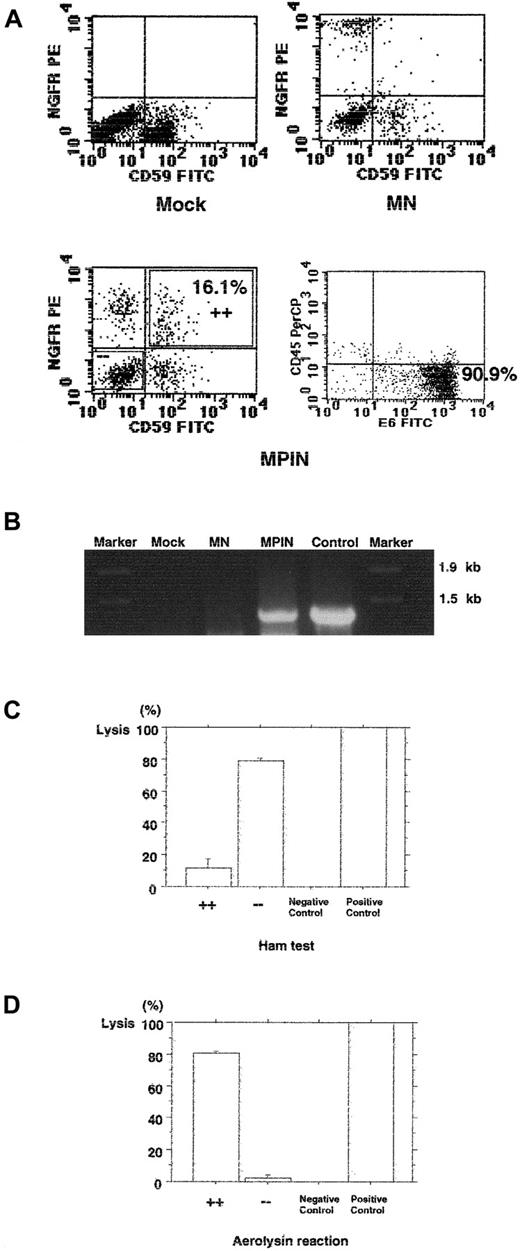
One of the primary clinical manifestations of PNH is increased sensitivity of red cells (RBCs) to hemolysis by complement. To test whether transduction with MPIN would restore resistance to hemolysis in PNH RBCs, CD59− BMMCs were isolated from a patient with PNH and transduced with MPIN. Cells were then cultured with flt-3 ligand, Epo, and IL-3, as previously described, to generate erythroid progeny.33 Multiparameter FACS analysis performed on day 12 of culture demonstrated that 16.1% (19.2% ± 2.0%; range, 16.1% to approximately 24.9%; n = 4) of transduced cells were CD59+NGFR+; 90.9% were positive for E6, an erythroid marker; and most of the cells were negative for CD45, a leukocyte marker (Figure 5A). The integrated region including PIG-A was detected only in MPIN-infected erythroid progeny (Figure 5B).
Gene transfer by MPIN into PNH-phenotype primary erythroid cells from a patient with PNH restores PIG-A function and resistance to hemolysis.
(A) GPI-AP (CD59), NGFR, E6, and CD45 surface expression of MPIN-transduced erythroid cells generated from GPI-AP−(CD59−) BMMCs on day 12. (B) Detection of the region including PIG-A in MPIN-transduced BMMCs by PCR. DNA was isolated from transduced (MN or MPIN) or untransduced (mock) BMMCs. A region including vector plus 5′ PIG-A was amplified by PCR. For positive control, the MPIN vector plasmid was used as a template, and Marker 6 was loaded as a marker. (C) (D) Small-scaled Ham test (panel C), and aerolysin assay (panel D). The percentage of lysis of CD59+NGFR+ (+ +) and CD59−NGFR− (− −) cells from MPIN-transduced BMMCs were compared. For 100% lysis, water was used in place of serum, and for 0% lysis, heat-inactivated serum was used. Results are shown as mean ± SE.
Gene transfer by MPIN into PNH-phenotype primary erythroid cells from a patient with PNH restores PIG-A function and resistance to hemolysis.
(A) GPI-AP (CD59), NGFR, E6, and CD45 surface expression of MPIN-transduced erythroid cells generated from GPI-AP−(CD59−) BMMCs on day 12. (B) Detection of the region including PIG-A in MPIN-transduced BMMCs by PCR. DNA was isolated from transduced (MN or MPIN) or untransduced (mock) BMMCs. A region including vector plus 5′ PIG-A was amplified by PCR. For positive control, the MPIN vector plasmid was used as a template, and Marker 6 was loaded as a marker. (C) (D) Small-scaled Ham test (panel C), and aerolysin assay (panel D). The percentage of lysis of CD59+NGFR+ (+ +) and CD59−NGFR− (− −) cells from MPIN-transduced BMMCs were compared. For 100% lysis, water was used in place of serum, and for 0% lysis, heat-inactivated serum was used. Results are shown as mean ± SE.
After the separation of CD59+NGFR+ and CD59−NGFR− cells, their sensitiviy to hemolytic action was measured by means of the Ham test. An average of 79.0% ± 1.7% (range; 75.7% to approximately 81.5%; n = 3) of CD59+NGFR+ cells were lysed, whereas only 11.9% ± 5.5% (range, 6.1% to approximately 22.9%; n = 3) of CD59+NGFR+ cells with restored expression of GPI-AP were lysed (P = .0003) (Figure 5C). Their sensitivity to aerolysin, a toxin capable of killing target cells by forming channels after binding to GPI-APs, was also measured. Only 2.2% ± 1.9% (range, 0.3% to approximately 4.1%; n = 2) of CD59−NGFR− cells were lysed, whereas 80.3% ± 1.5% (range, 78.8% to approximately 81.8%; n = 2) of CD59+NGFR+ cells with restored expression of GPI-AP were lysed (P = .001) (Figure 5D). Thus, MPIN restored resistance to hemolysis and the cytotoxic effectiveness of aerolysin, as well as expression of GPI-APs.
Retroviral vector gene transfer by MPIN into PNH-phenotype and normal BMMCs
BMMCs were also isolated from a patient with PNH and transduced with MPIN. Cells were then cultured in methylcellulose with Epo, SCF, GM-CSF, IL-3, and G-CSF to generate colonies and bursts. The numbers of colonies/bursts were scored on day 15 in culture, and the ratio of BFU-E to CFU-GM to CFU-mix was about 1:1:1 (data not shown). FACS analysis performed on day 15 of culture demonstrated that 13.6% of transduced cells were CD59+NGFR+ (Figure6A). The integrated region includingPIG-A was also detected only in MPIN-infected colonies/bursts (Figure 6B).
Retroviral vector MPIN restores PIG-A function in PNH-phenotype BMMCs.
(A) GPI-AP (CD59) and NGFR surface expression of the pooled colonies/bursts generated from PNH-phenotype BMMCs transduced with MN or MPIN. (B) Detection of the region including PIG-A in MPIN-transduced colonies/bursts generated from PNH-phenotype BMMCs by PCR. DNA was isolated from transduced (MN or MPIN) or untransduced (mock) colonies/bursts. A region including vector plus 5′PIG-A was amplified by PCR. For positive control, the MPIN vector plasmid was used as a template, and Marker 6 was loaded as a marker.
Retroviral vector MPIN restores PIG-A function in PNH-phenotype BMMCs.
(A) GPI-AP (CD59) and NGFR surface expression of the pooled colonies/bursts generated from PNH-phenotype BMMCs transduced with MN or MPIN. (B) Detection of the region including PIG-A in MPIN-transduced colonies/bursts generated from PNH-phenotype BMMCs by PCR. DNA was isolated from transduced (MN or MPIN) or untransduced (mock) colonies/bursts. A region including vector plus 5′PIG-A was amplified by PCR. For positive control, the MPIN vector plasmid was used as a template, and Marker 6 was loaded as a marker.
PB CD34+ cells were enriched from a G-CSF primed donor after mobilization by means of StemSep and were transduced with MPIN. Gene-transfer efficiency in CD34+ cells was about 6% as measured by NGFR+ cells on day 5 in culture (Figure7).
Retroviral vector gene transfer by MPIN into CD34+ cells from a normal donor.
GPI-AP (CD59) and NGFR surface expression of the transduced CD34+ MNCs from the peripheral blood of a normal donor on day 5.
Retroviral vector gene transfer by MPIN into CD34+ cells from a normal donor.
GPI-AP (CD59) and NGFR surface expression of the transduced CD34+ MNCs from the peripheral blood of a normal donor on day 5.
Discussion
BMT is currently the only available cure for PNH. Syngeneic and allogeneic BMTs, including eradication of the PNH clone, have been successful in several patients with PNH.39-41 In most, the indication for BMT was BM failure, and guidelines similar to those for AA have been proposed for the use of BMT in these patients. BMT was performed on one patient who had portal and hepatic vein thrombosis, with resolution of much of the thrombosis.42 Socie et al23 reported that thrombosis and hematopoietic failure were the major risk factors affecting survival. For example, patients with thrombosis at presentation have only a 40% survival rate at 4 years.23 Therefore, these patients are also good candidates for early and aggressive therapies.
Autologous BMT, with or without depletion of GPI−hematopoietic stem cells, has been proposed as a possible cure for PNH. However, the low number of hematopoietic stem cells in the BM of a patient with PNH may pose a problem, particularly in those with BM failure. In many patients, one cannot easily isolate the normal cells and repopulate the BM with them, because the patients have few normal cells remaining and/or have BM hypoplasia, including AA. Indeed, our preliminary data show that the mean fraction of circulating polymorphonuclear cells (PMNs) deficient in GPI-APs at the time of diagnosis was 70% and that 50% of patients have fewer than 20% normal PMNs. The fraction of deficient PMNs directly reflects the fraction of PIG-A mutant stem cells.
An alternative to BMT would be therapy designed to restore the surface expression of GPI-APs, such as infusion of GPI-APs. Kooyman et al43 demonstrated that GPI-APs expressed on the surface of transgenic mouse red cells were transferred in a functional form to endothelial cells in vivo. In addition, Dunn et al44created a murine knock-out embryonic stem cell via targeted disruption of the Pig-a gene and demonstrated transfer in situ of GPI-APs from normal to knock-out cells. Subsequently, Sloand et al45 demonstrated that enriched DAF and CD59 reincorporated into the membranes of PNH erythrocytes by means of high-density lipoprotein were functional. At this time, however, strategies to transfer therapeutic proteins to the GPI− PNH clone remain exploratory.
The discovery of PIG-A allows a new approach to therapy for patients with PNH, and molecular therapy to introduce a normalPIG-A gene into PIG-A mutant hematopoietic stem cells appears attractive. Autologous BMT after ex vivo transduction of a normal PIG-A gene into mutant hematopoietic stem cells could avoid both graft failure and graft-versus-host disease seen in allogeneic BMT. However, there remains the risk of recurrent PNH, caused by the re-establishment of clonal dominance by any residualPIG-A mutant stem cells. The mechanisms of clonal dominance of mutant stem cells in PNH are yet to be clarified. A possible mechanism is selection for PIG-A mutant cells, such as an immunological selection. For example, if cytotoxic T cells are involved in the autoimmunity, stem cells defective in the surface expression of GPI-APs may be less sensitive to the cytotoxic cells because the effector-target interaction may be inefficient owing to a lack of certain GPI-anchored types of adhesion molecules. In any case, selective killing of normal stem cells would result in selective survival and expansion of the GPI-deficient stem cell clone. Indeed, syngeneic BMT without conditioning in patients with PNH has not been universally successful because of an apparent survival advantage of mutant stem cells.46 47 If pretransplantation BM conditioning is effective in eliminating such selective conditions, then residual mutant stem cells would be eliminated or might not become dominant. The other possibility is that expanded clonal cells have a genetic abnormality in addition to PIG-A mutation and that the 2 in combination or the putative second abnormality may impart an ability to expand autonomously even in a normal bone marrow environment. In this case, the PIG-A–restored PNH stem cell clone might still maintain the ability to expand autonomously. More complete understanding of the pathogenesis of PNH is required as a scientific foundation for the rational design of further molecular therapeutic approaches. At this time, autologous BMT after the transduction of the normal PIG-A gene into mutant hematopoietic stem cells, along with depletion of residual mutant hematopoietic cells, seems the best molecular therapeutic approach to PNH.
As proof of the principle of gene therapy for PNH, a functional recombinant transmembrane form of CD59 was expressed in GPI-deficient PNH B cells by means of retroviral gene transfer.48 This restored surface expression of CD59 and resistance to complement. In this study, we demonstrated that MPIN, a retroviral vector containing the full coding sequence of PIG-A, was able to restore GPI-AP expression and was capable of long-term stable gene transfer into various cell lines expressing the PNH phenotype. Subsequently, we demonstrated that MPIN was capable of efficient and stable gene transfer into primary peripheral T cells and progenitor cells from a patient with PNH. In addition, MPIN was capable of efficient functional restoration of GPI-APs that confer resistance to hemolytic action, indicating that MPIN can transduce erythroid progenitors. Finally, we also demonstrated that MPIN can transduce CD34+ primitive human progenitors.
Taken together, our observations provide convincing evidence that stable and functional PIG-A gene transfer into human hematopoietic cells is possible. These findings set the stage for determining whether MPIN can restore PIG-A function in multipotential stem cells from patients with PNH. An additional advantage of MPIN vector is that the NGFR marker gene also permits easy determination of gene transfer efficiencies, as well as rapid isolation of transduced cells by means of a flow cytometric technique. Since we recently developed a PNH mouse model in which only the hematopoietic system has Pig-a–deficient cells,49 we could initiate the study for determining the restoration of the PIG-A function in multipotential stem cells using this mice model system, thereby providing a potential new therapeutic option in PNH.
We thank Dr Yasuhiko Horiguchi for advice on use of aerolysin; and Toshiyuki Hirota, Yvonne Ellis, Yuki Murakami, Keiko Kinoshita, and Reiko Fukuyama for their excellent technical assistance.
Partly supported by a grant from the Japan Intractable Diseases Research Foundation and The Osaka Medical Research Foundation for Incurable Diseases.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Jun-ichi Nishimura, Dept of Immunoregulation, Research Institute for Microbial Diseases, Osaka University, 3-1 Yamada-oka, Suita, Osaka 565-0871, Japan; e-mail:junnishi@acpub.duke.edu.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal