Abstract
In order to compare the outcomes of unrelated umbilical cord blood transplants (UCBTs) or bone marrow transplants, 541 children with acute leukemia (AL) transplanted with umbilical cord blood (n = 99), T-cell–depleted unrelated bone marrow transplants (T-UBMT) (n = 180), or nonmanipulated (UBMT) (n = 262), were analyzed in a retrospective multicenter study. Comparisons were performed after adjustment for patient, disease, and transplant variables. The major difference between the 3 groups was the higher number in the UCBT group of HLA mismatches (defined by serology for class I and molecular typing for DRB1). The donor was HLA mismatched in 92% of UCBTs, in 18% of UBMTs, and in 43% of T-UBMTs (P < .001). Other significant differences were observed in pretransplant disease characteristics, preparative regimens, graft-versus-host disease (GVHD) prophylaxis, and number of cells infused. Nonadjusted estimates of 2-year survival and event-free survival rates were 49% and 43%, respectively, in the UBMT group, 41% and 37% in the T-UBMT group, and 35% and 31% in the UCBT group. After adjustment, differences in outcomes appeared in the first 100 days after the transplantation. Compared with UBMT recipients, UCBT recipients had delayed hematopoietic recovery (Hazard ratio [HR] = 0.37; 95% confidence interval [95CI]: 0.27-0.52; P < .001), increased 100 day transplant-related mortality (HR = 2.13; 95CI: 1.20-3.76;P < .01) and decreased acute graft-versus-host disease (aGVHD) (HR = 0.50; 95CI: 0.34-0.73; P < .001). T-UBMT recipients had decreased aGVHD (HR = 0.25; 95CI: 0.17-0.36;P < .0001) and increased risk of relapse (HR = 1.96; 95CI: 1.11-3.45; P = .02). After day 100 posttransplant, the 3 groups achieved similar results in terms of relapse. Chronic GVHD was decreased after T-UBMT (HR = 0.21; 95CI: 0.11-0.37;P < .0001) and UCBT (HR = 0.24; 95CI: 0.01-0.66;P = .002), and overall mortality was higher in T-UBMT recipients (HR = 1.39; 95CI: 0.97-1.99; P < .07). In conclusion, the use of UCBT, as a source of hematopoietic stem cells, is a reasonable option for children with AL lacking an acceptably matched unrelated marrow donor.
Introduction
Allogeneic hematopoietic stem cell transplants play an important role in treating patients with high-risk acute leukemia (AL). However, 70% of the children who might benefit from this therapy lack an HLA identical sibling donor. Despite the establishment of bone marrow donor registries with more than 5 million unrelated volunteer donors worldwide, finding a fully HLA-matched unrelated donor remains a problem for many patients because of HLA polymorphism.1,2Because of this, efforts have turned toward using HLA partially mismatched unrelated or related donors3-5 and other sources of stem cells such as umbilical cord blood cells6,7 or granulocyte-colony stimulating factor (G-CSF)–mobilized T-cell–depleted peripheral blood hematopoietic stem cells provided by related haploidentical donors.8
With the establishment of cord blood banks, more than 30 000 cord blood units have been made available for transplantation9-12 and facilitated more than 1200 unrelated umbilical cord blood transplants (UCBT) for children and adults with either malignant or nonmalignant diseases.7,13-17 In children with AL, cord blood has potential advantages compared with bone marrow hematopoietic stem cells, namely the rapid availability of cells and less stringent requirements for HLA identity between donor and recipient because of the lower risk of acute and chronic graft-versus-host disease (GVHD).18 In addition, a previous Eurocord study has shown that unrelated HLA-mismatched UCBT in children with AL gives results comparable to those reported with other sources of stem cells.19
With better characterization of HLA types, improvements in GVHD prophylaxis, and treatment of infectious diseases, results of HLA-matched unrelated donor transplants have become comparable to HLA-matched sibling transplants in children with AL.20Also, T-cell–depleted HLA-matched and -mismatched UBMT5,21-23 and T-cell–depleted haploidentical related peripheral blood hematopoietic stem cell transplants in patients with AL have also shown promising results.24 Consequently, the number of allogeneic BMTs using alternative donors is increasing, as is the difficulty in choosing the best donor for a specific patient. In order to evaluate these different strategies, we compared the outcomes of 99 children with AL receiving a UCBT to those of 442 children receiving either a nonmanipulated UBMT (n = 262) or a T-UBMT (n = 180).
Materials and methods
Data collection and population
Eurocord is an international registry operating on behalf of the European Blood and Marrow Transplant (EBMT). Participation was open to European and non-European centers performing UCBT. Eurocord worked in close collaboration with Netcord banks9 and the EBMT database. Unrelated BMT data were collected from Eurocord centers and also from large centers not reporting UCBT in children with AL (participating centers and number of transplants reported by center are listed in the ). The median number of children reported by each center was 4.5 (range: 1-122). The study included consecutive patients receiving allogeneic UCBT or UBMT, who (1) were less than 16 years old at time of transplant; (2) had AL; and (3) received the transplant between January 1, 1994 and May 31, 1998. Patients who received peripheral blood hematopoietic stem cells were excluded. Data concerning patient and disease characteristics and transplant outcomes were collected by standardized questionnaire for each UCBT and UBMT recipient. Submitted data were reviewed by 2 physicians and computerized error checks were performed to ensure data quality. A total of 99 UCBT and 442 UBMT recipients from 51 centers satisfied the criteria. Sixty children receiving UCBT in this study were previously reported in a Eurocord analysis.19
Bone marrow donor registries, cord blood banks, and HLA typing
Searches for unrelated bone marrow donors were processed through the National Marrow Donor Program (n = 108), British Bone Marrow Registry (n = 88), Anthony Nolan Bone Marrow Trust (n = 77), German registries (n = 44), France Greffe de Moelle (n = 32), Italian Bone Marrow donor registry (n = 31), and 10 European (n = 33), Australian (n = 15), and Japanese (n = 14) registries. Forty-seven umbilical cord blood units came from the New York Cord Blood Bank (CBB), 23 from the Milan CBB, 16 from the Duesseldorf CBB, 5 from the Barcelona CBB, and 8 from other banks.
Donor-recipient histocompatibility was determined by serology for HLA-A and HLA-B antigens and by DNA typing for HLA-DRB1. Most HLA-DRB1 typing was performed by high molecular resolution allelic technique and only 15 (3%) donor-recipient pairs had low resolution molecular typing.
All HLA data were reviewed and queries concerning patient and donor HLA typing were verified in transplant centers, bone marrow donor registries, and cord blood banks. Transplants were classified as HLA-mismatched with 1, 2, 3, or 4 differences if disparities were detected in HLA-A, HLA-B, or HLA-DRB1 antigens or alleles. Blanks at the same locus were considered matches only if the paired allele was the same.
Outcomes
Hematopoietic recovery.
Neutrophil and platelet recoveries were analyzed separately, and defined by a neutrophil count of ≥ 0.5 × 109/L for 3 consecutive days and a nontransfused platelet count of ≥ 20 × 109/L for 7 consecutive days, respectively. Failure of engraftment was defined by the absence of blood counts recovery at day 60 or in cases of second transplant or hematopoietic reconstitution with autologous cells.
Graft-versus-host disease.
Acute graft-versus-host disease (aGVHD) was diagnosed and graded at each transplant center according to Seattle criteria.25All patients were considered at risk for developing aGVHD at day +1 after transplantation. The reason for this definition was that between day +1 to day +14 after transplantation, 20% of UBMT and 14% of UCBT recipients without neutrophil recovery had signs of aGVHD. Chronic GVHD (cGVHD) was defined according to standard criteria.26Patients surviving for more than 100 days after transplantation with sustained donor engraftment were considered as evaluable for cGVHD.
Relapse.
Relapse was defined on the basis of morphologic evidence of leukemia in bone marrow, or other extramedullary organs.
Early transplant-related mortality.
Early transplant-related mortality (TRM) was defined as all causes of nonleukemic deaths occurring within 100 days after transplantation.
Event-free survival.
Event-free survival (EFS) was defined as time interval from transplantation to first event (either relapse or death in complete remission).
Overall survival.
Overall survival was defined as time between transplantation and death.
Statistical analysis
Analysis used January 1, 1999, as the reference date, that is, the day on which all centers locked data on patient outcomes.
Patient-, disease-, and transplant-related variables of the 3 transplant groups were compared, using the Fisher exact test for categoric variables and the nonparametric Kruskal-Wallis test for continuous variables.
Since the outcomes following transplantation were all right-censored (neutrophil and platelet recoveries, acute and chronic GVHD, relapse, TRM, survival, and EFS), time to each endpoint was estimated by the Kaplan-Meier method. Cox models were used to evaluate the joint influence of patient-, disease-, and transplant-related variables (Table 1) on each endpoint, in each transplant group, separately. To ensure the availability of all input variables where and when prediction will be made, and owing to a strategy of data reduction, all variables with high rate of missing values (≥ 10%) were excluded from the analysis, namely peripheral blasts at diagnosis and cytogenetics. For the other variables, missing values were estimated using the median value on the whole sample.
Patient- and disease-related characteristics of nonmanipulated unrelated bone marrow (UBMT), T-cell–depleted unrelated bone marrow donor (T-UBMT), and umbilical unrelated cord blood transplants (UCBT)
| Characteristics . | UBMT (n = 262) . | T-UBMT (n = 180) . | UCBT (n = 99) . | P value* . |
|---|---|---|---|---|
| Patient-related | ||||
| Age, years | 8 (5-12) | 8 (6-12) | 6 (2.5-10) | .0004 |
| Missing data | 0 | 0 | 0 | |
| < 2 yr | 20 (8%) | 5 (3%) | 21 (21%) | .0001 |
| < 6 yr | 79 (30%) | 58 (32%) | 54 (55%) | .0001 |
| Gender | ||||
| Male | 159 (61%) | 118 (66%) | 58 (59%) | .44 |
| Female | 103 (39%) | 62 (34%) | 41 (41%) | |
| Weight, kg | 28 (20-42) | 28 (20-41) | 21 (13-34) | .0001 |
| Missing data | 3 (1%) | 11 (6%) | 0 | |
| Positive recipient CMV serology prior to transplant | 119 (46%) | 48 (27%) | 47 (48%) | .0001 |
| Missing data | 1 (0.4%) | 0 | 1 (1%) | |
| Disease-related | ||||
| Diagnosis | ||||
| ALL | 195 (74%) | 145 (81%) | 65 (66%) | .014 |
| AML | 49 (19%) | 24 (13%) | 30 (30%) | |
| Secondary leukemia | 18 (7%) | 11 (6%) | 4 (4%) | |
| Previous transplant for relapse† | 10 (4%) | 3 (2%) | 14 (14%) | .0001 |
| Missing data | 9 (3%) | 2 (1%) | 0 | |
| Immunophenotype (only for ALL) B (B + preB + null)/T/Hybrid (biphenotypic or others) | 153/23/8 | 110/27/6 | 46/10/7 | .11 |
| Missing data | 11 (4%) | 2 (1%) | 2 (2%) | |
| FAB (only for AML) M0 + M1 + M5 + M6 + M7 vs M3 + M4 | 39/7 | 15/7 | 20/9 | .17 |
| Missing data | 3 (1%) | 2 (1%) | 1 (1%) | |
| Karyotype | ||||
| Unfavorable t(9;22), 11q23, t(4;11), monosomy 7, 5q- | 48 (18%) | 31 (17%) | 19 (19%) | .93 |
| Intermediate (Others or normal) | 135 (52%) | 102 (57%) | 58 (59%) | |
| Favorable (hyperploidy + inv16 + t(8;21) + t(15,17) | 22 (8%) | 13 (7%) | 8 (8%) | |
| Missing data | 57 (22%) | 34 (19%) | 14 (14%) | |
| Time interval from diagnosis to transplantation, months | 20 (8-42) | 24 (8-41) | 15 (8-31) | .10 |
| Missing data | 0 | 0 | 0 | |
| Median days from last CR to transplantation (only for patients in CR at time of transplantation) | 113 (70-190) | 109 (76-156) | 84 (52-139) | .001 |
| (n = 210) | (n = 163) | (n = 81) | ||
| First relapse on therapy | 85 (45%) | 55 (42%) | 44 (58%) | .08 |
| First relapse off therapy | 105 (55%) | 75 (58%) | 32 (42%) | |
| Missing data | 1 (0.4%) | 2 (1%) | 0 | |
| Median days from first CR to first relapse | 650 (296-939) | 744 (388-1019) | 400 (184-814) | .001 |
| (n = 187) | (n = 131) | (n = 76) | ||
| Status at time of transplantation | ||||
| First CR | 59 (23%) | 43 (24%) | 18 (18%) | .04 |
| Second CR | 102 (39%) | 90 (50%) | 49 (49%) | |
| ≥ Third CR | 49 (19%) | 30 (17%) | 14 (14%) | |
| Advanced (refractory/partial response/relapse/first acute phase) | 52 (20%) | 17 (9%) | 18 (18%) | |
| Poor Risk (≥ 3CR + advanced) | 101 (39%) | 47 (26%) | 32 (32%) | .02 |
| Good risk (1CR + 2CR) | 161 (61%) | 133 (74%) | 67 (68%) |
| Characteristics . | UBMT (n = 262) . | T-UBMT (n = 180) . | UCBT (n = 99) . | P value* . |
|---|---|---|---|---|
| Patient-related | ||||
| Age, years | 8 (5-12) | 8 (6-12) | 6 (2.5-10) | .0004 |
| Missing data | 0 | 0 | 0 | |
| < 2 yr | 20 (8%) | 5 (3%) | 21 (21%) | .0001 |
| < 6 yr | 79 (30%) | 58 (32%) | 54 (55%) | .0001 |
| Gender | ||||
| Male | 159 (61%) | 118 (66%) | 58 (59%) | .44 |
| Female | 103 (39%) | 62 (34%) | 41 (41%) | |
| Weight, kg | 28 (20-42) | 28 (20-41) | 21 (13-34) | .0001 |
| Missing data | 3 (1%) | 11 (6%) | 0 | |
| Positive recipient CMV serology prior to transplant | 119 (46%) | 48 (27%) | 47 (48%) | .0001 |
| Missing data | 1 (0.4%) | 0 | 1 (1%) | |
| Disease-related | ||||
| Diagnosis | ||||
| ALL | 195 (74%) | 145 (81%) | 65 (66%) | .014 |
| AML | 49 (19%) | 24 (13%) | 30 (30%) | |
| Secondary leukemia | 18 (7%) | 11 (6%) | 4 (4%) | |
| Previous transplant for relapse† | 10 (4%) | 3 (2%) | 14 (14%) | .0001 |
| Missing data | 9 (3%) | 2 (1%) | 0 | |
| Immunophenotype (only for ALL) B (B + preB + null)/T/Hybrid (biphenotypic or others) | 153/23/8 | 110/27/6 | 46/10/7 | .11 |
| Missing data | 11 (4%) | 2 (1%) | 2 (2%) | |
| FAB (only for AML) M0 + M1 + M5 + M6 + M7 vs M3 + M4 | 39/7 | 15/7 | 20/9 | .17 |
| Missing data | 3 (1%) | 2 (1%) | 1 (1%) | |
| Karyotype | ||||
| Unfavorable t(9;22), 11q23, t(4;11), monosomy 7, 5q- | 48 (18%) | 31 (17%) | 19 (19%) | .93 |
| Intermediate (Others or normal) | 135 (52%) | 102 (57%) | 58 (59%) | |
| Favorable (hyperploidy + inv16 + t(8;21) + t(15,17) | 22 (8%) | 13 (7%) | 8 (8%) | |
| Missing data | 57 (22%) | 34 (19%) | 14 (14%) | |
| Time interval from diagnosis to transplantation, months | 20 (8-42) | 24 (8-41) | 15 (8-31) | .10 |
| Missing data | 0 | 0 | 0 | |
| Median days from last CR to transplantation (only for patients in CR at time of transplantation) | 113 (70-190) | 109 (76-156) | 84 (52-139) | .001 |
| (n = 210) | (n = 163) | (n = 81) | ||
| First relapse on therapy | 85 (45%) | 55 (42%) | 44 (58%) | .08 |
| First relapse off therapy | 105 (55%) | 75 (58%) | 32 (42%) | |
| Missing data | 1 (0.4%) | 2 (1%) | 0 | |
| Median days from first CR to first relapse | 650 (296-939) | 744 (388-1019) | 400 (184-814) | .001 |
| (n = 187) | (n = 131) | (n = 76) | ||
| Status at time of transplantation | ||||
| First CR | 59 (23%) | 43 (24%) | 18 (18%) | .04 |
| Second CR | 102 (39%) | 90 (50%) | 49 (49%) | |
| ≥ Third CR | 49 (19%) | 30 (17%) | 14 (14%) | |
| Advanced (refractory/partial response/relapse/first acute phase) | 52 (20%) | 17 (9%) | 18 (18%) | |
| Poor Risk (≥ 3CR + advanced) | 101 (39%) | 47 (26%) | 32 (32%) | .02 |
| Good risk (1CR + 2CR) | 161 (61%) | 133 (74%) | 67 (68%) |
P value: Fisher test for categoric variables and Kruskal-Wallis for continuous variables. For continuous variables, median (25th-75th percentiles) are given; for qualitative variables, sample size (percentages) are given within each strata.
Autologous or allogeneic transplant for relapse.
ALL indicates acute lymphoblastic leukemia; AML, acute myeloblastic leukemia; FAB, French-American-British classification; and CR, complete remission.
Model selection used the following steps for each endpoint. The first step was to fit models that contained each of the variables one at a time (univariable models). Continuous variables were dichotomized according to median values. For categoric variables, dummy variables for all but one category were created, taking on the value 1 for patients in that category and 0 otherwise. Hypotheses of proportional hazards were checked using time-varying coefficients. Variables considered were: recipient age (< or ≥ 6 years), weight (continuous), recipient and donor cytomegalovirus status, donor-recipient gender, ABO-match and HLA-match, leukemia type (acute lymphoblastic or acute myeloblastic leukemia), leukemia status at transplantation (good risk [first and second complete remission] versus poor risk [≥ third complete remission, relapse and refractory]), white blood cells at diagnosis (≥50.000 × 109/L), and nucleated cell dose (≥ or < 0.37 × 108/kg for cord blood and ≥ or < 3.7 × 108/kg for bone marrow transplants). However, variables that are not important on their own may become important in the presence of others. Thus, all variables were combined and those variables with a P value above .10 by the likelihood ratio test were omitted from the set. Once a variable was dropped, the effect of omitting each of the remaining variables in turn was examined, and those previously omitted were reconsidered. A final check was made to ensure that no term could have been omitted without significantly increasing the value of the likelihood, and no term included without significantly reducing this value. Hazard ratios (HR) were estimated with 95% confidence intervals (95CI). Comparisons of outcomes between transplant groups were then adjusted for variables of these final models. Finally, to incorporate potential differences in baseline hazards between EBMT centers or large centers (ie, having reported at least 15 transplants), we reran final models stratifying on these variables.
Statistical analysis used the SAS (Sas, Cary, NC) and S-Plus Software (MathSoft, Seattle, WA).
Results
Patient, donor, and disease characteristics
A total of 262 children with AL received UBMTs, 180 received T-UBMTs, and 99 received UCBTs. Table 1 and Table2 show the main characteristics of the 541 enrolled children. Compared with UBMT or T-UBMT recipients, recipients of UCBTs were younger (P = .004), were more likely to have acute myeloblastic leukemia (AML) (P = .014), were previously treated for relapses of leukemia with autologous (n = 12) or allogeneic stem cell transplants (n = 2) (P = .0001), and tended to have early relapses on therapy before transplant (P = .08). Eighteen children (18%) receiving a UCBT and 52 (20%) receiving a UBMT were transplanted in advanced stages of leukemia (refractory, relapse, or partial response), whereas in the T-UBMT group, only 17 (9%) patients were in an advanced stage of the disease (P = .04). One hundred and twenty-five patients with acute lymphoblastic leukemia (ALL) received transplants in first and second complete remission (CR1 and CR2) using a UBMT, 107 using a T-UBMT, and 45 using a UCBT. Thirty-six patients with AML in CR1 and CR2 received a transplant using a UBMT, 26 using a T-UBMT, and 22 using a UCBT. Median time from diagnosis to transplantation was 15 months in the UCBT group compared with 22 months in the UBMT group (P = .03). Results of HLA typing are shown in Table 3. Mismatches were mostly observed for class I in UBMT and T-UBMT and for any of class I and class II HLA antigens in the UCBT group.
Donor- and transplant-related characteristics of nonmanipulated unrelated bone marrow (UBMT), T-cell–depleted unrelated bone marrow transplants (T-UBMT), and umbilical unrelated cord blood recipients (UCBT)
| Characteristics . | UBMT (n = 262) . | T-UBMT (n = 180) . | UCBT (n = 99) . | P value3-150 . |
|---|---|---|---|---|
| Donor-related | ||||
| Gender match | 125 (48%) | 96 (53%) | 49 (49%) | .51 |
| Male donor to female recipient | 56 (21%) | 38 (21%) | 19 (19%) | .25 |
| Male donor to male recipient | 78 (30%) | 72 (40%) | 28 (28,5%) | |
| Female donor to male recipient | 80 (31%) | 46 (26%) | 28 (28,5%) | |
| Female donor to female recipient | 47 (18%) | 24 (13%) | 21 (21%) | |
| Missing data | 1 (0.4%) | 0 | 3 (4%) | |
| ABO compatible | 110 (42%) | 81 (45%) | 41 (41%) | .78 |
| Missing data | 1 (0.4%) | 3 (1.6%) | 0 | |
| ABO major incompatible | 89 (34%) | 53 (29%) | 36 (36%) | .44 |
| HLA disparities3-151 | ||||
| 0 | 211 (80.5%) | 97 (54%) | 8 (8%) | .0001 |
| 1 | 46 (17.6%) | 61 (34%) | 43 (43%) | |
| 2 | 1 (0.4%) | 10 (5.5%) | 40 (41%) | |
| 3 | — | 2 (1%) | 6 (6%) | |
| 4 | — | 1 (1%) | ||
| Missing data | 4 (1.5%) | 10 (5.5%) | 1 (1%) | |
| Donor positive CMV serology | 112 (43%) | 73 (41%) | 0 | .0001 |
| Donor's age | 36 (28-42) | 37 (31-43) | — | .103-152 |
| Missing data | 26 (10%) | 5 (3%) | ||
| Transplant-related | ||||
| Graft before January 1, 1996 | 107 (41%) | 85 (47%) | 10 (10%) | .0001 |
| Conditioning regimen | ||||
| BUCY | 12 (5%) | 6 (3%) | 13 (13%) | .002 |
| TBI + CY | 103 (39%) | 122 (68%) | 17 (17%) | .0001 |
| TBI + 2 or more drugs | 109 (42%) | 36 (20%) | 35 (35%) | .0001 |
| Anti-T cell antibodies | ||||
| No | 97 (37%) | 14 (8%) | 12 (12%) | .0001 |
| ALG/ATG | 130 (50%) | 44 (24%) | 82 (84%) | |
| Monoclonal antibody | 34 (13%) | 122 (68%) | 4 (4%) | |
| GVHD prophylaxis | ||||
| No | 0 | 2 | 0 | |
| CsA alone | 8 (3%) | 94 (52%) | 7 (7%) | |
| CsA + pred | 2 (0,8%) | 9 (5%) | 62 (63%) | .0001 |
| CsA + MTX | 180 (69%) | 53 (30%) | 9 (9%) | |
| CsA + MTX + pred ± ATG/ALG | 36 (14%) | 8 (4%) | 9 (9%) | |
| CsA + ATG/ALG ± pred | 4 (2%) | 9 (5%) | 6 (6%) | |
| Others | 32 (12%) | 5 (3%) | 6 (6%) | |
| T depletion (methods) | — | 180 | — | — |
| Campath | 132 (73%) | |||
| Elutriation | 12 (7%) | |||
| E-rosetting | 11 (6%) | |||
| CD34 positive selection | 16 (9%) | |||
| Others | 9 (5%) | |||
| Early growth factors (< day 8) | 96 (37%) | 42 (23%) | 54 (55%) | .0001 |
| Nucleated cells infused/kg (108) | 4.2 (3.0-6.0) | 3.8 (1.4-5.6) | 0.38 (0.24-3.6) | .0001 |
| Missing data | 8 (3%) | 11 (6%) | 6 (6%) | |
| Median follow up time, months | 30 (17-43) | 33 (18-47) | 19 (13-29) | .0001 |
| Lost to follow-up at 01/01/99 | 0 | 9 (5%) | 0 | .0001 |
| Characteristics . | UBMT (n = 262) . | T-UBMT (n = 180) . | UCBT (n = 99) . | P value3-150 . |
|---|---|---|---|---|
| Donor-related | ||||
| Gender match | 125 (48%) | 96 (53%) | 49 (49%) | .51 |
| Male donor to female recipient | 56 (21%) | 38 (21%) | 19 (19%) | .25 |
| Male donor to male recipient | 78 (30%) | 72 (40%) | 28 (28,5%) | |
| Female donor to male recipient | 80 (31%) | 46 (26%) | 28 (28,5%) | |
| Female donor to female recipient | 47 (18%) | 24 (13%) | 21 (21%) | |
| Missing data | 1 (0.4%) | 0 | 3 (4%) | |
| ABO compatible | 110 (42%) | 81 (45%) | 41 (41%) | .78 |
| Missing data | 1 (0.4%) | 3 (1.6%) | 0 | |
| ABO major incompatible | 89 (34%) | 53 (29%) | 36 (36%) | .44 |
| HLA disparities3-151 | ||||
| 0 | 211 (80.5%) | 97 (54%) | 8 (8%) | .0001 |
| 1 | 46 (17.6%) | 61 (34%) | 43 (43%) | |
| 2 | 1 (0.4%) | 10 (5.5%) | 40 (41%) | |
| 3 | — | 2 (1%) | 6 (6%) | |
| 4 | — | 1 (1%) | ||
| Missing data | 4 (1.5%) | 10 (5.5%) | 1 (1%) | |
| Donor positive CMV serology | 112 (43%) | 73 (41%) | 0 | .0001 |
| Donor's age | 36 (28-42) | 37 (31-43) | — | .103-152 |
| Missing data | 26 (10%) | 5 (3%) | ||
| Transplant-related | ||||
| Graft before January 1, 1996 | 107 (41%) | 85 (47%) | 10 (10%) | .0001 |
| Conditioning regimen | ||||
| BUCY | 12 (5%) | 6 (3%) | 13 (13%) | .002 |
| TBI + CY | 103 (39%) | 122 (68%) | 17 (17%) | .0001 |
| TBI + 2 or more drugs | 109 (42%) | 36 (20%) | 35 (35%) | .0001 |
| Anti-T cell antibodies | ||||
| No | 97 (37%) | 14 (8%) | 12 (12%) | .0001 |
| ALG/ATG | 130 (50%) | 44 (24%) | 82 (84%) | |
| Monoclonal antibody | 34 (13%) | 122 (68%) | 4 (4%) | |
| GVHD prophylaxis | ||||
| No | 0 | 2 | 0 | |
| CsA alone | 8 (3%) | 94 (52%) | 7 (7%) | |
| CsA + pred | 2 (0,8%) | 9 (5%) | 62 (63%) | .0001 |
| CsA + MTX | 180 (69%) | 53 (30%) | 9 (9%) | |
| CsA + MTX + pred ± ATG/ALG | 36 (14%) | 8 (4%) | 9 (9%) | |
| CsA + ATG/ALG ± pred | 4 (2%) | 9 (5%) | 6 (6%) | |
| Others | 32 (12%) | 5 (3%) | 6 (6%) | |
| T depletion (methods) | — | 180 | — | — |
| Campath | 132 (73%) | |||
| Elutriation | 12 (7%) | |||
| E-rosetting | 11 (6%) | |||
| CD34 positive selection | 16 (9%) | |||
| Others | 9 (5%) | |||
| Early growth factors (< day 8) | 96 (37%) | 42 (23%) | 54 (55%) | .0001 |
| Nucleated cells infused/kg (108) | 4.2 (3.0-6.0) | 3.8 (1.4-5.6) | 0.38 (0.24-3.6) | .0001 |
| Missing data | 8 (3%) | 11 (6%) | 6 (6%) | |
| Median follow up time, months | 30 (17-43) | 33 (18-47) | 19 (13-29) | .0001 |
| Lost to follow-up at 01/01/99 | 0 | 9 (5%) | 0 | .0001 |
P value: Fisher test for categoric variables and Kruskal-Wallis for continuous variables. For continuous variables, medians (25th-75th percentiles) are given; for qualitative variables, sample sizes (percentages) are given within each strata.
A and B by serology and allelic typing for DRB1.
P value corresponds to the comparison between UBMT and T-UBMT since the age of UCBT is nonsensical.
CMV indicates human cytomegalovirus; BU, busulfan; CY, cyclophosphamide; TBI, total body irradiation; ALG, antilymphocyte globulin; ATG, antihymocyte globulin; CsA, ciclosporine A; MTX, methotrexate; Pred, prednisone.
Donor recipient HLA compatibility and disparities among the 3 types of transplant; nonmanipulated unrelated bone marrow (UBMT), T-cell–depleted unrelated bone marrow donor (T-UBMT), and umbilical unrelated cord blood recipients (UCBT)
| HLA typing . | UBMT (n = 262) . | T-UBMT (n = 180) . | UCBT (n = 99) . | P value4-150 . |
|---|---|---|---|---|
| A (serology) | ||||
| Matched | 252 (96%) | 146 (81%) | 64 (65%) | .0001 |
| 1 difference | 10 (4%) | 34 (19%) | 35 (35%) | |
| B (serology) | ||||
| Matched | 254 (97%) | 144 (80%) | 50 (51%) | |
| 1 difference | 8 (3%) | 34 (19%) | 44 (44%) | .0001 |
| 2 differences | 0 | 2 (1%) | 5 (5%) | |
| DRB1 (allelic typing) | ||||
| Matched | 229 (89%) | 149 (88%) | 50 (51%) | |
| 1 difference | 28 (11%) | 21 (12%) | 40 (41%) | |
| 2 differences | 1 (0.4%) | 0 | 8 (8%) | .0001 |
| missing | 4 | 10 | 1 |
| HLA typing . | UBMT (n = 262) . | T-UBMT (n = 180) . | UCBT (n = 99) . | P value4-150 . |
|---|---|---|---|---|
| A (serology) | ||||
| Matched | 252 (96%) | 146 (81%) | 64 (65%) | .0001 |
| 1 difference | 10 (4%) | 34 (19%) | 35 (35%) | |
| B (serology) | ||||
| Matched | 254 (97%) | 144 (80%) | 50 (51%) | |
| 1 difference | 8 (3%) | 34 (19%) | 44 (44%) | .0001 |
| 2 differences | 0 | 2 (1%) | 5 (5%) | |
| DRB1 (allelic typing) | ||||
| Matched | 229 (89%) | 149 (88%) | 50 (51%) | |
| 1 difference | 28 (11%) | 21 (12%) | 40 (41%) | |
| 2 differences | 1 (0.4%) | 0 | 8 (8%) | .0001 |
| missing | 4 | 10 | 1 |
Fisher test.
Transplant characteristics: preparative regimens, GVHD prophylaxis, supportive treatment, and graft composition
Preparative regimens varied according to patient's age, disease status, and transplant center protocols (Table 2). Addition of an anti–T-cell antibody before transplantation was commonly given to patients receiving T-UBMTs or UCBTs (P = .0001). GVHD prophylaxis differed: most of the UBMT recipients (69%) received the combination of cyclosporine A (CsA) and methotrexate (MTX), 53% of the T-UBMT recipients received CsA alone, and 63% of UCBT recipients received CsA and corticosteroids. In the T-UBMT group, CAMPATH-1M22 was used for ex-vivo T-cell–depletion in 132 cases (73%). Supportive therapy, as well as prophylaxis and treatment of infections, varied among centers. Recombinant human granulocyte colony-stimulating factor (rHuG-CSF) or recombinant human granulocyte-macrophage colony-stimulating factor (rHuGM-CSF) were more frequently used early after UCBTs (P < .001). Finally and importantly, umbilical cord blood grafts contained one log fewer nucleated cells than bone marrow grafts (P < .001).
Outcomes: univariate analysis (nonadjusted for patient, disease, and transplant differences)
On January 1, 1999, the median follow-up was 29 months (range: 7-60 months); it was significantly shorter in the UCBT group (P < .001) since most of UCBTs (90%) were performed after January 1996.
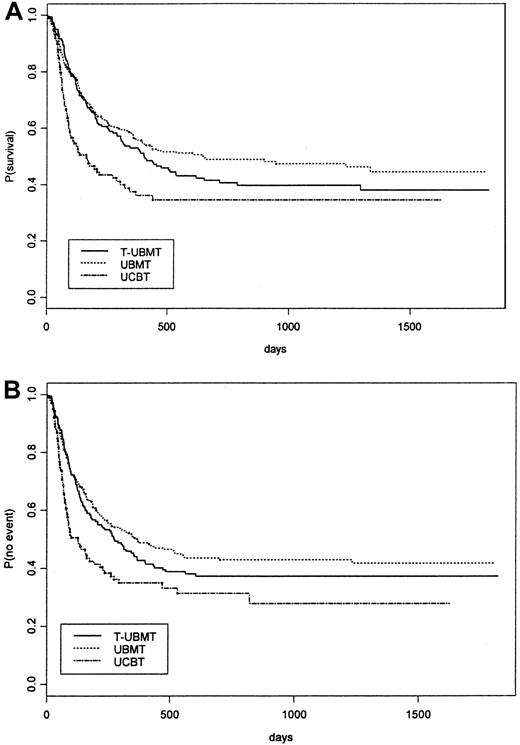
Table 4 lists probabilities of neutrophil and platelets recovery, acute and chronic GVHD, early transplant related mortality, relapse, and overall survival by transplant type not adjusted for differences in factors that influence transplant outcome. It showed a significant delay of neutrophil and platelet recovery in the UCBT group compared with the UBMT and the T-UBMT groups (P < .001). The incidence and severity of acute and chronic GVHD are shown in Table 4. Table 4 shows a significant reduction of aGVHD ≥ II and of cGVHD in T-UBMTs and UCBTs compared with UBMTs (P < .001). Early TRM was higher in the UCBT group compared with the other groups (P < .01). Nonadjusted estimates of 2-year survival and event-free survival in the 3 groups are listed in Table 4 and shown in Figure 1A,B. Causes of death before and after day 100 in the 3 transplant groups are listed in Table5.
Univariate analysis of outcomes (cumulative incidences—95% confidence interval) after unrelated bone marrow (UBMT), T-cell–depleted unrelated bone marrow (T-UBMT), and unrelated cord blood transplants (UCBT) nonadjusted for differences in prognostic factors
| Outcomes . | UBMT (n = 262) . | T-UBMT (n = 180) . | UCBT (n = 99) . |
|---|---|---|---|
| Neutrophil recovery at day 60 | 96% (95-97) | 90% (84-96) | 80% (70-90) |
| Median days (95Cl) | 18 (10-40) | 16 (9-40) | 32 (11-56) |
| Recoveries (n) | 243 | 151 | 70 |
| Platelet recovery at day 180 | 85% (79-91) | 85% (77-93) | 90% (80-100) |
| Median days (95Cl) | 29 (8-141) | 29 (8-165) | 81 (16-159) |
| Recoveries (n) | 201 | 129 | 49 |
| Early TRM at day 100 | 19% (14-24) | 14% (9-20) | 39% (29-48) |
| Nonleukemic deaths (n) | 49 | 25 | 38 |
| Acute GVHD (II-IV) at day 100 | 58% (51-63) | 20% (15-25) | 35% (24-45) |
| Grade 0 (n, %) | 51 (20%) | 97 (54%) | 43 (43%) |
| Grade I | 63 (24%) | 49 (27%) | 23 (23%) |
| Grade II | 71 (27%) | 20 (11%) | 12 (12%) |
| Grade III | 55 (21%) | 10 (6%) | 11 (11%) |
| Grade IV | 22 (8%) | 4 (2%) | 10 (10%) |
| Acute GVHD (II-IV) (n) | 148 | 34 | 33 |
| Acute GVHD (III-IV) | 30% (24-36) | 8% (0-16) | 22% (14-30) |
| Acute GVHD (n) | 77 | 14 | 21 |
| Chronic GVHD at 2 years | 46% (37-53) | 12% (6-17) | 25% (1-17) |
| Chronic GVHD (n/patients at risk5-150) | 86/201 (43%) | 14/124 (11%) | 5/43 (12%) |
| Relapse at 2 years | 39% (32-46) | 47% (39-55) | 38% (25-53) |
| relapses (n) | 75 | 66 | 23 |
| Survival at 2 years | 49% (43-55) | 41% (33-49) | 35% (25-45) |
| Deaths (n) | 133 | 104 | 63 |
| EFS at 2 years | 43% (37-49) | 37% (30-44) | 31% (21-41) |
| Deaths and or relapses (n) | 146 | 110 | 67 |
| Outcomes . | UBMT (n = 262) . | T-UBMT (n = 180) . | UCBT (n = 99) . |
|---|---|---|---|
| Neutrophil recovery at day 60 | 96% (95-97) | 90% (84-96) | 80% (70-90) |
| Median days (95Cl) | 18 (10-40) | 16 (9-40) | 32 (11-56) |
| Recoveries (n) | 243 | 151 | 70 |
| Platelet recovery at day 180 | 85% (79-91) | 85% (77-93) | 90% (80-100) |
| Median days (95Cl) | 29 (8-141) | 29 (8-165) | 81 (16-159) |
| Recoveries (n) | 201 | 129 | 49 |
| Early TRM at day 100 | 19% (14-24) | 14% (9-20) | 39% (29-48) |
| Nonleukemic deaths (n) | 49 | 25 | 38 |
| Acute GVHD (II-IV) at day 100 | 58% (51-63) | 20% (15-25) | 35% (24-45) |
| Grade 0 (n, %) | 51 (20%) | 97 (54%) | 43 (43%) |
| Grade I | 63 (24%) | 49 (27%) | 23 (23%) |
| Grade II | 71 (27%) | 20 (11%) | 12 (12%) |
| Grade III | 55 (21%) | 10 (6%) | 11 (11%) |
| Grade IV | 22 (8%) | 4 (2%) | 10 (10%) |
| Acute GVHD (II-IV) (n) | 148 | 34 | 33 |
| Acute GVHD (III-IV) | 30% (24-36) | 8% (0-16) | 22% (14-30) |
| Acute GVHD (n) | 77 | 14 | 21 |
| Chronic GVHD at 2 years | 46% (37-53) | 12% (6-17) | 25% (1-17) |
| Chronic GVHD (n/patients at risk5-150) | 86/201 (43%) | 14/124 (11%) | 5/43 (12%) |
| Relapse at 2 years | 39% (32-46) | 47% (39-55) | 38% (25-53) |
| relapses (n) | 75 | 66 | 23 |
| Survival at 2 years | 49% (43-55) | 41% (33-49) | 35% (25-45) |
| Deaths (n) | 133 | 104 | 63 |
| EFS at 2 years | 43% (37-49) | 37% (30-44) | 31% (21-41) |
| Deaths and or relapses (n) | 146 | 110 | 67 |
Patients at risk: survivors after day 100 with sustained engraftment.
GVHD indicates graft-versus-host disease; TRM, transplant-related mortality; and EFS, event-free survival.
Kaplan-Meier estimate of overall survival (A) and event-free survival (B) of all children with acute leukemia receiving unrelated stem cell transplants (UBMT, T-UBMT, and UCBT) nonadjusted for patient, disease, and transplant differences.
Kaplan-Meier estimate of overall survival (A) and event-free survival (B) of all children with acute leukemia receiving unrelated stem cell transplants (UBMT, T-UBMT, and UCBT) nonadjusted for patient, disease, and transplant differences.
Causes of death after unrelated bone marrow (UBMT), T-cell–depleted unrelated bone marrow (T-UBMT), and unrelated cord blood transplants (UCBT) before and after day 100 posttransplant
| Causes . | UBMT . | T-UBMT . | UCBT . | |||
|---|---|---|---|---|---|---|
| < 100 days N = 56 . | ≥ 100 days N = 77 . | < 100 days N = 37 . | ≥ 100 days N = 67 . | < 100 days N = 43 . | ≥ 100 days N = 20 . | |
| Relapse or progression | 7 (12.5%) | 55 (71.4% | 12 (32.5%) | 48 (71.5%) | 5 (11.6%) | 14 (70%) |
| Transplantation-related causes | 49 (87.5%) | 22 (28.6%) | 25 (67.5%) | 19 (28.5%) | 38 (88.4%) | 6 (30%) |
| GVHD | 18 (31.5%) | 5 (6.5%) | 3 (8%) | 6 (9%) | 6 (14%) | 0 |
| Toxicity6-150 | 12 (21%) | 5 (6.5%) | 7 (19%) | 0 | 9 (21%) | 3 (15%) |
| Hemorrhage | 0 | 1 (1.3%) | 1 (2.7%) | 0 | 0 | 0 |
| Rejection | 0 | 0 | 2 (5.4%) | 2 (3%) | 4 (9.3%) | 0 |
| Bacterial infection | 3 (5%) | 2 (2.6%) | 2 (5.4%) | 2 (3%) | 4 (9.3%) | 0 |
| Viral infection | 4 (7%) | 5 (6.5%) | 5 (13.5%) | 3 (4.5%) | 8 (18.6%) | 1 (5%) |
| EBV lymphoma | 2 (3.5%) | 0 | 2 (5.4%) | 1 (1.5%) | 0 | 1 (5%) |
| Fungal infection | 9 (16%) | 1 (1.3%) | 2 (5.4%) | 2 (3%) | 4 (9.3%) | 0 |
| Parasitic infection | 0 | 0 | 0 | 0 | 1 (2.3%) | 0 |
| Unknown | 2 (3.5%) | 3 (3.9%) | 1 (2.7%) | 3 (4.5%) | 2 (4.6%) | 1 (5%) |
| Causes . | UBMT . | T-UBMT . | UCBT . | |||
|---|---|---|---|---|---|---|
| < 100 days N = 56 . | ≥ 100 days N = 77 . | < 100 days N = 37 . | ≥ 100 days N = 67 . | < 100 days N = 43 . | ≥ 100 days N = 20 . | |
| Relapse or progression | 7 (12.5%) | 55 (71.4% | 12 (32.5%) | 48 (71.5%) | 5 (11.6%) | 14 (70%) |
| Transplantation-related causes | 49 (87.5%) | 22 (28.6%) | 25 (67.5%) | 19 (28.5%) | 38 (88.4%) | 6 (30%) |
| GVHD | 18 (31.5%) | 5 (6.5%) | 3 (8%) | 6 (9%) | 6 (14%) | 0 |
| Toxicity6-150 | 12 (21%) | 5 (6.5%) | 7 (19%) | 0 | 9 (21%) | 3 (15%) |
| Hemorrhage | 0 | 1 (1.3%) | 1 (2.7%) | 0 | 0 | 0 |
| Rejection | 0 | 0 | 2 (5.4%) | 2 (3%) | 4 (9.3%) | 0 |
| Bacterial infection | 3 (5%) | 2 (2.6%) | 2 (5.4%) | 2 (3%) | 4 (9.3%) | 0 |
| Viral infection | 4 (7%) | 5 (6.5%) | 5 (13.5%) | 3 (4.5%) | 8 (18.6%) | 1 (5%) |
| EBV lymphoma | 2 (3.5%) | 0 | 2 (5.4%) | 1 (1.5%) | 0 | 1 (5%) |
| Fungal infection | 9 (16%) | 1 (1.3%) | 2 (5.4%) | 2 (3%) | 4 (9.3%) | 0 |
| Parasitic infection | 0 | 0 | 0 | 0 | 1 (2.3%) | 0 |
| Unknown | 2 (3.5%) | 3 (3.9%) | 1 (2.7%) | 3 (4.5%) | 2 (4.6%) | 1 (5%) |
Including interstitial pneumonitis, veno-occlusive disease, cardiac toxicity, and Acute Respiratory Distress Syndrome.
We separately analyzed all the outcome variables on time scale, using day 100 posttransplantation as the cut-off, since the estimated relative effect of the UCBT group over the T-UBMT and UBMT groups was not proportional over time (P = .017) with decreased relative hazards after approximately day 100.
We thus distinguished 2 types of outcomes, namely early outcomes within the first 100 days posttransplantation (neutrophil and platelet recoveries, aGVHD, early relapse, TRM), and long-term outcomes in survivors at day 100 posttransplantation (cGVHD, late relapse, overall survival, and EFS).
Multivariable analysis
Prognostic factors.
We first attempted to select the variables that could be associated with each outcome separately in each transplant group. Table6 reports the prognostic value of the variables retained after a stepwise selection procedure, at the 10% level, when jointly introduced into Cox models.
Multivariable analysis for main outcomes measured in each transplant group: unrelated bone marrow (UBMT), T-cell–depleted unrelated bone marrow (T-UBMT), and unrelated cord blood transplants (UCBT) for early and long term outcomes
| . | UBMT (n = 262) . | T-UBMT (n = 180) . | UCBT (n = 99) . | |||
|---|---|---|---|---|---|---|
| Model . | HR (95% CI);P value . | Model . | HR (95% CI); Pvalue . | Model . | HR (95% CI); P value . | |
| Early outcomes7-150 | ||||||
| Neutrophils recovery | Age < 6 years | 1.50 (1.15-2.00); .003 | Cell dose ≥ 3.7 × 108/kg | 1.42 (1.015-2.00); .04 | Cell dose ≥ 0.37 × 108/kg | 1.65 (1.03-2.66), .04 |
| HLA compatibility | 1.39 (0.99-1.96); .06 | |||||
| Platelets recovery | Good risk | 1.61 (1.20-2.17); .002 | Cell dose ≥ 3.7 × | 2.03 (1.41-295); .001 | Cell dose ≥ 0.37 × | 2.29 (1.28-4.11); .006 |
| ↓ weight | 1.01 (1.01-1.02); .009 | 108/kg | 108/kg | |||
| Acute GVHD | — | — | Cell dose < 3.7 × 108/kg | 2.12 (1.03-4.34); .04 | — | — |
| Positive recipient CMV serology | 2.23 (1.102-4.525); .03 | |||||
| Relapse during the first 100 days | Positive recipient CMV serology | 2.88 (1.18-7.05); .02 | — | — | AML | 4.10 (1.1-15.87); .04 |
| Poor risk | 3.15 (0.95-10.43); .06 | |||||
| Poor risk | 2.66 (1.14-6.21); .02 | Age > 6 years | 7.75 (0.98-50); .05 | |||
| Gender (D/R) match | 2.87 (1.17-7.01); .02 | |||||
| TRM | ↑weight | 1.02 (1.00-1.03); .05 | HLA incompatibility | 2.86 (1.23-6.67); .01 | — | — |
| Positive recipient CMV serology | 1.74 (0.97-3.10); .06 | Gender (D/R) match | 4.34 (1.61-11.75); .004 | |||
| Long-term outcomes7-151 | ||||||
| Relapse after day 1007-152 | ABO incompatibility | 1.73 (1.11-2.94); .03 | WBC at diagnosis ≥ 50 G/L | 1.87 (1.08-3.23); .03 | ↓weight | 1.08 (1.04-1.14); .003 |
| Poor risk | 2.18 (1.37-3.45); .001 | Poor risk | 2.74 (1.52-4.93); .0007 | Poor risk | 2.98 (1.27-7); .012 | |
| Positive recipient CMV serology | 1.79 (1.13-2.83); .03 | HLA incompatibility | 1.60 (1.07-2.39); .02 | |||
| Death after day 100 | Poor risk | 1.93 (1.22-3.05); .005 | Poor risk | 2.28 (1.32-3.92); .003 | Poor risk | 3.23 (1.3-7.8); .009 |
| Positive recipient CMV serology | 1.72 (1.08-2.74); .02 | HLA incompatibility | 1.81 (1.21-2.69); .004 | |||
| ↑weight | 1.02 (1.01-1.03); .002 | |||||
| Chronic GVHD7-153 | ↑ weight | 1.02 (1.00-1.03); .006 | — | — | — | — |
| . | UBMT (n = 262) . | T-UBMT (n = 180) . | UCBT (n = 99) . | |||
|---|---|---|---|---|---|---|
| Model . | HR (95% CI);P value . | Model . | HR (95% CI); Pvalue . | Model . | HR (95% CI); P value . | |
| Early outcomes7-150 | ||||||
| Neutrophils recovery | Age < 6 years | 1.50 (1.15-2.00); .003 | Cell dose ≥ 3.7 × 108/kg | 1.42 (1.015-2.00); .04 | Cell dose ≥ 0.37 × 108/kg | 1.65 (1.03-2.66), .04 |
| HLA compatibility | 1.39 (0.99-1.96); .06 | |||||
| Platelets recovery | Good risk | 1.61 (1.20-2.17); .002 | Cell dose ≥ 3.7 × | 2.03 (1.41-295); .001 | Cell dose ≥ 0.37 × | 2.29 (1.28-4.11); .006 |
| ↓ weight | 1.01 (1.01-1.02); .009 | 108/kg | 108/kg | |||
| Acute GVHD | — | — | Cell dose < 3.7 × 108/kg | 2.12 (1.03-4.34); .04 | — | — |
| Positive recipient CMV serology | 2.23 (1.102-4.525); .03 | |||||
| Relapse during the first 100 days | Positive recipient CMV serology | 2.88 (1.18-7.05); .02 | — | — | AML | 4.10 (1.1-15.87); .04 |
| Poor risk | 3.15 (0.95-10.43); .06 | |||||
| Poor risk | 2.66 (1.14-6.21); .02 | Age > 6 years | 7.75 (0.98-50); .05 | |||
| Gender (D/R) match | 2.87 (1.17-7.01); .02 | |||||
| TRM | ↑weight | 1.02 (1.00-1.03); .05 | HLA incompatibility | 2.86 (1.23-6.67); .01 | — | — |
| Positive recipient CMV serology | 1.74 (0.97-3.10); .06 | Gender (D/R) match | 4.34 (1.61-11.75); .004 | |||
| Long-term outcomes7-151 | ||||||
| Relapse after day 1007-152 | ABO incompatibility | 1.73 (1.11-2.94); .03 | WBC at diagnosis ≥ 50 G/L | 1.87 (1.08-3.23); .03 | ↓weight | 1.08 (1.04-1.14); .003 |
| Poor risk | 2.18 (1.37-3.45); .001 | Poor risk | 2.74 (1.52-4.93); .0007 | Poor risk | 2.98 (1.27-7); .012 | |
| Positive recipient CMV serology | 1.79 (1.13-2.83); .03 | HLA incompatibility | 1.60 (1.07-2.39); .02 | |||
| Death after day 100 | Poor risk | 1.93 (1.22-3.05); .005 | Poor risk | 2.28 (1.32-3.92); .003 | Poor risk | 3.23 (1.3-7.8); .009 |
| Positive recipient CMV serology | 1.72 (1.08-2.74); .02 | HLA incompatibility | 1.81 (1.21-2.69); .004 | |||
| ↑weight | 1.02 (1.01-1.03); .002 | |||||
| Chronic GVHD7-153 | ↑ weight | 1.02 (1.00-1.03); .006 | — | — | — | — |
Early outcomes: events occurring during the first 100 days after transplantation.
Long-term outcomes in patients surviving at day 100 posttransplant.
Patients alive and free of relapse at day 100.
Patients alive with sustained engraftment.
Good risk means patients who received a transplant in first or second complete remission (CR); poor risk means patients who received a transplant in relapse or primary refractoriness to chemotherapy or after second CR. CMV indicates human cytomegalovirus; AML, acute myeloid leukemia; WBC, white blood cells; ↑, increasing weight (continuous variable); ↓, decreasing weight (continuous variable).
Early outcomes.
Briefly, neutrophil and platelet recoveries were associated with cell dose in T-UBMTs and UCBTs and not in UBMTs. Relapse during the first 100 days was associated with the recipient's positive cytomegalovirus (CMV) serology, advanced leukemia at transplantation, and gender match in the UBMT group, whereas it was associated with younger patients, AML, and advanced stage of the disease in the UCBT group. We did not find any prognosis factor for relapse in the T-UBMT group. In the T-UBMT group, increased TRM at 100 days was associated with HLA incompatibility and sex match. We could not identify prognosis factors for TRM in the UCBT group.
Long-term outcomes.
The risk of relapse and death increased in all the groups of patients transplanted for leukemia in advanced stage of the disease. The risk of death increased in the T-UBMT group receiving an HLA-mismatched transplant but not in the other groups.
Outcomes comparison (adjusted for prognostic factors)
After selection of predictors for each endpoint in the 3 transplant groups, we used these predictors to adjust transplant group comparisons on outcomes. UBMTs defined the reference group, that is, with a baseline hazard ratio of 1.0.
Early outcomes.
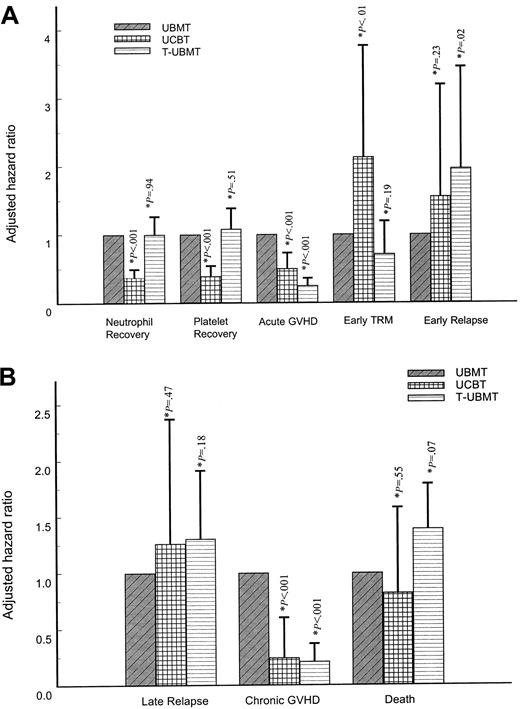
Although T-UBMT and UBMT groups did not differ in terms of time to hematopoietic recovery and treatment-related mortality, the main findings that emerged from these adjusted comparisons were the poor results in the UCBT group regarding these outcomes (Figure 2A). Indeed, hematopoietic recoveries were delayed and less frequent, either in terms of neutrophil or platelet recoveries (P = .00001, each), and an increased TRM was observed (P < .01). Conversely, the UCBT and T-UBMT groups less frequently experienced grade II-IV acute GVHD. Finally, whereas UBMT and UCBT groups experienced similar risks of early relapse, there was a higher risk of relapse in the T-UBMT group (P = .02) (Figure 2A).
Adjusted hazard ratios of each outcome for T-cell–depleted unrelated bone marrow transplant (T-UBMT) and unrelated cord blood transplant (UCBT) distinguishing early (A) and long-term outcomes (B), using the nonmanipulated unrelated bone marrow transplant (UBMT) as the reference group (hazard ratio of 1.0).
Error bars represent the 95% upper confidence limit of each hazard ratio. *P value refers to the likelihood ratio test of the transplant group (either T-UBMT or UCBT), when adjusting for confounders (see Table 6).
Adjusted hazard ratios of each outcome for T-cell–depleted unrelated bone marrow transplant (T-UBMT) and unrelated cord blood transplant (UCBT) distinguishing early (A) and long-term outcomes (B), using the nonmanipulated unrelated bone marrow transplant (UBMT) as the reference group (hazard ratio of 1.0).
Error bars represent the 95% upper confidence limit of each hazard ratio. *P value refers to the likelihood ratio test of the transplant group (either T-UBMT or UCBT), when adjusting for confounders (see Table 6).
Long-term outcomes.
The UBMT group was unfavorable in terms of risk of cGVHD compared with the T-UBMT group (P = .0001) and the UCBT group (P = .002) (Figure 2B). By contrast, whereas the outcome of the 3 groups was comparable in terms of long-term relapse, mortality after day 100 was increased in the T-UBMT group (P = .07) and comparable in the UBMT and UCBT groups (Figure 2B). Of note is that the poor outcome of the T-UBMT group was influenced by the past occurrence within the first 100 days posttransplantation of the lack of engraftment (P = .055), early relapse (P < .0001), and grade II-IV aGVHD (P = .0006) (data not shown).
These findings were slightly modified after stratifying on either EBMT centers or large centers, although the over-mortality in the T-depletion group after day 100 posttransplantation became statistically significant when stratifying on the EBMT centers (HR = 1.57, 95CI: 1.07-2.30; P = .02) (data not shown).
In summary, the main differences in adjusted outcomes between the 3 transplant groups appeared in the first 100 days after the transplant. Indeed, delayed and failure of engraftment, and increased treatment-related mortality after UCBT must be compared with the higher risk of aGVHD after UBMT and to the higher risk of relapse after T-UBMT. In contrast, after day 100, the 3 transplant groups achieved similar results in terms of relapse, but cGVHD occurred more frequently after UBMT and death after T-UBMT.
Discussion
This registry-based analysis included a large number of children receiving an allogeneic hematopoietic stem cell transplant for AL using an alternative donor. The objective of our study was to retrospectively compare the outcome of transplantations using unrelated bone marrow or cord blood as a source of hematopoietic stem cells in 541 children with AL. We compared outcomes after adjustment for patient-, disease-, and transplant-related factors based on separate multivariable prognostic analyses. We found several differences between unrelated BMT recipients and UCBT recipients. First, among the unrelated BMT recipients, we had to separately analyze nonmanipulated unrelated bone marrow transplants (UBMT) and T-cell–depleted UBMT. We thus compared 3 types of transplants. The first group of 262 patients received UBMTs; most of the donors were HLA matched for class I by serology and molecular typing for DRB1. As shown in the literature and in this study, this group experienced a high rate of acute and chronic GVHD and a low rate of relapse.23 The second group received T-UBMTs. Despite the fact that there were more class II mismatches, the patients in the second group experienced less acute and chronic GVHD and more rejections,21,22 more relapses,27 and delayed immune reconstitution.28,29 The group of UCBT patients had the highest number of HLA mismatches. These patients commonly experienced delayed hematologic reconstitution probably because they received one log less nucleated cells in the graft than the other groups. They also had less acute and chronic GVHD.18
Many pretransplant differences were observed among children receiving UBMTs, T-UBMTs, and UCBTs that probably influenced our ability to detect both advantages and disadvantages associated with each approach. The most important difference was related to HLA disparity since almost all UCBT patients had class I and class II HLA incompatibilities. However, the role of HLA mismatches was difficult to analyze because of the limitation of HLA typing methods which until recently did not take into consideration allelic variations and because most of the molecular HLA class I mismatches were not considered for the choice of donor recipient pairs. This has been changing recently as many centers are now using molecular techniques for both class I and class II typing.30-32
Since cord blood units were only recently available, UCBT patients had shorter follow-up than UBMT patients. Cord blood recipients were more likely to have adverse prognostic factors than the other transplant groups including early relapse before transplantation, shorter time interval from diagnosis to transplantation, and more patients receiving UCBT as a second transplant following relapse after a first autologous or allogeneic BMT. In order to take into account the potential measurable differences in patients according to center, we adjusted treatment comparison on baseline characteristics possibly related to the outcome and to the center. Although these differences were accounted for in the multivariable analyses, many other important baseline differences were observed among the 3 groups that were expected to modify the transplant outcome, including conditioning and GVHD prevention. Also, differences in supportive care and transplant center effect might have influenced our results. In a recent analysis of the EBMT group, center effect was an important factor influencing the outcome of HLA-identical bone marrow transplantation for AML in first complete remission.33 In addition, inequality in the type of patients contributed to the study by each center taken together with center-specific differences in coding GVHD may have contributed to some of the differences observed. Therefore, to incorporate potential difference in baseline hazard on either outcome between EBMT centers and others, as well as between large centers (that is, centers having reported at least 15 transplants, whatever the transplant group) and others, we finally stratified transplant group comparisons on these 2 variables, separately, without markedly modifying our results.
The principal difference in adjusted outcomes observed was more transplant-related deaths in the UCBT group in the first 100 days. After day 100, relapse rates were nearly identical but cGVHD occurred more frequently in UBMT patients and more deaths occurred after T-UBMT. Nevertheless, the low number of exposed patients in surviving patients and the shorter follow-up should be considered, and further studies based on larger samples size are required for definitive conclusions.
The major complication after UCBT was delayed neutrophil and platelet recovery. Others and we have shown that a cord blood nucleated cell dose above 0.37 × 108/kg was associated with increased probability of engraftment.7,16,17 In a previous report, patients with AL receiving a UBMT, a marrow cell dose above 3.65 × 108/kg had a better survival rate.34 In our study, patients who received more than 3.7 × 108 marrow nucleated cells infused per recipient's weight (one log higher than cord blood cells) engrafted more rapidly than patients receiving less. Our results confirm our previous recommendation that cord blood units should be selected on the basis of a number of nucleated cells > 0.37 × 108/kg recipient body weight after thawing.16 However, the minimum number of nucleated cells necessary for engraftment has not yet been established. The cause for delayed recovery after cord blood transplant might be due to the low number of cells infused or to other factors such as the immaturity of stem cells, which might need more cell divisions before differentiation to marrow progenitors, or to the lack of subpopulations facilitating engraftment.35 Whether current approaches being explored to speed hematopoietic recovery after cord blood transplantation, such as ex-vivo expansion, will result in decreased TRM is unknown.36
The incidence of grade II-IV aGVHD was lower after T-UBMT and intermediate with UCBT compared with UBMT. Since the majority of UCBT patients were mismatched, it was not possible to compare matched UBMT patients with matched UCBT patients; however, after adjustment for prognostic factors, aGVHD was reduced even in mismatched UCBT patients. The incidence of cGVHD was identical in both UCBT patients and T-UBMT patients, both being significantly lower than after UBMT. We showed also that the incidence of severe grade III-IV GVHD was reduced after UCBT and T-BMT. This confirms our previous observations that acute and chronic GVHD were significantly reduced when comparing HLA-identical sibling bone marrow and HLA-identical cord blood transplants.18 This study shows that the decreased incidence of acute and chronic GVHD after UCBT is still observed in the presence of major class I and class II HLA differences. This observation lends support to the hypothesis that umbilical cord blood differs from adult bone marrow in its alloreactive potential. The hypothesis that reduced GVHD results from fewer T cells infused is plausible since T-cell depletion of bone marrow transplants leads to a similarly lower GVHD risk. However, the number of T cells infused with umbilical cord blood transplants is on the order of 8 × 106/kg and it is known that GVHD can be induced by as few as 106 CD3 cells/kg and even fewer in HLA-mismatched situations.8 Since aGVHD results from activation, clonal expansion, and proliferation of donor-derived T lymphocytes that recognize alloantigens presented by either host or donor antigen-presenting cells, the lower GVHD risk after UCBT might be due to an impairment of these functions in umbilical cord blood cells. Therefore, identifying units with complete HLA identity does not seem to be an absolute prerequisite for a successful UCBT, as we did not find any correlation between the number of HLA mismatches and the outcome of UCBT. The number of HLA mismatches was an adverse prognostic factor for engraftment and survival after T-UBMT but not after UBMT.
Because the interaction between lower risk of GVHD and higher risk of leukemic relapse is known, we expected a higher risk in the UCBT and T-UBMT groups than in the UBMT group. In the present study, we did not find any difference between the adjusted risk of relapse in the UBMT group and the UCBT group. The probability of early relapse was higher in the T-UBMT group but more follow-up and more patients in defined risk groups are necessary for a better comparison.
In conclusion, we show that results were similar in the 3 groups of patients but the type of complications differed with more acute and chronic GVHD in the UBMT group, more relapses in the T-UBMT group, and more early deaths in the UCBT group. These findings show that both UBMT and UCBT represent alternatives for children with AL lacking a matched sibling donor. Developing the donor stem cell pool with bone marrow donors typed with high molecular resolution techniques to decrease the severity of GVHD1 2 and also increasing the number of cord blood units stored through international accredited cord blood banks should both result in an improved cure rate of children with AL given an unrelated hematopoietic stem cell transplant.
At this stage, we recommend simultaneously searching bone marrow donor registries and cord blood banks. The final choice of stem cell source must take into account the degree of HLA identity, the availability of the donor, the urgency of the transplant, and the cell dose in the cord blood unit.
We would like to thank the Netcord banks: G. Koegler at Dusseldorf CBB; P. Rebulla at Milano CBB; S. Querol and J. Garcia at Barcelona CBB; S. Armitage and M. Contreras at London CBB; and JP Marolleau and M. Benbunan at Paris CBB. We also thank all bone marrow donors registries, and the data managers from all centers for their input in collecting data and answering our queries.
Transplant centers reporting unrelated bone marrow transplants and/or umbilical cord blood transplants in children with acute leukemia are listed in the following table.
Participating centers and number of transplants reported in children with AL (from 01/94 to 05/98)
| Centers . | UBMT . | T-UBMT . | UCBT . | Total . |
|---|---|---|---|---|
| Centers reporting 3 types of transplants | ||||
| University of Lowain, Dr B. Brichard/Dr C. Vermylen, Belgique | 2 | 1 | 1 | 4 |
| Children's Hospital Medical Center, Dr A. Filipovich, USA* | 8 | 16 | 8 | 32 |
| MD Anderson Cancer Center, Dr. K.-W. Chan, USA* | 5 | 5 | 3 | 13 |
| Hôpital Pédiatrique La Timone, Pr G. Michel, France | 4 | 4 | 9 | 17 |
| Hôpital Saint Louis, Pr E. Gluckman, France | 6 | 6 | 2 | 14 |
| Hadassah University Hospital, Dr A. Nagler, Israel | 4 | 2 | 1 | 7 |
| Hospital Infantil Vall D'Hebron, Dr J. Ortega, Spain | 5 | 3 | 3 | 11 |
| Centers reporting only cord blood transplants | ||||
| Children's Associated Medical Group, Dr W. Spruce/J. Allen, USA* | 0 | 0 | 1 | 1 |
| Roswell Park Cancer Institute, Dr B. Bambach, USA* | 0 | 0 | 1 | 1 |
| Hôpital Saint Jacques, Dr E. Plouvier, France | 0 | 0 | 1 | 1 |
| Hôpital Saint Antoine, Dr J.P. Laporte, France | 0 | 0 | 2 | 2 |
| Hospital Santa Creu i San Pau, Dr I. Badell-Serra, Spain | 0 | 0 | 2 | 2 |
| Hôpital La Miletrie, Dr A. Sadoun, France | 0 | 0 | 1 | 1 |
| University Hospital Uppsala, Dr M. Bengtsson, Sweden | 0 | 0 | 1 | 1 |
| University Hospital Lund, Dr A. Bekassy, Sweden | 0 | 0 | 1 | 1 |
| Clinica Oncoematologia Pediatrica, Dr Zanesco/Dr C. Messina, Italy | 0 | 0 | 3 | 3 |
| Inst Portugues Oncologia, Dr M. Abecassis/A. Machado, Portugal | 0 | 0 | 3 | 3 |
| Heinrich-Heine-Universitat, Dr W. Numberger, Germany | 0 | 0 | 3 | 3 |
| Ospedale di Careggi, Dr R. Saccardi/Dr A. Bosi, Italy | 0 | 0 | 1 | 1 |
| Hospital Israelita A. Einstein, Dr E. Ferreira, Brazil*,† | 0 | 0 | 2 | 2 |
| Clinica Puerta de Hierro, Dr M.N. Fernandez, Spain | 0 | 0 | 1 | 1 |
| Hospital Nino Jesus of Madrid, Dr L.M. Madero, Spain | 0 | 0 | 4 | 4 |
| Hospital Infantil La Paz, Dr A.M. Martinez-Rubio, Spain | 0 | 0 | 2 | 2 |
| BMT Unit Schneider Children's, Dr I. Yaniv/Dr J. Stein, Israel | 0 | 0 | 1 | 1 |
| University of Bologna, Dr A. Pession, Italy | 0 | 0 | 2 | 2 |
| Centers reporting only UBMT | ||||
| Hospital de Clinicas, Dr R. Pasquini/Dr M. Bittencourt, Brazil* | 8 | 0 | 0 | 8 |
| Keio University School of Medicine, Dr A. Kinsohita, Japan* | 3 | 0 | 0 | 3 |
| St Sophia Children's Hospital, Dr S. Grafakos/Dr J. Peristeri, Greece | 2 | 0 | 0 | 2 |
| Hôpital Robert Debrè, Dr E. Vilmer, France | 9 | 0 | 0 | 9 |
| University Hospital Eppendorf, Dr A. Zander/P. Mundhenk, Germany | 11 | 0 | 0 | 11 |
| Hôpital Debrouosse, Dr G. Souillet, France | 10 | 0 | 0 | 10 |
| Centers reporting T-UBMT and/or UBMT | ||||
| Hôpital Civil, Dr P. Lutz, France | 0 | 4 | 0 | 4 |
| Royal Hospital for Sick Children, Dr B. Gibson, UK | 4 | 3 | 0 | 7 |
| Sheffield Children's Hospital, Dr A. Vora, UK | 3 | 4 | 0 | 7 |
| Tokai University, Dr S. Kato, Japan* | 9 | 2 | 0 | 11 |
| Huddinge University Hospital, Olle Ringden/Dr M. Remberger, Sweden‡ | 18 | 4 | 0 | 22 |
| Bristol Hospital for Sick Children, Dr J. Cornish/Dr A. Oakill, UK | 5 | 117 | 0 | 122 |
| St. Anna Kinderspital, Dr C. Peters, Austria | 21 | 2 | 0 | 23 |
| University Hospital Motol, Dr J. Stary, Czech Republic | 4 | 2 | 0 | 6 |
| Centers reporting T-UBMT or UBMT and UCBT | ||||
| FHCRC Seattle, Dr E. Sievers/A. Mellon, USA | 48 | 0 | 2 | 50 |
| Royal Children's Hospital, Dr K. Tiedemann, Australia* | 14 | 0 | 2 | 16 |
| Sydney Children's Hospital, Pr M. Vowels/C. Oswald, Australia* | 0 | 5 | 6 | 11 |
| E Ematologia, Univ. La Sapienza, Dr W. Arcese, Italy | 3 | 0 | 13 | 16 |
| Hôp/Cantonal Universitaire, Dr B. Chapuis, Switzerland | 1 | 0 | 1 | 2 |
| Institute G. Gaslini, Dr D. Giorgio/Dr S. Dallorso, Italy | 15 | 0 | 1 | 16 |
| Hôpital Claude Huriez, Dr J.P. Jouet, France | 3 | 0 | 2 | 5 |
| Ospedale Regine Margherita, Dr A. Busca/Dr R. Miniero, Italy | 12 | 0 | 3 | 15 |
| University of Pavia, Pediatric, Dr F. Locatelli/Dr G. Giorgani, Italy | 17 | 0 | 5 | 22 |
| Hospital Infantil La Fe, Dr A. Verdeguer/Dr V. Castel, Spain | 2 | 0 | 1 | 3 |
| The New Children's Hospital, Dr P. Shaw, Australia | 5 | 0 | 2 | 7 |
| University of Pisa, Dr C. Favre, Italy | 1 | 0 | 2 | 3 |
| Total | 262 | 180 | 99 | 541 |
| Centers . | UBMT . | T-UBMT . | UCBT . | Total . |
|---|---|---|---|---|
| Centers reporting 3 types of transplants | ||||
| University of Lowain, Dr B. Brichard/Dr C. Vermylen, Belgique | 2 | 1 | 1 | 4 |
| Children's Hospital Medical Center, Dr A. Filipovich, USA* | 8 | 16 | 8 | 32 |
| MD Anderson Cancer Center, Dr. K.-W. Chan, USA* | 5 | 5 | 3 | 13 |
| Hôpital Pédiatrique La Timone, Pr G. Michel, France | 4 | 4 | 9 | 17 |
| Hôpital Saint Louis, Pr E. Gluckman, France | 6 | 6 | 2 | 14 |
| Hadassah University Hospital, Dr A. Nagler, Israel | 4 | 2 | 1 | 7 |
| Hospital Infantil Vall D'Hebron, Dr J. Ortega, Spain | 5 | 3 | 3 | 11 |
| Centers reporting only cord blood transplants | ||||
| Children's Associated Medical Group, Dr W. Spruce/J. Allen, USA* | 0 | 0 | 1 | 1 |
| Roswell Park Cancer Institute, Dr B. Bambach, USA* | 0 | 0 | 1 | 1 |
| Hôpital Saint Jacques, Dr E. Plouvier, France | 0 | 0 | 1 | 1 |
| Hôpital Saint Antoine, Dr J.P. Laporte, France | 0 | 0 | 2 | 2 |
| Hospital Santa Creu i San Pau, Dr I. Badell-Serra, Spain | 0 | 0 | 2 | 2 |
| Hôpital La Miletrie, Dr A. Sadoun, France | 0 | 0 | 1 | 1 |
| University Hospital Uppsala, Dr M. Bengtsson, Sweden | 0 | 0 | 1 | 1 |
| University Hospital Lund, Dr A. Bekassy, Sweden | 0 | 0 | 1 | 1 |
| Clinica Oncoematologia Pediatrica, Dr Zanesco/Dr C. Messina, Italy | 0 | 0 | 3 | 3 |
| Inst Portugues Oncologia, Dr M. Abecassis/A. Machado, Portugal | 0 | 0 | 3 | 3 |
| Heinrich-Heine-Universitat, Dr W. Numberger, Germany | 0 | 0 | 3 | 3 |
| Ospedale di Careggi, Dr R. Saccardi/Dr A. Bosi, Italy | 0 | 0 | 1 | 1 |
| Hospital Israelita A. Einstein, Dr E. Ferreira, Brazil*,† | 0 | 0 | 2 | 2 |
| Clinica Puerta de Hierro, Dr M.N. Fernandez, Spain | 0 | 0 | 1 | 1 |
| Hospital Nino Jesus of Madrid, Dr L.M. Madero, Spain | 0 | 0 | 4 | 4 |
| Hospital Infantil La Paz, Dr A.M. Martinez-Rubio, Spain | 0 | 0 | 2 | 2 |
| BMT Unit Schneider Children's, Dr I. Yaniv/Dr J. Stein, Israel | 0 | 0 | 1 | 1 |
| University of Bologna, Dr A. Pession, Italy | 0 | 0 | 2 | 2 |
| Centers reporting only UBMT | ||||
| Hospital de Clinicas, Dr R. Pasquini/Dr M. Bittencourt, Brazil* | 8 | 0 | 0 | 8 |
| Keio University School of Medicine, Dr A. Kinsohita, Japan* | 3 | 0 | 0 | 3 |
| St Sophia Children's Hospital, Dr S. Grafakos/Dr J. Peristeri, Greece | 2 | 0 | 0 | 2 |
| Hôpital Robert Debrè, Dr E. Vilmer, France | 9 | 0 | 0 | 9 |
| University Hospital Eppendorf, Dr A. Zander/P. Mundhenk, Germany | 11 | 0 | 0 | 11 |
| Hôpital Debrouosse, Dr G. Souillet, France | 10 | 0 | 0 | 10 |
| Centers reporting T-UBMT and/or UBMT | ||||
| Hôpital Civil, Dr P. Lutz, France | 0 | 4 | 0 | 4 |
| Royal Hospital for Sick Children, Dr B. Gibson, UK | 4 | 3 | 0 | 7 |
| Sheffield Children's Hospital, Dr A. Vora, UK | 3 | 4 | 0 | 7 |
| Tokai University, Dr S. Kato, Japan* | 9 | 2 | 0 | 11 |
| Huddinge University Hospital, Olle Ringden/Dr M. Remberger, Sweden‡ | 18 | 4 | 0 | 22 |
| Bristol Hospital for Sick Children, Dr J. Cornish/Dr A. Oakill, UK | 5 | 117 | 0 | 122 |
| St. Anna Kinderspital, Dr C. Peters, Austria | 21 | 2 | 0 | 23 |
| University Hospital Motol, Dr J. Stary, Czech Republic | 4 | 2 | 0 | 6 |
| Centers reporting T-UBMT or UBMT and UCBT | ||||
| FHCRC Seattle, Dr E. Sievers/A. Mellon, USA | 48 | 0 | 2 | 50 |
| Royal Children's Hospital, Dr K. Tiedemann, Australia* | 14 | 0 | 2 | 16 |
| Sydney Children's Hospital, Pr M. Vowels/C. Oswald, Australia* | 0 | 5 | 6 | 11 |
| E Ematologia, Univ. La Sapienza, Dr W. Arcese, Italy | 3 | 0 | 13 | 16 |
| Hôp/Cantonal Universitaire, Dr B. Chapuis, Switzerland | 1 | 0 | 1 | 2 |
| Institute G. Gaslini, Dr D. Giorgio/Dr S. Dallorso, Italy | 15 | 0 | 1 | 16 |
| Hôpital Claude Huriez, Dr J.P. Jouet, France | 3 | 0 | 2 | 5 |
| Ospedale Regine Margherita, Dr A. Busca/Dr R. Miniero, Italy | 12 | 0 | 3 | 15 |
| University of Pavia, Pediatric, Dr F. Locatelli/Dr G. Giorgani, Italy | 17 | 0 | 5 | 22 |
| Hospital Infantil La Fe, Dr A. Verdeguer/Dr V. Castel, Spain | 2 | 0 | 1 | 3 |
| The New Children's Hospital, Dr P. Shaw, Australia | 5 | 0 | 2 | 7 |
| University of Pisa, Dr C. Favre, Italy | 1 | 0 | 2 | 3 |
| Total | 262 | 180 | 99 | 541 |
Non-EBMT centers; however, Eurocord centers.
This center has never performed an unrelated bone marrow transplant.
Only this center has never reported a cord blood transplant in the Eurocord registry. All the other centers have reported their transplants for other patients not included in the present study.
Supported by an EEC grant for Eurocord BIOMED II QLRT-1999-00380, by Etablissement Français des Greffes and PHRC 96, Ministry of Health, Association pour la Recherche contre le Cancer (ARC9085).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Eliane Gluckman, Eurocord Registry—Hospital Saint Louis AP/HP, Department of Hematology Bone Marrow Transplantation 1, Ave Claude Vellefaux, 75010 Paris, France; e-mail:eliane.gluckman@sls.ap-hop-paris.fr.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal